REVIEW: Novel Mitochondria-Targeted Compounds Composed of Natural Constituents: Conjugates of Plant Alkaloids Berberine and Palmatine with Plastoquinone
B. V. Chernyak1*, Y. N. Antonenko1, E. R. Galimov1, L. V. Domnina1, V. B. Dugina1, R. A. Zvyagilskaya2, O. Yu. Ivanova1, D. S. Izyumov1, K. G. Lyamzaev1, A. V. Pustovidko1, T. I. Rokitskaya1, A. G. Rogov2, I. I. Severina1, R. A. Simonyan1, M. V. Skulachev1, V. N. Tashlitsky1, E. V. Titova1, T. A. Trendeleva2, and G. S. Shagieva1
1Belozersky Institute of Physico-Chemical Biology and Center of Mitoengineering, Lomonosov Moscow State University, 119991 Moscow, Russia; fax: (495) 939-3181; E-mail: fxb@belozersky.msu.ru2Bach Institute of Biochemistry, Russian Academy of Sciences, Leninsky pr. 33, 119071 Moscow, Russia; fax: (495) 954-2732; E-mail: inbi@inbi.ras.ru
* To whom correspondence should be addressed.
Received April 20, 2014
Novel mitochondria-targeted compounds composed entirely of natural constituents have been synthesized and tested in model lipid membranes, in isolated mitochondria, and in living human cells in culture. Berberine and palmatine, penetrating cations of plant origin, were conjugated by nonyloxycarbonylmethyl residue with the plant electron carrier and antioxidant plastoquinone. These conjugates (SkQBerb, SkQPalm) and their analogs lacking the plastoquinol moiety (C10Berb and C10Palm) penetrated across planar bilayer phospholipid membrane in their cationic forms and accumulated in isolated mitochondria or in mitochondria in living human cells in culture. Reduced forms of SkQBerb and SkQPalm inhibited lipid peroxidation in isolated mitochondria at nanomolar concentrations. In isolated mitochondria and in living cells, the berberine and palmatine moieties were not reduced, so antioxidant activity belonged exclusively to the plastoquinol moiety. In human fibroblasts, nanomolar SkQBerb and SkQPalm prevented fragmentation of mitochondria and apoptosis induced by exogenous hydrogen peroxide. At higher concentrations, conjugates of berberine and palmatine induced proton transport mediated by free fatty acids both in model and in mitochondrial membrane. In mitochondria this process was facilitated by the adenine nucleotide carrier. As an example of application of the novel mitochondria-targeted antioxidants SkQBerb and SkQPalm to studies of signal transduction, we discuss induction of cell cycle arrest, differentiation, and morphological normalization of some tumor cells. We suggest that production of oxygen radicals in mitochondria is necessary for growth factors–MAP-kinase signaling, which supports proliferation and transformed phenotype.
KEY WORDS: mitochondria-targeted antioxidants, SkQ, berberine, palmatine, phospholipid membranes, mitochondria, tumor cellsDOI: 10.1134/S0006297912090040
Abbreviations: ANT, adenine nucleotide translocase; AMVN, 2,2′-azodi(2,4′-dimethylvaleronitrile); BLM, bilayer phospholipid membrane; C10Berb, 13-(decyloxycarbonylmethyl)berberine; CM-DCF-DA, 5-(-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate; C10Palm, 13-(decyloxycarbonylmethyl)palmatine; C12TPP, dodecyltriphenylphosphonium; FA, fatty acid; FCCP, trifluoromethoxycarbonylcyanide phenylhydrazone; ROS, reactive oxygen species; SkQ1, 10-(6′-plastoquinonyl)decyltriphenylphosphonium; SkQBerb, 13-[9-(6′-plastoquinonyl)nonyloxycarbonylmethyl]berberine; SkQPalm, 13-[9-(6′-plastoquinonyl)nonyloxycarbonylmethyl]palmatine; SkQR1, 10-(6′-plastoquinonyl)decylrhodamine 19; TPP+, triphenylphosphonium; Δψ, transmembrane electric potential difference.
The idea of Peter Mitchell [1] on a key role of
transmembrane electric potential (Δψ) in coupling of
respiration and ATP synthesis has revolutionized the field of
bioenergetics since the 1960s. After almost a decade, the idea of E. A.
Liberman and V. P. Skulachev [2] to apply membrane
penetrating artificial ions for measurement of Δψ in
mitochondria resulted in experimental evidence of Mitchell’s
hypothesis. Applications of penetrating ions in the in the new
millennium flourished with the development of a new class of compounds
that selectively accumulate in mitochondria of living cells (for review
see [3]). Rapid progress in this field allows us to
hope that the new mitochondria-targeted drugs will be effective in
treatment of a variety of human pathologies including diseases of the
elderly and aging in particular [4].
The most popular representative of the family of penetrating ions has been and remains the tetraphenylphosphonium cation. Its main advantages: (i) high permeability through lipid bilayer in combination with high solubility in water; and (ii) low reactivity and low sorption the cell components. Tetraphenylphosphonium and its derivatives – conjugates of triphenylphosphonium (TPP+) – are widely used to measure membrane potential and for delivery to mitochondria of various compounds. Among the “cargo” to be delivered to the mitochondria the most often used are various antioxidants and indicators of reactive oxygen species (ROS). This focus is based on the idea that mitochondria are a major source of ROS in the cell, and excessive production of ROS causes the development of various pathologies and aging. The mitochondria-targeted antioxidants, TPP+ conjugates to ubiquinone (MitoQ) [5], plastoquinone (SkQ) [6], tocopherol (MitoVitE) [7], lipoic acid (MitoL) [8], ebselen (MitoPeroxidase) [9], and to spin traps (MitoPBN, MitoCP, Mito-TEMPO) [10-12] have been studied in various models. Mitochondria-targeted ROS indicators are represented by TPP+ conjugates with dihydroethidine (MitoSOX) [13] and boronate derivatives (MitoPY) [14].
In the second place in popularity are derivatives of rhodamines – fluorescent penetrating cations introduced into the practice of bioenergetics by Lan Bo Chen [15] and by V. P. Skulachev [16]. Various rhodamines and their esters are widely used for visualization of mitochondria and measurement of Δψ in cells. Rhodamine-19 conjugated decylplastoquinone (SkQR1) is one of the most effective antioxidants in vivo [17]. Dihydrorhodamine is used to measure mitochondrial ROS, but it acquires a positive charge and accumulates in the mitochondria only after oxidation, making difficult interpretation of its effects [18]. In 2005, compounds bearing two or more guanidine groups were proposed as mitochondria-targeted cations [19]. The mechanism of accumulation of these substances in the mitochondria is not clear, and probably some carrier proteins are involved in their transport since the permeability of multicharged ions through the lipid bilayer is extremely small [20].
In conclusion of this historical overview, it should be noted that the family of penetrating cations includes: fuscin, Janus green, Cristal violet, and other histological dyes that were used for visualization of mitochondria from the middle of the XIX century. Their direct descendants are the various MitoTracker dyes, which are widely used (for the same purpose) in modern studies.
Recently the ability of TPP+ and rhodamine conjugates to catalyze transfer of protons across the lipid bilayer was described [21]. In the case of TPP+ derivatives, this effect was related to acceleration of transport of fatty acid (FA) anions, which limited protonophorous activity of FA in phospholipid membranes. A similar effect of tetraphenylphosphonium (at much higher concentrations) has been known for almost 20 years [22]. Protonophorous activity of rhodamine-19 derivatives did not depend on FA and was related to protonation-deprotonation of the rhodamine residue [23]. Cations in use as mitochondria-targeted uncouplers of oxidative phosphorylation are of great interest as a possible tool to treat obesity. The main advantage of the cations over conventional protonophores is dependence of their activity on Δψ. It could be expected that the decrease in Δψ by the cationic uncouplers will be limited since at the same time the driving force for their accumulation in mitochondria will be decreased.
Neither the derivatives of TPP+ nor rhodamines are natural compounds. This fact was important for their application for measurements of Δψ, as it excluded participation of specific mechanisms of transport through the mitochondrial membrane. Now, when the possibility of long-term use of derivatives of these cations becomes real, their artificial nature is a matter of concern. In search of natural penetrating cations our attention was attracted by berberine and palmatine, which are isoquinoline alkaloids derived from plants of the Berberidaceae family. Extracts containing berberine and palmatine have been used in traditional Chinese medicine for many centuries. Pharmacological studies of these alkaloids revealed their therapeutic effects against diabetes, inflammatory disorders, arrhythmia, hypertension, etc. Anticancer activity of berberine mediated by its antiproliferative and antiangiogenic properties was also reported [24]. The mechanisms of therapeutic action of the alkaloids are not known, but in vivo studies have shown a pronounced antioxidant effect of berberine [25] or its metabolites [26]. In cell-free models, berberine inhibits lipid peroxidation, but only in the reduced form [27]. It was shown that berberine can accumulate in mitochondria of living cells. Experiments on bilayer phospholipid membranes (BLM) have shown that berberine and palmatine penetrate the bilayer in the cationic form [28].
In 2010, we synthesized conjugates of berberine and palmatine with decylplastoquinone (SkQBerb and SkQPalm), as well as their analogs lacking the quinone residue, C10Berb and C10Palm (Fig. 1). The conjugates with different length of aliphatic chain -(CH2)n, where n ranged from 4 to 10, were also synthesized. Lipophilicity of these compounds was estimated using reversed-phase chromatography (HPLC). Previously it was shown that this analysis gave results corresponding to the partition coefficients in the octanol–water system for conjugates of TPP+ (SkQ1 and MitoQ) [30]. SkQBerb and SkQPalm, as well as C10Berb and C10Palm, were selected as the closest to SkQ1 in lipophilicity (partition coefficient in the octanol–water system in the range 2000-3000 : 1).
The results of studies of berberine and palmatine conjugates were published in part by us [29]. The goal of the present work was to describe a complete experimental system for testing of such substances in vitro in models with increasing complexity from BLM and liposomes to living cells. We would like to stress that these studies are a logical extension of ideas of Yefim Arsentievich Liberman, to whose blessed memory we dedicate it.Fig. 1. Chemical structures of the studied compounds.
ARTIFICIAL LIPID MEMBRANES
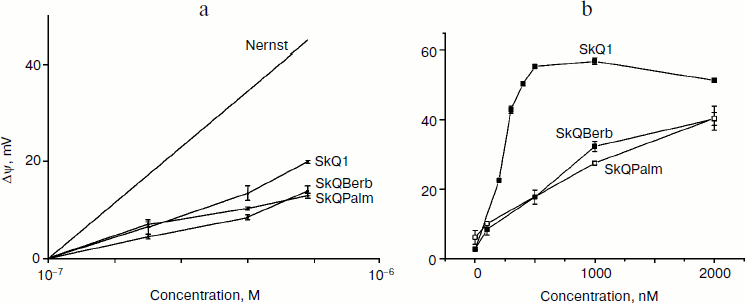
Tests for permeability of BLM for alkaloid conjugates were performed using the method developed with direct participation of E. A. Liberman as described in detail previously [31]. The BLM was formed from a solution of Escherichia coli lipids in decane at the aperture (0.6 mm) in a Teflon wall separating the chamber into two compartments. When gradient of penetrating ion between the compartments was created, the electrical potential difference (Δψ), which is measured by the electrometer, appeared across the BLM. In theory (according to the Nernst equation) the value of Δψ is 60 mV for a tenfold gradient of a singly charged ion. In practice, this value is usually lower because at a low rate of ion transfer the effect is blunted by ions in the medium (K+, Cl–), whose permeability is very low, but their concentration is high. As can be seen from Fig. 2a, Δψ, generated by a gradient of SkQBerb or SkQPalm, was significantly below the theoretical and somewhat lower than that observed for SkQ1. Thus, permeability of the BLM for the alkaloid conjugates was slightly lower than for similar derivatives of TPP+.
A similar approach was used to assess the protonophorous activity of the berberine and palmatine conjugates. In the same chamber, when a pH gradient was created between the compartments, addition of protonophore caused the formation of Δψ (“minus” in the compartment with lower pH). The protonophorous effect of SkQBerb, SkQPalm, C10Berb, and C10Palm (as in the case SkQ1) was observed only when the fatty acid (palmitate) was added to the BLM. Again, as in the experiment in Fig. 2a, the activity and SkQBerb and SkQPalm was lower than that of SkQ1 (Fig. 2b). A similar difference was observed between C10Berb and C10Palm on one side and C12TPP on the other. Apparently, the lower protonophorous activity of the berberine and palmatine conjugates was related to their lower permeability in comparison with derivatives of TPP+.Fig. 2. Tests of the novel mitochondria-targeted compounds in BLM. a) Permeability of BLM for the tested compounds. Two compartments of the experimental chamber separated by a BLM initially contained equal concentrations (10–7 M) of the cations. Δψ was measured after addition of increasing cation concentration into one compartment. The theoretical dependence of Δψ on the gradient of a monovalent penetrating cation is shown. b) Protonophorous activity of the tested compounds in BLM. The BLM was formed from synthetic diphytanoylphosphatidylcholine (DPhPC) and contained 1 mg palmitate per 20 mg of lipid. The compartments of the chamber contained equal concentrations of the cations. Δψ was measured after shift of pH from 7.0 to 8.0 by addition of KOH on one side of the BLM.
Induction of proton conductivity by the new compounds was investigated also in liposomes as described previously [32]. Liposomes were loaded with K+ and treated with the selective K+ ionophore valinomycin in a medium without this ion. The Δψ generated by the exit of K+ from the liposomes was measured using the fluorescent dye DiS-C3-(5). Conjugates of berberine and palmatine induced slow decrease in Δψ in liposomes but only in the presence of palmitate, indicating that their protonophorous activity was mediated by transfer of FA (not shown).
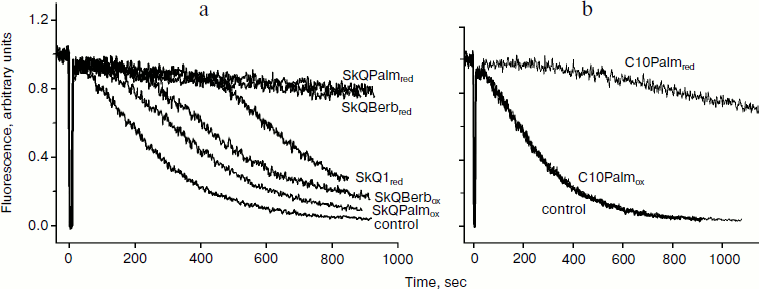
Also, liposomes were used for testing of the antioxidant activity of the berberine and palmatine conjugates. It was found previously that oxidation of cardiolipin plays a key role in oxidative damage to mitochondria. In experiments with isolated mitochondria, it was shown that cardiolipin was oxidized significantly earlier than the other phospholipids, and SkQ1 effectively protected it from oxidation [6]. Lipid peroxidation in liposomes containing 20% cardiolipin was induced with the azo-initiator AMVN and measured with lipophilic fluorescent probe BODIPY 581/591C11 as described previously [33]. Oxidized SkQBerb and SkQPalm had no effect on lipid peroxidation, and oxidized SkQ1 was ineffective as well. After reduction with NaBH4, SkQBerb and SkQPalm inhibited peroxidation of liposomes, and their effect was even more pronounced than in the case of reduced SkQ1 (Fig. 3). These data suggest that the berberine and palmatine residues as well as quinol exhibited antioxidant activity. Indeed, it was found that reduced C10Berb and C10Palm effectively inhibited lipid peroxidation in the same model. Thus, both of the components of the new compounds in reduced form demonstrate antioxidant activity.
Fig. 3. Antioxidant activity of SkQBerb, SkQPalm, and C10Palm in liposomes (following the method used in [29]). Liposomes were prepared from a mixture of phosphatidylcholine and cardiolipin in proportion 4 : 1 w/w. Lipid oxidation was induced by the azo-initiator AMVN (0.25 mM) and monitored as a decrease in fluorescence of BODIPY 581/591 C11 at 60°C, pH 7.4. The conjugates of berberine and palmatine (2 µM final concentration) were reduced with NaBH4 at neutral pH. The curves with reduced and oxidized compounds are indicated as “red” and “ox”, respectively.
ISOLATED MITOCHONDRIA
Berberine and palmatine fluoresce brightly at 530 nm (excitation at 350 nm), and upon reduction their fluorescence drops sharply. Taking advantage of this property, we estimated both the kinetics of accumulation of berberine and palmatine conjugates in mitochondria and their possible reduction. It was found that all the conjugates accumulated in mitochondria of rat heart (in the presence of respiratory substrates), and the process was completed within 15-20 min. Dissipation of membrane potential using the uncoupler FCCP significantly reduced accumulation of the conjugates, which confirmed the electrophoretic nature of their accumulation. Incomplete inhibition of accumulation was apparently related by the high distribution coefficient of these compounds in the membrane–water system.
The possibility of reduction of berberine and palmatine residues was studied under conditions of maximal reduction of the respiratory chain and matrix components of mitochondria. To do this, mitochondria were incubated with NAD-dependent substrates of respiration (pyruvate, glutamate, malate) or succinate in the presence of rotenone or cyanide, but no decrease in the fluorescence of alkaloids was observed, i.e. they were not reduced. The reduction of plastoquinone residues of SkQBerb and SkQPalm (analyzed at 274 nm) appeared with the same kinetics as in the case of SkQ1 [29]. Thus, the antioxidant action of these compounds in mitochondria could be associated only with the plastoquinone residues.
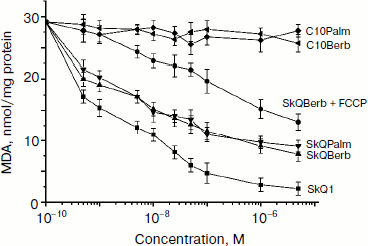
To evaluate the antioxidant activity of berberine and palmatine conjugates in mitochondria, we measured accumulation of lipid peroxidation products (mainly malondialdehyde) reacting with thiobarbituric acid (TBARS). Oxidation was induced by Fe2+ in the presence of ascorbate. We previously observed rapid oxidation of cardiolipin under these conditions and strong protective effect of SkQ1 [6]. It is evident from Fig. 4 that SkQBerb and SkQPalm were almost as effective as SkQ1 in this model. The uncoupler FCCP inhibited the effect of SkQBerb and SkQPalm, consistent with the potential-dependent accumulation of these compounds in mitochondria. C10Berb and C10Palm, conjugates lacking a plastoquinone residue, did not inhibit lipid peroxidation, confirming the absence of reduction of berberine and palmatine residues in mitochondria (Fig. 4).
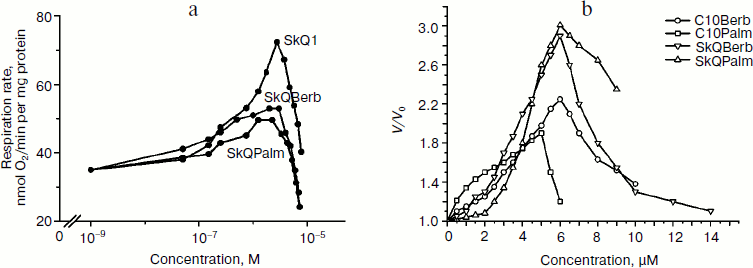
Protonophorous action of the conjugates in the mitochondria was assessed by measuring respiration. In the absence of ADP (substrate of phosphorylation), protonophores stimulate respiration. This effect was pronounced in the case of SkQBerb and SkQPalm, and was slightly weaker for C10Berb and C10Palm (Fig. 5a). At higher concentrations of these compounds decreased the rate of respiration, which is typical also for SkQ1 [21], SkQR1 [23], and for almost all protonophoric uncouplers. When succinate was replaced by a mixture of NAD-dependent substrates, inhibition of respiration by berberine and palmatine conjugates was significantly increased. These results are consistent with data on the inhibition of complex I of the respiratory chain by berberine [34]. Stimulation of respiration by SkQBerb and SkQPalm was also observed in mitochondria of the yeast Yarrowia lipolytica, which contains highly active NADH-quinone oxidoreductase (complex I) in the respiratory chain [35]. SkQBerb and SkQPalm strongly stimulated oxidation of NAD-dependent substrates, while C10Berb and C10Palm (as in heart mitochondria) were less effective (Fig. 5b). Measurements of mitochondrial membrane potential demonstrated that in heart mitochondria as well as in mitochondria of yeast, the different conjugates induced similar decreases in Δψ (Fig. 6, a and b). Thus, we assume that C10Berb and C10Palm have similar protonophorous activity, but more effectively inhibit respiration than SkQBerb and SkQPalm.Fig. 4. Antioxidant activity of SkQBerb and SkQPalm in isolated mitochondria [29]. Mitochondria were isolated from rat heart and incubated with the antioxidants for 20 min. Lipid peroxidation was initiated by addition of 10 mM ascorbate and 0.1 mM FeSO4. Accumulation of TBARS was measured and expressed as nanomoles of malondialdehyde (MDA) per mg protein. FCCP (1 µM) was added where indicated simultaneously with SkQBerb.
Fig. 5. Stimulation of respiration in isolated mitochondria by SkQBerb, SkQPalm, C10Berb, and C10Palm. Respiration was measured as oxygen consumption using a Clark electrode. a) Mitochondria from rat heart were in medium supplemented with 5 mM succinate, 2 µM rotenone, and 1 µg/ml oligomycin. b) Yeast mitochondria were incubated in medium supplemented with 20 mM pyruvate and 5 mM malate. The ratio of stimulated rate of respiration (V) to the initial rate (V0) is shown.
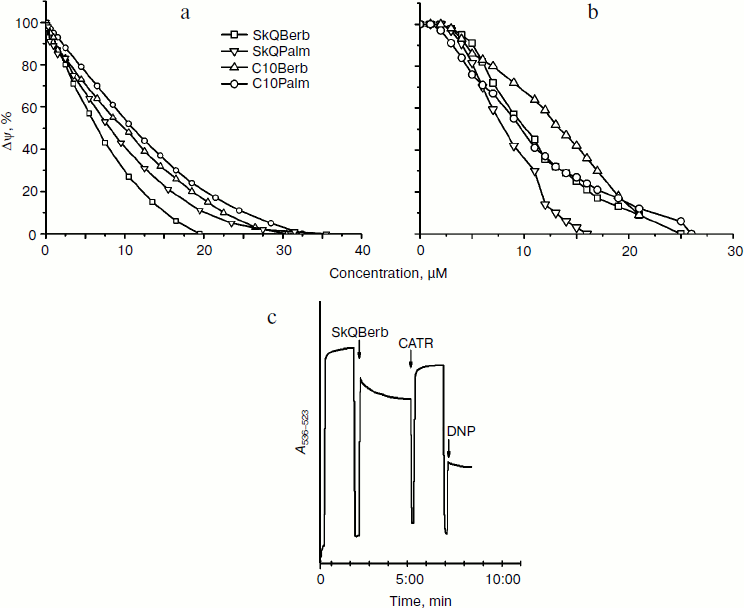
Experiments with phospholipid membranes revealed that protonophorous activity of berberine and palmatine conjugates was pronounced only in the presence of fatty acids. One might suppose that in mitochondria (as well as in BLM) cationic conjugates stimulate transfer of FA anion through the membrane and thus facilitate transport of protons on the protonated form of FA. However, in mitochondrial membrane transport of FA anions is catalyzed by adenine nucleotide translocase (ANT), and (to a lesser extent) by other anion transporters [36]. Experiments on heart mitochondria showed that the selective inhibitor of ANT carboxyatractyloside (CATR) partially restored the membrane potential, reduced by the conjugates (Fig. 6c). This effect decreased with increasing concentration of the conjugates. Apparently, at low concentrations they stimulate transport of FA anions mediated by ANT, while at higher concentrations dominated transport of FA through the bilayer. The ability to stimulate ANT-dependent transport of FA has been shown previously for tetraphenylphosphonium [37]. It can be assumed that penetrating cations form ion pairs with FA anions and stimulate their binding to the positively charged residues in the nucleotide-binding site of ANT. The X-ray structure of ANT [38] indicates that the access to this center is open primarily from the aqueous phase. Perhaps the cations contribute to release of FA from the membrane bilayer and thus accelerate their binding to ANT. It is possible also that the cations neutralize the negative charges at the surface of ANT that prevent penetration of FA anions into the nucleotide-binding site. One way or another, the described effect of CATR indicated that protonophorous action of the berberine and palmatine conjugates in mitochondria depends on transfer of FA anions.Fig. 6. Decrease in membrane potential difference by SkQBerb, SkQPalm, C10Berb, and C10Palm. Δψ was measured by the optical density of safranin O at 555 nm (reference at 523 nm). a, c) Mitochondria from rat heart were incubated in the same medium as in Fig. 5a. b) Yeast mitochondria were incubated in the same medium as in Fig. 5b. Additions of 5 µM SkQBerb, 1 µM CATR, and 40 µM 1,4-dinitrophenol (DNP) were made as indicated.
CELLS IN CULTURE
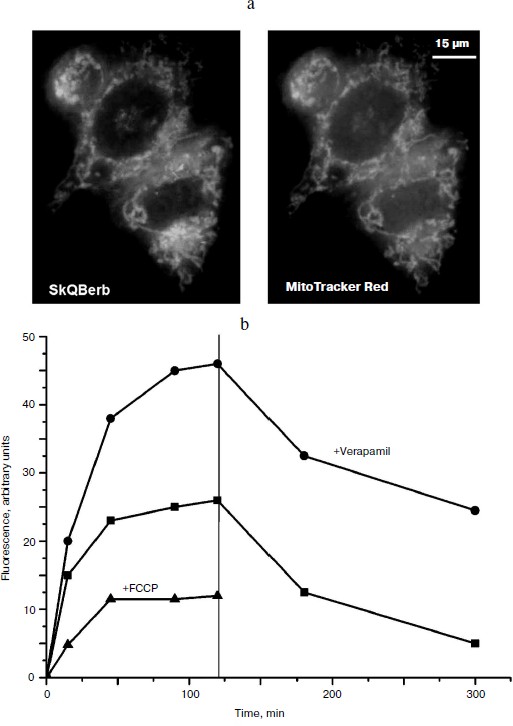
Accumulation of the berberine and palmatine conjugates in cells was measured taking advantage of the fluorescence of these compounds. It was shown that all of the conjugates accumulated in the cells within 1-1.5 h, and the fluorescence did not change for following incubation, indicating that the alkaloid residues were not reduced in the cells as well as in isolated mitochondria. Uncoupler FCCP prevented the accumulation of the conjugates in the cells and accelerated their release into the medium (Fig. 7a). Thus, Δψ at the mitochondrial membrane was the main driving force for accumulation of these compounds in the cell. Fluorescence microscopy demonstrated mitochondrial localization of the berberine and palmatine conjugates (Fig. 7b), as their fluorescence was co-localized with the fluorescence of MitoTracker red. It is important to note that the conjugates almost completely left the cell, indicating the absence of irreversible binding to cellular components.
In HeLa carcinoma cells accumulation of the berberine and palmatine conjugates was stimulated after inhibition of P-glycoprotein with verapamil or other specific inhibitors. The effect of verapamil was not observed in normal human fibroblasts, where the content of P-glycoprotein was very low. The conjugates are apparently substrates of multidrug resistance enzyme(s) (MDR), which pump out of the cell compounds of various structure including chemotherapeutic drugs. Previously we showed that SkQ1 and SkQR1 are also substrates of MDR enzyme [39].Fig. 7. Accumulation of SkQBerb in HeLa cells [29]. a) Cells were incubated with 0.5 μM SkQBerb for 60 min and with 200 nM MitoTracker Red for 15 min, and then analyzed with the fluorescence microscope. b) Cells were incubated with 5 μM SkQBerb. Verapamil (50 μM) or 10 μM FCCP was added 15 min before SkQBerb where indicated. After 2 h the medium was changed to similar medium but without SkQBerb (indicated with vertical line). Fluorescence was measured after lysis of the cells with a detergent mixture.
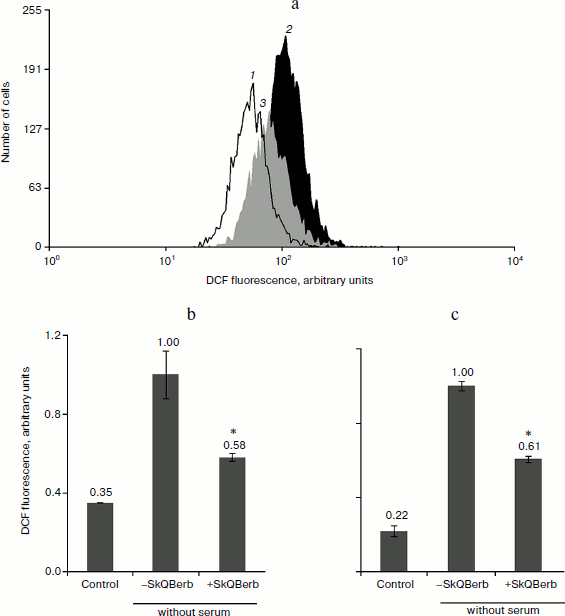
To study the antioxidant activity of the new compounds, we used a model of oxidative stress induced by hydrogen peroxide. Previously we have shown that mitochondria are the main source of endogenous ROS under these conditions [6, 40]. Cultivation of cells in medium without serum served as another model of oxidative stress. The data on the nature of oxidative stress in this model were contradictory. We found (Fig. 8) that SkQBerb (but not C10Berb) at a concentration of 2 nM prevented the accumulation of ROS (measured using fluorescent probe CM-DCF-DA) in carcinoma RKO cells and in human fibroblasts under these conditions. Thus, ROS production in mitochondria underlies the oxidative stress induced by deprivation of growth factors in normal as well as in cancer cells.
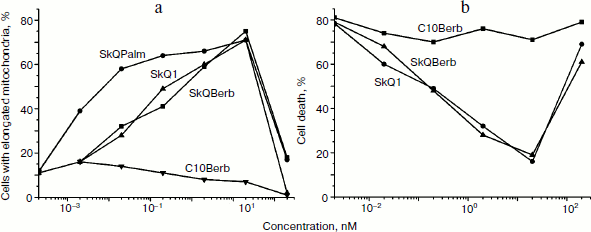
One of the earliest effects of oxidative stress is extensive fragmentation of mitochondria. We previously showed that SkQ1 at nanomolar concentrations prevented fragmentation of mitochondria induced by hydrogen peroxide [6]. This effect was also observed during the tests of SkQBerb and SkQPalm (Fig. 9a), and they were as effective as SkQ1. C10Berb and C10Palm (as previously found for C12TPP) did not protect mitochondria. SkQBerb and SkQPalm protected cells from death induced by hydrogen peroxide, and again their effectiveness differed little from that of SkQ1 (Fig. 9b). Interestingly, for the protection of the mitochondria 2 h preincubation with the antioxidants was sufficient, whereas prevention of ROS accumulation (measured by CM-DCF-DA in cytoplasm) and protection against cell death required at least 24 h. We suggested that fragmentation of mitochondria is the result of oxidation of some components of the inner membrane, which depends on accumulation of ROS in mitochondria. At the same time, cell death was initiated only after the accumulation of ROS in cytoplasm. Delayed protective effect of the mitochondria-targeted antioxidants may be due to their slow redistribution through the mitochondrial population in the cell. A small fraction of mitochondria with low potential could slowly accumulate cationic conjugates and at the same time produce (under oxidative stress) the bulk of ROS in the cell [41]. These “bad” mitochondria probably determined the fate of cells under stress.Fig. 8. Antioxidant action of SkQBerb in cells upon serum starvation. Colon carcinoma RKO cells (a, b) and human fibroblasts (c) were cultivated with 2 nM SkQBerb for 48 h and transferred into medium without serum for the next 24 h. The cells were incubated with 5 µM CM-DCF-DA (15 min at 37°C) and analyzed by flow cytometry. a) Fluorescence of CM-DCF in RKO cell population. 1) Control; 2) without serum; 3) without serum plus SkQBerb. b, c) Mean values of CM-DCF fluorescence.
An alternative explanation for the slow development of the protective effect is based on possible induction of expression of endogenous antioxidant systems by the mitochondria-targeted antioxidants. This effect is typical of various prooxidants. Mitochondrial antioxidants of the SkQ family (including SkQBerb and SkQPalm) exhibit prooxidant properties at much higher (micromolar) concentrations than their antioxidant action. No significant increase in ROS was found and no induction of expression of antioxidant enzymes was detected at nanomolar concentrations of SkQBerb and SkQPalm.
Fig. 9. Protective action of the novel mitochondria-targeted compounds against oxidative stress in human fibroblasts [29]. a) SkQBerb and SkQPalm prevent fragmentation of mitochondria in fibroblasts treated with 0.4 mM H2O2 for 3 h. The cells were preincubated with antioxidants for 2 h. Mitochondria were visualized with MitoTracker Green, and cells with elongated mitochondria were counted. b) SkQBerb and SkQPalm prevented cell death of fibroblasts treated with 0.4 mM H2O2 for 24 h. Cells were preincubated with the antioxidants for 24 h. Cell viability was determined with CellTiter-Blue reagent (Promega, USA).
EXAMPLES OF APPLICATIONS
One of the main areas of application of mitochondria-targeted compounds in cell biology is studies of the role of mitochondria in intracellular signaling. Below we present examples illustrating the possibility of application of berberine and palmatine conjugates to studies on the role of mitochondrial ROS production in physiology of cancer cells. It is known that the level of ROS in transformed cells is significantly elevated. These cells are adapted to such state and perhaps even depend on continuous endogenous oxidative stress. These considerations were confirmed by numerous experiments in which various antioxidants inhibited proliferation of tumor (and to a lesser extent normal) cells.
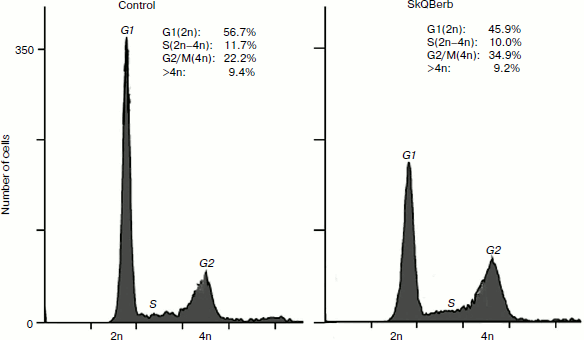
The role of mitochondrial ROS in cell cycle regulation was studied in fibrosarcoma cell line HT1080. These cells bear a mutant form of the oncogene N-ras, and for this type of transformation antiproliferative and “normalizing” effects of antioxidants were first demonstrated [42, 43]. The traditional antioxidant N-acetylcysteine (NAC) inhibited cell proliferation at millimolar concentrations, while SkQBerb and SkQPalm caused a similar effect at concentrations of 100,000 to 1 million times lower. C10Berb and C10Palm did not have this effect. Cytometric analysis of the cell cycle revealed accumulation of cells with doubled DNA content (Fig. 10). Microscopic analysis showed that this population consisted of binuclear cells, and dynamic observations indicated that they were arrested at the exit from mitosis. The exit from mitosis could be affected by modification of anaphase-promoting complex (APC), which is sensitive to redox regulation [44]. It can be assumed that mitochondrial ROS are required for the functioning of APC in fibrosarcoma cells.
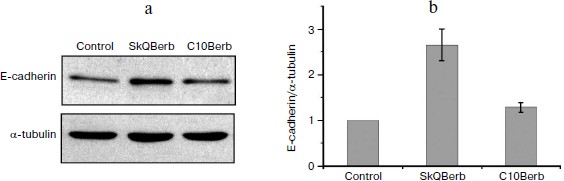
Cervical carcinoma cells SiHa represent a model where SkQBerb and SkQPalm not only inhibit proliferation, but also change the morphology of the cells. The cells largely regained the morphology of normal epithelium: they lose their individual mobility, restore the circular bundle of actin filaments, and form typical epithelioid isles. Expression of E-cadherin, a major protein of epithelial cell–cell contacts, was increased, and this protein was localized at intercellular contact sites (Fig. 11). In general, the observed changes indicate differentiation of carcinoma cells and their partial normalization. C10Berb and C10Palm did not cause such changes, and the traditional antioxidant NAC had a similar effect but only at much higher (millimolar) concentrations. SkQ1 and SkQR1 in nanomolar concentrations induced similar changes in carcinoma cells [45].Fig. 10. SkQBerb induces changes in the cell cycle of fibrosarcoma HT1080 cells. The cells were incubated with 20 nM SkQBerb for 4 days. Cell cycle phases were analyzed by flow cytometry after staining of DNA with propidium iodide. The fraction of cells at G1, S, and G2/M phases and polyploid cells are shown.
Thus, it can be assumed that mitochondrial ROS in carcinoma cells are involved in maintenance of transformed phenotype. Further experiments showed that the effects of mitochondria-targeted antioxidants are associated with inhibition of signaling pathways initiated by extracellular growth factors. These molecules bind to receptors on the cell surface and activate the cascade of kinases (MAP kinases), which define fast proliferation and dedifferentiation of cells. It was activation of MAP kinases that was one of the first established signaling pathways critically dependent on intracellular ROS [46]. These signaling pathways are targeted by a variety of modern anticancer drugs such as Iressa (Gefitinib). Differentiation cancer therapy and chemoprevention has attracted growing interest among oncologists in recent years. Our data suggest that mitochondria-targeted antioxidants may be an important component of comprehensive anticancer therapy.
This study was supported by a research contract with the Russian Ministry of Education and Science (“Centers for Science and Education” No. 14.740.11.0010) and by the Russian Foundation for Basic Research (grant Nos. 09-04-01238, 11-04-12050, 12-04-00538).Fig. 11. SkQBerb induces “normalization” of cervical carcinoma SiHa cells. Cells were incubated with 20 nM SkQBerb for 7 days. a) SkQBerb induces increase in E-cadherin content. Western blotting. b) Level of E-cadherin relative to tubulin content.
REFERENCES
1.Mitchell, P. (1961) Nature, 191,
144-148.
2.Liberman, E. A., Topaly, V. P., Tsofina, L. M.,
Jasaitis, A. A., and Skulachev, V. P. (1969) Nature, 222,
1076-1078.
3.Yousif, L. F., Stewart, K. M., and Kelley, S. O.
(2009) Chembiochem: Eur. J. Chem. Biol., 10,
1939-1950.
4.Skulachev, M. V., Antonenko, Y. N., Anisimov, V.
N., Chernyak, B. V., Cherepanov, D. A., Chistyakov, V. A., Egorov, M.
V., Kolosova, N. G., Korshunova, G. A., Lyamzaev, K. G., Plotnikov, E.
Y., Roginsky, V. A., Savchenko, A. Y., Severina, I. I., Severin, F. F.,
Shkurat, T. P., Tashlitsky, V. N., Shidlovsky, K. M., Vyssokikh, M. Y.,
Zamyatnin, A. A., Jr., Zorov, D. B., and Skulachev, V. P. (2011)
Curr. Drug Targets, 12, 800-826.
5.Kelso, G. F., Porteous, C. M., Coulter, C. V.,
Hughes, G., Porteous, W. K., Ledgerwood, E. C., Smith, R. A., and
Murphy, M. P. (2001) J. Biol. Chem., 276, 4588-4596.
6.Antonenko, Y. N., Avetisyan, A. V., Bakeeva, L. E.,
Chernyak, B. V., Chertkov, V. A., Domnina, L. V., Ivanova, O. Y.,
Izyumov, D. S., Khailova, L. S., Klishin, S. S., Korshunova, G. A.,
Lyamzaev, K. G., Muntyan, M. S., Nepryakhina, O. K., Pashkovskaya, A.
A., Pletjushkina, O. Y., Pustovidko, A. V., Roginsky, V. A.,
Rokitskaya, T. I., Ruuge, E. K., Saprunova, V. B., Severina, I. I.,
Simonyan, R. A., Skulachev, I. V., Skulachev, M. V., Sumbatyan, N. V.,
Sviryaeva, I. V., Tashlitsky, V. N., Vassiliev, J. M., Vyssokikh, M.
Y., Yaguzhinsky, L. S., Zamyatnin, A. A., Jr., and Skulachev, V. P.
(2008) Biochemistry (Moscow), 73, 1273-1287.
7.Smith, R. A., Porteous, C. M., Coulter, C. V., and
Murphy, M. P. (1999) Eur. J. Biochem./FEBS, 263,
709-716.
8.Brown, S. E., Ross, M. F., Sanjuan-Pla, A., Manas,
A. R., Smith, R. A., and Murphy, M. P. (2007) Free Rad. Biol.
Med., 42, 1766-1780.
9.Filipovska, A., Kelso, G. F., Brown, S. E., Beer,
S. M., Smith, R. A., and Murphy, M. P. (2005) J. Biol. Chem.,
280, 24113-24126.
10.Murphy, M. P., Echtay, K. S., Blaikie, F. H.,
Asin-Cayuela, J., Cocheme, H. M., Green, K., Buckingham, J. A., Taylor,
E. R., Hurrell, F., Hughes, G., Miwa, S., Cooper, C. E., Svistunenko,
D. A., Smith, R. A., and Brand, M. D. (2003) J. Biol. Chem.,
278, 48534-48545.
11.Dhanasekaran, A., Kotamraju, S., Karunakaran, C.,
Kalivendi, S. V., Thomas, S., Joseph, J., and Kalyanaraman, B. (2005)
Free Rad. Biol. Med., 39, 567-583.
12.Ban, S., Nakagawa, H., Suzuki, T., and Miyata, N.
(2007) Bioorg. Med. Chem. Lett., 17, 2055-2058.
13.Robinson, K. M., Janes, M. S., Pehar, M.,
Monette, J. S., Ross, M. F., Hagen, T. M., Murphy, M. P., and Beckman,
J. S. (2006) PNAS, 103, 15038-15043.
14.Dickinson, B. C., and Chang, C. J. (2008) J.
Am. Chem. Soc., 130, 9638-9639.
15.Johnson, L. V., Walsh, M. L., and Chen, L. B.
(1980) PNAS, 77, 990-994.
16.Skulachev, V. P. (1988) Membrane
Bioenergetics, Springer-Verlag, Berlin-New York.
17.Plotnikov, E. Y., Silachev, D. N., Chupyrkina, A.
A., Danshina, M. I., Jankauskas, S. S., Morosanova, M. A., Stelmashook,
E. V., Vasileva, A. K., Goryacheva, E. S., Pirogov, Y. A., Isaev, N.
K., and Zorov, D. B. (2010) Biochemistry (Moscow), 75,
145-150.
18.Koide, Y., Urano, Y., Kenmoku, S., Kojima, H.,
and Nagano, T. (2007) J. Am. Chem. Soc., 129,
10324-10325.
19.Fernandez-Carneado, J., van Gool, M., Martos, V.,
Castel, S., Prados, P., de Mendoza, J., and Giralt, E. (2005) J. Am.
Chem. Soc., 127, 869-874.
20.Ross, M. F., Da Ros, T., Blaikie, F. H., Prime,
T. A., Porteous, C. M., Severina, I. I., Skulachev, V. P., Kjaergaard,
H. G., Smith, R. A., and Murphy, M. P. (2006) Biochem. J.,
400, 199-208.
21.Severin, F. F., Severina, I. I., Antonenko, Y.
N., Rokitskaya, T. I., Cherepanov, D. A., Mokhova, E. N., Vyssokikh, M.
Y., Pustovidko, A. V., Markova, O. V., Yaguzhinsky, L. S., Korshunova,
G. A., Sumbatyan, N. V., Skulachev, M. V., and Skulachev, V. P. (2010)
PNAS, 107, 663-668.
22.Schonfeld, P. (1992) FEBS Lett.,
303, 190-192.
23.Antonenko, Y. N., Avetisyan, A. V., Cherepanov,
D. A., Knorre, D. A., Korshunova, G. A., Markova, O. V., Ojovan, S. M.,
Perevoshchikova, I. V., Pustovidko, A. V., Rokitskaya, T. I., Severina,
I. I., Simonyan, R. A., Smirnova, E. A., Sobko, A. A., Sumbatyan, N.
V., Severin, F. F., and Skulachev, V. P. (2011) J. Biol. Chem.,
286, 17831-17840.
24.Lin, C. C., Ng, L. T., Hsu, F. F., Shieh, D. E.,
and Chiang, L. C. (2004) Clin. Exp. Pharm. Physiol., 31,
65-69.
25.Hwang, J. M., Wang, C. J., Chou, F. P., Tseng, T.
H., Hsieh, Y. S., Lin, W. L., and Chu, C. Y. (2002) Arch.
Toxicol., 76, 664-670.
26.Shia, C. S., Hou, Y. C., Juang, S. H., Tsai, S.
Y., Hsieh, P. H., Ho, L. C., and Chao, P. D. (2009) Evidence-Based
Complementary and Alternative Medicine: eCAM.
27.Shirwaikar, A., Rajendran, K., and Punitha, I. S.
(2006) Biol. Pharm. Bul., 29, 1906-1910.
28.Severina, I. I., Muntyan, M. S., Lewis, K., and
Skulachev, V. P. (2001) IUBMB Life, 52, 321-324.
29.Lyamzaev, K. G., Pustovidko, A. V., Simonyan, R.
A., Rokitskaya, T. I., Domnina, L. V., Ivanova, O. Y., Severina, I. I.,
Sumbatyan, N. V., Korshunova, G. A., Tashlitsky, V. N., Roginsky, V.
A., Antonenko, Y. N., Skulachev, M. V., Chernyak, B. V., and Skulachev,
V. P. (2011) Pharm. Res., 28, 2883-2895.
30.Martyushin, A. A., Tcarev, D. A., Grigorenko, M.
A., Fedorov, I. I., Ramenskaya, G. V., Tashlitsky, V. N., and
Skulachev, V. P. (2008) Farmatsiya, 5, 23-29.
31.Severina, I. I. (1982) Biochim. Biophys.
Acta, 681, 311-317.
32.Konishi, T., Murakami, N., Hatano, Y., and
Nakazato, K. (1986) Biochim. Biophys. Acta, 862,
278-284.
33.Naguib, Y. M. (1998) Anal. Biochem.,
265, 290-298.
34.Pereira, G. C., Branco, A. F., Matos, J. A.,
Pereira, S. L., Parke, D., Perkins, E. L., Serafim, T. L., Sardao, V.
A., Santos, M. S., Moreno, A. J., Holy, J., and Oliveira, P. J. (2007)
J. Pharm. Exp. Therap., 323, 636-649.
35.Kovaleva, M. V., Sukhanova, E. I., Trendeleva, T.
A., Zyl’kova, M. V., Ural’skaya, L. A., Popova, K. M.,
Saris, N. E., and Zvyagilskaya, R. A. (2009) J. Bioenerg.
Biomembr., 41, 239-249.
36.Andreyev, A., Bondareva, T. O., Dedukhova, V. I.,
Mokhova, E. N., Skulachev, V. P., Tsofina, L. M., Volkov, N. I., and
Vygodina, T. V. (1989) Eur. J. Biochem./FEBS, 182,
585-592.
37.Dedukhova, V. I., Mokhova, E. N., Starkov, A. A.,
and Leikin, Yu. N. (1993) Biochem. Mol. Biol. Int., 30,
1161-1167.
38.Pebay-Peyroula, E., Dahout-Gonzalez, C., Kahn,
R., Trezeguet, V., Lauquin, G. J., and Brandolin, G. (2003)
Nature, 426, 39-44.
39.Fetisova, E. K., Avetisyan, A. V., Izyumov, D.
S., Korotetskaya, M. V., Chernyak, B. V., and Skulachev, V. P. (2010)
FEBS Lett., 584, 562-566.
40.Chernyak, B. V., Izyumov, D. S., Lyamzaev, K. G.,
Pashkovskaya, A. A., Pletjushkina, O. Y., Antonenko, Y. N., Sakharov,
D. V., Wirtz, K. W., and Skulachev, V. P. (2006) Biochim. Biophys.
Acta, 1757, 525-534.
41.Izyumov, D. S., Domnina, L. V., Nepryakhina, O.
K., Avetisyan, A. V., Golyshev, S. A., Ivanova, O. Y., Korotetskaya, M.
V., Lyamzaev, K. G., Pletjushkina, O. Y., Popova, E. N., and Chernyak,
B. V. (2010) Biochemistry (Moscow), 75, 123-129.
42.Irani, K., Xia, Y., Zweier, J. L., Sollott, S.
J., Der, C. J., Fearon, E. R., Sundaresan, M., Finkel, T., and
Goldschmidt-Clermont, P. J. (1997) Science, 275,
1649-1652.
43.Alexandrova, A. Y., Kopnin, P. B., Vasiliev, J.
M., and Kopnin, B. P. (2006) Exp. Cell Res., 312,
2066-2073.
44.Havens, C. G., Ho, A., Yoshioka, N., and Dowdy,
S. F. (2006) Mol. Cell. Biol., 26, 4701-4711.
45.Agapova, L. S., Chernyak, B. V., Domnina, L. V.,
Dugina, V. B., Efimenko, A. Y., Fetisova, E. K., Ivanova, O. Y.,
Kalinina, N. I., Khromova, N. V., Kopnin, B. P., Kopnin, P. B.,
Korotetskaya, M. V., Lichinitser, M. R., Lukashev, A. L., Pletjushkina,
O. Y., Popova, E. N., Skulachev, M. V., Shagieva, G. S., Stepanova, E.
V., Titova, E. V., Tkachuk, V. A., Vasiliev, J. M., and Skulachev, V.
P. (2008) Biochemistry (Moscow), 73, 1300-1316.
46.Sundaresan, M., Yu, Z. X., Ferrans, V. J., Irani,
K., and Finkel, T. (1995) Science, 270, 296-299.