Method for isolation of intact titin (connectin) molecules from Mammalian cardiac muscle
I. M. Vikhlyantsev1*, A. D. Okuneva1,2, U. V. Shumilina1, N. N. Salmov1, A. G. Bobylev1, N. V. Molochkov1, and Z. A. Podlubnaya1,2
1Institute of Theoretical and Experimental Biophysics, Russian Academy of Sciences, 142290 Pushchino, Moscow Region, Russia; fax: (4967) 33-0553; E-mail: vikhlyantsev@iteb.ru2Pushchino State Institute of Natural Sciences, pr. Nauki 3, 142290 Pushchino, Moscow Region, Russia
* To whom correspondence should be addressed.
Received November 27, 2012; Revision received January 20, 2013
Cardiac titin was isolated from rabbit and ground squirrel ventricular muscles by a method that was used earlier to obtain myofibrils with intact minor proteins located in A-bands of sarcomeres (Podlubnaya, Z. A., et al. (1989) J. Mol. Biol., 210, 655-658). Small pieces of cardiac muscle were incubated for 2-3 weeks at 4°C in Ca2+-depleting solution before their homogenization to decrease activity of Ca2+-dependent proteases. Then the muscle was homogenized, and titin was isolated by the method of Soteriou, A., et al. (1993) J. Cell Sci., 14, 119-123. In control experiments, titin was isolated from cardiac muscle without its preincubation in Ca2+-depleting solution. Sometimes control titin preparations contained only T2-fragment, but generally they contained ~5-20% N2B-isoform of titin along with its T2-fragment. Preparations of titin obtained from rabbit cardiac muscle by our method contained ~30-50% of N2BA- and N2B-titin isoforms along with its T2-fragment. The content of α-structures in titin isolated by our method was increased. Actomyosin ATPase activity in vitro increased in the presence of titin preparations containing more intact molecules. This result confirms the significant role of titin in the regulation of actin–myosin interaction in muscles. The method used by us to preserve titin might be used for isolation of other proteins that are substrates of Ca2+-dependent proteases.
KEY WORDS: striated muscles of mammals, NT-, N2A-, N2BA- and N2B-isoforms of titin, T2-fragments, actin-activated ATPase activity of myosin, Ca2+-depleting solutionDOI: 10.1134/S0006297913050039
Abbreviations: ATPase, adenosine triphosphatase; CD, circular dichroism; CaD-solution, Ca2+-depleting solution; LV, left ventricle; MHC, myosin heavy chains; μ, ionic strength; S1, myosin subfragment 1; SR, sarcoplasmic reticulum.
Titin (connectin) [1, 2] is a
giant elastic protein in the sarcomeres of striated muscles of
vertebrates. It plays an important role in the formation and
maintenance of a highly ordered sarcomeric structure, and it is also
involved in the regulation of actin–myosin interactions and
intracellular signaling processes [3, 4]. Titin molecules cover half of the sarcomere from
the M-line to the Z-disc and form the third filament system in
myofibrils. Titin is bound to myosin filaments in the sarcomere A-zone
[5]. This part of the titin molecule was named T2.
Titin interacts with thin filaments in the sarcomere I-zone close to
the Z-disc [6], but the greater part of its
molecule passes freely in this zone, forming a flexible connection
between the ends of the myosin filaments and the Z-membrane.
The discovery of titin stimulated studies of the structure and functional properties of this protein. The first in vitro studies of molecular parameters of titin, its secondary structure, amino acid composition, degree of phosphorylation, solubility, proteolytic degradation, interaction with contractile proteins, and influence on actin-activated myosin ATPase activity were conducted in the 1980s on T2-fragments of the protein (β-connectin, MW ~ 2000 kDa) [7-16]. In particular, an activating effect of titin on actomyosin ATPase was found in vitro [7]. This result pointed to the possible involvement of titin in the regulation of actin–myosin interaction in muscle.
Later, some authors reported the extraction of intact titin-1 (T1 or α-connectin, MW ~ 3000 kDa); studies were conducted to determine its amino acid composition, secondary structure, interaction with a number of sarcomeric proteins, and its influence on actomyosin ATPase [17-24]. The content of sulfhydryl groups in titin-1 [21] and its molecular parameters [20, 25, 26] were studied. In particular, electron microscopy showed the length of the straightened individual T1 molecules to be 900-1000 nm [20, 26]. However, the length of α-connectin molecule (T1) was assumed to be up to 1250 nm [25]. Further studies confirmed this assumption. Some titin molecules from rabbit striated muscles were shown to be ~1300 nm long [26].
A number of T1 isoforms, i.e. N2B, N2BA, and N2A with MW 3000-3700 kDa were later discovered in mammalian striated muscles [3, 4]. Recently, titin isoforms with molecular weight ~3750-3900 kDa were discovered in muscles of mutant rats [27, 28]. Our own research conducted on mammalian striated muscles has shown the presence of titin isoforms of higher molecular weight (NT-isoforms) along with the known N2B-, N2BA-, and N2A-isoforms [4, 29-31]. We previously extracted different titin isoforms from mammalian striated muscles by method [20] to study their structural and functional properties. We expected to get titin preparations containing different amounts of its isoforms and T2-fragments. However, the results of our electron-microscopic and electrophoretic studies showed the preparations of skeletal muscle titin with the majority of molecules being about 900-1000 nm long, and individual molecules being over 1 µm, to contain only T2-fragments [32] (Fig. 1). Cardiac titin preparations contained, besides T2, no more than 7% of its N2B isoform [31, 32]. Our subsequent research focused on the search for approaches to the isolation of intact titin molecules in an amount sufficient to study their structural and functional properties. In this paper, we suggest a new method for isolation of titin from mammalian heart muscle that provides protein preparations containing ~50% of N2BA- and N2B-isoforms.
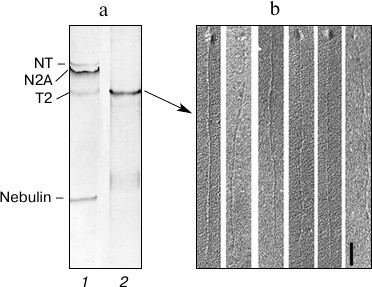
Fig. 1. Isolation of titin from rabbit skeletal muscle and preparation of straightened protein molecules. a) Electrophoretic analysis of titin in 1.9% polyacrylamide gel strengthened by agarose: 1) rabbit m. psoas; 2) titin preparation isolated from rabbit m. psoas. b) Electron micrographs of straightened titin molecules obtained by method [26]. Scale, 100 nm.
MATERIALS AND METHODS
We suggest pre-incubating muscle tissue for 2-3 weeks at 4°C in Ca2+-depleting solution to reduce the activity of Ca2+-dependent calpain proteases (prior to homogenization). Preparations of rabbit heart muscle myofibrils with transverse bands of myosin-binding protein C (MyBP-C) were first obtained by using this approach [33]. Titin, as well as MyBP-C, is known to be a substrate of Ca2+-dependent calpain proteases [34, 35]. Calcium ions were suggested to play an important role in the process of muscle protein turnover, which starts with a Ca2+-dependent splitting of titin and other proteins of thick and thin filaments [35]. Given this, we assumed that prolonged incubation of muscle tissue in a Ca2+-depleting solution would reduce substantially titin proteolysis prior to homogenization as well as in the process of its isolation, which would result in preparations of this protein with high content of intact molecules. The Ca2+-depleting solution (CaD-solution) contained 100 mM NaCl, 2 mM MgCl2, 2 mM KCl (Panreac, Spain), 4.3 mM Na2HPO4, 1.7 mM NaH2PO4 (Reakhim, Russia), 2 mM EGTA, 0.1% (w/v) glucose, 0.2-0.3 μl/ml complex of protease inhibitors (Sigma, USA), 2 mM DTT (DIA-M, Germany), pH 7.0.
Striated muscles of rabbit and long-tailed ground squirrel (Spermophilus undulatus) were used for the experiments. Animals were kept in a vivarium in individual cages under natural photoperiod. Food, water and nesting material were provided ad libitum. All the procedures with experimental animals were approved by the Commission on Biomedical Ethics of the Institute of Theoretical and Experimental Biophysics, Russian Academy of Sciences. The following muscles were used for titin isolation: left ventricle (LV), the m. psoas fiber bundles, 5.0 × 0.5 cm strips of back muscle (m. longissimus dorsi), whole muscles soleus and gastrocnemius. These skeletal muscles as well as LV pieces (~2 × 2 mm) were incubated for 1-21 days in CaD-solution at 4°C. In most experiments, stretched skeletal muscles were fixed on glass rods, and in some cases they were not fixed. The solution to muscle ratio was 25 : 1 (v/w). The CaD-solution was changed every 1.0-1.5 h during the first day of the experiment and later once a day. After the end of the incubation, the muscle tissue was homogenized, and titin was isolated by the method described in [20]. Titin was purified on a 1 × 60 cm column with Sepharose CL-2B (Sigma).
Macroporous 1.9-2.3% polyacrylamide gel in the presence of SDS containing 0.5-0.6% agarose prepared by method [36] with our modifications [4] was used for electrophoretic separation of high molecular weight titin isoforms. The purity of isolated titin preparations was tested by electrophoresis in the aforementioned macroporous gel as well as in 6.5-7.0% polyacrylamide gel in the presence of SDS by the method described in [37]. Electrophoretic samples were prepared by the method described in [4], which does not include boiling. Electrophoresis was performed in devices from Helicon (Russia) and C.B.S. Scientific Co. (USA) with vertically positioned gel of 8 × 10 cm. Gels stained with Coomassie Brilliant Blue (G-250 and R-250, a 1 : 1 mixture) were scanned; these scans were later processed using a densitometric approach using the Total Lab v. 1.11 computer program.
Circular dichroism (CD) spectra were registered on a J-815 CD spectrometer (Jasco, Japan). Measurements were performed at 4°C in a quartz cell with optical path length of 1 mm in buffer containing 0.6 M KCl, 30 mM KH2PO4, 1 mM DTT, pH 7.0. The volume of the solution was 200 μl. The titin concentration in preparations used for the experiments was 0.09-0.12 mg/ml. The molecular weight of one amino acid was assumed to be 100 Da. Difference spectra for titin N2B isoform were obtained by subtracting the CD spectrum characteristic for T2 from the CD spectrum of the protein preparation containing approximately equal amounts of T2 fragment and N2B isoform of this protein.
The effect of cardiac titin on actin-activated myosin ATPase activity in vitro was studied by a previously described method [31].
RESULTS AND DISCUSSION
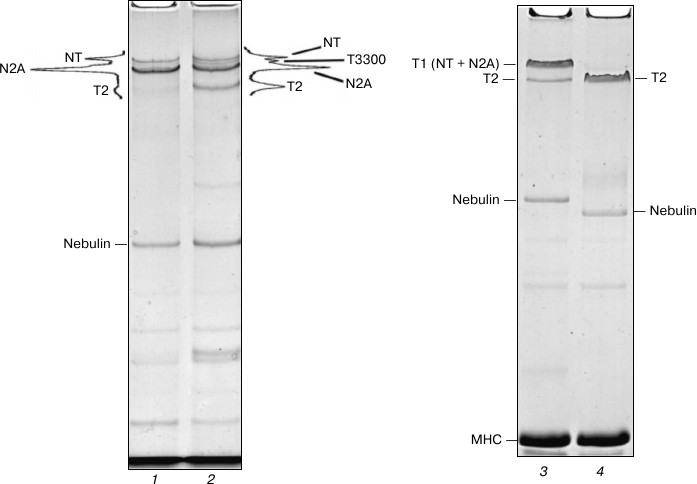
Destruction of NT and N2A titin isoforms during incubation of skeletal muscles in CaD-solution. We believed that prolonged incubation of skeletal muscles in the Ca2+-depleting solution, aimed at reduction of post mortem proteolysis of NT and N2A titin isoforms, would help us obtain preparations of this protein with high content of intact molecules. However, the results did not confirm this assumption. In particular, we discovered a nearly complete degradation of NT titin isoform and significant decrease (by 2-4 times) of its N2A isoform content in small pieces of skeletal muscles (weighing 50-100 mg) after the first day of their incubation in a CaD-solution (data not shown). Given these data, for further experiments we used not shredded samples of muscle tissue, but fiber bundles of psoas muscle, strips of back muscle longissimus dorsi, and intact soleus and gastrocnemius muscles. This, however, also did not give positive results. In particular, we observed a significant degradation of NT and N2A titin isoforms in these muscles after the third day of incubation, this phenomenon being accompanied by the appearance of a ~3300 kDa fragment (T3300) and 3-4-fold increase in the content of titin T2 fragments on electrophoregrams (Fig. 2, lanes 1 and 2). Complete destruction of NT and N2A titin isoforms to T2 fragments was observed in the studied skeletal muscles after eight days of incubation (Fig. 2, lanes 3 and 4). Therefore, there seems to be needed a further search for approaches that would reduce post mortem proteolysis of skeletal titin isoforms, which, in turn, would make it possible to isolate them.
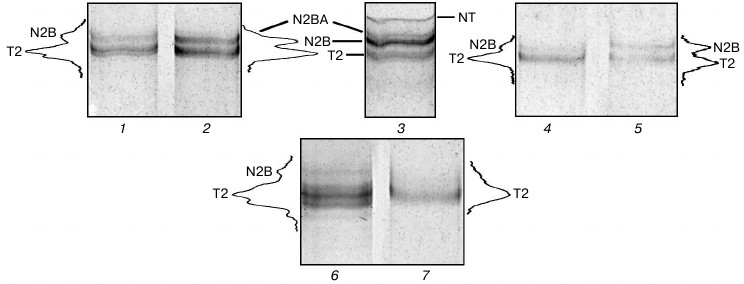
Fig. 2. Destruction of NT and N2A titin isoforms during incubation of rabbit and ground squirrel skeletal muscles in CaD-solution. Electrophoresis was performed in 2.0-2.1% polyacrylamide gel strengthened by agarose: 1) ground squirrel m. soleus (control); 2) ground squirrel m. soleus (3-day-long incubation; the muscle was fixed in a stretched position on a glass rod); 3) rabbit m. longissimus dorsi (control); 4) rabbit m. longissimus dorsi (14-day-long incubation). T3300 – supposedly a fragment of NT titin isoform (~3300 kDa). For more detailed information on the estimates of molecular weight of titin isoforms and their fragments see [4, 29, 30].
To conclude this part of the presentation of our experimental data, we would like to note an interesting fact. We discovered that NT and N2A titin isoforms in skeletal muscles degraded more slowly when being fixed on glass rods in a stretched position (in contrast to non-fixed muscles, data not shown). So far we have not been able to find an explanation for this experimental fact.
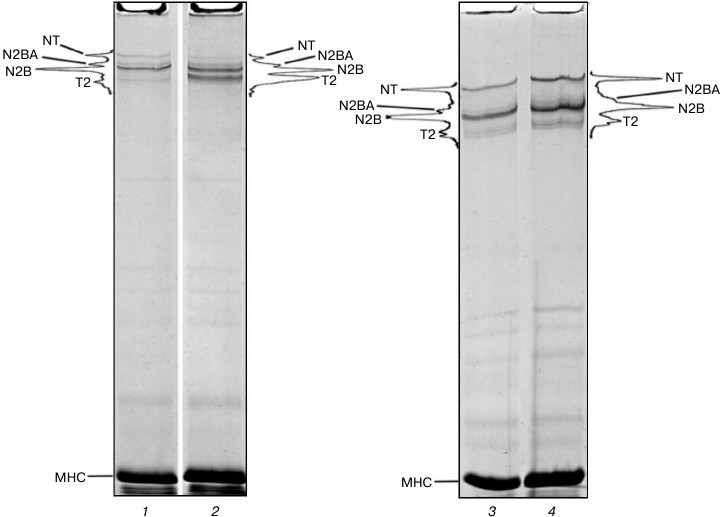
Preservation of titin isoforms in cardiac muscle incubated in CaD-solution. Positive results were obtained in experiments with cardiac muscle. In some cases (n = 2) no reliable changes in the content of NT, N2BA, and N2B titin isoforms were found after 2-3-week-long incubation of myocardial samples in CaD-solution (Fig. 3, lanes 3 and 4). In most cases (n = 4) still there was a decrease (2-2.5-fold) in the relative content of N2BA and NT titin isoforms, the process being accompanied by increase in the content of T2 fragments of this protein (Figs. 3 (lanes 1 and 2) and 4a (lanes 1-3)). However, the total content of cardiac titin isoforms decreased by no more than ~1.5 times, and all three isoforms were clearly visible on electrophoregrams (Figs. 3 (lane 2) and 4a (lanes 2 and 3)).
Fig. 3. Preservation of NT, N2BA, and N2B titin isoforms in rabbit and ground squirrel left ventricle after prolonged incubation of the muscle tissue in CaD-solution. Electrophoresis was performed in 2.2% polyacrylamide gel strengthened by agarose: 1) rabbit LV (control); 2) rabbit LV (14-day-long incubation); 3) ground squirrel LV (control); 4) ground squirrel LV (14-day-long incubation).
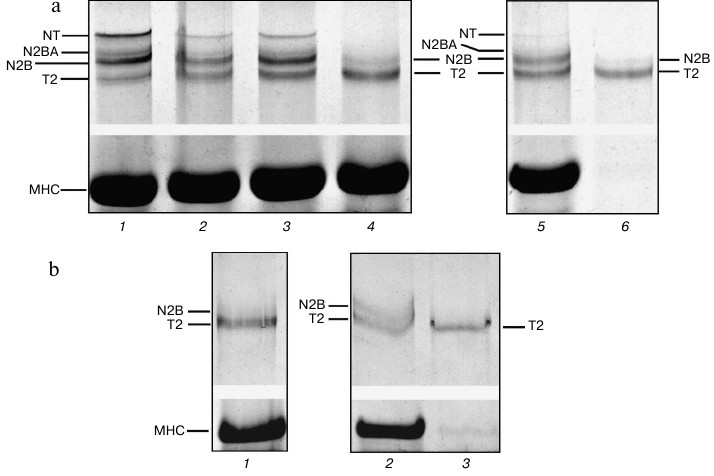
Fig. 4. Isolation of ground squirrel cardiac muscle titin. Electrophoresis was performed 2.1-2.3% polyacrylamide gel strengthened by agarose. a) Muscle incubated in CaD-solution: 1) LV (control); 2) LV (21-day-long incubation); 3) cardiac muscle homogenate (ionic strength ~0.05); 4) extract (ionic strength of the solution ~0.6), protein bands of T2 fragment and N2B titin isoform can be seen; 5) muscle residuum after extraction, protein bands of T2 fragment, N2B, N2BA, and NT titin isoforms can be seen; 6) titin preparation not purified on the column, containing T2 fragment and N2B titin isoform. b) Muscle not incubated in CaD-solution: 1) extract (ionic strength ~0.6), protein bands of T2 fragment can be seen. The content of N2B titin isoform is very low; 2) muscle residuum after extraction, protein bands of N2BA and NT titin isoforms are absent; 3) titin preparation not purified on the column, containing only T2 fragment.
What can explain the difference between titin preservation in cardiac muscle and its destruction in skeletal muscles during their incubation in CaD-solution? This difference seems likely to arise from different types of electromechanical coupling in these muscles. In particular, in the case of skeletal muscles calcium release from the sarcoplasmic reticulum (SR) follows after depolarization of the outer membrane. In heart muscle, this process is Ca2+-dependent (CICR, calcium-induced calcium release). That is, in cardiac cells calcium release from SR through ryanodine receptors occurs after uptake of external calcium through activated potential-dependent calcium channels of L-type located in the outer membrane. In our experiments, incubation of muscle in a Ca2+-depleting solution is likely to have caused a significant decrease in Ca2+ release from SR in cardiomyocytes while having no effect on its diffusion from SR in skeletal muscles. This could be the reason for the more pronounced proteolysis of titin isoforms from skeletal than from cardiac muscle (Figs. 2 and 3).
Isolation of cardiac titin isoforms and study of their structural and functional properties. Cardiac titin isoforms remaining after prolonged incubation of muscles in CaD-solution were isolated by the method of Soteriou et al. [20]. Several titin isolations from cardiac muscle without its prior incubation in CaD-solution were used for control experiments. Results of these experiments showed the possibility of obtaining crude (pre-columnar) and purified by gel filtration on a column with Sepharose CL-2B (columnar) preparations of rabbit cardiac muscle titin, which along with T2 fragments contained ~5-10% (n = 3) and ~17-20% (n = 1) of N2B isoform of this protein (Fig. 5, lanes 1, 4, and 6). In some cases (n = 2) purified preparations of rabbit cardiac titin (Fig. 5, lane 7) as well as preparations of crude and purified ground squirrel titin (Fig. 4b, lane 3, n = 2) contained only T2 fragments. Thus, no titin preparations containing its NT and N2BA isoforms were obtained in control experiments.
Fig. 5. Isolation of titin isoforms from rabbit cardiac muscle. Electrophoresis was performed in 2.1-2.2% polyacrylamide gel strengthened by agarose: 1, 4, 6, 7) control preparations of crude (1, 6) and purified on Sepharose CL-2B column (4, 7) titin isolated from rabbit LV by method [20] without muscle tissue pre-incubation in CaD-solution; 2, 5) preparations of crude (2) and purified on Sepharose CL-2B column (5) titin isolated from rabbit LV after muscle tissue incubation in CaD-solution; 3) rabbit LV (control). Electrophoretic samples containing titin preparations (1, 2, 4-7) consisted of 3-7 parts of sample solution (1.0-1.2% SDS, 2% β-mercaptoethanol or 65-75 mM DTT, 10% glycerol, 8-10 μg/ml leupeptin or E64, 8-10 mM Tris-HCl, pH 6.8-7.0) and one part of protein dissolved in 0.6 M KCl, 30 mM KH2PO4, 1 mM DTT, pH 7.0.
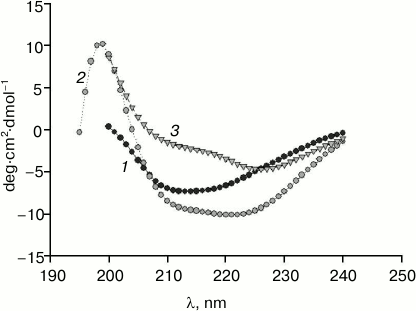
Crude preparations of rabbit cardiac muscle titin, containing not only N2B isoform, but also N2BA isoform of this protein, were obtained by our method (Fig. 5, lane 2). In the case of purified titin preparations obtained by our method, only N2B titin isoform was present (in addition to T2 fragments; Fig. 5, lane 5). However, its content was ~30-35% (n = 2) and ~47% (n = 1) (Fig. 5, lane 5), which was ~2-5-fold higher than the content of this isoform in control titin preparations (Fig. 5, lanes 4 and 7). Preparations of pre-columnar and columnar titin from ground squirrel cardiac muscle obtained by our method contained about 10-15% of N2B isoform (n = 2) (Fig. 4a, lane 6) in contrast to control preparations containing only T2 (Fig. 4b, lane 3). Unfortunately, we could not obtain titin preparations containing NT isoform of this protein. However, in contrast to the method of Soteriou et al. [20], our approach allowed us to obtain preparations of cardiac titin with a higher content of intact molecules. The relatively high content of N2B isoform in columnar preparations of rabbit cardiac muscle titin made it possible to study its secondary structure and functional properties in comparison with T2 fragment. In particular, difference spectra for titin N2B isoform were obtained by circular dichroism, showing an increase in α-structure in N2B isoform (Fig. 6, spectrum with triangles). Sites of N2B titin isoform located in the I-zone of the sarcomere are likely to contribute to these changes.
Fig. 6. CD spectra of rabbit cardiac muscle titin: 1) titin preparation containing only T2 fragment; 2) titin preparation containing N2B isoform (~47%) and T2 fragment (~53%); 3) difference spectra for titin N2B isoform.
Higher ATPase actomyosin activity in vitro was found in the presence of titin preparations with higher content of intact molecules. In particular, titin preparations with more than 30% of N2B isoform were shown to activate ATPase activity of actomyosin by factor of 1.75-1.85 (n = 2). Titin preparations with no more than 20% of N2B isoform increased actin-activated myosin enzymatic activity to a lesser degree (1.30-1.45 times, n = 3). What is the possible explanation for these differences? Titin is known to bind with filaments of not only myosin [7, 12], but also actin [23]. In this case, two processes will support actin–myosin interaction in vitro: ATP-sensitive interaction of myosin subfragment-1 (S1) with actin, and actin–titin–myosin interaction. The latter will facilitate actin and myosin filaments staying close to each other during the ATP hydrolysis cycle, despite periodic dissociation of S1–actin complex, which is likely to cause an increase in actomyosin enzymatic activity. Taking this into account, we assume that the found differences in titin effects on actomyosin ATPase to be caused by N2B titin isoform (primarily its sites located in the sarcomere I-zone) binding to actin filaments stronger than T2 fragment binding to the same filaments. Some data confirm this assumption: immunoglobulin-like (IgC2) titin domains located mainly in I-zone [38], as well as PEVK-domain located in this sarcomere zone [39-41], were shown to bind actin. Our results do not contradict the cited data and indicate the important role of titin in regulation of actin–myosin interaction in muscles.
However, there remains a question why we could not isolate NT titin isoform and obtain columnar preparations containing N2BA isoform of this protein in our experiments. To answer this question, we performed comparative electrophoretic testing of titin content changes in the course of isolation of this protein from ground squirrel cardiac muscle by two different methods. The results of control experiments revealed significant degradation of titin isoforms at the stage of protein extraction from muscle homogenate (μ ~ 0.6) (Fig. 4b, lanes 1 and 2). In particular, we observed complete destruction of NT and N2BA titin isoforms, which were absent both in the muscle residuum (Fig. 4b, lane 2) and muscle extract (Fig. 4b, lane 1), containing T2 fragment and a small amount of N2B isoform. We obtained similar data confirming the destruction of N2A and NT titin isoforms at the stage of extraction (and not homogenization) in the course of isolation of this protein from skeletal muscles of the tested animals (data not shown). There was one more important result obtained in this series of experiments that seems to be worth noting. A significant amount of T2 fragment as well as N2B titin isoform was found to be lost with the precipitate at the stage of removal of actomyosin from muscle extract (μ ~ 0.2). To reduce losses of titin at this stage of isolation, we suggest precipitating the protein not at 20,000g as suggested by the authors of [20], but rather at 5000g.
Isolation of titin by our method – after incubation of cardiac muscle in CaD-solution – preserved high content of all titin isoforms in muscle residuum after protein extraction (Fig. 4a, lane 5). However, only T2 fragment and N2B titin isoform were present in the extract (there were no NT and N2BA isoforms) (Fig. 4a, lane 4).
Thus, on one hand, our results have shown that incubation of cardiac muscle in CaD-solution before homogenization to reduce titin proteolytic degradation during isolation allows obtaining these protein preparations with high content of intact molecules. We believe that this method should be used for isolation of other proteins that are substrates for Ca2+-dependent proteases, in particular, nebulin, tropomyosin, troponin and myosin-binding C- and M-proteins [35]. On the other hand, our results, in particular, those presented in Fig. 4, have set a new research problem: the search for approaches to increase the extraction of titin intact molecules from muscle tissue. In our future research we will concentrate on solution of this scientific task as well as further search for approaches reducing titin proteolysis, especially in skeletal muscles.
This work was supported by grants from the Russian Foundation for Basic Research (10-04-00141, 11-04-01026) and a grant of the Federal Target Program “Research and Scientific-Pedagogical Personnel of Innovative Russia” (grant agreement No. 8600) with the use of equipment of the Shared Access Center of the Institute of Theoretical and Experimental Biophysics of the Russian Academy of Sciences.
REFERENCES
1.Wang, K., McClure, J., and Tu, A. (1979) Proc.
Natl. Acad. Sci. USA, 76, 3698-3702.
2.Maruyama, K., Kimura, S., Ohashi, K., and Kuwano,
Y. (1981) J. Biochem., 89, 701-709.
3.Kruger, M., and Linke, W. A. (2011) J. Biol.
Chem., 286, 9905-9912.
4.Vikhlyantsev, I. M., and Podlubnaya, Z. A. (2012)
Biochemistry (Moscow), 77, 1515-1535.
5.Houmeida, A., Holt, J., Tskhovrebova, L., and
Trinick, J. (1995) J. Cell Biol., 131, 1471-1481.
6.Trombitas, K., Pollack, G. H., Wright, J., and
Wang, K. (1993) Cell Motil. Cytoskeleton, 24,
274-283.
7.Kimura, S., Maruyama, K., and Huang, Y. P. (1984)
Biochem. J., 96, 499-506.
8.Wang, K., Ramirez-Mitchell, R., and Palter, D.
(1984) Proc. Natl. Acad. Sci. USA, 81, 3685-3689.
9.Trinick, J., Knight, P., and Whiting, A. (1984)
J. Mol. Biol., 180, 331-356.
10.Maruyama, K., Kimura, S., Yoshidomi, H., Sawada,
H., and Kikuchi, M. (1984) J. Biochem., 95,
1423-1433.
11.Wang, K. (1985) Cell Muscle Motil.,
6, 315-369.
12.Maruyama, K. (1986) Int. Rev. Cytol.,
104, 81-114.
13.Itoh, Y., Kimura, S., Suzuki, T., Ohashi, K., and
Maruyama, K. (1986) J. Biochem., 100, 439-447.
14.Maruyama, K., Hu, D. H., Suzuki, T., and Kimura,
S. (1987) J. Biochem., 101, 1339-1346.
15.Murayama, T., Nakauchi, Y., Kimura, S., and
Maruyama, K. (1989) J. Biochem., 105, 323-326.
16.Nave, R., Furst, D. O., and Weber, K. (1989)
J. Cell. Biol., 109, 2177-2187.
17.Kimura, S., and Maruyama, K. (1989) J.
Biochem., 106, 952-954.
18.Matsuura, T., Kimura, S., Ohtsuka, S., and
Maruyama, K. (1991) J. Biochem., 110, 474-478.
19.Kimura, S., Matsuura, T., Ohtsuka, S., Nakauchi,
Y., Matsuno, A., and Maruyama, K. (1992) J. Muscle Res. Cell
Motil., 13, 39-47.
20.Soteriou, A., Gamage, M., and Trinick, J. (1993)
J. Cell Sci., 14, 119-123.
21.Pan, K. M., Damodaran, S., and Greaser, M. L.
(1994) Biochemistry, 33, 8255-8261.
22.Vikhlyantsev, I. M., Makarenko, I. V., Khalina,
Ya. N., Udaltsov, S. N., Malyshev, S. L., and Podlubnaya, Z. A. (2000)
Biophysics, 45, 805-809.
23.Podlubnaya, Z. A., Shpagina, M. D., Vikhlyantsev,
I. M., Malyshev, S. L., Udaltsov, S. N., Ziegler, C., and Beinbrech, G.
(2003) Insect Biochem. Mol. Biol., 33, 789-793.
24.Vikhlyantsev, I. M., and Podlubnaya, Z. A. (2003)
Biophysics, 48, 471-476.
25.Suzuki, J., Kimura, S., and Maruyama, K. (1994)
J. Biochem., 116, 406-410.
26.Tskhovrebova, L., and Trinick, J. (1997) J.
Mol. Biol., 265, 100-106.
27.Greaser, M. L., Warren, C. M., Esbona, K., Guo,
W., Duan, Y., Parrish, A. M., Krzesinski, P. R., Norman, H. S.,
Dunning, S., Fitzsimons, D. P., and Moss, R. L. (2008) J. Mol. Cell
Cardiol., 44, 983-991.
28.Li, S., Guo, W., Schmitt, B., and Greaser, M. L.
(2012) J. Cell Biochem., 113, 1265-1273.
29.Vikhlyantsev, I. M., and Podlubnaya, Z. A. (2006)
Biophysics, 51, 842-848.
30.Vikhlyantsev, I. M., and Podlubnaya, Z. A. (2008)
Biophysics, 53, 592-597.
31.Vikhlyantsev, I. M., Okuneva, A. D.,
Shpagina, M. D., Shumilina, Yu. V., Molochkov, N. V., Salmov, N.
N., and Podlubnaya, Z. A. (2011) Biochemistry (Moscow),
76, 1312-1320.
32.Vikhlyantsev, I. M. (2011) Polymorphism of
Striated Muscle Titin in Norm, Adaptation and Pathology: Doctoral
dissertation [in Russian], Institute of Theoretical and Experimental
Biophysics, Pushchino.
33.Podlubnaya, Z. A., Shpagina, M. D., and Lednev,
V. V. (1989) J. Mol. Biol., 210, 655-658.
34.Verburg, E., Murphy, R. M., Stephenson, D. G.,
and Lamb, G. D. (2005) J. Physiol., 564, 775-789.
35.Goll, D. E., Neti, G., Mares, S. W., and
Thompson, V. F. (2008) J. Anim. Sci., 86, E19-E35.
36.Tatsumi, R., and Hattori, A. (1995) Anal.
Biochem., 224, 28-31.
37.Fritz, J. D., Swartz, D. R., and Greaser, M. L.
(1989) Analyt. Biochem., 180, 205-210.
38.Jin, J. P. (2000) Adv. Exp. Med. Biol.,
481, 319-333, discussion 334-335.
39.Kulke, M., Fujita-Becker, S., Rostkova, E.,
Neagoe, C., Labeit, D., Manstein, D. J., Gautel, M., and Linke, W. A.
(2001) Circ. Res., 89, 874-881.
40.Bianco, P., Nagy, A., Kengyel, A., Szatmari, D.,
Martonfalvi, Z., Huber, T., and Kellermayer, M. S. (2007) Biophys.
J., 93, 2102-2109.
41.Chung, C. S., Bogomolovas, J., Gasch, A.,
Hidalgo, C. G., Labeit, S., and Granzier, H. L. (2011) J. Biomed.
Biotechnol., 2011, Article ID 310791, 8 pages, Epub 2011 Nov
15, doi: 10.1155/2011/310791.