Extracellular Phosphomannan as a Phosphate Reserve in the Yeast Kuraishia capsulata
L. P. Lichko, T. V. Kulakovskaya*, and I. S. Kulaev
Skryabin Institute of Biochemistry and Physiology of Microorganisms, Russian Academy of Sciences, pr. Nauki 5, 142290 Pushchino, Moscow Region, Russia; fax: (495) 956-3370; E-mail: alla@ibpm.pushchino.ru* To whom correspondence should be addressed.
Received February 21, 2013; Revision received February 26, 2013
We have found that extracellular phosphomannan is the main phosphate reserve in the yeast Kuraishia capsulata, in contrast to other yeast species effectively absorbing Pi. Under nitrogen starvation, K. capsulata absorbed essentially all Pi from the medium containing 240 mM glucose, 2.5 mM MgSO4, and 11 mM KH2PO4. Inorganic polyphosphate level in the cells was about 14% of the Pi absorbed. Most of the Pi (~60%) was found in the fraction of extracellular phosphomannan that can be used as a carbon and phosphorus source by this yeast in deficient media.
KEY WORDS: inorganic polyphosphate, phosphomannan, phosphate accumulation, nitrogen deficit, yeast, Kuraishia capsulataDOI: 10.1134/S0006297913060138
Phosphorus is a vital element and, therefore, microorganisms are able to reserve phosphorus to use it under phosphate depletion in the medium. Reserve phosphate compounds are highly variable in both chemical nature and localization in microorganisms. Thus, the halophilic archae form extracellular poorly soluble salts MgPO4OH·4H2O, and bacteria of the genus Brevibacterium accumulate NH4MgPO4·6H2O within their cells [1, 2]. In Acetobacter xylinum, orthophosphate or high molecular weight inorganic polyphosphates dominate as reserve phosphorus compounds depending on cultivation conditions [3]. Many bacteria and yeast accumulate inorganic polyphosphates as reserve phosphorus compounds [4]. In contrast to prokaryotes, the biodiversity of reserve phosphorus compounds in yeasts is still poorly studied.
The objective of the present work was to study the peculiarities of reserving phosphorus compounds by the yeast Kuraishia capsulata capable of synthesizing extracellular phosphomannan.
MATERIALS AND METHODS
Strains and culture conditions. The object of research was the yeast Kuraishia (Hansenula) capsulata VKM Y-2514 from the All-Russian Collection of Microorganisms. The yeast kept on malt agar slants was grown at 30°C on a circulatory shaker at 140 rpm for 24 h (stationary phase) in the YPD medium (2% glucose, 1% yeast extract, 2% peptone). Then the biomass was separated from the culture medium by 15-min centrifugation at 13,000g, washed with 1% KCl solution, weighed, and used in experiments for determination of Pi uptake.
The cells of K. capsulata (10 g of wet biomass per liter) were placed in 50 ml of the medium containing 240 mM glucose, 2.5 mM MgSO4, and KH2PO4 (concentrations are indicated in the tables and figures) and incubated at 30°C and 140 rpm. After the incubation, the cells were harvested by 15-min centrifugation at 13,000g, washed with 1% KCl solution, and used for analysis. The resulting supernatant was used for the assay of phosphorus compounds, for isolation of phosphomannan, and as a carbon and phosphate source in the experiments described below.
Phosphomannan utilization was studied by cultivating K. capsulata cells at 30°C and 140 rpm in a medium (1 liter) supplemented with 3 g of (NH4)2SO4, 0.7 g of MgSO4, 0.4 g of Ca(NO3)2, 1.31 g of K2SO4, 0.5 ml of microelement solution [5], and 0.5 g of yeast extract from which Pi had been removed by the method described earlier [6]. Glucose or phosphomannan-containing supernatant was added as a carbon source.
Phosphomannan isolation. The cells of K. capsulata were incubated for 24 h in a shaker (140 rpm) at 30°C in the medium containing 240 mM glucose, 2.5 mM MgSO4, and 10 mM KH2PO4. Phosphomannan was isolated from the supernatant obtained after precipitation of the cells according to the method described earlier [7]. Sodium borate (8 ml of 5% solution) and cetavlon (8 ml of 5% solution) were added to 20 ml of the supernatant. The precipitate was washed with water, dissolved in 5 ml of concentrated acetic acid, lyophilized, and weighed for phosphomannan quantification.
Analysis of phosphorus compounds. Several polyphosphate fractions were isolated from the biomass. Acid-soluble and salt-soluble fractions were obtained by a known method [5]. Acid-insoluble polyphosphates were extracted by adding 10 ml of 0.5 N HClO4 to residual biomass, followed by boiling for 30 min. The suspension was centrifuged at 5000g for 20 min, and polyphosphate level in the supernatant was estimated by the amount of Pi. The levels of Pi and labile phosphorus were determined in the fractions of acid-soluble and salt-soluble polyphosphates [8]. The polyphosphate levels can be judged by the amount of labile phosphorus. The level of total phosphorus was determined in the biomass. Inorganic Pi and labile and total phosphorus were determined in the supernatant after incubation of the cells with glucose, MgSO4, and KH2PO4. The samples were mineralized in 32% HClO4 at 150°C to assay total phosphorus by the level of released Pi. The optical density of the culture was determined at 600 nm in a 1-cm cell. The biomass weight was determined after 15-min centrifugation of the cells at 13,000g.
The mean values of three independent experiments are presented in the tables and figures.
RESULTS AND DISCUSSION
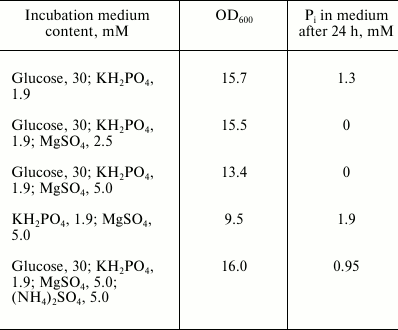
The model conditions used previously to study this process in bacteria and other yeasts [2, 9] were used to analyze the Pi absorption capacity of the yeast K. capsulata in minimal media. Similar to other yeast species [9], K. capsulata needed the carbon and Mg2+ sources for Pi uptake (Table 1). On addition of a nitrogen source, Pi uptake decreased.
Table 1. Compositions of incubation media
for the study of Pi accumulation by the cells of K.
capsulata. The initial optical density at 600 nm was 10.5.
Incubation time was 24 h
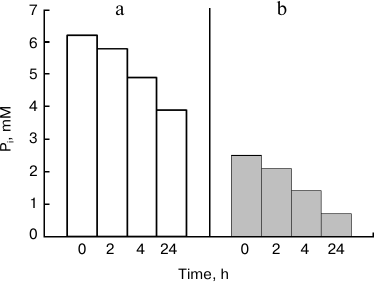
Under the used model conditions, Pi concentration in the medium (Fig. 1) decreased more slowly than in other yeast species studied previously [9]. Substantial decrease in Pi concentration was reached after 24 h of incubation. Incubation of cells of Saccharomyces cerevisiae and Cryptococcus humicola under the same conditions was accompanied by decrease in Pi concentration (5 mM) by 80% during 5 h [9]. The 24-h incubation period was chosen for further experiments with K. capsulata.
Fig. 1. Influence of incubation time on decrease in Pi concentration under incubation of K. capsulata cells in the presence of 30 mM glucose and 5 mM MgSO4: a) the initial Pi concentration was 6.2 mM; b) the initial Pi concentration was 2.5 mM.
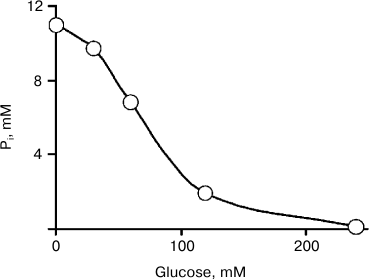
The dependence of Pi concentration on glucose concentration in the medium is shown in Fig. 2. In the medium containing 240 mM glucose and 2.5 mM Mg2+, Pi concentration decreased from 11 to 0.12 mM in 24 h. The cells of K. capsulata effectively decreased Pi in the medium, similar to the cells of other species [9].
Fig. 2. Dependence of Pi uptake by K. capsulata cells on glucose concentration. The incubation medium contained 2.5 mM MgSO4. The incubation time was 24 h.
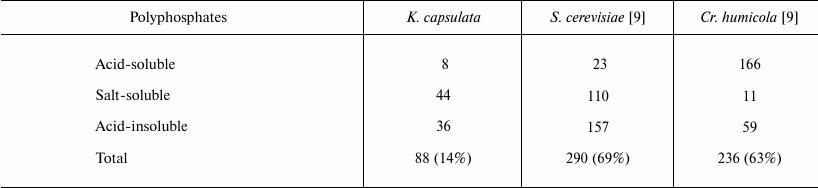
We tried to reveal the phosphorus compounds accumulated in K. capsulata. Table 2 demonstrates the data on the content of different polyphosphate fractions in K. capsulata under nitrogen starvation. Polyphosphate level in the cells of K. capsulata was lower compared to those of S. cerevisiae and Cr. humicola (Table 2). The distinctive feature of this yeast was a salt-soluble fraction representing the greater part of the polyphosphates. The polyphosphate content was more than 60% of the accumulated Pi in S. cerevisiae and Cr. humicola but only 14% in K. capsulata under the same model conditions (Table 2). Therefore, polyphosphates cannot be considered as the main phosphorus reserve of K. capsulata.
Table 2. Polyphosphate content in different
fractions (µmol/g wet weight) of K. capsulata, S.
cerevisiae, and Cr. humicola under the model conditions with
carbon and phosphate excess and nitrogen starvation. The total polyP
percentage of the initial Pi in the medium is given in
parentheses. The incubation medium contained 240 mM glucose,
11 mM Pi, and 2.5 mM Mg2+; incubation
time was 24 h
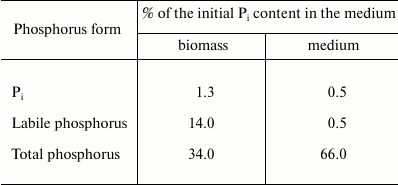
It is known that a special feature of this yeast is formation of extracellular phosphomannan [10]. We suggested that Pi was used for the synthesis of this polymer under growth-limiting nitrogen starvation, since the medium was rich in the carbon source and Pi. Different phosphorus forms were determined in the biomass and supernatant after the incubation of K. capsulata to test this assumption (Table 3). The content of Pi in the biomass and supernatant was low compared to that in the starting medium. In contrast to the biomass, the incubation medium contained no labile phosphorus, indicating the absence of polyphosphates. It is notable that the medium has a high content (66%) of organic phosphorus compounds, the phosphorus of which can be determined only after mineralization. We suggested that the total phosphorus of the medium was present as phosphomannan.
Table 3. Distribution of Pi,
labile and total phosphorus between the biomass and the incubation
medium containing 240 mM glucose, 2.5 mM Mg2+, and
10 mM Pi; incubation time was 24 h
Phosphomannan preparation was isolated from the incubation medium using a known method [7], and its amount was 34 mg/ml under the above conditions, i.e. much above the amount produced by K. (Hansenula) capsulata in the optimal media used by other authors, 1.9 mg/ml [11]. In the work cited, a slightly higher glucose concentration (360 mM) and a substantially higher Pi concentration (36 mM) were used. It seems likely that the greater amount of phosphomannan under our conditions was a result of nitrogen starvation. These conditions are of certain biotechnological interest, since extracellular polysaccharides of the yeast can be considered as promising immunomodulators [12] and dental preparations [13].
The phosphomannan preparation obtained in our experiments contained 3.35 µmol of total phosphorus per gram of the preparation, making ~57% of total phosphorus in the incubation medium. The phosphomannan preparation contained neither Pi nor labile phosphate. Under hydrolysis of this preparation with 2 N HCl at 100°C, 25% of phosphorus was released as Pi in 2 h, followed by 64% release in 6 h. Such character of hydrolysis is in agreement with the literature data [10].
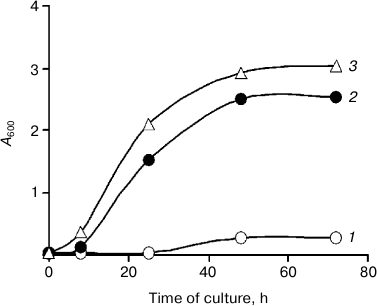
The question of the function of extracellular phosphomannan has not been discussed in other works [7, 10, 11]. We have tested the ability of K. capsulata to use phosphomannan for its growth. Kuraishia capsulata was cultivated in a Pi-deficient medium (0.1 mM Pi) with addition of a supernatant containing ~1 g of phosphomannan as the only carbon source (Fig. 3). This substrate had little effect on growth compared to glucose (Fig. 3), and K. capsulata absorbed no less than 50% of the total phosphomannan phosphorus.
Fig. 3. Growth curves of K. capsulata: 1) medium with 0.1 mM Pi and without a carbon source; 2) 120 mM glucose was added to medium (1); 3) the supernatant containing ~1 g of phosphomannan was added to medium (1).
The findings suggest that the extracellular phosphomannan of K. capsulata is a secondary metabolite that can be used by cells as a carbon and phosphorus reserve.
We are grateful to V. I. Golubev for kindly donating the strain K. capsulata.
This work was supported by the Russian Foundation for Basic Research (grant 11-04-01009) and the Program of Presidium of the Russian Academy of Sciences “Problems of Life Origin and Biosphere Formation”.
REFERENCES
1.Smirnov, A., Suzina, N., Chudinova, N.,
Kulakovskaya, T., and Kulaev, I. (2005) FEMS Microbiol. Ecol.,
52, 129-137.
2.Ryazanova, L. P., Smirnov, A. V., Kulakovskaya, T.
V., and Kulaev, I. S. (2007) Microbiology, 76,
663-669.
3.Ryazanova, L. P., Suzina, N. E., Kulakovskaya, T.
V., and Kulaev, I. S. (2009) Arch. Microbiol., 191,
467-471.
4.Kulaev, I. S., Vagabov, V. M., and Kulakovskaya, T.
V. (2004) The Biochemistry of Inorganic Polyphosphates, Wiley,
Chichester.
5.Vagabov, V. M., Trilisenko, L. V., and Kulaev,
I. S. (2000) Biochemistry (Moscow), 65, 349-354.
6.Rubin, G. M. (1973) J. Biol. Chem.,
11, 3860-3875.
7.Cawley, T. N., Harrington, M. G., and Letters, R.
(1972) Biochem. J., 129, 711-720.
8.Lichko, L., Kulakovskaya, T., Pestov, N., and
Kulaev, I. (2006) Biosci. Rep., 26, 45-54.
9.Breus, N. A., Ryazanova, L. P., Dmitriev, V. V.,
Kulakovskaya, T. V., and Kulaev, I. S. (2012) FEMS Yeast Res.,
12, 617-624.
10.Slodki, M. E., Wickerham, L. J., and Cadmus, M.
C. (1961) J. Bacteriol., 82, 269-274.
11.Avigad, G., and Kalina, M. (1979) FEMS
Microbiol. Lett., 6, 111-114.
12.Ferro, V., Fewings, K., Palermo, M. C., and Li,
C. (2001) Carbohydr. Res., 332, 183-189.
13.Shimotoyodome, A., Kobayashi, H., Nakamura, J.,
Tokimitsu, I., Hase, T., Inoue, T., Matsukubo, T., and Takaesu, Y.
(2006) Biofouling, 22, 261-268.