REVIEW: Polypotency of the Immunomodulatory Effect of Pectins
S. V. Popov* and Yu. S. Ovodov
Institute of Physiology, Komi Science Center, Ural Branch of the Russian Academy of Sciences, Pervomaiskaya ul. 50, 167982 Syktyvkar, Russia; fax: +7 (8212) 241-001; E-mail: popov@physiol.komisc.ru* To whom correspondence should be addressed.
Received February 25, 2013; Revision received March 6, 2013
Pectins are the major component of plant cell walls, and they display diverse biological activities including immunomodulation. The pectin macromolecule contains fragments of linear and branched regions of polysaccharides such as homogalacturonan, rhamnogalacturonan-I, xylogalacturonan, and apiogalacturonan. These structural features determine the effect of pectins on the immune system. The backbones of pectic macromolecules have immunosuppressive activity. Pectins containing greater than 80% galacturonic acid residues were found to decrease macrophage activity and inhibit the delayed-type hypersensitivity reaction. Branched galacturonan fragments result in a biphasic immunomodulatory action. The branched region of pectins mediates both increased phagocytosis and antibody production. The fine structure of the galactan, arabinan, and apiogalacturonan side chains determines the stimulating interaction between pectin and immune cells. This review summarizes data regarding the relationship between the structure and immunomodulatory activity of pectins isolated from the plants of the European north of Russia and elucidates the concept of polypotency of pectins in native plant cell walls to both stimulate and suppress the immune response. The possible mechanisms of the immunostimulatory and anti-inflammatory effects of pectins are also discussed.
KEY WORDS: pectic polysaccharides, structure–activity relations, immunomodulatory effect, polypotencyDOI: 10.1134/S0006297913070134
Pectins are complicated heteropolysaccharides belonging to the group of acidic plant polysaccharides, glycanogalacturonans [1]. Pectic substances are components of plant cell walls and are found in the intercellular space of flowering land plants, phanerogams, and some freshwater algae [2]. Pectins create a matrix that binds cellulose microfibrils and are involved in ion transport and water regimen. They also influence germination of seeds, growth, and vegetation of plants and play a defensive role between plants and phytopathogens [3]. Overall, functions of pectin in plants have been extensively investigated.
Pectins were first identified by the French scientists Braconnot and Payen in 1825, and the physiological activity of these plant cell polysaccharides have received much attention since then. Humans consume pectins in the form of food and medicines. The daily consumption of pectins by an individual is 1-7 g, corresponding to 10-100 mg/kg body weight [4]. Because crude plant food has been consumed by humans for millions of years [5], it is hypothesized that the human gastrointestinal tract is evolutionarily adapted to pectins. Epidemiological and clinical investigations demonstrated that a deficiency of pectic polysaccharides causes severe diseases.
Studies of the effects of pectin on immunity are of a great interest because of the importance of the immune system in humans and animals. The regulation of immunological surveillance may lead to prophylactic treatments and possibly cures for various illnesses and diseases. Therefore, substances that increase weakened immunity or decrease undesirable immune reactions have been extensively studied.
Preliminary interest in pectins that influence immunity was due to the observation that dietary pectins decreased the risk of cancer. The frequency of cancer in Seventh Day Adventists is two times lower than for Californians. Members of this religion are vegetarians, and they consume two to three times more pectin [6, 7]. There is extensive clinical and experimental data regarding the antitumor, anti-infectious, and anti-allergic properties of pectins [8-16].
Pectins are resistant to digestive enzymes and maintain macromolecular structural patterns of their sugar chains in the stomach and small intestine [17, 18]. Therefore, special consideration has been focused on the elucidation of the relation between structural features and immunomodulatory activity of pectic polysaccharides. The structure of pectic substances depends on numerous parameters, and they may substantially change during growth and vegetation of the plant, whereas the dynamic character of pectin structures is ensured by the non-regular structural pattern of the sugar chain containing various macromolecular fragments in the linear and branched regions [19].
In this review, the immunomodulatory activity of pectins with respect to the structure of the macromolecule is analyzed. The activity of pectins isolated from edible plants, plants of the European north of Russia, and pectins used in the food industry are described. Phagocytosis and the antigen-specific cellular and humoral immune responses of mice treated orally with pectin solutions are considered to be targets of pectin action.
STRUCTURAL PATTERN OF PECTINS
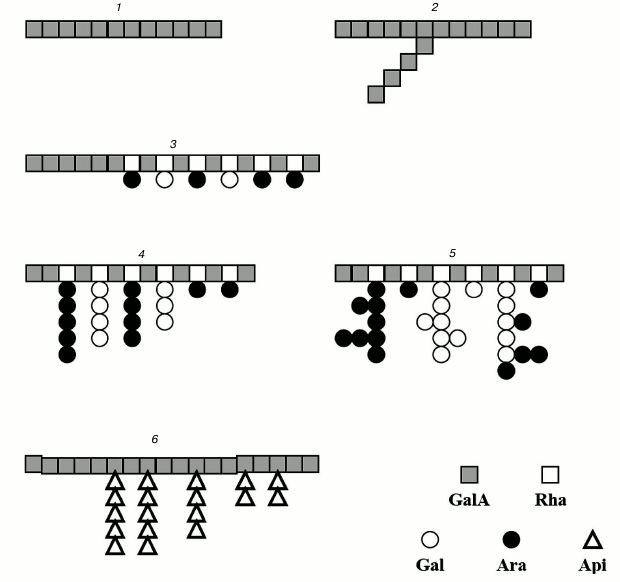
Pectin macromolecules include various fragments of linear and ramified regions that appear to be covalently connected [20, 21]. The linear region consists of units of 1,4-α-D-galacturonan, which represent the backbone of all pectins. These units are bound to each other with one or two α-L-rhamnopyranose residues by 1,2-linkages. The ramified region is represented by different heteropolysaccharides. The structural constituents of the macromolecule include a heterogeneous mixture of pectic polysaccharides that have been obtained by isolating pectins from plant tissue. Homogalacturonan, rhamnogalacturonan-I (RG-I), xylogalacturonan, and apiogalacturonan are the primary pectic polysaccharides [19, 22]. The structural features of these polysaccharides differ significantly from each other and have various physiological effects.
In addition, the pectin polysaccharides are different for various plants. Galacturonan differs in the degree of methyl esterification and molecular weight, rhamnogalacturonan differs in the fine structure of the branched chains that consist of galactose, arabinose, and other sugars, and xylo- and apiogalacturonans differ in the degree of branching.
A set of polysaccharides with different chemical characteristics has been obtained from more than 50 plants of the European north of Russia and the corresponding cell (callus) cultures [23]. A modified procedure for isolating biologically active polysaccharides from raw plant materials by extraction [24, 25] allowed the isolation of pectins with structures that are closely related to native pectins.
The isolated pectins were shown to have common chemical structural features. The structural patterns of these pectins differed in the content of galacturonic acid residues (from 50 to 90%) and side chain structures of the ramified regions.
The pectin backbones consist of 1,4-linked α-D-galactopyranosyl uronic acid residues. Comaruman, pectin of the marsh cinquefoil Comarum palustre L., appears to have a branched core of galacturonan [26, 27].
The majority of pectins contain RG-I; however, they show individual differences in the structure of the ramified regions. In particular, the structure of the tanacetan macromolecule, a pectin from the tansy Tanacetum vulgare L., contains blocks of arabinogalactan, arabinan, and galactan. Arabinogalactan consists of branched side chains of 1,5-α-L-arabinofuranan joined by 1,3- and 1,4-linkages to the linear chains of 1,4-β-D-galactopyranan. The arabinofuranose residues in arabinan are linked by α-1,2- and α-1,5-linkages, and the branch points are 2,5-di-O- and 3,5-di-O-substituted α-L-arabinofuranose. The galactose residues in galactan are connected by β-1,6-linkages, and the 4,6-di-O-substituted β-D-galactopyranose residues of long sugar chains with a high degree of branching are present at the branch points [28].
The ramified region of silenan, a pectin from the campion Silene vulgaris Moench (Garke) (Oberna behen L.), differs from tanacetan in the occurrence of small RG-I blocks [29, 30]. The side chains of silenan consist of 1,5-linked residues of α-arabinofuranose and 1,3-, 1,4-, and 1,6-linked β-galactopyranose. The β-1,3-galactopyranan appears to be bound to α-1,5-arabinofuranan via branch points of 2,3-di-O-substituted galactopyranose residues. In addition, the presence of branch points with 3,6- and 4,6-di-O-substituted galactopyranose residues and 3,5-di-O-substituted arabinofuranose residues confirmed a covalent bond between the fragments of arabinan and galactopyranan in the side chains of silenan.
The ramified regions of comaruman contain rhamnogalacturonan with side chains consisting of 1,6- and 1,4-linked residues of β-D-galactopyranose, 3-O-substituted galactopyranose, and 5-O-subtituted arabinofuranose. The branch points of the side chains are 3,4- and 4,6-di-O-substituted galactopyranose residues. Some residues of the terminal galactopyranose provide single side chains and/or occur on the non-reduced ends of the side chains [26, 27].
The ramified regions of apiogalacturonan and heteroglycanogalacturonans are present in lemnan, a pectin from the duckweed Lemna minor L. [31, 32]. Using NMR spectroscopy, the side chains of the lemnan macromolecule were shown to comprise single and/or 1,5-linked D-apiofuranose residues attached to the 2- and 3-positions of the galacturonic acid residues of the backbone. In addition, the ramified region of lemnan contains a small amount of heteroglycanogalacturonan. The main neutral sugar residues of lemnan are apiose, arabinose, galactose, and xylose. The L-arabinofuranose residues are the primary terminal residues of the side chains of the ramified region of the lemnan macromolecule, and significantly fewer D-galactopyranose and D-xylopyranose (or 2-O-methyl-xylopyranose) residues occupy the terminal positions. Additionally, greater amounts of 1,3-linked D-galactopyranose residues and some 1,6- and possibly 1,4-linked residues are present in the galactan side chains.
The macromolecule of bergenan, a pectin from Siberian tea Bergenia crassifolia L., contains a small number of RG-I branched units. The side chains of RG-I are attached by 1,4-linkages to the rhamnopyranose residues of the backbone and consist of terminal residues of arabinofuranose and α-1,5-linked arabinofuranose, β-1,4- and β-1,6-linked galactopyranose residues. The branch points are 3,4- and 3,6-di-O-substituted galactose residues [23]. Bergenan is a mixture of two acid polysaccharides that differ in galacturonic acid content.
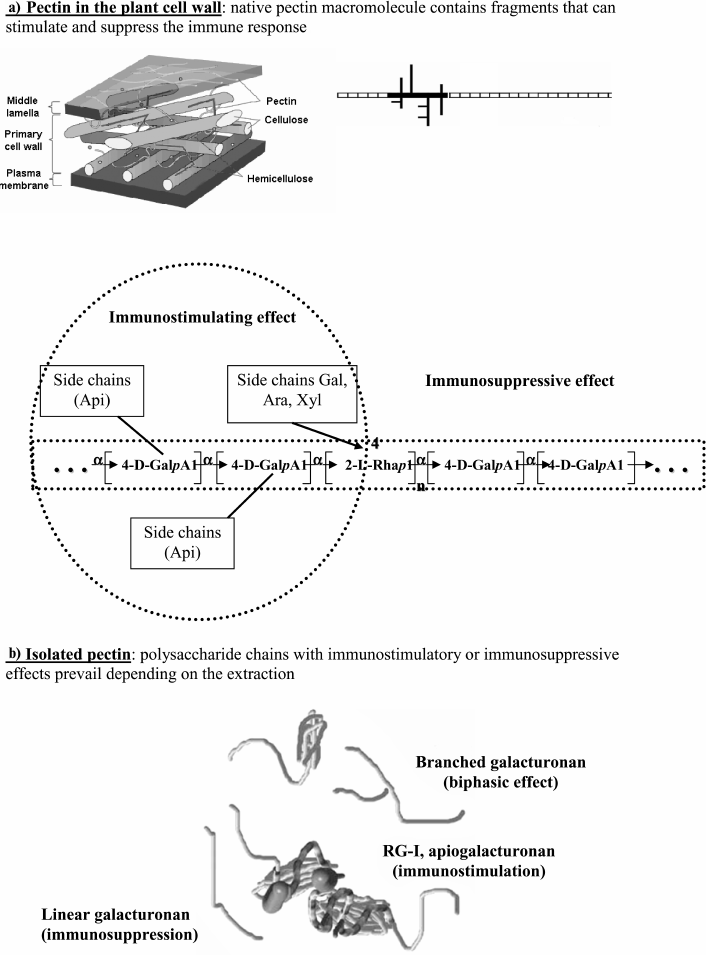
The structures of the ramified regions of some pectins are presented in Fig. 1.
Fig. 1. Structure of pectin sugar chain fragments: 1) linear galacturonan; 2) branched galacturonan (comaruman); 3) poorly branched region of RG-I (bergenan); 4, 5) highly branched region of RG-I (silenan and tanacetan, respectively); 6) apiogalacturonan (lemnan).
IMMUNOMODULATORY ACTION OF PECTIN DEPENDS ON STRUCTURE OF THE
PECTIN BACKBONE
The structural differences in the pectin galacturonic chain determine their ability to decrease immune reactivity. This statement is based on the following data: 1) pectins with high galacturonic acid residue content exhibit immunosuppressive activity; 2) galacturonan fragments obtained using acid hydrolysis of any pectin show anti-inflammatory action; and 3) the structural differences in galacturonan, such as branching, degree of methyl esterification, and molecular weight, influence the ability of pectins to inhibit the functional activity of leukocytes.
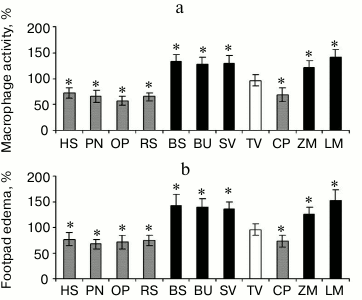
Effect of pectins having various galacturonic acid residue content on activity of phagocytes. The immunomodulatory action of pectins depends on the galacturonic acid residue contents. Pectins containing greater than 80% galacturonic acid residues were found to decrease the accumulation of macrophages caused by the injection of zymosan. The pectins containing greater than 80% galacturonic acid residues are as follows: heracleuman, a pectin from hogweed Heracleum sibiricum (HS); potamogetonan, a pectin from pondweed Potamogeton natans L. (PN); oxycoccusan, a pectin from cranberry Vaccinium oxycoccos L. (or Oxycoccus palustris Pers.) (OP); and rauwolfian, a pectin from callus of snakeroot Rauwolfia serpentina (RS). Pectins containing less than 75% galacturonic acid residues do not decrease the accumulation of macrophages caused by the injection of zymosan; however, some of these pectins, such as lemnan (LM), butomusan, a pectin from grass rush Butomus umbellatus (BU), and silenan (SV) increase cellular activity (Fig. 2a).
Fig. 2. a) Accumulation of peritoneal macrophages induced by injection of zymosan. b) Delayed-type hypersensitivity reaction of mice orally administered pectin for 28 days. HS, heracleuman, a pectin from hogweed Heracleum sibiricum L.; PN, potamogetonan, a pectin from pondweed Potamogeton natans L.; OP, oxycoccusan, a pectin from cranberry Vaccinium oxycoccos L. (or Oxycoccus palustris Pers.); RS, rauwolfian, a pectin from callus of snakeroot Rauwolfia serpentina L.; BC, bergenan, a pectin from Siberian tea Bergenia crassifolia L.; BU, butomusan, a pectin from grass rush Butomus umbellatus L.; SV, silenan, a pectin from the campion Silene vulgaris Moench (Garke) (Oberna behen L.); TV, tanacetan, a pectin from the tansy Tanacetum vulgare L.; CP, comaruman, a pectin of the marsh cinquefoil Comarum palustre L.; ZM, zosteran, a pectin from the phanerogam Zostera marina L.; LM, lemnan, a pectin from the duckweed Lemna minor L. GalA content (%): HS, 81; PN, 80; OP, 82; RS, 82; BC, 83; BU, 75; SV, 74; TV, 60; CP, 65; ZM, 60; LM, 64. * Significant differences compared to control at p < 0.05.
The increasing and decreasing effect of pectin on macrophages led to a corresponding change in the antigen-specific immune response as identified by delayed hypersensitivity. Injection of ovalbumin in the footpad of mice that were previously sensitized by subcutaneous injection of the same antigen in Freund’s adjuvant produced edema. The amount of footpad edema was increased for mice treated with lemnan, silenan, bergenan, butomusan, and zosteran, a pectin from the phanerogam Zostera marina (ZM). Furthermore, these pectins inhibited the activity of macrophages and inhibited the delayed hypersensitivity to ovalbumin (Fig. 2b).
Bergenan and comaruman were found to be an exception to this finding. Bergenan contains 85% galacturonic acid residues, and rather than decreasing the activity of leukocytes, it increased their activity. Comaruman inhibited cellular functions, although it contains nearly 60% galacturonic acid residues. These pectins have unique structures that differ from other pectins. Bergenan is characterized by a high degree of methyl esterification of the galacturonic acid residues. Ramified regions of the branched galacturonan are present in the comaruman macromolecule in addition to linear galacturonan.
Pectin fragments consisting of galacturonic acid residues only (98-100%) were obtained by partial acid hydrolysis with trifluoroacetic acid. This procedure is based on the different resistances of glycosidic bonds in polysaccharides to acid [33]; therefore, it is possible to obtain a pectic galacturonan without the sugar side chains composed of neutral sugars [34]. All galacturonans decrease the reactivity of leukocytes independently from the activity of the parent pectins. In particular, pectic galacturonic fragments with anti-inflammatory activity [35-38] were shown to inhibit inflammation similarly to the parent pectins (table). The ability to inhibit leukocyte activity was identified for galacturonic fragments of inactive pectins and for the fragments of pectins such as bergenan, butomusan, silenan, lemnan, and zosteran that stimulated the activity of leukocytes.
Anti-inflammatory effect* of pectins and galacturonans
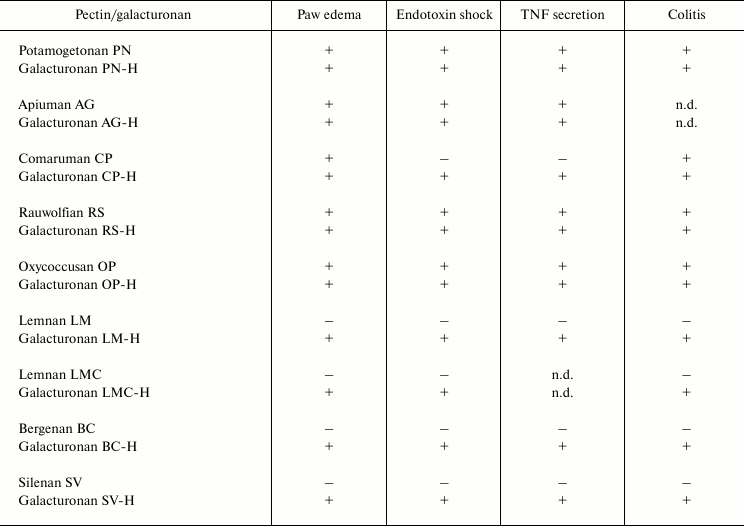
* Anti-inflammatory action is defined in the mouse models as follows:
paw edema induced by injection of carrageenan; endotoxin shock caused
by intraperitoneal injection of lipopolysaccharide; secretion of tumor
necrosis factor (TNF) by blood leukocytes; acute ulcerative colitis
induced by rectal administration of acetic acid; (+) and
(–), effect is present or absent, respectively; n.d., not
determined.
Effect of pectins with different degrees of methyl esterification on activity of phagocytes was shown for both food plant and commercial pectins. Capsicuman, a pectin from the sweet pepper Capsicum annum, is 25% methyl esterified and was found to inhibit the functional activity of leukocytes [39]. Pectins from carrots, onions, and cabbage with 51, 60, and 67% methyl esterification, respectively, failed to influence the activity of leukocytes.
Pectins isolated from fruits and vegetables with aqueous hydrochloric acid are known to have a high degree of methyl etherification. In particular, the degree of methyl etherification of pectins from citrus, sugar beets, and squash is 50, 55, and 60%, respectively [40-42]. Pepsin-comprised extragent is hypothesized to inhibit activity of methyl esterases that occur in plant tissue.
Citrus and apple pectins with 34-38% methyl esterification inhibit the activity of leukocytes, whereas similar pectins with a high degree of methyl esterification (68-76%) failed to show this effect [43]. Notably, the ability to inhibit intracellular mediators of inflammation in vitro increased inversely with the increased degree of pectin methyl esterification [44].
These data indicate that free carboxyl groups of the galacturonic acid residues are necessary for the inhibitory activity of pectins and galacturonans on leukocytes. A high degree of methyl esterification of the galacturonic acid residues of the backbone (more than 50%) hindered the inhibitory action of pectins on leukocyte activity. Pectins with a low degree of esterification penetrate the thick mucin layer of the intestine wall, whereas highly esterified pectins form a gel on its surface [45]. The pectin molecules with low degree of esterification have negative charge because of free carboxyl groups and are repelled from negatively charged mucin molecules, thus preventing the formation of hydrogen bonds and mucin–pectic aggregates. Mucin–pectic particle sizes were approximately 400 and 1000 nm for lowly and highly methyl esterified citric pectins, respectively. These are closely related to the pectins used in the present study [46]. Penetration of the mucin layer promotes interaction of lowly methyl esterified pectins with the intestinal epithelium.
Galacturonan fragments with a very high degree of methyl esterification are synthesized in the Golgi apparatus of plant cells followed by inclusion in the cell wall [47]. Pectin was found to partially lose methoxyls of the galacturonic acid residues during vegetative growth, consistent with the necessities of the plant. The degree of pectin methyl esterification is determined by the quality and composition of the parent raw materials. Similarly, the chemical composition and degree of methyl esterification appears to depend on the method of pectin isolation [48-50]. Extraction is accompanied by the removal of side sugar chains and methyl ester groups of galacturonic acid.
Ramified galacturonan blocks cause two phases of immunomodulatory action of pectin, the decreasing and increasing activity (Fig. 3).
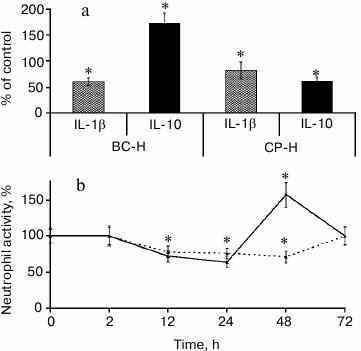
Fig. 3. Biphasic leukocyte reaction caused by the branched galacturonic fragments of comaruman. Effect of the galacturonic fragments from bergenan (BC-H) and comaruman (CP-H) on cytokine production (a) and the number of neutrophils at the site of inflammation (b). * Significant differences compared to control at p < 0.05.
A comparison of the activity of galacturonans from comaruman and bergenan on inflammation revealed differences in the effect of the ramified regions of these compounds. Galacturonan from bergenan, which does not contain sugar chain branches, was shown to alter the balance of cytokines towards an anti-inflammatory response. Specifically, this galacturonan decreased the concentration of interleukin-1β (IL-1β) and increased the concentration of interleukin-10 (IL-1). As a result, the number of attracted neutrophils decreased in the inflammatory nidus. Galacturonan from comaruman consists of linear and branched molecules and decreases the concentration of IL-10, which is accompanied by an increase in neutrophils. These data indicate that the action of the parent comaruman during the first and second days of inflammation is reflected by the influence of the linear and ramified regions of the galacturonan on the production of cytokines. Fragments of the ramified galacturonan cause a more pronounced action on the target cells than the linear sugar chains. As a result, the mechanisms that compensate for the inhibitory activity of this compound begin to cause a transitory increase in the activity of leukocytes. The parent comaruman showed anti-inflammatory action despite the average number of galacturonic acid residues because the ramified galacturonan has high activity.
The occurrence of a ramified galacturonan appears to be a rare property of the pectin macromolecule. Previously, based on the elucidation of the macromolecule architectonics using atomic force microscopy, it was suggested that tomato pectins contained side chains comprising long regions of galacturonan attached to the core with non-established linkages [51]. Notably, other pectins, which contain ramified galacturonan, were not detected except comaruman and tomato pectin. However, branched galacturonan as the backbone of other pectins may be identified when atomic force microscopy is more widely used in the future for structural carbohydrate chemistry.
The molecular weight of galacturonans that inhibit leukocyte activity exceeds 300 kDa. Moreover, galacturonans with molecular weights ranging from 100-300 and 50-100 kDa do not possess this ability. The studied pectins consist of polymer homologs with molecular weights ranging from 20 to 700 kDa. Polysaccharides tend to aggregate in aqueous solutions, which results in an equilibrium between low and high molecular weight polymer homologs. The molecular weights of the aggregates are related to the number and average molecular weights of the polysaccharides. Low molecular weight fragments stretch lengthwise as ribbons and have less outer surface area compared to aggregates formed as globules.
The ability of pectins to decrease immune reactivity appears to depend on the correlation between the high and low molecular weight polysaccharide chains. This correlation depends on the method of pectin isolation and its biotransformation in the gastrointestinal tract.
The choice of extraction conditions is usually intended to obtain a higher yield of pectin from the raw plant materials. Therefore, harsh chemical procedures, in particular, high temperature, are used for complete pectin isolation. As a result, these pectins have molecular weights no greater than 100 kDa. Our results demonstrate the need to use methods that maintain high molecular weight sugar chains to isolate pectins that display immunomodulatory action.
Pectins are assumed to exhibit anti-inflammatory action if galacturonan fragments with molecular weights greater than 300 kDa are formed during biotransformation of pectin in the gastrointestinal tract. However, modifications of the pectin structures in the intestine have not been sufficiently investigated.
Cleavage of 10-40% of the glycosidic bonds in the side sugar chains of the pectin macromolecule occurred in artificial gastric medium and was dependent on the structural differences. Treatment of the pectins with hydrochloric acid at pH 1-2 caused changes in the sugar composition and molecular weight of the pectins [52]. As a result of this treatment, the number of fragments with molecular weights between 300-400 kDa increased simultaneously with the decreasing number of fragments with molecular weights between 400-600 kDa. These data confirmed that the partial biotransformation of pectins appears to begin in the upper sections of the gastrointestinal tract. In particular, only 50-80% of the pectin consumed with food is detected in the feces of patients with an ileostomy [53]. The contents of the small intestine of these patients are removed via a special aperture in the abdominal wall; therefore, the food does not reach the large intestine and cannot be treated with symbiont microflora. A change in the chemical properties of the pectins during biotransformation in the intestine leads to changes in the physiological activity of the pectins. In particular, the quantities of phenolic admixtures in the pectins are decreased with simultaneously decreasing antioxidant activity [52].
IMMUNOMODULATORY ACTION OF PECTINS DEPENDS ON STRUCTURE OF
RAMIFIED REGION OF THE PECTIC MACROMOLECULE
Differences in the structure of the branched region appear to determine the ability of pectins to increase immune reactivity. This statement is based on the following data: 1) pectins containing less than 75% galacturonic acid residues and characterized by a developed ramified region were shown to possess an immunostimulatory effect; 2) the structural features of the side sugar chains in the RG-I fragment of the branched region of the pectic macromolecule were found to play an important role in stimulating the immune response; 3) the immunostimulatory effect of lemnan, which contains apiogalacturonan as the branched region, was significantly different than the pectins with RG-I as the branched region; and 4) the immunostimulatory activity of pectins is retained after the linear region is removed and the branched region is maintained.
Comparison of the immunomodulatory action of pectins reveals that only pectins containing less than 75% galacturonic acid residues can increase immunity (Fig. 2). These pectins are characterized by a large branched region, which is represented by RG-I and apiogalacturonan in the macromolecules bergenan, silenan, butomusan, and lemnan, respectively. Consistent with these results, immunostimulatory ability of the pectins was less common than inhibitory activity for immune reactions. Lemnan, silenan, bergenan, and butomusan can be considered as immunostimulatory pectins.
Indication of the immunostimulatory ability of pectin is based on data showing the improvement of patients with neoplastic and infectious diseases. However, pectins should improve the condition of the patient without affecting the immune system. The antitumor effect of modified citrus pectin is due to the inhibition of key stages of tumor metastasis [54]. Modified citrus pectin inhibits primary tumor cells from penetrating into the lumen of the blood and lymph vessels, thereby preventing the cells from localizing to a new site and extravasation.
In addition, the antitumor effect of pectin may also be because pectins bind to carcinogens in the gut, as well as substances that promote carcinogenesis, such as insulin and insulin-like growth factor-1. Pectins inhibit carcinogenesis in the colon by increasing apoptosis [55, 56], slowing the proliferation of colonocytes [57], reducing the activity of β-glucuronidase [58], stimulating the growth of bifidobacteria, and producing short-chain fatty acids.
Anti-infection properties of pectins are mainly associated with a decrease in microbial aggression by improving the composition of the colon microflora [59], inhibiting the adhesion of pathogens to epithelial cells [60], inhibiting bacterial colonization [61], and binding bacterial toxins [62].
The glycosidic linkages between neutral sugar residue side chains are known to be more labile than the 1,4-linked residues of α-D-galacturonic acid. Therefore, the ability of pectin to maintain its native structure and immunostimulatory effect in the gastrointestinal tract is expected to be lower than the inhibitory effect, which is mediated by the more stable linear galacturonan.
The structural features of the side chains appear to determine the ability of pectins to stimulate cellular activity. Silenan and tanacetan were shown to have similar structural patterns; however, silenan was found to stimulate phagocytes, whereas tanacetan did not. The branched region of silenan is primarily linear 1,4-linked β-galactan and 1,5-α-arabinan chains, whereas the side chains of tanacetan contain large regions of branched blocks of arabinans and galactan [28, 30]. The bergenan macromolecule is similar to silenan and contains mainly unbranched regions of 1,5-linked α-arabinofuranose and 1,4- and 1,6-linked β-galactopyranose residues in the rhamnogalacturonan region [23]. It is unclear why tanacetan does not affect the immune response. The branched sugar chains of arabinan and galactan are hypothesized to prevent the linear fragments of arabinan and galactan from interacting with target cells. It is also possible that there are not enough arabinan and galactan chains in tanacetan for exhibition of immunostimulatory effect.
The role of the ramified region of pectin in enhancing the immune response has been demonstrated by cirsiuman, a pectin from the edible thistle Cirsium esculentum Siev., which belongs to the class of rhamnogalacturonan pectins. The cirsiuman fragment obtained by enzymatic hydrolysis was found to contain fewer galacturonic acid residues and greater number of neutral monosaccharide residues. The fragment was shown to stimulate the immune response against orally co-administered antigen similarly to the native cirsiuman [63].
The role of the ramified region in the immunostimulatory effect of pectins has also been demonstrated in vitro [64, 65]. Modification of the fine structure of the branched region of Centella asiatica pectin increased the immunostimulatory activity [66]. Rhamnogalacturonan-containing sugar side chain is a fragment of bupleuran, a pectin from the roots of Bupleurum falcatum, and was suggested to mediate a stimulatory effect on macrophages, lymphocytes, and intestinal epithelial cells [67-69]. Galactan chains with terminal residues of β-D-glucuronic acid appeared to be necessary for the stimulatory effect of the branched pectin from Astragalus mongholicus on the immunocompetent cells of Peyer’s patches [70]. The branched fragment, which is similar but not identical to typical rhamnogalacturonan II, was found to determine the immunostimulatory effects of pectin from the rhizomes of Atractylodes lancea [71]. Pectic polysaccharides from different types of cabbage Brassica oleracea have been shown to activate the complement system in vitro. Higher activity was reported to correlate with increased amount of neutral monosaccharide residues in the side chains and was not dependent on the molecular weight of the pectin [72].
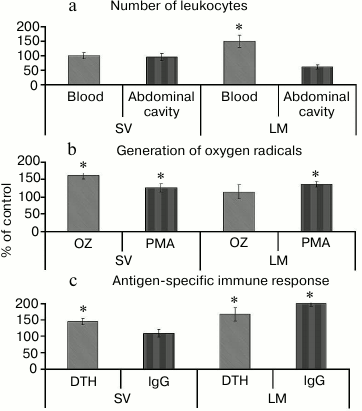
The stimulatory effect of lemnan [73, 74] is different from pectins with RG-I type ramified regions. First, lemnan causes the redistribution of leukocytes between tissues and blood. The number of neutrophils in the blood of mice increased, whereas the number of peritoneal cells was reduced after oral administration of lemnan. Silenan did not affect the number of cells (Fig. 4a).
Fig. 4. Effect of pectin containing RG-I (silenan SV) and apiogalacturonan (lemnan LM) on the number (a) and functional activity of leukocytes (b) and antigen-specific immune response (c). OZ, opsonized zymosan; PMA, phorbol myristate acetate; DTH, delayed-type hypersensitivity reaction. * Significant differences compared to control at p < 0.05.
Second, lemnan stimulates resting phagocytes and increases their response to phorbol myristate. Silenan increases the activity of cells induced by both zymosan and phorbol myristate (Fig. 4b). The data demonstrate that oral administration of lemnan changes the physical and chemical properties of the plasma membrane of phagocytes and increases its permeability to small molecule activators, such as phorbol myristate. Silenan is postulated to affect the functional activity of cellular receptors because zymosan stimulates cells by binding to receptors on the cell surface. Differences in the structure of the ramified region of the pectin macromolecules were also found to determine the effect of pectin on the antigen-specific immune response. Lemnan, silenan, and bergenan [75] increase the intensity of delayed-type hypersensitivity against protein antigens that are orally administered. In addition, lemnan stimulates the production of antibodies (Fig. 4c).
Moreover, the immunostimulatory effect of lemnan has been found to be different from that of zosteran, a pectin that also contains a significant amount of apiose residues in the branched region. Previously [76], the structural features of lemnan and zosteran were determined using autohydrolysis of the polysaccharides that was induced by its own carboxyl groups. This was followed by spectroscopic analysis of the fragments of the resulting macromolecule. In contrast to the 1,5-linkages between the β-D-apiose residues in the apiobioside fragment of lemnan [32], the zosteran oligosaccharide has 1,2-glycosidic bonds between the same sugars. Consequently, the nature of relationships in the branched apiogalacturonanic fragments from similar pectins is thought to be significant for the immunostimulatory activity.
These data suggest that pectins stimulate immunity in at least two ways. One method provides an increase in cellular reactions only (silenan, bergenan), and the second method leads to the stimulation of antibody production, as demonstrated by lemnan. In both cases, the pectin side chains are involved in the stimulatory effect.
The minimal domain of lemnan that possesses immunostimulatory activity is an apiogalacturonan region that is resistant to 1,4-α-D-galactopyranosyluronase [77]. Apiogalacturonan was found to constitute 45% of the lemnan molecule and consists of 76% galacturonic acid and 18% apiose residues. The apiogalacturonanic fragment co-administered with ovalbumin stimulates antigen-specific IgG antibodies and enhances delayed-type hypersensitivity. It also stimulates the production of secretory IgA in the intestinal mucosa. Fragments of the lemnan macromolecule similar to the parent lemnan did not affect the blood levels of IgE. Enhancement of the immune response with the apiogalacturonanic fragment of lemnan was comparable to the parent pectin and the effect of cholera toxin. Partial removal of the apiogalacturonan side chains using acid hydrolysis leads to the formation of a fragment that does not have an immunostimulatory effect. This indicates that the branched region of the pectin macromolecule mediates the immunostimulatory effect of lemnan.
The study by Gloaguen et al. [76] demonstrates an essential role for the apiose residues of the side chains for the antitumor activity of zosteran. Removal of the apiose and oligo-apiose fragments abrogated the cytotoxic effects of zosteran on tumor cells.
The apiose residues of lemnan are hypothesized to contribute to the formation of inter- and intramolecular networks, the latter in the presence of metal ions. As a result, the surface area of the molecule and nonspecific binding of the polysaccharide to other biopolymers (antigens) and the plasma membrane of the target cells are increased. In addition, lemnan is expected to be more resistant to pectinolysis due to the presence of the apiogalacturonan, which most likely provides additional stability to lemnan during digestion compared to other pectins.
Notably, the immunostimulatory effect of pectins is postulated to involve polysaccharide chains with molecular weight of 20-100 kDa. Pectic fractions from silenan, bergenan, and butomusan deprived of polysaccharide chains of molecular weight less than 100 kDa were obtained using ultrafiltration. These fractions did not exhibit immunostimulatory effects, unlike the parent pectins. In addition, the immunostimulatory effect of pectin has been shown to depend on contamination with monosaccharides, which is common in commercial pectins [78].
STRUCTURAL POLYPOTENCY IS A UNIQUE PROPERTY OF PECTIN
MACROMOLECULES
Pectic polysaccharides demonstrate a wide range of immunomodulatory activities mediated by the structure of both the backbone and branched regions of the macromolecule. Analysis of the relationship between the structure and immunomodulatory activity suggests that the pectin macromolecule contains fragments that can either decrease or increase the activity of leukocytes. Therefore, native pectins in the plant cell wall have the potential to both stimulate and suppress immunity (Fig. 5a).
Fig. 5. Diagram illustrating the polypotency of pectin macromolecules.
Thus, pectins appear to demonstrate structural polypotency with regard to their effect on the immune system.
In general, the pattern of immunomodulatory activity of isolated pectins is defined by the ratio of galacturonic chains (linear and branched) and branched fragments (RG-I and apiogalacturonan) (Fig. 5b). Isolated pectin exhibits immunosuppressive activity when macromolecular fragments of galacturonan with free carboxyl groups are prevalent. Fragments of branched galacturonan partially prevent the linear galacturonan from stimulating the production of anti-inflammatory cytokines. The resulting effect of pectins containing these fragments changes with time. Pectin containing fragments of the ramified region, such as RG-I and apiogalacturonan, exhibits immunostimulatory effects. However, the stimulatory effect of the branched chains will manifest if it is not prevented by the inhibitory effect of galacturonan. In some inactive pectins, the branched and linear fragments appear to compensate for the action of each other, similar to that which most likely occurs with tanacetan.
The immunostimulatory effect of bergenan is thought to correspond with the predominance of the stimulatory activity of RG-I fragments over the inhibitory activity of galacturonan because of the high degree of methyl esterification. The presence of pectic fragments resistant to enzyme digestion, such as apiogalacturonan, should allow pectins to interact with protein antigens and stimulate the humoral immune response.
Notably, the side chains of pectins with immunostimulatory effects, such as silenan and bergenan, are more resistant to the gastrointestinal medium than pectins that decrease immune reactivity, such as comaruman and potamogetonan. However, the glycosidic bonds between the side chains of neutral monosaccharide residues are known to be more labile than the 1,4-linkage between α-D-galacturonic acid residues. Therefore, the immunostimulatory effect, which requires the native structure of the pectin macromolecule, is reduced after the pectin reaches the gastrointestinal tract, whereas the inhibitory effect of pectins is mediated by the more stable linear galacturonan.
Based on existing data, it can be hypothesized that activity of the immune system of animals depends on the chemical properties of food pectins. The identified patterns are expected to be inherent to monogastric animals, including the humans and mice used in studies. The immune response of rodents and humans to plant polysaccharides appears to be the same because of the similarity of the structure and function of the immune systems.
The multiple actions of pectins on immune reactivity may be caused by structural polypotency. Pectic fragments are extracted during the digestion of plant foods. Immune resistance through the functional activity of leukocytes appears to depend on the ratio of the polysaccharide chains of galacturonan, rhamnogalacturonan, apiogalacturonan, and other fragments.
The leukocyte system is the first to react after the administration of pectic polysaccharides. Pectins with immunomodulatory abilities do not cause significant changes in homeostasis and do not stress the body. However, pectins were shown to change the functional activity of leukocytes both in the intestinal wall and in tissues. Because of their high molecular mass, pectins do not penetrate into the systemic circulation; therefore, they do not harm the internal environment of the body. However, the mammalian immune system recognizes the structural features of pectins. The phagocyte response to pectins can be explained by the suggestion that neutrophils and macrophages interact with pectin during their life cycle. The intestinal wall should be the only place where such contact occurs followed by penetration of the tissue barrier by leukocytes, which affects immune reactions away from the digestive tract [79]. Phagocytes are known to be highly mobile cells [80, 81]. Non-digestible polysaccharides are thought to attach to the intestinal wall because of carbohydrate-recognizing receptors expressed on the surface of phagocytes, whereas other foreign molecules are washed away by the mucus that coats the epithelium [82].
The gastrointestinal medium that affects the physical and chemical properties of pectin is likely to fluctuate. The acidity and composition of the gastric juice are known to change during digestion and also depend on the type of food and physiological state of the animal. The pectic macromolecule is most stable at pH 4, whereas partial depolymerization of the polysaccharide chain or de-esterification of the galacturonic acid residues could occur with either an increase or decrease in the acidity of the medium [83]. The enzymatic activity of the digestive system and the composition and pectin-hydrolyzing activity of the microbes inhabiting the lower intestine are also reported to vary widely.
Thus, on one hand, the molecular activity of plant-derived food to affect the immune system appears to be dependent on the structural polypotency of pectins. On the other hand, there are mechanisms to implement these effects on the immune system. Therefore, the data regarding the immunomodulatory effects of pectins that are orally administered likely reflect the evolutionary patterns of the immune response to the presence of pectic polysaccharides in the gastrointestinal tract. For the integrated (holistic) picture of the organic world, mammals are suggested to use polypotent pectins by choosing plants for food and changing the properties of the gastrointestinal medium. Moreover, the structural features of pectins provide information on the species composition of plants in the habitat of the animal, and this information is recognized by the immune system.
The proposed concept is hypothesized to complement the modern paradigm of nutrition and food assimilation. The essence of the paradigm first proposed by Academician A. M. Ugolev is that there is a vital need for the delivery of nutrients, hormones, bacterial metabolites, and secondary nutrients from the intestinal medium to the internal environment of the body [84]. According to this scheme, pectins represent a portion of roughage, the only physiological role of which is the formation of a gel-like bolus, and they affect the speed of passage through the intestine, the absorption of nutrients, and the stimulation of the growth of symbiotic microorganisms. The immunomodulatory activity of pectins reveals the existence of one more streams of nutrients. These compounds may cause a physiological effect without penetrating into the circulation.
The immunomodulatory effects of orally administered pectin depend on the structure of the pectin macromolecule. The mammalian immune system may recognize the structural features of pectins from different plants. The development of the immune response depends on the ratio of polysaccharide chains of galacturonan, rhamnogalacturonan, apiogalacturonan, and other fragments produced from plant foods during digestion. In addition, the biotransformation of pectic macromolecules passing through the gastrointestinal tract may influence the action of pectin on immune reactivity. Elucidation of the molecular mechanisms of the immunomodulatory properties of pectins that are dependent on their structure is a promising task for future research efforts.
This work was supported by the program of the Presidium of the Urals Branch of the Russian Academy of Sciences “Molecular and Cell Biology” (No. 12-P-4-1033) and the program of the joint research of the Urals and Far East Branches of the Russian Academy of Sciences (No. 12-S-4-1016).
REFERENCES
1.Ovodov, Yu. S. (2009) Russ. J. Bioorg.
Chem., 35, 269-284.
2.Popper, Z. A. (2008) Curr. Opin. Plant
Biol., 11, 286-292.
3.Cabrera, J. C., Boland, A., Messiaen, J., Cambier,
P., and Cutsen, P. V. (2008) Glycobiology, 18,
473-482.
4.Linseisen, J., Schulze, M. B., Saadatian-Elahi, M.,
Kroke, A., Miller, A. B., and Boeing, H. (2003) Ann. Nutr.
Metab., 47, 37-46.
5.Ungar, P. S., and Sponheimer, M. (2011)
Science, 334, 190-193.
6.Kurup, P. A., Jayakumari, N., and Indira, M. (1984)
Am. J. Clin. Nutr., 40, 942-946.
7.Ross, J. K., Pusateri, D. J., and Shultz, T. D.
(1990) Am. J. Clin. Nutr., 51, 365-370.
8.Loenko, Yu. N., Artyukov, A. A., Kozlovskaya, E.
P., Miroshnichenko, V. A., and Elyakov, G. B. (1997) Zosterin
[in Russian], Dalnauka, Vladivostok.
9.Komisarenko, S. N., and Spiridonov, V. N. (1998)
Plant Resources, 34, 111-119.
10.Khotimchenko, Yu. S., Odintsova, M. V., and
Kovalev, V. V. (2001) Polysorbovite [in Russian], NTL Press,
Tomsk.
11.Buisseret, P. (1980) Am. J. Clin. Nutr.,
33, 865-871.
12.Nie, Y., Li, Y., and Wu, H. (1999)
Helicobacter, 4,128-134.
13.Rabbani, G. H., Tera, T., and Zaman, B. (2001)
Gastroenterology, 21, 554-560.
14.Guess, B. W., Scholz, M. C., Strum, S. B., Lam,
R. Y., Johnson, H. J., and Jennrich, R. I. (2003) Prost. Canc.
Prost. Dis., 6, 301-304.
15.Kobayashi, M., Matsushita, H., Yoshida, K.,
Tsukiyama, R., Sugimura, T., and Yamamoto, K. (2004) Int. J. Mol.
Med., 14, 879-884.
16.Kobayashi, M., Matsushita, H., Tsukiama, R. I.,
Saito, M., and Sugita, T. (2005) Int. J. Mol. Med., 15,
463-467.
17.Knaup, B., Kempf, M., Fuchs, A., Valotis, A.,
Kahle, K., Oehme, A., Richling, E., and Schreier, P. (2008) Mol.
Nutr. Fd. Res., 52, 840-848.
18.Gulfi, M., Arrigoni, E., and Amado, R. (2007)
Carbohydr. Polym., 67, 410-416.
19.Voragen, A. G. J., Coenen, G. J., Verhoef, R. P.,
and Schols, H. A. (2009) Struct. Chem., 20, 263-275.
20.Coenen, G. J., Bakx, E. J., Verhoef, R. P.,
Schols, H. A., and Voragen, A. G. J. (2007) Carbohydr. Polym.,
70, 224-235.
21.Caffall, K. H., and Mohnen, D. (2009)
Carbohydr. Res., 344, 1879-1900.
22.Round, A. N., Rigby, N. M., MacDougall, A. J.,
and Morris, V. J. (2010) Carbohydr. Res., 345,
487-497.
23.Ovodov, Yu. S., Golovchenko, V. V., Gunter, E.
A., and Popov, S. V. (2009) Pectic Substances of Plants of the
European North of Russia [in Russian], UrB RAS, Yekaterinburg.
24.Ovodova, R. G., Bushneva, O. A., Golovchenko, V.
V., Popov, S. V., and Ovodov, Yu. S. (2000) RF Patent No. 2149642,
Byul. Izobret., No. 15.
25.Polle, A. Ya., Ovodova, R. G., and Ovodov, Yu. S.
(2001) RF Patent No. 2176515, Byul. Izobret., No. 34.
26.Ovodova, R. G., Bushneva, O. A., Shashkov, A. S.,
Chizhov, A. O., and Ovodov, Yu. S. (2005) Biochemistry (Moscow),
70, 867-877.
27.Ovodova, R. G., Popov, S. V., Bushneva, O. A.,
Golovchenko, V. V., Chizhov, A. O., Klinov, D. V., and Ovodov, Yu. S.
(2006) Biochemistry (Moscow), 71, 538-542.
28.Polle, A. Ya., Ovodova, R. G., Shashkov, A. S.,
and Ovodov, Yu. S. (2002) Carbohydr. Polym., 49,
337-344.
29.Ovodova, R. G., Bushneva, O. A., Shashkov, A. S.,
and Ovodov, Yu. S. (2000) Bioorg. Khim., 26, 686-692.
30.Bushneva, O. A., Ovodova, R. G., Shashkov, A. S.,
and Ovodov, Yu. S. (2002) Carbohydr. Polym., 49,
471-478.
31.Ovodova, R. G., Golovchenko, V. V., Shashkov, A.
S., Popov, S. V., and Ovodov, Yu. S. (2000) Russ. J. Bioorg.
Chem., 26, 61-67.
32.Golovchenko, V. V., Ovodova, R. G., Shashkov, A.
S., and Ovodov, Yu. S. (2002) Phytochemistry, 60,
89-97.
33.Aspinall, G. O. (1982) The
Polysaccharides, Academic Press Inc., London.
34.Thibault, J. F., Renard, C. M. G. C., Axelos, M.
A. V., Roger, P., and Crepeau, M. J. (1993) Carbohydr. Res.,
238, 271-286.
35.Ovodova, R. G., Golovchenko, V. V., Popov, S. V.,
Popova, G. Yu., Paderin, N. M., Shashkov, A. S., and Ovodov, Yu. S.
(2009) Food Chem., 114, 610-615.
36.Popov, S. V., Popova, G. Yu., Koval, O. A.,
Paderin, N. M., Ovodova, R. G., and Ovodov, Yu. S. (2007) Phytother.
Res., 21, 609-614.
37.Popov, S. V., Markov, P. A., Nikitina, I. R.,
Petrishev, S., Smirnov, V., and Ovodov, Yu. S. (2006) World J.
Gastroenterol., 12, 6646-6651.
38.Popov, S. V., Vinter, V. G., Patova, O. A.,
Markov, P. A., Nikitina, I. R., Ovodova, R. G., Popova, G. Yu.,
Shashkov, A. S., and Ovodov, Yu. S. (2007) Biochemistry
(Moscow), 72, 778-784.
39.Popov, S. V., Ovodova, R. G., Golovchenko, V. V.,
Popova, G. Yu., Viatyasev, F. V., Shashkov, A. S., and Ovodov, Yu. S.
(2011) Food Chem., 124, 309-315.
40.Phatak, L., Chang, K. C., and Brown, G. (1988)
J. Fd. Sci., 53, 830-833.
41.Mesbahi, G., Jamalian, J., and Farahnaky, A.
(2005) Fd. Hydrocoll., 19, 731-738.
42.Ptichkina, N. M., Markina, O. A., and
Rumyantseva, G. N. (2008) Fd. Hydrocoll., 22,
192-195.
43.Popov, S. V., Markov, P. A., Popova, G. Yu.,
Nikitina, I. R., Efimova, E., and Ovodov, Yu. S. (2013) Biomed.
Prevent. Nutr., 3, 59-63.
44.Chen, C. H., Sheu, M. T., Chen, T. F., Wang, Y.
C., Hon, W. C., Liu, D. Z., Chung, T. C., and Liang, Y. C. (2006)
Biochem. Pharmacol., 72, 1001-1009.
45.Liu, L., Fishman, M. L., Hicks, K. B., and Kende,
M. (2005) Biomaterials, 26, 5907-5916.
46.Sriamornsak, P., Wattanakorn, N., and Takeuchi,
H. (2010) Carbohydr. Pol., 79, 54-59.
47.Gorshkova, T. A. (2007) Plant Cell Wall as
Dynamic System [in Russian], Nauka, Moscow.
48.Yeoh, S., Shi, J., and Langrish, T. A. G. (2008)
Desalination, 218, 229-237.
49.Koubala, B. B., Kansci, G., Mbome, L. I.,
Crepeau, M. J., Thibault, J. F., and Ralet, M. C. (2008) Fd.
Hydrocoll., 22, 1345-1351.
50.Wai, W., Alkarkhi, A. F. M., and Easa, A. M.
(2010) Fd. Bioprod. Proc., 88, 209-214.
51.Round, A. N., Rigby, N. M., MacDougall, A. J.,
Ring, S. G., and Moriss, V. J. (2001) Carbohydr. Res.,
331, 337-342.
52.Michaleva, N. Ya., Borisenkov, M. F., Gunter, E.
A., Popeyko, O. V., and Ovodov, Yu. S. (2010) Chem. Plant Raw
Material, 3, 29-36.
53.Holloway, W. D., Tasman-Jones, C., and Maher, K.
(1983) Am. J. Clin. Nutr., 37, 253-255.
54.Glinsky, V. V., and Raz, A. (2009) Carbohydr.
Res., 344, 1788-1791.
55.Sanders, L. M., Henderson, C. E., and Hong, M. Y.
(2004) J. Nutr., 134, 3233-3238.
56.Vanamala, J., Glagolenko, A., Yang, P., Carroll,
R. J., Murphy, M. E., Newman, R. A., Ford, J. R., Braby, L. A.,
Chapkin, R. S., Turner, N. D., and Lupton, J. R. (2008)
Carcinogenesis, 29, 790-796.
57.Umar, S., Morris, A. P., Kourouma, F., and
Sellin, J. H. (2003) Cell Prolif., 36, 361-375.
58.Rao, C. V., Chou, D., Simi, B., Ku, H., and
Reddy, B. (1998) Carcinogenesis, 19, 1815-1819.
59.Fluer, F. S., Kuznetzova, G. G., and Batisheva,
S. Yu. (2006) Vopr. Pitaniya, 4, 46-49.
60.Ganan, M., Collins, M., Rastal, R., Hotchkiss, A.
T., Chan, H. K., Carrascosa, A. V., and Martinez-Rodriguez, A. J.
(2010) Int. J. Fd. Microbiol., 137, 181-185.
61.Larsen, J. L. (1981) Nord. Vet. Med.,
33, 218-223.
62.Olano-Martin, E., Williams, M. R., Gibson, G. R.,
and Rastall, R. A. (2003) FEMS Microbiol. Lett., 218,
101-105.
63.Golovchenko, V. V., Khramova, D. S., Shashkov, A.
S., Otgonbayar, D., Chimidsogzol, A., and Ovodov, Y. S. (2012)
Carbohydr. Res., 356, 265-272.
64.Zhao, Z., Li, J., Wu, X., Dai, H., Gao, X., Liu,
M., and Tu, P. (2006) Fd. Res. Int., 39, 917-923.
65.Wang, J. H., Luo, J. P., and Zha, X. Q. (2010)
Carbohydr. Polym., 81, 1-7.
66.Wang, X. S., Dong, Q., Zuo, J. P., and Fang, J.
N. (2003) Carbohydr. Res., 338, 2393-2402.
67.Matsumoto, N., Cyong, L. C., Kiyohara, H.,
Matsui, H., Abe, A., Hirano, M., Danbara, H., and Yamada, H. (1993)
Int. J. Immunopharmacol., 15, 683-693.
68.Sakurai, M. H., Matsumoto, T., Kiyohara, H., and
Yamada, H. (1999) Immunology, 97, 540-547.
69.Matsumoto, T., Moriya, M., Sakurai, M. H.,
Kiyohara, H., Tabuchi, Y., and Yamada, H. (2008) Int.
Immunopharmacol., 8, 581-588.
70.Kiyohara, H., Uchida, T., Takakiwa, M.,
Matsuzaki, T., Hada, N., Takeda, T., Shibata, T., and Yamada, H. (2010)
Phytochemistry, 71, 280-293.
71.Yu, K. W., Kiyohara, H., Matsumoto, T., Yang, H.
C., and Yamada, H. (2001) Carbohydr. Polym., 46,
125-134.
72.Samuelsen, A. B., Westereng, B., Yousif, O.,
Holtekjolen, A. K., Michaelsen, T. E., and Knutsen, S. H. (2007)
Biomacromolecules, 8, 644-649.
73.Ovodova, R. G., Golovchenko, V. V., Shashkov, A.
S., Popov, S. V., and Ovodov, Yu. S. (2000) Russ. J. Bioorg.
Chem., 26, 743-751.
74.Popov, S. V., Golovchenko, V. V., Ovodova, R. G.,
Smirnov, V. V., Popova, G. Yu., and Ovodov, Yu. S. (2006)
Vaccine, 24, 5413-5419.
75.Popov, S. V., Popova, G. Yu., Nikolaeva, S. Yu.,
Golovchenko, V. V., and Ovodova, R. G. (2005) Phytother. Res.,
19, 1052-1056.
76.Gloaguen, V., Brudieux, V., Closs, B., Barbat,
A., Krausz, P., Sainte, Catherine, O., Kraemer, M., Maes, E., and
Guerardel, Y. (2010) J. Nat. Prod., 73, 1087-1092.
77.Popov, S. V., Ovodova, R. G., and Ovodov, Yu. S.
(2006) Phytother. Res., 20, 403-407.
78.Khramova, D. S., Popov, S. V., Golovchenko, V.
V., Vityazev, F. V., Paderin, N. M., and Ovodov, Yu. S. (2009)
Nutrition, 5, 226-232.
79.Vazques-Torres, A., Jones-Carson, J., and
Baumler, A. (1999) Nature, 401, 804-807.
80.Soesatyo, M., Thepen, T., and Ghufron, M. (1993)
in Dendritic Cells in Fundamental and Clinical Immunology
(Kamperdijk, K., ed.) Plenum Press, N. Y., pp. 321-326.
81.Bloom, P. D., and Boedeker, E. C. (1996)
Semin. Gastrointest. Dis., 7, 151-166.
82.Sharma, R., Van Damme, E. J. M., and Peumans, W.
J. (1996) Histochem. Cell Biol., 105, 459-465.
83.Eriksson, I., Andersson, R., and Aman, P. (1997)
Carbohydr. Res., 301, 177-185.
84.Ugolev, A. M. (1985) Evolution of the
Digestion and Principles of Evolution of Functions: Elements of Modern
Functionalism [in Russian], Nauka, Leningrad.