REVIEW: Spatial Structure of Plant Cell Wall Polysaccharides and Its Functional Significance
T. A. Gorshkova*, L.V. Kozlova, and P. V. Mikshina
Kazan Institute of Biochemistry and Biophysics, Kazan Scientific Center, Russian Academy of Sciences, Lobachevsky str., 2/31, 420111 Kazan, Russia; fax: +7 (843) 292-7347; E-mail: gorshkova@mail.knc.ru; gorshkova1953@mail.ru* To whom correspondence should be addressed.
Received February 19, 2013; Revision received March 5, 2013
Plant polysaccharides comprise the major portion of organic matter in the biosphere. The cell wall built on the basis of polysaccharides is the key feature of a plant organism largely determining its biology. All together, around 10 types of polysaccharide backbones, which can be decorated by different substituents giving rise to endless diversity of carbohydrate structures, are present in cell walls of higher plants. Each of the numerous cell types present in plants has cell wall with specific parameters, the features of which mostly arise from the structure of polymeric components. The structure of polysaccharides is not directly encoded by the genome and has variability in many parameters (molecular weight, length, and location of side chains, presence of modifying groups, etc.). The extent of such variability is limited by the “functional fitting” of the polymer, which is largely based on spatial organization of the polysaccharide and its ability to form supramolecular complexes of an appropriate type. Consequently, the carrier of the functional specificity is not the certain molecular structure but the certain type of the molecules having a certain degree of heterogeneity. This review summarizes the data on structural features of plant cell wall polysaccharides, considers formation of supramolecular complexes, gives examples of tissue- and stage-specific polysaccharides and functionally significant carbohydrate–carbohydrate interactions in plant cell wall, and presents approaches to analyze the spatial structure of polysaccharides and their complexes.
KEY WORDS: higher plants, cell wall, polysaccharides, spatial structure, cellulose, xylan, rhamnogalacturonan IDOI: 10.1134/S0006297913070146
The major portion of biosphere organic matter is concentrated within plants. Its main components are polysaccharides localized within a special compartment of the plant cell – the cell wall – whose multifunctional structure largely determines the specificity of plant biology. Complexity of structure and dynamic rearrangements of cell wall are to a large extent based on the presence of various polysaccharide structures, which can be significantly changed in the course of plant cell development (both due to synthesis and to modification of molecules within cell wall).
Polysaccharide synthesis, as distinct from that of proteins and nucleic acids, is not organized on a template that dictates strict uniformity of primary structure. One and the same function can be performed by non-identical polysaccharides; though the variations in structure are limited, the concept of polysaccharide specificity differs from that of proteins and nucleic acids whose strict correlation between structure and performed function is supposed. The bearer of functional specificity is not a single structure, but its certain type [1]. This type may be specified as strictly as the single structure in the case of protein or nucleic acid; its characteristics are largely based on the parameters of spatial organization.
FEATURES OF SPATIAL ORGANIZATION OF POLYSACCHARIDES
To describe macromolecules, the concepts of primary, secondary, tertiary, and quaternary structures have been developed as the most convenient way to specify different levels of spatial organization. According to currently accepted ideas [2]: primary structure of a polymer – covalently linked chemical sequence of units within a chain, plus all inter- and intramolecular links; secondary structure – locally ordered arrangement of units (often helical); tertiary structure – overall spatial organization of an object, which cannot be split without cleavage of covalent bonds; quaternary structure – arrangement of single units of tertiary structure within a complex built by non-covalent interactions (hydrogen bonds, van der Waals forces, etc.).
However, to describe polysaccharides, the terms that characterize various levels of organization are often used with somewhat different interpretation [3]. A single molecule is described only in terms of primary and secondary structures. Tertiary structure is defined as a complex of non-covalently linked molecules, and quaternary structure – as a “complex of complexes”, which form the extended three-dimensional network. This approach to characterizing polysaccharides is convenient in the case of regular homopolymers, like cellulose or polygalacturonic acid, but while describing the structure of complex polysaccharides with irregular structure (e.g. rhamnogalacturonans with complicated side chains) it leaves without consideration the level of individual molecule spatial organization, at which the locally ordered parts are linked by unordered ones. Differences in terminology cause some confusion, but we will keep the universal concept of organization levels for various nature macromolecules by referring to complexes of noncovalently interacting polysaccharides as “quaternary structure”.
To determine the primary structure of polysaccharides, it is necessary to know the degree of polymerization, the structure (including absolute configuration) of individual monosaccharides, the sequence of their linkage to each other, the points of branching, the type of substitution, the character and distribution of modifying groups (e.g. phosphates, sulfates, methyl- and acetyl-groups), and the configuration (α- or β-) of glycosidic bonds.
In this review, we will concentrate on the cell wall polymers of higher plants, which form relatively few basic structures constituting the backbones or long side chains of polysaccharides (Table 1). Among them, β-(1→4) linkages are the most widespread: β-d-(1→4)-Glcp, β-d-(1→4)-Xylp, β-d-(1→4)-Manp, β-d-(1→4)-Galp. Neutral chains may also contain β-d-(1→3)-Glcp, β-d-(1→3)-Galp and β-d-(1→6)-Galp, α-l-(1→5)-Araf. Also, there are two basic structures that which contain uronic acids: α-d-(1→4)-GalpA and dimer [→4)-α-d-GalpA -(1→2)-α-l-Rhap(1→]; polymers based on them are collectively named “pectins”.
Table 1. Variability of the main plant cell
wall polysaccharides
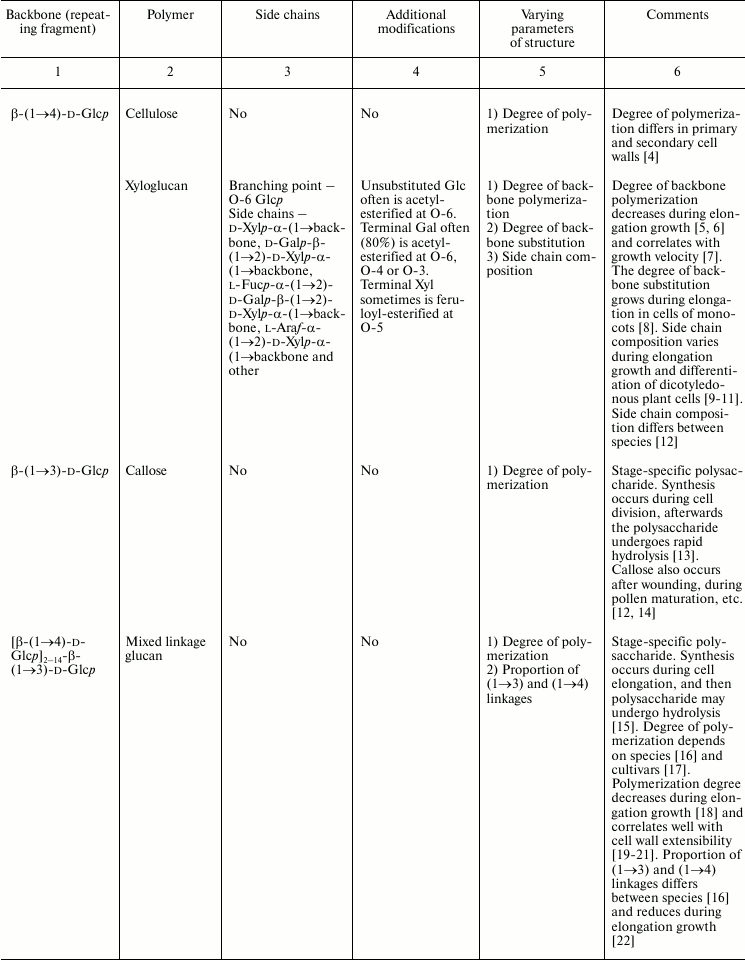
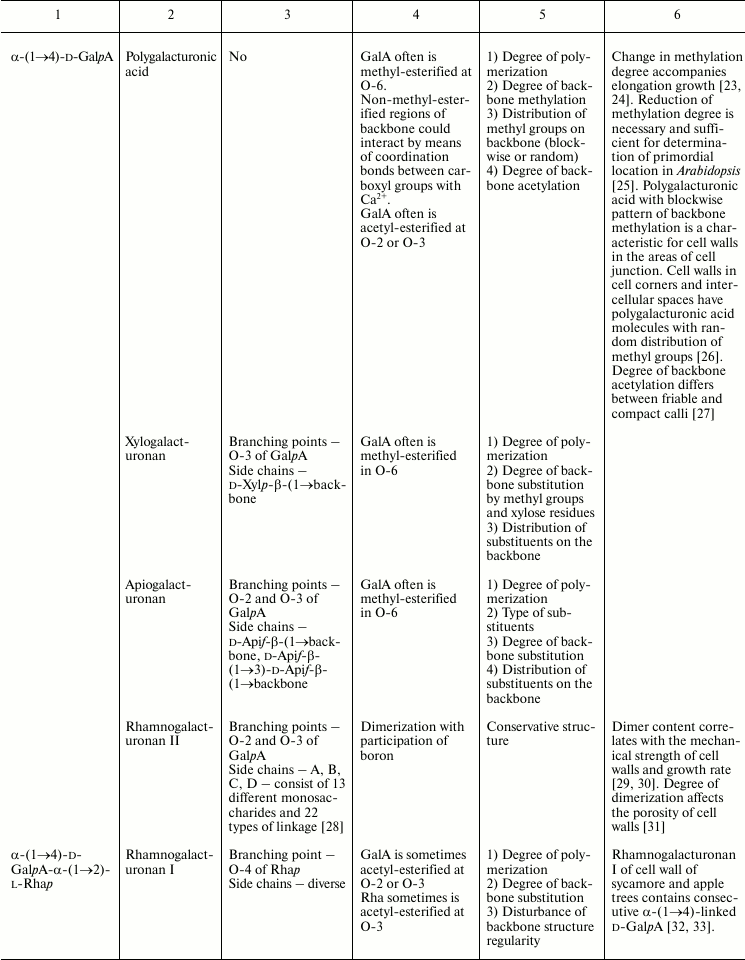
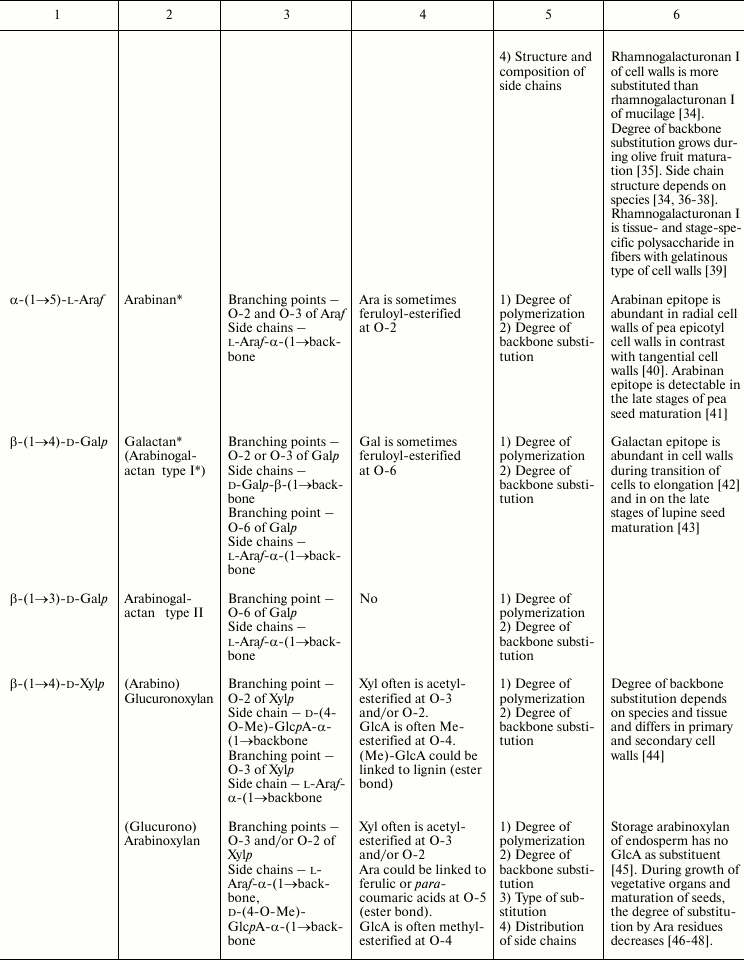
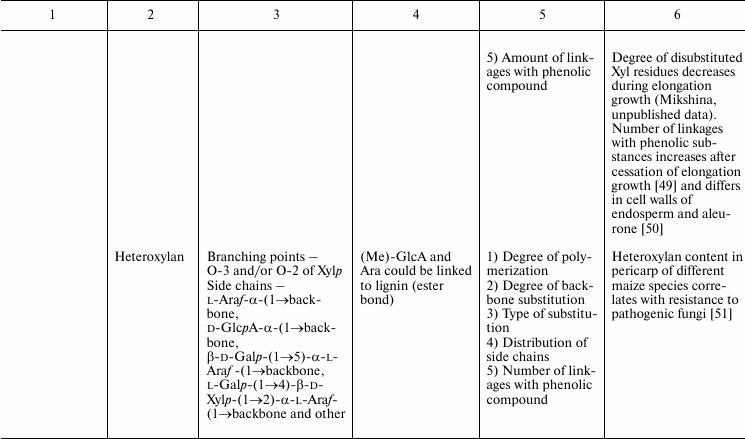
* Could be side chains of rhamnogalacturonan I.
The degrees of polymerization of various cell wall polysaccharides usually lie within the limits of three- to five-digit numbers. Most of the plant cell wall polysaccharides have well-defined backbone. The branched structures, like glycogen or amylopectin, in which the distribution of branching points does not permit definition of some chain as the backbone, are virtually absent. An exception is arabinogalactan of type II, in which the various combinations of β-d-(1→3)-Galp and β-d-(1→6)-Galp may lead to the formation of complex branched structures. Side chains of the polysaccharides with well-defined backbone, if any, are usually short (1-3 monomers). Long side chains may be present in rhamnogalacturonan I, but not always.
Plant can make polymers with different properties and functions based on the same type of backbone. General routes to diversify the primary structure of polysaccharides built from the listed basic structures together with the most widespread types of polysaccharides present in cell walls of higher plants (cellulose, xylan, xyloglucan, polygalacturonic acid, rhamnogalacturonan, etc.) are presented in Table 1. The character of primary structures (branched and linear, homo- and hetero-, one or several types of linkages) determines the higher levels of spatial organization, influences the various properties like water-solubility, aggregation and crystallization, gel viscosity, and biological functions (structural, reserve, defense, signal, etc.).
Secondary structure of polysaccharide chains is dictated, first of all, by the conformation of individual monosaccharide residues and by the geometry of their linkage to each other by glycosidic bonds [52, 53]. It is known that the conformation of an individual monosaccharide ring is usually rather rigid. For example, it was shown that the length of the within-cycle linkages, the valence, and torsion angles of the monosaccharides in pyranose form are only weakly dependent on the type of oligosaccharide [54]. The major factor in the final conformation of a polysaccharide is made by the relative orientation of monomers. In cases when the glycosidic linkage is made with participation of the hydroxyl belonging to the carbon atom of a pyranose or furanose ring (cyclic hydroxyl), the possibilities for conformational rearrangements are limited by two angles of torsion around the glycosidic bond C-1–O (φ) and O–C (ψ). In cases when the linkage is formed with participation of exocyclic hydroxyl (at C-6 in pyranoses and at C-5 in furanoses), three torsion angles exist (the torsion angle around the linkage between C-5 and C-6 for hexoses in pyranose form, or between C-4 and C-5 for pentoses in furanose form); this increases flexibility of the molecule. Polymers having elongated chains of the latter type are rarely found in plant cell walls. An exception is arabinan, which consists of furanose residues of l-Ara linked by α-(1→5)-bonds.
Low degree of conformational mobility leads to determination of secondary structure of regularly built polysaccharidic chains. Several major variants that are mainly helical are distinguishable. Key parameters to describe helical conformations are the number of monomers per turn of helix (n) and the length of the projection of each residue on the helix axis (h). Four types of secondary structures are distinguished for linear homopolysaccharides [55]; these may be partially related to the combination of equatorial and axial linkages.
Type A – flat ribbon-like structures with n = 2 ± 4 (negative number designates left-handed structure) and h value close to the absolute length of the residue. Conformation of flat ribbon emerges as a consequence of the fact that equatorial linkages O–C-4 and C-1–O are parallel to each other and lie almost within the same straight line relative to the ring plane. Among the basic structures of higher plant cell wall, cellulose, xyloglucan backbone, β-(1→4)-d-xylan, and β-(1→4)-d-mannan belong to type A.
Type B – normal helix with a wide range of values of n = 2 ± 10 and approximately zero h value. Such conformation, which is also called “U-turned hollow helix”, is formed when the glycosidic linkages of the pyranose ring are not parallel – one is axial, while the other is equatorial. Structures of B type are formed by β-(1→4)-d-galactan and β-(1→3)-d-glucan (callose).
Type C – crumpled ribbon. Structures of this type are formed by axial linkages that are almost parallel to each other but are displaced relative to the pyranose ring plane and do not fit the same straight line; due to this, at each monomer a zigzag step is formed in the polysaccharide chain. α-(1→4)-d-galacturonan – the most widespread component of pectins – represents type C structure since it has diaxial linkages.
Type D – flexible random coil. Homopolymers of type D are formed by linkages involving an exocyclic hydroxyl, like α-(1→5)-l-arabinan.
For rhamnogalacturonan I heteropolymer, the repeating unit in which is a dimer (Table 1), data on possible secondary structure are limited and contradictory. The only the notion is that unambiguously accepted is that the structure is helical with three monosaccharide residues per turn [56-59].
Tertiary structure is defined by packing of geometrically regular chains (if they are present in the polysaccharide) into a single whole. The following major factors determining the polysaccharide tertiary structure can be listed: interactions with nearby monomers along the molecular chain, distal three-dimensional interactions, entropic effects, and interactions with the environment. Tertiary structure is stabilized by hydrogen bonds and in the presence of electrically charged groups by electrostatic forces. Packing of an individual polysaccharide chain within a certain volume has not been described in the literature; considerably more forces of various characters should be present to form and stabilize such a structure [60, 61]. Well-ordered forms of regularly-built homopolymers restrict sharp bending of chains, preventing folding of a molecule. In complex branched polysaccharides, approach and interaction of the monomers remote from each other along the chain is possible. However, the absence of crystal structure is usually characteristic for macromolecules of branched polysaccharides. This gives rise to such property of branched polymers as water solubility despite molecular mass reaching several million Daltons.
The tertiary structure of the majority of higher plant cell wall polysaccharides is characterized extremely poorly. In most of them, regular structures, if present, are more complicated than unbranched homopolymers. Information on the influence of various substitutions and their distribution along the backbone on the overall shape of the molecule is also very scarce.
Quaternary structure is formed by association of individual molecules; pronounced quaternary structure is characteristic for many plant cell wall polysaccharides. Ribbon-like structures of type A lie especially well over each other and generate supramolecular formations due to numerous hydrogen bonds and van der Waals forces. As the result, structures that are regular in three dimensions are formed, and these are characteristic of crystals. The capacity to form double and triple helixes and aggregations of helixes and ribbons has been demonstrated for several polysaccharidic chains [62-65]. One chain may pass through several places of association, giving rise to three-dimensional nets or gels. Many structures, especially cellulose microfibrils and gels of polygalacturonic acid have been characterized in detail from both the chemical and biological viewpoints. There are also some papers on supramolecular structures of xylan [66], glucomannan [67], galactan [68, 69], and callose [70]. Examples of high levels of spatial organization of cell wall polysaccharides will be given in the following chapters.
VARIABILITY OF PLANT CELL WALL POLYSACCHARIDE STRUCTURE
Populations of molecules of the majority of plant cell wall polysaccharides, the same as for other polymers with non-template synthesis, have some heterogeneity. The degree of heterogeneity varies for different plant cell wall polysaccharides; the range of variability is not always proportional to the complexity of the molecular structure (Table 1). Virtually all polysaccharides have variable molecular mass. In branched polymers, the position of branching points, their frequency and regularity, the composition and structure of side chains, etc. may vary. Sometimes the regularity of the backbone structure is disturbed. For example, the ratio of rhamnose to galacturonic acid in the backbone of rhamnogalacturonan I is often not equal to 1 : 1, since the backbone may include insertions of α-d-GalpA sequential residues connected by (1→4)-linkages [32, 33]. Having in mind the combination of many variable parameters in the structure of most polysaccharides (Table 1) and high degree of polymerization, it is reasonable to assume that it is impossible to find two identical molecules of the same polysaccharide within the cell wall of an individual cell and within the whole plant (an exception is rhamnogalacturonan II).
Let us consider various xylans and rhamnogalacturonans I as examples illustrating variations in polysaccharide composition. Xylans of higher plants can be subdivided into three groups according to the character of backbone substitution: (arabino)glucuronoxylans, (glucurono)arabinoxylans, and heteroxylans [45]; the name of the main substituent is put just before the name of the backbone, while the variable substituent, which can even be absent, is put in brackets.
(Glucurono)arabinoxylans have the residues of α-l-Araf as the major substituent; they are attached to xylose at the O-3 position in the case of monosubstitution and at O-2 and O-3 in the case of disubstitution. As comes from the name of the polymer, glucuronic acid can be present in the polysaccharide structure; it is attached to xylose at the O-2 position [45, 66]. The range of xylan structural variability is characterized by the ratio Ara/Xyl, which may be equal to null as in the case of glucuronoxylan, and may be over 1, as in arabinoxylans from wheat endosperm [71, 72]. The suggestion of blockwise character of xylan substitution, which is based both on experimental data and modeling, is periodically put forward [73-75]. Arabinose residues can be linked with ferulic or para-coumaric acids [76, 77].
As distinct from (arabino)glucuronoxylans and (glucurono)arabinoxylans, which have only Ara, GlcA, and Ac as substituents, heteroxylans may have Gal and Xyl within their side chains, sometimes in unique combinations [78] (Table 1). It is for heteroxylans that so-called weak self-associations were demonstrated for the carbohydrate–carbohydrate system; the importance of such interactions is well known for enzyme–substrate and antigen–antibody systems and for the mechanism of cellular recognition. The revealed interaction has hydrophobic nature; its affinity differs for heteroxylans of various origins; however, attempts to determine the relationship between certain structural elements of a polysaccharide and its ability for weak self-association have not yet been successful [79].
The range of rhamnogalacturonan I variability is mainly based on the number and diversity of side chains of various length and structure rather than on the above-mentioned irregularity of backbone structure. Side chains are attached to rhamnose at the O-4 position, and degree of rhamnose substitution can vary from 20 to 96%. Side chains may be single residues or linear and branched arabinans, galactans, and arabinogalactans [36, 80, 81]; their presence, proportion, length, and structural details differ depending on the plant source and localization within plant tissues. Besides the above-listed widespread types of rhamnogalacturonan I side chains, xylogalacturonan (chains of (1→4)-linked α-d-GalpA units with attached single Xylp residues or two Xylp connected by (1→4)-linkage at the O-3 position) was also described as a side chain of rhamnogalacturonan I [82]. Xylogalacturonan can also be a part of the polysaccharide backbone [33].
Besides branching with side chains, rhamnogalacturonans I may be partially acetylated at position O-2 [83] and/or O-3 of α-d-GalpA [84] and/or at O-3 of α-l-Rhap [85]. It was shown that acetyl groups take part in the association of rhamnogalacturonan I due to hydrophobic interactions; the sizes of such aggregates may reach 200 nm [86].
It is considered that α-d-GalpA within rhamnogalacturonan I backbone does not have side chains, but there is a paper demonstrating that ~2% of this residue in the backbone of rhamnogalacturonan I isolated from sugar beet is substituted at O-3 position by a single residue of β-d-GlcpA [87]. Residues of α-l-Fucp and 4-O-methyl-β-d-GlcpA [88], like ferulic and para-coumaric acids [89], may also be present within the molecules of rhamnogalacturonan I.
Variability of cell wall polysaccharide structure makes their study one of the most challenging tasks of modern biochemistry. Any of the structural analysis methods well established for the investigation of the components of living matter face problems that are often quite difficult to solve when applied to polysaccharides. Attempts to identify the higher level of spatial organization of carbohydrate molecules meet many challenges. As a result, the process of matching the structure and function of particular polysaccharides moves very slowly. Nevertheless, it is possible to find examples of successful application of various approaches to characterize spatial organization of carbohydrates and their complexes [90, 91]. The main methods used for these tasks, the type of information obtained during analysis, major difficulties in the application of a given approach, and examples of its use to characterize polysaccharides of plant cell wall are given in Table 2.
Table 2. Methods for characterization of
polysaccharides and their supramolecular complexes
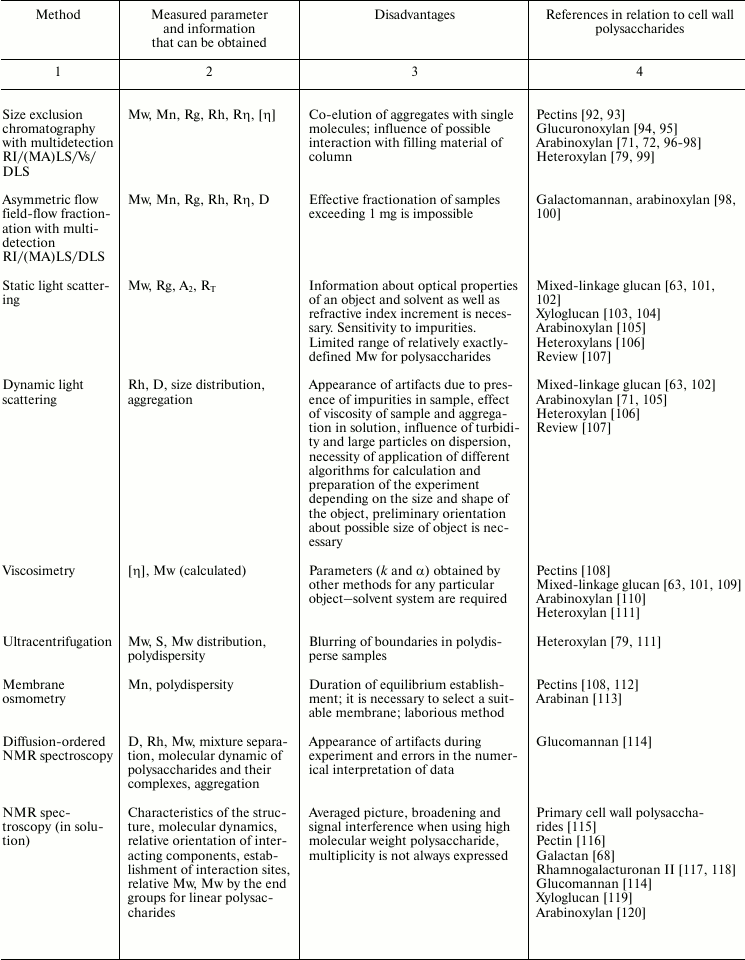
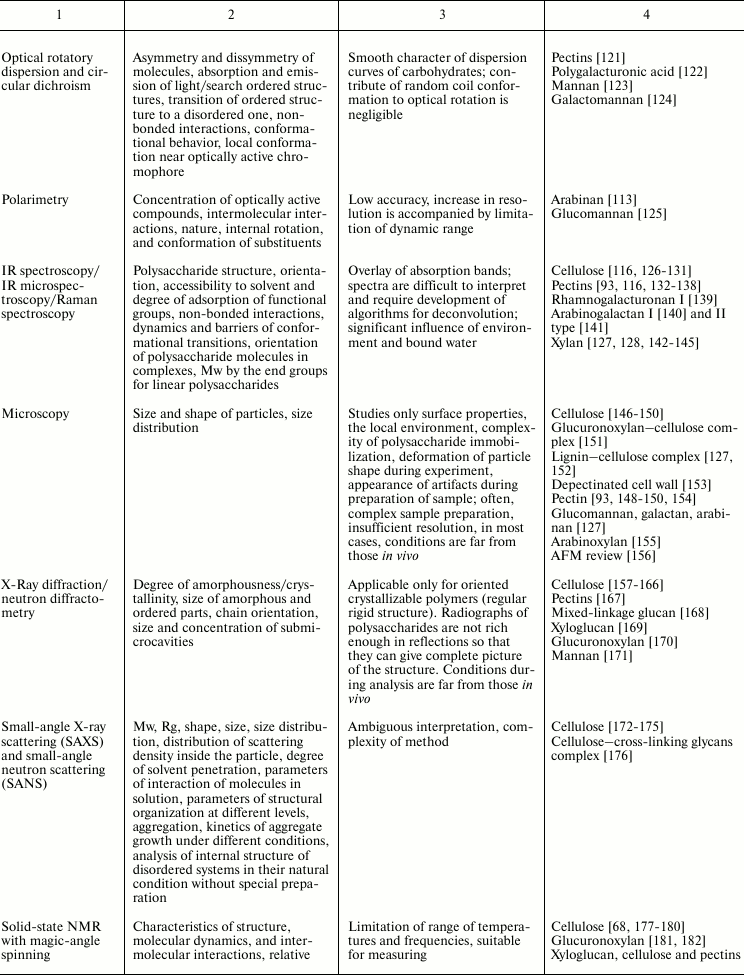
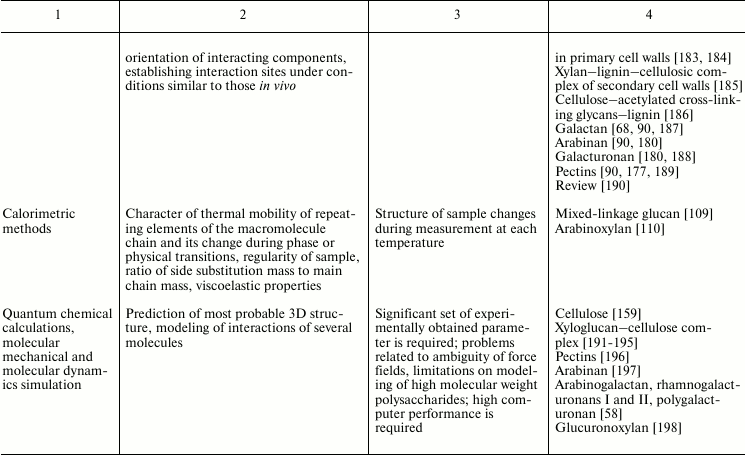
Note: RI, refractive index; (MA)LS, multi-angle light scattering; Vs,
viscometry; DLS, dynamic light scattering; Mw, weight-average molar
mass; Mn, number-average molar mass; Rg, radius of gyration; D,
diffusion coefficient; S, sedimentation coefficient; Rh, hydrodynamic
radius; Rη, viscometric radius; RT, thermodynamic
radius; A2, second virial coefficient; [η], intrinsic
viscosity.
ORIGIN OF STRUCTURAL VARIABILITY OF PLANT CELL WALL
POLYSACCHARIDES
Using various approaches, the heterogeneity of polysaccharides isolated from plant samples are established. Some examples are given in Table 1.
Does variability in the structure of polysaccharides that a given cell synthesizes at certain stage of its development exist? Though the answer seems to be obvious, it is rather difficult to obtain conclusive data. The point is that plant samples used to analyze cell wall polysaccharides are usually heterogeneous. They include cells of various tissues, each of which has specificity in cell wall composition; even within one tissue, the cells are not synchronized by stage of development, and this may also affect the features of the polysaccharides synthesized. Moreover, within one cell polysaccharides are present that were recently deposited into cell wall and those that have been there long enough to be modified by enzymes of the apoplast.
Proof of the presence of variability even within one type of cells can be obtained from the data on cell cultures (especially if they are synchronized), and the results of pulse-chase experiments, which can distinguish the polysaccharides synthesized in the course of the experiment and those existing before the experiment. In such experiments, data on variability of the degree of polymerization of cellulose [199] and xyloglucan [200] were obtained. A seldom used but effective approach is analysis of tissue- and stage-specific polysaccharides such as complex rhamnogalacturonan with ling galactan chains present in phloem fibers of flax. An additional advantage of this model system is the possibility of isolating the polysaccharide of interest before its incorporation into the cell wall. The broad peak obtained on gel filtration of the polysaccharide indicates variability in polymerization degree [36].
There are two general ways to reach a certain primary structure of plant cell wall polysaccharide that is necessary to fulfill its function: 1) in the course of synthesis, by the action of corresponding glycosyl-, methyl-, acetyl-, and feruloyl-transferases – definitely the major way, and 2) modification of polysaccharides that have already been deposited into cell wall, by enzymatic and nonenzymatic processes. The picture gets more complicated due to the formation of covalent linkages between polymers already within the Golgi apparatus – the place of synthesis of all cell wall polysaccharides except cellulose and callose. The presence of such linkages was demonstrated, for example, for xyloglucan and rhamnogalacturonan I [201], and also for arabinoxylan and rhamnogalacturonan I [202]. Many studies considered that the components of pectic substances – polygalacturonic acid, rhamnogalacturonan I, and rhamnogalacturonan II – are covalently linked to each other [34, 203].
Cell wall polysaccharides are synthesized via exceptionally complex mechanisms that are poorly characterized not only in details, but also in the basic “characters in the play”, such as the involved glycosyltransferases. As distinct from many bacterial polysaccharides, which are formed by a combination of similar oligosaccharide units [204], plant cell wall polysaccharides are synthesized by step-by-step addition of single activated monomer to the growing polysaccharidic chain. All glycosyltransferases that are involved in the process are membrane-bound enzymes and are organized into complex multicomponent complexes, as shown for several polymers – xyloglucan [205], xylan [75, 206], and pectic substances [34, 207]. Even the synthesis of chemically very simple polysaccharide, like cellulose – a linear homopolymer, in which all the monomers are linked by the same type of linkage, is provided by a very sophisticated multicomponent complex that has dimensions similar to the ribosome [4]. Despite massive efforts, enzymes involved in formation of many types of linkages in plant cell wall polysaccharides have not yet been determined [28, 34, 207].
Formation of the “necessary” primary sequence of the polymer is mainly achieved by functioning of a particular set of glycosyltransferases in combination, if necessary, with methyl-, acetyl-, or feruloyl-transferases. So, the change of the being formed cell wall type from primary to the secondary one in the course of cell development is coupled to corresponding changes in the activities of the enzymes involved in the formation of specific polysaccharides [208]. Replacements in the synthesis of such enzymes on change in the being formed cell wall type are revealed by large-scale gene transcription analysis [209].
Variability in a certain type of polysaccharide structure can hardly be explained by imperfectness of the machinery of their synthesis or by errors in its work. “Reliability” of glycosyltransferase complex work can be controlled quite fully. For instance, the structure of rhamnogalacturonan II, which due to the wide diversity of monomers and of the linkage types is considered to be one of the most complex compounds of biological origin, is very conservative not only in various tissues of the same plant, but also in evolutionary and taxonomically remote plant species [28, 210].
Polysaccharides present in the Golgi apparatus are less variable than after incorporation into cell wall, where the modifications due to the action of numerous enzymes and non-enzymatic factors are possible. Cell wall enzymes, mainly glycanases and esterases, are encoded by exceptionally large multigenic families. This fact was noticed on the very first description of the whole plant genome sequence [211]. For instance, in the Arabidopsis genome 66 genes annotated as pectinmethylesterases are present [212]. Though the specificity in action of the enzymes by different representatives of the multigenic family has not been completely characterized for any type of glycanase or esterase modifying plant cell wall polysaccharide, it is known that their products and/or substrates can differ. For example, there are pectinmethylestarases, the action of which leads to methyl group removal either from prolonged sequences of polygalacturonic acid, or in single, randomly located spots. As a result, homogalactan with blockwise or randomly localized methyl groups is formed [212]. Glycanases with tissue- or stage-specific character of expression have been revealed [15, 213, 214].
TISSUE- OR STAGE-SPECIFIC POLYSACCHARIDES
Each of around 40 cell types present in plants is characterized by cell wall specificity [12], which is largely based on the features of polysaccharide structure. The only polysaccharide that is always present in any type of cell in a plant organism is cellulose. However, there are differences in its structure at different stages of cell development. In “young” plant cells with so-called primary cell wall, which is distinguished by the ability to grow by expansion, the cellulose is formed with considerably lower degree of polymerization than in the secondary cell wall; the latter is formed in the cells which have stopped expansion growth [4]. Machinery specialized for primary and secondary cell wall cellulose synthesis exists: different genes of cellulose synthesizing catalytic subunits are expressed in one and the same cell at different stages of its development. These multienzyme complexes concurrently have three types of closely related but distinct catalytic subunits; their sets for the stages of primary and secondary cell wall formation are completely different [215].
An example of tissue-specific polysaccharide is callose; its quaternary structure is formed by weak interactions of coaxial triple helixes [70]. This polysaccharide is usually not present in cell wall. It can be formed in certain specific situations (like mechanical injury), but it plays a major role at the stage of plant cell division, when the cell plate is formed between two daughter cells. It is callose that serves as the key polymer in such “newly born” cell plate. This function is underlain both by a special mechanism of synthesis and by special properties of the polysaccharide supramolecular structure. Callose synthesis occurs at plasma membrane and is characterized by high rate since it is limited neither by the crystallization process, as in the case of cellulose, nor by a long duration of secretion, like in the case of all other cell wall matrix polysaccharides, which are synthesized exclusively in Golgi apparatus [14]. After the being formed from the center to the cell periphery, cell plate reaches the cell wall of the mother cell, and callose is degraded by special enzymes [13]. We can assume that the necessity for callose degradation is dictated by the poor ability of this polysaccharide supramolecular structure for extension. Since the division of a plant cell is followed by a well-pronounced stage of cell expansion, callose has to be degraded.
Another example of stage-specific polysaccharide is the low-substituted glucuronoxylan synthesized at the stage of secondary cell wall formation. In the backbone of glucuronoxylans, on average, one of ten xylopyranoses is substituted at O-2 by a residue of α-d-GlcpA, which is usually methylated at O-4. Also, xylose can be acetylated at the O-2 and/or O-3 positions [65]. This polymer forms left-handed helixes of type A; a single turn of the helix is formed by three xylose residues located towards each other at an angle of 120°. This leads to more uniform than in cellulose distribution of hydroxyl groups along the molecule, and as a result the xylan chain is less tightly packed and is more water-soluble than cellulose [170, 216]. The conformation of a β-d-(1→4)-Xylp residue resembles that of β-d-(1→4)-Glcp, but the fifth atom of carbon is substituted not by hydroxymethyl group, but by hydrogen; this may lead to a decrease in the possibility for intra- and intermolecular associations due to hydrogen bonds. This makes the xylan molecule more flexible and able to exist in other conformations [170].
The thickness of the xylan type secondary cell wall is at least an order of magnitude greater than that of primary cell wall. In higher plants, it is comprised from three major polymers – cellulose, glucuronoxylan, and lignin. The main function of the secondary cell wall is mechanical; thus, “good” interactions between key components must be provided. Cellulose and lignin do not interact with each other. They are interconnected by glucuronoxylan, part of its molecules forming covalent linkages with lignin, mainly through glucuronic acid residues [217, 218]. The study of isolated lignin–carbohydrate complexes showed higher tendency of these compounds for self-association and for adsorption on the surface of cellulose microfibrils as compared to non-lignified xylans [219, 220].
Analysis of the energy of xylan and cellulose interaction suggested that it is largely based on hydrogen and van der Waals bonds [221-223]. In water solution, xylan has the conformation of ribbon-like helix with n = 3. On the basis of data of solid-state 13C NMR-spectroscopy with magic angle spinning, arguments were suggested indicating that upon interaction of cellulose the length of the xylan helix turn decreases to two residues, i.e. it becomes similar to that of cellulose [224]. This suggestion is confirmed by molecular dynamic simulation [225]. However, Paananen et al. [151] questioned the importance of hydrogen bonds and suggested that the driving forces of interaction between xylan and cellulose are the increase in entropy upon exclusion of solvent and of weak van der Waals bonds.
The structure of glucuronoxylan is so well adapted by evolution that it is quite similar in the secondary cell walls of dicotyledonous plants of various taxonomical groups [45, 226]. However, the same xylan backbone may be the basis for synthesis of polysaccharides with a very different set of substituents and frequencies of their presence.
Variations in xylan composition are well exemplified by analysis of different tissues in seeds of grasses. Arabinoxylans of endosperm are almost exclusively composed of arabinose and xylose with average molecular ratio around 0.5. The amounts of ferulic and glucuronic acids are negligibly small [66]. Reserve function in addition to the mechanical one is considered to be the main function of these polysaccharides; also, due their considerable water-holding capacity, they are able to regulate water balance [227, 228]. It was shown that in water-soluble arabinoxylans from wheat endosperm the presence of even rather prolonged blocks of non-substituted xylose residues does not lead to interactions of chains with each other [229], as often suggested. Thus, endosperm arabinoxylans are highly hydrated and do not tend to form associates; features of their structure also do not give them the possibility to make covalent linkages with other cell wall components (due to the absence of ferulic and glucuronic acids). The character of xylan substitution with arabinose leaves enough sites for the enzyme action to cleave the polymer upon seed germination.
The presence of phenolic substances in the arabinoxylans from cell wall of the aleuronic layer can be considered as their main difference from the arabinoxylans of endosperm [50, 230]. This peculiarity in structure permits to the molecules of arabinoxylans from cell wall of the aleuronic layer to interact with each other through ferulic acid bridges between polymer chains. The strength of the formed structure increases not only due to formation of covalent linkages, but also due to the processes of entangling of polymeric chains [110]. The tendency of arabinoxylans from cell wall of aleuronic layer to make supramolecular complexes on the basis of covalent linkages (including those with cell wall proteins through tyrosine [231]) allows them to form more rigid cell walls that are resistant to the action of degradation factors, thus fulfilling defensive function. Accordingly, the amount of feruloylated arabinoxylans in the aleuronic layer and pericarp of seeds is proportional to the resistance of different maize cultivars to the pathogenic fungi Fusarium graminearum [51]. Study of saccharification of plant raw materials from various sources led to the conclusion that the presence of phenolic acid esters in arabinoxylans leads to decrease in effectiveness of degradation of these polysaccharides by the enzymes of fungi or bacteria [232].
In the pericarp of wheat [230], maize [51], and barley [233], heteroxylans were found in which the ratio Ara/Xyl is above 1, and galactose, xylose, and glucuronic acids are found in the side chains; significant is also the amount of hydroxycinnamic acids. Information about the features of heteroxylan spatial organization and functional role is quite limited. It is possible that high degree of backbone decoration in heteroxylans burdens their enzymatic hydrolysis, and the presence of variable substituents opens additional possibilities for interactions with other cell wall polymers.
Xylans play a specific function during one of the key processes in plants – expansion growth. However, the participation of xylans is not characteristic for all higher plants, but only for specific groups, mainly grasses (in other plants, a similar role is played by xyloglucan). In primary cell walls of the growing plant organs, the key matrix glycan is glucuronoarabinoxylan distinguished by high degree of branching with arabinose residues [46, 234, 235]. Treatment with endoxylanases, which needs three consecutive unsubstituted xylose residues, revealed the distant orderliness of glucuronoarabinoxylans [235]. Molecules of the polysaccharide may be conventionally subdivide into domains differing in the degree of backbone substitution to such extent that one of the domains (Ara/Xyl < 0.3) seems to interact with cellulose, while the opposite is the highly substituted part of the polymer (Ara/Xyl > 0.7) not linked to microfibrils. The proportion of these domains changes during expansion growth: at the beginning of the process, most of the xylose is within highly substituted domain, while after completion of growth it interacts with the cellulose domain [236]. These data correlate well with the known regularity noticed for expansion growth of monocotyledonous plants – decrease in Ara/Xyl ratio in cell walls during this process [8, 46, 49]. High correlation of the features of glucuronoarabinoxylan structure with plant cell growth rate suggests direct involvement of this polymer in expansion growth [235].
Another polymer with the described tissue-specific variant is rhamnogalacturonan I. Rhamnogalacturonans I with all differences in their structure is usually considered to be a component of the thin primary cell walls, where it plays the role of special “glue” to keep neighboring cells together [82, 237-240]. The presence of polysaccharide of this type was also demonstrated for mucilages secreted by seeds [37, 241, 242]. A tissue- and stage-specific variant of rhamnogalacturonan I has still been characterized only for one cell type – for plant fibers that form the so-called “gelatinous” cell wall type after elongation growth is completed. Characteristic features of the gelatinous cell wall are contractile properties, exclusive width (up to 15 µm), axial orientation of all cellulose microfibrils, and absence of xylan and lignin [39, 243].
It is not yet possible to relate the set of rhamnogalacturonan I structural elements with the performed function. However, it is obvious that the structure of the polymer differs depending on its location. For example, in flax plants it is possible to distinguish at least three populations of rhamnogalacturonan I molecules: 1) those from primary cell walls; 2) from mucilage secreted by seeds; 3) from fibers that form a peculiar type of secondary cell wall.
In primary cell wall rhamnogalacturonan I, there are three GalpA residues per two Rhap residues, and neutral monosaccharides are mainly represented by galactose and to the lesser extent by arabinose; the acetylation degree is high [244]. Epitopes of (1→6)- and (1→4)-galactans were immunocytochemically revealed in flax root tips [245].
Rhamnogalacturonan I from mucilage secreted by flax seeds contains the rare monosaccharide l-Galp, which is attached to Rhap at the O-3 position; also, terminal residues of Fucp and d-Galp attached to rhamnose at the O-3 position are present; the ratio of branched at O-3 and linear rhamnose is around seven. It was shown that rhamnogalacturonan I increases the viscosity of mucilage [37].
Tissue- and stage-specific rhamnogalacturonan I from flax phloem fibers can be isolated before and after incorporation into cell wall. The polymer synthesized in flax phloem fibers elutes on gel filtration at time corresponding to molecular mass 700-2000 kDa, the GalA/Rha ratio is close to 1, and the α-l-Rhap substitution degree is very high (up to 96%); the side chains of various length consist mainly of β-d-(1→4)-Galp and are sometimes decorated by single arabinose residues [36, 246].
After incorporation into cell wall, the polymer is modified by tissue-specific galactosidase leading to transformation of cell wall supramolecular organization that is necessary to form the specific mechanical properties of fibers [214]. Part of the rhamnogalacturonan I molecules get entrapped by laterally interacting cellulose microfibrils. The entrapped cellulose microfibrils of rhamnogalacturonan I elutes on gel filtration at the time corresponding to molecular mass 100-400 kDa and has GalA/Rha ratio close to 1; the degree of α-l-Rhap substitution is 72%. Most of the side chains are single residues of Galp, but there are also longer chains with average degree of polymerization 14 [38, 247]. It is assumed that this polysaccharide is able to self-associate due to galactose side chain interactions; as a result, compact complex is formed with charged backbone located at the surface [248]. If this polysaccharide is entrapped between laterally interacting cellulose microfibrils, such spatial organization of rhamnogalacturonan I helps to effectively form the tension of microfibrils; this is considered to be the origin of contractile properties [249].
Thus, variability that is inherent in almost all plant cell wall polysaccharides can reach a level of specialization for a certain function. This gives rise to the challenge of identifying the crucial parameters of the type of the polysaccharide molecule population (less important parameters may vary within a certain range among individual molecules), which make this population appropriate to fulfill specific biological functions. Though such tasks were formulated several decades ago [1], only the first steps have been achieved in this direction. The special challenge originates from the difference in polysaccharide structure in solution and within cell wall. The concentrations of polysaccharides within the cell wall are so high and the various interactions so widespread that the structure of polysaccharides there may differ considerably from that in solution. An additional reason for the differences in interactions between the polymers in vivo and in vitro is the mechanism of individual polymer formation, which dictates to a certain extent the sequence and conditions for polysaccharide chains to interact. Thus, investigations of the spatial organization and functional specialization of cell wall polysaccharides will long be needed.
The authors appreciate the partial financial support for the work on the topic of the paper from the Russian Foundation for Basic Research (project Nos. 11-04-01016, 11-04-01602, 12-04-97077).
REFERENCES
1.Bochkov, A. N., Afanas’ev, V. A., and Zaikov,
G. E. (1980) Carbohydrates [in Russian], Nauka, Moscow.
2.IUPAC (1997) Gold Book, Blackwell Scientific
Publications, Oxford.
3.Wang, Q., and Cui, S. W. (2005) in Food
Carbohydrates. Chemistry, Physical Properties, and
Applications (Cui, S. W., ed.) CRC Press, London, pp. 219-261.
4.Somerville, C. (2006) Annu. Rev. Cell Devel.
Biol., 22, 53-78.
5.Nishitani, K., and Masuda, Y. (1983) Plant Cell
Physiol., 24, 345-355.
6.Talbott, L. D., and Ray, P. M. (1992) Plant
Physiol., 98, 369-379.
7.Takeda, T., Furuta, Y., Awano, T., Mizuno, K.,
Mitsuishi, Y., and Hayashi, T. (2002) Proc. Natl. Acad. Sci.
USA, 99, 9055-9060.
8.Gibeaut, D. M., Pauly, M., Bacic, A., and Fincher,
G. B. (2005) Planta, 221, 729-738.
9.Hoson, T., Sone, Y., Misaki, A., and Masuda, Y.
(1995) Physiol. Plant., 87, 142-147.
10.Pauly, M., Qin, Q., Greene, H., Albersheim, P.,
Darvill, A., and York, W. S. (2001) Planta, 212,
842-850.
11.Gunl, M., Gille, S., and Pauly, M. (2010) J.
Visual. Exp., 40, 2046.
12.Carpita, N. C., and McCann, M. C. (2000) in
Biochemistry and Molecular Biology of Plants (Buchanan, B. B.,
Gruissem, W., and Jones, R., eds.) American Society for Plant
Physiologists, Rockville, MD, pp. 52-109.
13.Zabotin, A. I., Barysheva, T. S., Trofimova, O.
I., Lozovaya, V. V., and Widholm, J. (2002) Russ. J. Plant
Physiol., 49, 792-798.
14.Chen, X. Y., and Kim, J.-Y. (2009) Plant
Signal Behav., 4, 489-492.
15.Kim, J. B., Olek, A. T., and Carpita, N. C.
(2000) Plant Physiol., 123, 471-485.
16.Stone, B. A. (2009) in Chemistry,
Biochemistry and Biology of (1→3)-β-Glucans and Related
Polysaccharides (Bacic, A., Fincher, G., and Stone, B., eds.)
Elsevier, San Diego, pp. 5-46.
17.Beer, M. U., Wood, P. J., and Weisz, J. (1997)
Cereal Chem., 74, 476-480.
18.Huber, D. J., and Nevins, D. J. (1980) Plant
Physiol., 65, 768-773.
19.Soga, K., Harada, K., Wakabayashi, K., Hoson, T.,
and Kamisaka, S. (1999) J. Plant Res., 112, 273-278.
20.Hoson, T., Soga, K., Mori, R., Saiki, M.,
Nakamura, Y., Wakabayashi, K., and Kamisaka, S. (2002) Plant Cell
Physiol., 43, 1067-1071.
21.Nakamura, Y., Wakabayashi, K., and Hoson, T.
(2003) Physiol. Plant., 118, 597-604.
22.Kozlova, L. V., Snegireva, A. V., and Gorshkova,
T. A. (2012) Russ. J. Plant Physiol., 59, 339-347.
23.Rockel, N., Wolf, S., Kost, B., Rausch, T., and
Greiner, S. (2008) Plant J., 53, 133-143.
24.Derbyshire, P., McCann, M. C., and Roberts, K.
(2007) BMC Plant Biol., 7, 1-12.
25.Peaucelle, A., Louvet, R., Johansen, J. N.,
Hofte, H., Laufs, P., Pelloux, J., and Mouille, G. (2008) Curr.
Biol., 18, 1943-1948.
26.Willats, W. G. T., Orfila, C., Limberg, G.,
Buchholti, H. C., van Alebeek, G. J. W. M., Voragen, A. G. J., Marcus,
S. E., Christensen, T. M. I. E., Mikkelsen, J. D., Murray, B. S., and
Knox, J. P. (2001) J. Biol. Chem., 276, 19404-19413.
27.Liners, F., Gaspar, T., and van Cutsem, P. (1994)
Planta, 192, 545-556.
28.Bar-Peled, M., Urbanowicz, B. R., and
O’Neill, M. A. (2012) Front. Plant Sci., 3,
1-12.
29.Ryden, P., Sugimoto-Shirasu, K., Smith, A. C.,
Findlay, K., Reiter, W. D., and McCann, M. C. (2003) Plant
Physiol., 132, 1033-1040.
30.O’Neill, M. A., Ishii, T., Albersheim, P.,
and Darvill, A. G. (2004) Annu. Rev. Plant Biol., 55,
109-139.
31.Fleischer, A., O’Neill, M. A., and Ehwald,
R. (1999) Plant Physiol., 121, 829-838.
32.McNeil, M., Darvill, A. G., and Albersheim, P.
(1980) Plant Physiol., 66, 1128-1134.
33.Schols, H. A., Posthumus, M. A., and Voragen, A.
G. J. (1990) Carbohydr. Res., 206, 117-129.
34.Caffall, H. K., and Mohnen, D. (2009)
Carbohydr. Res., 344, 1879-1900.
35.Vierhuis, E., Schols, H. A., Beldman, G., and
Voragen, A. G. J. (2000) Carbohydr. Polym., 43,
11-21.
36.Gorshkova, T. A., Wyatt, S. E., Salnikov, V. V.,
Gibeaut, D. M., Ibragimov, M. R., Lozovaya, V. V., and Carpita, N. C.
(1996) Plant Physiol., 110, 721-729.
37.Naran, R., Chen, G., and Carpita, N. C. (2008)
Plant Physiol., 148, 132-141.
38.Gurjanov, O. P., Ibragimova, N. N., Gnezdilov, O.
I., and Gorshkova, T. A. (2008) Carbohydr. Polym., 72,
719-729.
39.Gorshkova, T. A., Gurjanov, O. P., Mikshina, P.
V., Ibragimova, N. N., Mokshina, N. E., Salnikov, V. V., Ageeva, M. V.,
Amenitskii, S. I., Chernova, T. E., and Chemikosova, S. B. (2010)
Russ. J. Plant Physiol., 57, 328-341.
40.Willats, W. G. T., Marcus, S. E., and Knox, J. P.
(1998) Carbohydr. Res., 308, 149-152.
41.McCartney, L., and Knox, J. P. (2002) J. Exp.
Bot., 53, 707-713.
42.McCartney, L., Steele-King, C. G., Jordan, E.,
and Knox, J. P. (2003) Plant J., 33, 447-454.
43.McCartney, L., Ormerod, A. P., Gidley, M. J., and
Knox, J. P. (2000) Plant J., 22, 105-113.
44.Thornber, J. P., and Northkote, D. H. (1962)
Biochem. J., 82, 340-346.
45.Ebringerova, A., Hromadkova, Z., and Heinze, T.
(2005) Adv. Polym. Sci., 186, 1-67.
46.Gibeaut, D. M., and Carpita, N. C. (1991)
Plant Physiol., 97, 551-561.
47.Suzuki, K., Kitamura, S., Kato, Y., and Itoh, T.
(2000) Plant Cell Physiol., 41, 948-959.
48.Philippe, S., Saulnier, L., and Guillon, F.
(2006) Planta, 224, 449-461.
49.Obel, N., Porchia, A. C., and Scheller, H. V.
(2002) Phytochemistry, 60, 603-610.
50.Philippe, S., Tranquet, O., Utille, J. P.,
Saulnier L., and Guillon, F. (2007) Planta, 225,
1287-1299.
51.Bily, A. C., Reid, L. M., Taylor, J. H.,
Johnston, D., Malouin, C., Burt, A. J., Bakan, B., Regnault-Roger, B.,
Pauls, K. P., Arnason, J. T., and Philogene, B. J. R. (2003)
Phytopathology, 93, 712-719.
52.Stoddart, J. F. (1971) Stereochemistry of
Carbohydrates, Wiley-Interscience, New York.
53.Grachev, A. A., Gerbst, A. G., Shashkov, A. S.,
and Nifant’ev, N. E. (2009) Russ. Chem. Rev.,
78, 776-795.
54.Lipkind, G. M. (1991) Conformational Analysis
of Carbohydrate Chains: Doctoral thesis [in Russian], IOCh USSR AS,
Moscow.
55.Tsai, C. S. (2006) Biomacromolecules:
Introduction to Structure, Function and Informatics, John
Wiley & Sons Inc., Hoboken.
56.Cros, S., Garnier, C., Axelos, M. A. V., Imberty,
A., and Perez, S. (1996) Biopolymers, 39, 339-352.
57.Engelsen, S. B., Cros, S., Mackie, W., and Perez,
S. (1996) Carbohydr. Biopolym., 39, 417-433.
58.Perez, S., Mazeau, K., and Herve du Penhoat, C.
(2000) Plant Physiol. Biochem., 38, 37-55.
59.Choi, J. K., Lee, B. H., Chae, C. H., and Shin,
W. (2004) Proteins: Structure, Function, and
Bioinformatics, 55, 22-33.
60.Rees, D. A., and Welsh, E. J. (1977) Angew.
Chem. Int. Ed. Engl., 16, 214-224.
61.Eggleston, G., and Doyle, J. P. (2006) ACS
Symp. Ser., 935, 19-35.
62.Lang, P., and Kajiwara, K. (1993) J. Biomater.
Sci., Polymer Edn., 4, 517-528.
63.Grimm, A., Kruger, E., and Burchard, W. (1995)
Carbohydr. Polym., 27, 205-214.
64.McIntire, T. M., and Brant, D. A. (1998) J.
Am. Chem. Soc., 120, 6909-6919.
65.Kohnke, T. (2010) Adsorption of Xylans on
Cellulosic Fibers. Influence of Xylan Composition on Adsorption
Characteristics and Kraft Pulp Properties: PhD thesis, Chalmers
University of Technology, Goteborg.
66.Saulnier, L., Sado, P.-E., Branlard, G., Charmet,
G., and Guillon, F. (2007) J. Cereal Sci., 46,
261-281.
67.Wang, C., Zhang, Yu. Y., Huang, H. X., Chen, M.
B., and Li, D. S. (2011) Adv. Mater. Res.,
197-198, 96-104.
68.Foster, T. J., Ablett, S., McCann, M. C., and
Gidley, M. J. (1996) Biopolymers, 39, 51-66.
69.Fukushima, A., Tanaka, F., Azuma, J., and Iwata,
T. (1997) Wood Res., 84, 37-38.
70.Gidley, M. J., and Nishinari, K. (2009) in
Chemistry, Biochemistry, and Biology of
(1→3)-β-Glucans and Related Polysaccharides (Bacic, A.,
Fincher, G., and Stone, B., eds.) Elsevier, San Diego, pp. 47-118.
71.Dervilly, G., Saulnier, L., Roger, P., and
Thibault, J. F. (2000) J. Agr. Food Chem., 48,
270-278.
72.Dervilly-Pinel, G., Thibault, J. F., and
Saulnier, L. (2001) Carbohydr. Res., 330, 365-372.
73.Izydorczyk, M. S., and Biliaderis, C. G. (1994)
Carbohydr. Polym., 24, 61-71.
74.Dervilly-Pinel, G., Tran, V., and Saulnier, L.
(2004) Carbohydr. Polym., 55, 171-177.
75.Faik, A. (2010) Plant Physiol.,
153, 396-402.
76.Mueller-Harvey, I., and Hartley, R. D. (1986)
Carbohydr. Res., 148, 71-85.
77.Grabber, J. H., Hatfield, R. D., Ralph, J., Zon,
J., and Amrhein, N. (1995) Phytochemistry, 40,
1077-1082.
78.Appeldoorn, M. M., Kabel, M. A., Eylen, D.,
Gruppen, H., and Schols, H. A. (2010) J. Agric. Food Chem.,
58, 11294-11301.
79.Patel, T. R., Harding, S. E., Ebringerova, A.,
Deszczynski, M., Hromadkova, Z., Togola, A., Paulsen, B. S., Morris, G.
A., and Rowe, A. J. (2007) Biophys. J., 93, 741-749.
80.Albersheim, P., Darvill, A. G., O’Neill, M.
A., Schols, H. A., and Voragen, A. G. J. (1996) in Pectins and
Pectinases (Visser, J., and Voragen, A. G. J., eds.) Elsevier
Science, Amsterdam, pp. 47-55.
81.Ridley, B. L., O’Neill, M. A., and Mohnen,
D. (2001) Phytochemistry, 57,
929-967.
82.Oechslin, R., Lutz, M. V., and Amado, R. (2003)
Carbohydr. Polym., 51, 301-310.
83.Ishii, T. (1997) Plant Physiol.,
113, 1265-1272.
84.Perrone, F. M., Dell’Anno, A., Danovaro,
R., Della Croce, N., and Thurston, M. H. (2002) J. Marine Biol.
Assoc. UK, 82, 419-425.
85.Sengkhamparn, N., Bakx, E. J., Verhoef, R.,
Schols, H. A., Sajjaanantakul, T., and Voragen, A. G. J. (2009)
Carbohydr. Polym., 344, 1842-1851.
86.Sengkhamparn, N., Sagis, L. M. C., de Vries, R.,
Schols, H. A., Sajjaanantakul, T., and Voragen, A. G. J. (2010) Food
Hydrocolloids, 24, 35-41.
87.Renard, C. M. C. G., Crepeau, M. J., and
Thibault, J. F. (1999) Eur. J. Biochem., 266,
566-574.
88.O’Neill, M., Albersheim, P., and Darvill,
A. (1990) in Methods in Plant Biochemistry, Vol. 2 (Dey, D. M.,
ed.) Academic Press, London, pp. 415-441.
89.Saulnier, L., and Thibault, J. F. (1999) J.
Sci. Food Agric., 79, 396-402.
90.Jarvis, M. C., and McCann, M. C. (2000) Plant
Physiol. Biochem., 38, 1-13.
91.Yang, L. Q., and Zhang, L. M. (2009)
Carbohydr. Polym., 76, 349-361.
92.Mazumder, S., Morvan, C., Thakur, S., and Ray, B.
(2004) J. Agr. Food Chem., 52, 3556-3562.
93.Pose, S., Kirbyc, A. R., Mercado, J. A., Morris,
V. J., and Quesada, M. A. (2012) Carbohydr. Polym., 88,
882-890.
94.Saake, B., Kruse, T., and Puls, J. (2001)
Bioresource Technol., 80, 195-204.
95.Roubroeks, J. P., Saake, B., Glasser, W., and
Gatenholm, P. (2004) ACS Symp. Ser., 864, 167-183.
96.Pitkanen, L., Tuomainen, P., Virkki, L., Aseyev,
V., and Tenkanen, M. (2008) J. Agr. Food Chem., 56,
5069-5077.
97.Pitkanen, L., Virkki, L., Tenkanen, M., and
Tuomainen, P. (2009) Biomacromolecules, 10,
1962-1969.
98.Pitkanen, L., Tenkanen, M., and Tuomainen, P.
(2011) Analyt. Bioanalyt. Chem., 399, 1467-1472.
99.Ebringerova, A., Hromadkova, Z., Alfoldi, J., and
Berth, G. (1992) Carbohydr. Polym., 19, 99-105.
100.Pitkanen, L. (2011) The Effect of Structure
on the Dilute Solution Properties of Branched Polysaccharides Studied
with SEC and AsFlFFF: PhD thesis, University of Helsinki,
Helsinki.
101.Cui, W., and Wang, Q. (2006)
Biomacromolecules, 7, 446-452.
102.Gomez, C., Navarro, A., Horta, P., and
Carbonell, J. V. (1997) Carbohydr. Polym., 32,
7-15.
103.Gidley, M. J., Lillford, P. J., Rowlands, D.
W., Lang, P., Dentini, M., Creszenzi, V., Edwards, M., Fanutti, C., and
Reid, J. S. G. (1991) Carbohydr. Res., 214, 229-314.
104.Nishinari, K., Zhang, H., and Ikeda, S. (2000)
Curr. Opin. Colloid Interface Sci., 5, 195-201.
105.Ebringerova, A., Hromadkova, Z., Buchard, W.,
Dolega, R., and Vorwerg, W. (1994) Carbohydr. Polym., 24,
161-169.
106.Chanliaud, E., Roger, P., Saulnier, L., and
Thibault, J. F. (1996) Carbohydr. Polym., 31, 41-46.
107.Buchard, W. (2008) in Soft Matter
Characterization (Borsali, R., and Pecora, R., eds.)
Springer-Verlag, Berlin, pp. 463-603.
108.Rees, D. A., and Steele, I. W. (1966)
Biochemistry, 5, 3108-3110.
109.Lazaridou, A., and Biliaderis, C. G. (2007)
J. Cereal Sci., 46, 101-118.
110.Carvajal-Millan, E., Landillon, V., Morel,
M.-H., Rouau, X., Doublier, J. L., and Micard, V. (2005)
Biomacromolecules, 6, 309-317.
111.Dhami, R., Harding, S. E., Elizabeth, N. J.,
and Ebringerova, A. (1996) Carbohydr. Polym., 28,
113-119.
112.Rinaudo, M. (2009) Struct. Chem.,
20, 277-289.
113.Karacsonyi, S. (1993) Chem. Papers,
47, 400-405.
114.Hsieh, Y. S., Chien, C., Liao, S. K., Liao, S.
F., Hung, W. T., Yang, W. B., Lin, C. C., Cheng, T. J., Chang, C. C.,
Fang, J. M., and Wong, C. H. (2008) Bioorg. Med. Chem.,
16, 6054-6068.
115.MacKay, A. L., Wallace, J. C., Sasaki, K., and
Taylor, I. E. P. (1998) Biochemistry, 27, 1467-1473.
116.Fenwick, K. M., Jarvis, M. C., and Apperley, D.
C. (1997) Plant Physiol., 115, 587-592.
117.Herve du Penhoat, C., Gey, C., Pellerin, P.,
and Perez, S. (1999) J. Biomol. NMR, 14, 253-271.
118.Rodriguez-Carvajal, M. A., Herve du Penhoat,
C., Mazeau, K., Doco, T., and Perez, S. (2003) Carbohydr. Res.,
338, 651-671.
119.Hoffman, M., Jia, Z. H., Pena, M. J., Cash, M.,
Harper, A., Blackburn, A. R., Darvill, A., and York, W. S. (2005)
Carbohydr. Res., 340, 1826-1840.
120.Apirattananusorn, S. (2007) Arabinoxylans
from Job’s Tears (Coix Lachryma-Jobi L.): Chemical,
Molecular and Structural Characterization: PhD Thesis, Suranaree
University of Technology, Nankhon Ratchisima.
121.Bystricky, S., Fric, I., Stanek, J., Capek, K.,
Jary, J., and Blaha, K. (1979) Coll. Czech. Chem. Commun.,
44, 174-182.
122.Stoddart, R. W., Spires, I. P. C., and Tipton,
K. F. (1969) Biochem. J., 114, 863-870.
123.Duda, C. A., and Stevens, E. S. (1992)
Carbohydr. Res., 228, 333-338.
124.Buffington, L. A., Stevens, E. S., Morris, E.
R., and Rees, D. A. (1980) Int. J. Biol. Macromol., 2,
199-203.
125.Capek, P. (2009) Carbohydr. Polym.,
75, 356-359.
126.Cael, J. J., Gardner, K. H., Koenig, J. L., and
Blackwell, J. (1975) J. Chem. Phys., 975, 1145-1153.
127.Marchessault, R. H. (1962) Pure Appl.
Chem., 5, 107-129.
128.Marchessault, R. H., and Liang, C. Y. (1962)
J. Polym. Sci., 59, 357-378.
129.Eichhorn, S. J., Sirichaisit, J., and Young, R.
J. (2001) J. Materials Sci., 36, 3129-3135.
130.Kong, K., and Eichhorn, S. J. (2005)
Polymer, 46, 6380-6390.
131.Peetla, P., Schenzel, K. C., and Diepenbrock,
W. (2006) Appl. Spectrosc., 60, 682-691.
132.McCann, M. C., Hammouri, M., Wilson, R. H.,
Belton, P., and Roberts, K. (1992) Plant Physiol., 100,
1940-1947.
133.McCann, M. C., Chen, L., Roberts, K., Kemsley,
E. K., Sene, C., Carpita, N. C., Stacey, N. J., and Wilson, R. H.
(1997) Physiol. Plant., 100, 729-738.
134.Morikawa, H., Hayashi, R., and Senda, M. (1978)
Plant Cell Physiol., 19, 1151-1159.
135.Sene, C. F. B., McCann, M. C., Wilson, R. H.,
and Grinter, R. (1996) Plant Physiol., 106,
1623-1631.
136.Chen, L., Wilson, R. H., and McCann, M. C.
(1997) J. Mol. Struct., 408, 257-260.
137.Wellner, N., Kacurakova, M., Malovokova, A.,
Wilson, R. H., and Belton, P. S. (1998) Carbohydr. Res.,
308, 123-131.
138.Ovodova, R. G., Golovchenko, V. V., Popov, S.
V., and Ovodov, Yu. S. (2010) Proc. Komi Sci. Center UD RAS,
3, 37-45.
139.Capek, P., Rosik, J., Kardosova, A., and Toman,
R. (1987) Carbohydr. Res., 164, 443-452.
140.Capek, P., and Kardosova, A. (1995) Coll.
Czech. Chem. Commun., 60, 2112-2118.
141.Odonmazig, P., Ebringerova, A., Machova, E.,
and Alfoldi, J. (1994) Carbohydr. Res., 252, 317-324.
142.Coimbra, M. A., Barros, A., Barros, M.,
Rutledge, D. N., and Delgadillo, I. (1999) Carbohydr. Res.,
317, 145-154.
143.Kacurakova, M., Wellner, N., Ebringerova, A.,
Wilson, R. H., and Belton, P. S. (1999) Food Hydrocolloids,
13, 35-41.
144.Kacurakova, M., and Wilson, R. H. (2001)
Carbohydr. Polym., 44, 291-303.
145.Sun, R. C., Fang, J. M., Rowlands, P., and
Bolton, J. (1998) J. Agr. Chem., 46, 2804-2809.
146.Thomson, C. I., Lowe, R. M., and Ragauskas, A.
J. (2007) Carbohydr. Polym., 69, 799-804.
147.Baker, A. A., Helbert, W., Sugiyama, J., and
Miles, M. J. (1997) J. Struct. Biol., 119, 129-138.
148.Marszalek, P. E., Oberhauser, A. F., Pang,
Y.-P., and Fernandez, J. M. (1998) Nature, 396,
661-664.
149.Marszalek, P. E., Pang, Y.-P., Li, H., El
Yazal, J., Oberhauser, A. F., and Fernandez, J. M. (1999) Proc.
Natl. Acad. Sci. USA, 96, 7894-7898.
150.McCann, M. C., Dimitra, M. B., Sado, M. P.,
Stacey, N. J., Catchpole, G., Defernez, M., Carpita, N. C., Hofte, H.,
Ulvskov, P., Wilson, R. H., and Roberts, K. (2001)
Phytochemistry, 57, 811-821.
151.Paananen, A., Osterberg, M., Rutland, M.,
Tammelin, T., Saarinen, T., Tappura, K., and Stenius, P. (2004) ACS
Symp. Ser., 864, 269-290.
152.Donaldson, L. A., and Knox, J. P. (2012)
Plant Physiol., 158, 642-653.
153.McCann, M. C., Wells, B., and Roberts, K.
(1990) J. Cell Sci., 96, 323-334.
154.Round, A. N., MacDougall, A. J., Ring, S. G.,
and Morris, V. J. (1997) Carbohydr. Res., 303,
251-253.
155.Gunning, A. P., Mackie, A. R., Kirby, A. R.,
Kroon, P., Williamson, G., and Morris, V. J. (2000)
Macromolecules, 33, 5680-5685.
156.Morris, V. J., Gunning, A. P., Kirby, A. R.,
Round, A., Waldron, K., and Ng, A. (1997) Int. J. Biol.
Macromol., 21, 61-66.
157.Gardivert, K. H., and Blackwell, J. (1974)
Biopolymers, 13, 1975-2001.
158.Kolpak, F. J., and Blackwell, J. (1976)
Macromolecules, 9, 273-278.
159.Hardy, B. J., and Sarko, A. (1996)
Polymer, 37, 1833-1839.
160.Wada, M., Okano, T., and Sugiyama, J. (1997)
Cellulose, 4, 221-232.
161.Lai Kee Him, J., Chanzy, H., Muller, M.,
Putaux, J. L., Imai, T., and Bulone, V. (2002) J. Biol. Chem.,
277, 36931-36939.
162.Muller, M., Hori, R., Itoh, T., and Sugiyama,
J. (2002) Biomacromolecules, 3, 182-186.
163.Nishiyama, Y., Langan, P., and Chanzy, H.
(2002) J. Am. Chem. Soc., 124, 9074-9082.
164.Nishiyama, Y., Sugiyama, J., Chanzy, H., and
Langan, P. (2003) J. Am. Chem. Soc., 125,
14300-14306.
165.Langan, P., Narayanasami, S., Yoshiharu, N.,
and Chanzy, H. (2005) Cellulose, 12, 551-562.
166.Leppanen, K., Andersson, S., Torkkeli, M.,
Knaapila, M., Kotelnikova, N., and Serimaa, R. (2009) Cellulose,
16, 999-1015.
167.Walkinshaw, M. D., and Struther, A. (1981)
J. Mol. Biol., 153, 1075-1085.
168.Tvaroska, K., Ogawa, Y., Deslandesa, N., and
Marchessault, D. R. H. (1983) Canad. J. Chem., 61,
1608-1616.
169.Taylor, I. E. P., and Atkins, E. D. T. (1985)
FEBS Lett., 181, 300-302.
170.Atkins, E. D. T. (1992) in Xylans and
Xylanases. Progress in Biotechnology (Visser, J., Beldman, G.,
Kusters-van Someren, M. A., and Voragen, A. G. J., eds.) Elsevier,
Amsterdam, pp. 39-50.
171.Putaux, J. L. (2005) Macromol. Symp.,
229, 66-71.
172.Jakob, H. F., Fratzl, P., and Tschegg, S. E.
(1994) Struct. Biol., 113, 13-22.
173.Reiterer, A., Jakob, H. F., StanzI-Tschegg, S.
E., and Fratzl, P. (1998) Wood Sci. Technol., 32,
335-345.
174.Lichtenegger, H., Reiterer, A., Tschegg, S.,
and Fratzl, P. (1997) in Microfibril Angle in Wood, Proc.
IAWA/IUFRO Int. Workshop on the Significance of Microfibril Angle to
Wood Quality, Westport, New Zealand, pp. 140-156.
175.Thomas, L. H., Forsyth, V. T., Sturcova, A.,
Kennedy, C. J., May, R. P., Altaner, C. M., Apperley, D. C., Wess, T.
J., and Jarvis, M. C. (2013) Plant Physiol., 161,
465-476.
176.Garvey, J., Mikkelsen, D., Dykes, G. A., and
Gidley, M. (2006) in ANSE-ANBUG Neutron Scattering Symp., Lucas
Heights, Australia, Vol. 5, E4.
177.Jarvis, M. C., Fenwick, K. M., and Apperley, D.
C. (1996) Carbohydr. Res., 288, 1-14.
178.Koh, T. H., Melton, L. D., and Newman, R. H.
(1997) Canad. J. Bot., 75, 1957-1964.
179.Larsson, P. T., Hult, E. L., Wickholm, K.,
Pettersson, E., and Iversen, T. (1999) Solid State Nucl. Magnet.
Reson., 15, 31-40.
180.Renard, C. M. G. C., and Jarvis, M. C. (1999)
Plant Physiol., 119, 1315-1322.
181.Teleman, A., Larsson, P. T., and Iversen, T.
(2001) Cellulose, 8, 209-215.
182.Liitia, T., Maunu, L. S., Hortling, B.,
Tamminen, T., Pekkala, O., and Varhimo, A. (2003) Cellulose,
10, 307-316.
183.Dick-Perez, M., Wang, T., Salazar, A.,
Zabotina, O. A., and Hong, M. (2012) Magnet. Reson. Chem.,
50, 539-550.
184.Wang, T., Zabotina, O., and Hong, M. (2012)
Biochemistry, 51, 9846-9856.
185.Foston, M., Katahira, R., Gjersing, E., Davis,
M. F., and Ragauskas, A. J. (2012) J. Agr. Food Chem.,
60, 1419-1427.
186.Newman, R. H. (1992) Holzforschung,
46, 205-210.
187.Zykwinska, A., Rondeau-Mouro, C., Garnier, C.,
Thibault, J. F., and Ralet, M. C. (2006) Carbohydr. Polym.,
65, 510-520.
188.Jarvis, M. C., and Apperley, D. C. (1995)
Carbohydr. Res., 275, 131-145.
189.Ha, M. A., Jardine, W. G., and Jarvis, M. C.
(1997) J. Agr. Food Chem., 45, 117-119.
190.Bootten, T. J., Harris, P. J., Melton, L. D.,
and Newman, R. H. (2011) in The Plant Cell Wall: Methods and
Protocols, Methods in Molecular Biology, Vol. 715 (Popper,
Z. A., ed.) Springer Science, London, pp. 179-196.
191.Hayashi, T., Takeda, T., Ogawa, K., and
Mitsuishi, Y. (1994) Plant Cell Physiol., 35,
893-899.
192.Finkenstadt, V. L., Hendrixson, T. L., and
Millane, R. P. (1995) J. Carbohydr. Chem., 14,
601-611.
193.Levy, S., York, W. S., Struike-Prill, R.,
Meyer, B., and Staehelin, L. A. (1991) Plant J.,
1, 195-215.
194.Hanus, J., and Mazeau, K. (2006)
Biopolymers, 82, 59-73.
195.Zhang, Q., Brumer, H., Egren, H., and Tu, Y.
(2011) Carbohydr. Res., 346, 2595-2602.
196.Braccini, I., Grasso, R. P., and Perez, S.
(1999) Carbohydr. Res., 317, 119-130.
197.Cros, S., Imberty, A., Bouchemal, N., Herve du
Penhoat, C., and Perez, S. (1994) Biopolymers, 34,
1433-1447.
198.Leeflang, B. R., van Kuik, J. A., and
Kroon-Bratenburg, L. M. J. (2006) ACS Symp. Ser., 930,
133-155.
199.Blaschek, W., Koehler, H., Semler, U., and
Franz, G. (1982) Planta, 154, 550-555.
200.Thompson, J. E., Smith, R. C., and Fry, S. C.
(1997) Biochem. J., 327, 699-708.
201.Popper, Z. A., and Fry, S. C. (2008)
Planta, 227, 781-794.
202.Tan, L., Eberhard, S., Pattathil, S., Warder,
C., Glushka, J., Yuan, C., Hao, Z., Zhu, X., Avci, U., Miller, J. S.,
Baldwin, D., Pham, C., Orlando, R., Darvill, A., Hahn, M. G.,
Kieliszewski, M. J., and Mohnen, D. (2013) Plant Cell,
25, 270-287.
203.Morris, G., Ralet, M. C., Bonnin, E., Thibault,
J. F., and Harding, S. E. (2010) Carbohydr. Polym., 82,
1161-1167.
204.Rehm, B. H. A. (2010) Nature Rev.
Microbiol., 8, 578-592.
205.Zabotina, O. A. (2012) Front. Plant
Sci., 3, 134.
206.Doering, A., Lathe, R., and Persson, S. (2012)
Mol. Plant, 5, 769-771.
207.Harholt, J., Suttangkakul, A., and Scheller, V.
H. (2010) Plant Physiol., 153, 384-395.
208.Dalessandro, G., and Northcote, D. H. (1977)
Biochem. J., 162, 267-279.
209.Andersson-Gunneras, S., Mellerowicz, E. J.,
Love, J., Segerman, B., Ohmiya, Y., Coutinho, P. M., Nilsson, P.,
Henrissat, B., Moritz, T., and Sundberg, B. (2006) Plant J.,
45, 144-165.
210.Matsunaga, T., Ishii, T., Matsumoto, S.,
Higuchi, M., Darvill, A., Albersheim, P., and O’Neill, M. A.
(2004) Plant Physiol., 134, 339-351.
211.Arabidopsis Genome Initiative (2000)
Nature, 408, 796-815.
212.Wolf, S., Mouille, G., and Pellouc, J. (2009)
Mol. Plant, 2, 851-860.
213.Hrmova, M., and Fincher, G. B. (2009) in
Chemistry, Biochemistry, and Biology of
(1→3)-β-Glucans and Related Polysaccharides (Bacic, A.,
Fincher, G., and Stone, B., eds.) Elsevier, San Diego, pp. 119-171.
214.Roach, M. J., Mokshina, N. Y., Snegireva, A.
V., Hobson, N., Deyholos, M. K., and Gorshkova, T. A. (2011) Plant
Physiol., 156, 1351-1363.
215.Lei, L., Li, S., and Gu, Y. (2012) Front.
Plant Sci., 3, 1-6.
216.Settineri, W. J., and Marchessault, R. H.
(1965) J. Polym. Sci., 11, 253-264.
217.Takahashi, N., and Koshijima, T. (1988) Wood
Sci. Technol., 22, 231-241.
218.Koshijima, T., and Watanabe, T. (2003)
Association Between Lignin and Carbohydrates in Wood and Other Plant
Tissues, Springer-Verlag, Berlin.
219.Esker, A., Becker, U., Jamin, S., Beppu, S.,
Renneckar, S., and Glasser, W. (2004) ACS Symp. Ser.,
864, 198-219.
220.Gradwell, S. E., Renneckar, S., Esker, A. R.,
Heinze, T., Gatenholm, P., Vaca-Garcia, C., and Glasser, W. (2004)
Comptes Rendus Biol., 327, 945-953.
221.Clayton, D. W., and Phelps, G. R. (1965) J.
Polym. Sci., 11, 197-220.
222.Hansson, J.-A. (1970) Svensk
Papperstidning, 73, 49-53.
223.Danielsson, S., and Lindstrom, M. E. (2005)
Nordic Pulp Paper Res. J., 20, 436-441.
224.Tenkanen, M., Kroon, J., Gilli, G., Larsson,
T., and Biely, P. (2000) Final Report FAIR CT96-1624.
225.Leeflang, B. R., van Kuik, J. A., and
Kroon-Bratenburg, L. M. J. (2006) ACS Symp. Ser., 930,
133-155.
226.Scheller, H. V., and Ulvskov, P. (2010)
Annu. Rev. Plant Biol., 61, 263-289.
227.Gruppen, H., Kormelink, F. J. M., and Voragen,
A. G. J. (1993) J. Cereal Sci., 18, 129-143.
228.Wang, M., Oudgenoeg, G., van Vliet, T., and
Hamer, R. J. (2003) J. Cereal Sci., 38, 95-104.
229.Saulnier, L., Marot, C., Chanliaud, E., and
Thibault, J. F. (1995) Carbohydr. Polym., 26,
279-287.
230.Parker, M. L., Ng, A., and Waldron, K. W.
(2005) J. Sci. Food Agr., 85, 2539-2547.
231.Rhodes, D. I., Sadek, M., and Stone, B. A.
(2002) J. Cereal Sci., 36, 67-81.
232.Buanafina, M. M. O. (2009) Mol. Plant,
2, 861-872.
233.Hoije, A., Grondahl, M., Tommeraas, K., and
Gatenholm, P. (2005) Carbohydr. Polym., 61, 266-275.
234.Kato, Y., and Nevins, D. J. (1984) Plant
Physiol., 75, 745-752.
235.Kozlova, L. V., Mikshina, P. V., and Gorshkova,
T. A. (2012) Biochemistry (Moscow), 77, 395-403.
236.Kozlova, L. V. (2012) Mixed Linkage Glucan
and Glucuronoarabinoxylan during Elongation Growth of Maize (Zea mays
L.) Coleoptiles and Roots: PhD Thesis [in Russian], KIBB KSC RAS,
Kazan.
237.Oomen, R. J. F. J., Doeswijk-Voragen, C. H. L.,
Bush, M. S., Vincken, J. P., Borkhardt, B., van den Broek, L. A. M.,
Corsar, J., Ulvskov, P., Voragen, A. G. J., McCann, M. C., and Visser,
R. G. F. (2002) Plant J., 30, 403-413.
238.Abdel-Massih, R. M., Rizkallah, H. D., Al-Din,
R. S., Baydoun, E. A. H., and Brett, C. T. (2007) J. Plant
Physiol., 164, 1-10.
239.Wu, Y., Ai, L., Wu, J., and Cui, S. W. (2013)
Int. J. Biol. Macromol., http://dx.doi.org/10.1016/j.ijbiomac.2013.01.005.
240.Khodaei, N., and Karboune, S. (2013) Food
Chem., http://dx.doi.org/10.1016/j.foodchem.2013.01.110.
241.Western, T. L., Skinner, D. J., and Haughn, G.
W. (2000) Plant Physiol., 122, 345-355.
242.Qian, K. Y., Cui, S. W., Nikiforuk, J., and
Goff, D. H. (2012) Carbohydr. Res., 132,
47-55.
243.Gorshkova, T., Brutch, N., Chabbert, B.,
Deyholos, M., Hayashi, T., Lev-Yadun, S., Mellerowicz, E. J., Morvan,
C., Neutelings, G., and Pilate, G. (2012) Crit. Rev. Plant Sci.,
31, 201-228.
244.Rihouey, C., Morvan, C., Borissova, I.,
Jauneau, A., Demarty, M., and Jarvis, M. (1995) Carbohydr.
Polym., 28, 159-166.
245.Vicre, M., Jauneau, A., Knox, J. P., and
Driouich, A. (1998) Protoplasma, 203, 26-34.
246.Gur’janov, O. P., Gorshkova, T. A.,
Kabel, M., Schols, H. A., and van Dam, J. E. G. (2007) Carbohydr.
Polym., 67, 86-96.
247.Mikshina, P. V., Gurjanov, O. P., Mukhitova, F.
K., Petrova, A. A., Shashkov, A. S., and Gorshkova, T. A. (2012)
Carbohydr. Polym., 87, 853-861.
248.Mikshina, P. V., Chernova, T. E., Chemikosova,
S. B., Ibragimova, N. N., Mokshina, N. Y., and Gorshkova, T. A. (2013)
Cellulose, http://dx.doi.org/10.5772/51941.
249.Mellerowicz, E. J., and Gorshkova, T. A. (2012)
J. Exp. Bot., 63, 551-565.