Proteasomes in the Brain of β2-Microglobulin Knockout Mice
Yu. V. Lyupina1*, M. E. Bogatyrev1, A. Sh. Orlova1, E. V. Marjukhnich2, D. B. Kazansky2, and N. P. Sharova1
1Koltsov Institute of Developmental Biology, Russian Academy of Sciences, ul. Vavilova 26, 119334 Moscow, Russia; fax: +7 (499) 135-8012; E-mail: yulial@bk.ru2Blokhin Russian Oncological Research Center, Russian Academy of Medical Sciences, Kashirskoe Shosse 23, 115478 Moscow, Russia; fax: +7 (495) 324-1205; E-mail: kazansky1@yandex.ru
* To whom correspondence should be addressed.
Received May 13, 2013
MHC class I molecules play an important role in synaptic plasticity of the mammalian nervous system. Proteolytic complexes (proteasomes) produce oligopeptides that are presented on cell surfaces in complexes with MHC class I molecules and regulate many cellular processes beside this. The goal of the present work was to study peculiarities in functioning of proteasomes and associated signaling pathways along with evaluation of NeuN and gFAP expression in different sections of the brain in mice with knockout of β2-microglobulin, a constituent of MHC class I molecules. It was found that the frontal cortex and the brainstem, structures with different ratio of NeuN and gFAP expression, are characterized by opposite changes in the proteasome pool under constant total proteasome levels in B2m-knockout mice in comparison with those in control animals. ChTL-activity as well as expression of LMP7 immune subunit and PA28 regulator of proteasomes was elevated in the cortex of B2m-knockout mice, while these indicators were decreased in the brainstem. The concentrations of the signaling molecules nNOS and HSP70 in B2m-knockout mice were increased in the cortex, while being decreased in the brainstem, and this indicates the possibility of control of expression of the LMP7 subunit and the regulator PA28 by these molecules. Changes in the proteasome pool observed in striatum of B2m-knockout mice are similar to those observed in the brainstem. At the same time, the cerebellum is characterized by a specific pattern of proteasome functioning in comparison with that in all other brain structures. In cerebellum the expression of immune subunits LMP7 and LMP2 and the regulator PA28 was increased, while expression of regulator PA700 was decreased. Deficiency of NeuN and gFAP was revealed in most brain compartments of B2m-knockout mice. Thus, increased expression of the above-mentioned immune subunits and the proteasome regulator PA28 in the cortex and cerebellum may compensate disturbances revealed in the brain structures and the absence of MHC class I molecules. Apparently, this promotes production of peptides necessary for cell-to-cell interactions and maintains nervous system plasticity in B2m-knockout mice.
KEY WORDS: immune proteasomes, proteasome regulators, proteasome activity, major histocompatibility complex class I molecules, synaptic plasticity, brain, β2-microglobulin knockout miceDOI: 10.1134/S0006297913100064
Abbreviations: B2m, β2-microglobulin; ChTL activity, chymotrypsin-like activity; gFAP, glial fibrillar acidic protein; HSP70, heat shock protein 70; mAb, monoclonal antibodies; MHC I, major histocompatibility complex class I; NeuN, neuronal nuclear protein; nNOS, neuronal NO-synthase; pAb, polyclonal antibodies; PBS, phosphate-buffered saline; TNT, mixture of Tris-HCl, NaCl, and Tween-20.
Synaptic plasticity is an important factor of adaptive changes in the
brain and is necessary for compensation of disruption in functioning of
neuronal networks in inherited and acquired neurodegenerative
pathologies [1]. Presently, the hypothesis about
the leading role of neurodegenerative processes in disorders of
mobility, cognitive functions, and memory is being replaced by the
concept of prevailing importance of disorders in formation of neuronal
networks and cell-to-cell contacts, and also of alterations in nervous
system plasticity as a reason for functional disruptions. According to
the modern viewpoints, central nervous system plasticity, i.e. any
change in synaptic structures (formation of spines, changes in size of
a synaptic cleft), is accompanied by changes at molecular levels, i.e.
in structures of receptors, adhesion molecules, regulatory and
structural proteins, and also proteins that participate in various
signaling cascades. The memorizing process is eventually concluded in
changes in structure and formation of new synapses. Investigation of
mechanisms underlying brain plasticity is of crucial importance in
neurobiology [2, 3]. In this
connection, investigation of multiple forms of proteasomes,
“ubiquitous” proteolytic systems that participate in
numerous cellular processes including formation of synapses, is
considered to be very promising [4].
Proteasomes perform selective proteolysis in cells of the central nervous system and other organs. Mammalian proteasomes can be divided into two groups on the basis of the set of proteolytic subunits they contain: constitutive and immune. Immune proteasomes contain proteolytic subunits LMP7(β5i), LMP2(β1i), and MECL1(β2i) instead of subunits X(β5), Y(β1), and Z(β2) in constitutive proteasomes [5, 6]. Both immune and constitutive proteasomes are capable of hydrolyzing foreign (mutant or viral) and their own cellular proteins. Replacement of constitutive subunits by immune ones takes place during assembling of new proteasomes. Subunits LMP2 and MECL1 are jointly inserted, but independently from LMP7. Although incorporation of subunit LMP7 is facilitated in the presence of subunits LMP2 and MECL1, this process can also proceed in the absence of the latter ones [7]. Hydrolysis of proteins by immune proteasomes leads to changes in preferential sites for hydrolysis that affects the structure of the peptides produced. Predominantly, peptide bonds next to basic or hydrophobic amino acid residues are cleaved due to conformational changes of substrate-binding sites in the proteolytic chamber of immune proteasomes. The oligopeptides produced are of 8-10 amino acid residues in length with “proper” C-terminus and are transferred to the endoplasmic reticulum, where they bind to MHC class I molecules. The latter molecules are composed of heavy α-chain that forms the Bjorkman cleft, where an oligopeptide is introduced, and also of a B2m molecule [6, 8, 9]. The latter is necessary for complete maturation of MHC class I molecules in endoplasmic reticulum and for delivery of membrane vesicles that contain the entire complex to the cell surface.
In cells of the central nervous system immune proteasomes and MHC class I molecules, in addition to their participation in adaptive cellular immune response, play an important role in development and providing synaptic plasticity [10-12]. The density of MHC class I molecules on pre- and post-synaptic membranes is of great importance because it regulates neuronal activity and, possibly, represents a structural basis for long-term potentiation. MHC class I molecules serve as reporter molecules in nervous cells and inform them about products of proteolysis performed, predominantly, by immune proteasomes. The specificity of functioning of immune proteasomes depends on many factors, particularly on the presence of regulatory subcomplexes that include PA700 (19S) and PA28 (11S) activators, flanking the 20S-“core” of proteasome [13-15]. The subcomplex PA700 is an ATP-dependent activator and provides binding and subsequent hydrolysis of ubiquitinated proteins by proteasomes. The heptameric regulator PA28 consists of α and β subunits and activates hydrolysis of unstructured proteins and short peptides. In proteasomes that contain one subunit of PA700 and one subunit of PA28, production of peptides with hydrophobic C-ends are often increased even in the absence of proteolytic immune subunits. The concentrations of both the regulatory PA28 subunit and heat shock proteins HSP70 are increased during oxidative stress and neurodegenerative processes in brain cells [16, 17], so it is possible to suggest their cooperative action in elimination of oxidatively damaged proteins. Heat shock proteins along with NO-synthase are activators of expression of proteasomal immune subunits [18, 19].
It should be noted that MHC class I molecules are necessary not only for maintenance of central nervous system plasticity, but also for migration of neuronal predecessors from the ventricle lining along the axons of radial glial cells to the future primordium of the gray substance during the developmental process [20]. Absence of B2m protein leads to disappearance of MHC class I molecules from the cell surface that, in turn, disturbs brain development and changes in parameters of synaptic transmission.
The goal of the present work was to study the features in functioning of proteasomes and associated signaling pathways and also to evaluate expression of NeuN (neuron marker) and gFAP (astroglia marker) proteins in the brain of mice with knockout of B2m, for revealing compensatory mechanisms of plasticity and developmental disorders of the central nervous system. In this connection, it was important to study frontal cortex, cerebellum, and striatum, which play leading roles in organization of motional activity and the learning process [21-23], along with the brainstem that contains nuclei of all vital centers of the body.
MATERIALS AND METHODS
Animals. Tissues of different brain compartments from adult male B2m-knockout mice, line C57BL/6J-B2mtm1Unc, and control mice, line C57BL/6, were used in our investigations. The animals were obtained from the department of breeding of laboratory animals of the Blokhin Russian Oncological Research Center, Russian Academy of Medical Sciences, and work with them were conducted in accordance with requirements of the Commission on Bioethics in the Institute of Developmental Biology, Russian Academy of Sciences.
Antibodies. The following monoclonal (mAb) and polyclonal (pAb) antibodies were used in this work: murine mAb against α1, 2, 3, 5, 6, and 7 subunits and proteasome LMP2 subunit, against subunit Rpt6 of proteasome regulator PA700; rabbit pAb against proteasome subunit LMP7 and subunit PA28α of proteasome regulator PA28 (Biomol, UK), murine mAb against β-actin, FITC-labeled antibodies against rat IgG (Santa Cruz Biotechnology, USA), murine mAb against gFAP and NeuN (Millipore, USA); rabbit pAb against nNOS (Abcam, UK), rat mAb against HSP/HSC70s (Lindquist Lab, USA) and peroxidase-conjugated antibodies against murine, rat, and rabbit IgG (Amersham Biosciences, UK), Alexa 546-labeled antibodies against rabbit IgG, Alexa 633-labeled antibodies against murine IgG (Invitrogen, USA).
Preparation of cleared homogenates of brain tissues. Frozen tissue was homogenized in a homogenizer (glass–glass) in 50 mM Tris-HCl buffer (pH 7.5) containing 100 mM NaCl, 1 mM EDTA, 1 mM dithiothreitol, 10% glycerol, 5 mM MgCl2, 1 mM ATP, 10 mM Na2S2O5, in ratio 1 : 3 (w/v). The homogenates were centrifuged at 15,400g for 30 min. All procedures were performed at 4°C. Supernatants (cleared homogenates) were used for further experiments. Protein concentrations in cleared homogenates were determined according to the Lowry method [24].
Determination of ChTL activity of proteasomes. Chymotrypsin-like (ChTL) activity of proteasomes was determined in cleared homogenates of brain tissues of B2m-knockout and control mice using commercial fluorogenic oligopeptide Suc-LLVY-AMC (Sigma, USA) as a substrate. Reaction mixtures contained 20 mM Tris-HCl (pH 7.5) and 1 mM dithiothreitol, 30 µM Suc-LLVY-AMC, 5 mM MgCl2, and 1 mM ATP. To exclude the contribution of admixture proteolytic activities, an inhibitor of ChTL activity in proteasomes, MG132 (Z-leucyl-leucyl-leucinal) (Sigma), was added to some samples. Reactions were performed at 37°C for 20 min after addition of 1-5 μl of cleared homogenate to the reaction mixture (to final volume of 100 μl) and stopped by adding 1% SDS. The products of reaction were registered on fluorometer (Bio-Rad VersaFluor Fluorometer, USA) at excitation wavelength 380 nm and emission wavelength 440 nm. Final calculations were performed on the basis of the difference between total and residual activities in the presence of 5 µM MG132. Activity of proteasomes was expressed in relative units of fluorometer indications and normalized in relation to concentration of β-actin in samples, which was determined by Western blotting.
Western blotting. After electrophoresis in 8 or 13% SDS-PAGE (15 μl/94-102 μg of cleared homogenate protein per line), the polypeptides were transferred from the gel to Hybond-ECL nitrocellulose membrane (Amersham Biosciences, UK) with pore diameter of 0.45 μm. The membranes were incubated for 2 h at 20°C in TNT, then for 16 h in TNT containing 5% defatted milk and the first murine mAb against either subunits α1, 2, 3, 5, 6, 7 (1 : 2000), or LMP2 (1 : 1000), or Rpt6 (1 : 2000), or β-actin (1 : 500), or gFAP (1 : 500), or NeuN (1 : 1000), or rabbit pAb against either LMP7 (1 : 1000), or PA28α (1 : 1000), or nNOS (1 : 500), or rat mAb to HSP/HSC70s (1 : 1000) at temperature 4°C, washed a few times in TNT, and incubated for 1 h in TNT containing 5% defatted milk and peroxidase-conjugated second antibodies against murine, rabbit, or rat IgG (1 : 2500). After washing in TNT, the membranes were treated by the standard ECL-system (Amersham Biosciences). Optical densities of bands on the X-ray film were analyzed using the standard program ImageJ. We preliminarily drew graphs of dependence of optical density on total protein amount subjected to Western blotting. For further experiments, we used the range of protein amounts for which the dependence has linear character on the graph. Results were normalized against β-actin content in samples.
Perfusion, fixation, and immunofluorescent labeling of cells. Animals were perfused through the heart with standard 0.02 M PBS to wash out blood from vessels and then with 4% paraformaldehyde solution in 0.02 M PBS for 15 min. Then the brain was isolated from the animals and fixed by immersion (by loading into solution) in the same fixing solution for 2-4 h at room temperature. Then the brain was placed in 25% sucrose for cryoprotection at 4°C. After that the brain tissues were frozen in n-hexane (–45°C) and kept at –20°C for not more than one month. Cryostat sectioning (cryostat-microtome Leica CM1900, Germany) of the brain tissues was performed with sections 12 μm in width that were placed on glasses and subjected to immunofluorescent labeling. The cryostat sections placed on glasses were consecutively incubated in 0.5% Triton X-100 with 5% bovine serum in PBS for 40 min at 20°C; then with first rat monoclonal antibodies to HSP70 (1 : 100), murine mAb to NeuN (1 : 1000), rabbit pAb to LMP7 (1 : 500) prepared in PBS with addition of 0.5% Triton X-100 and 5% BSA for 18 h at room temperature; then they were washed for 5 min in PBS and incubated with FITC-labeled antibodies against rat IgG (1 : 100), Alexa 633-labeled antibodies to murine IgG (1 : 800), and Alexa 546-labeled antibodies to rabbit IgG (1 : 600), prepared in PBS with addition of 0.3% Triton X-100 and 5% bovine serum for 2 h at 20°C. Then the sections were washed in PBS and placed in Mowiol (Calbiochem, Germany) to be analyzed using a Leica DM RXA2 fluorescent microscope (Germany) or Leica TCS 5 SP confocal microscope (Germany).
Specificity of the first antibodies was confirmed using control experiments in which the reaction was performed in the absence of the first antibodies. The possibility of cross-reactivity between the first and the second antibodies was excluded by introduction of additional controls for specificity of antibodies in which each type of first antibodies was incubated with second antibodies against proteins of other animals. The absence of fluorescent labeling indicated the reaction specificity. The obtained images were analyzed using the standard computer program ImageJ. Not less than 300 cells of B2m-knockout and control animals were analyzed.
Statistical analysis. Results are presented as mean value of data measured for four parallel samples in one experiment and obtained in four experiments ± standard error. Weight of differences was measured using the Mann–Whitney nonparametric criterion, and significant differences were considered to be at p < 0.05.
RESULTS
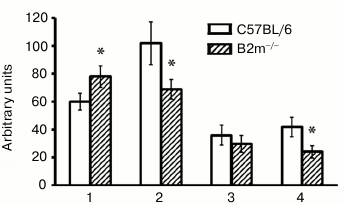
ChTL activity of proteasomes in brain of B2m-knockout mice. A significant increase in ChTL activity of proteasomes in the frontal cortex along with decrease in its activity in striatum and in the brainstem were observed in B2m-knockout mice in comparison with that in the same brain compartments of control animals (Fig. 1). The greatest decrease in ChTL activity was observed in the brainstem of B2m-knockout mice. Thus, the frontal cortex and the brainstem, being absolutely opposite to each other in ratio of neurons to glial cells, exhibit the most oppositely directed effects in changing ChTL activity of proteasomes in B2m-knockout mice in comparison with the control animals. The ChTL activity of proteasomes in cerebellum of B2m-knockout and control mice was not reliably different (Fig. 1).
Fig. 1. ChTL activity of proteasomes in different brain compartments in C57BL/6 mice and B2m-knockout mice. Mean values ± standard error are presented. 1) Cortex; 2) striatum; 3) cerebellum; 4) stem. * Significant difference from control at p < 0.05 (n ≥ 8).
What is the basis for such very different demonstrations of ChTL activity of proteasomes in different brain compartments in B2m-knockout animals? This may be caused first by changes in total amount of proteasomes, second by changes in expression of immune subunits of proteasomes, and third by changes in the ratio of regulators PA28 and PA700 [8, 25]. It should be noted that immune subunit LMP7 demonstrates ChTL activity, while immune subunit LMP2 exhibits caspase-like activity; however, the presence of the latter instead of the constitutive subunit Y influences ChTL activity [26]. So, our next task was to determine how these parameters are changed in the brain compartments under study in B2m-knockout mice in comparison with control animals.
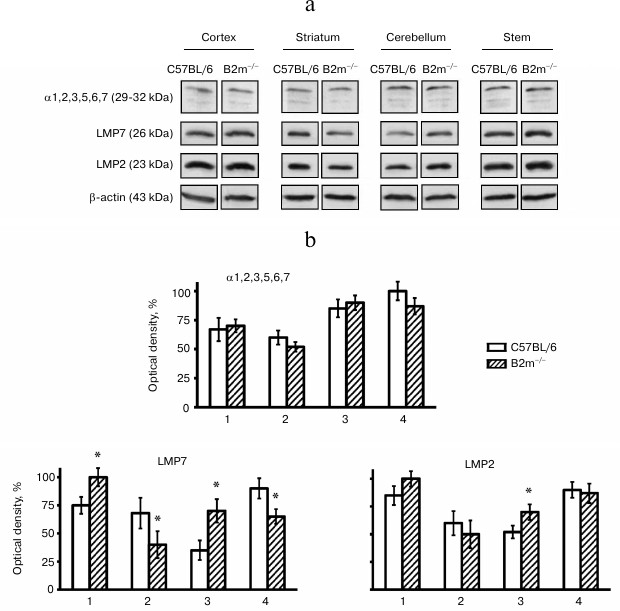
Expression of immune subunits, activators, and the entire pool of proteasomes in brain of B2m-knockout mice. Expression of the entire pool of proteasomes was evaluated by determination of concentrations of subunits α1, 2, 3, 5, 6, and 7 that are components of all forms of proteasomes, while the expression of regulators PA28 and PA700 was evaluated by the amount of their subunits PA28α and Rpt6, correspondingly. Levels of expression of immune subunits LMP7 and LMP2 were used for evaluation of different forms of immune proteasomes that contain LMP7 and/or LMP2 subunits.
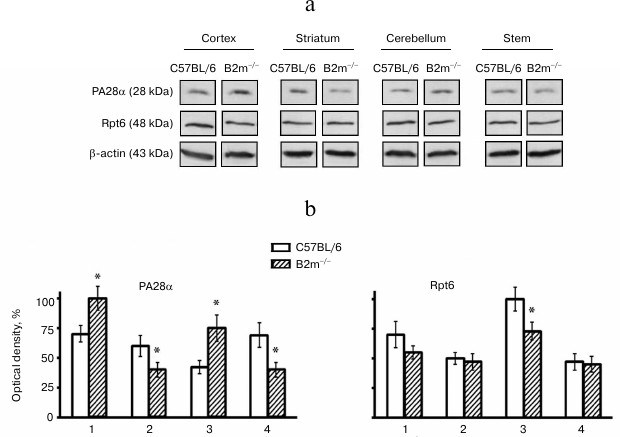
Unexpectedly, our results indicated no changes in levels of the entire pool of proteasomes in all studied brain compartments of B2m-knockout mice in comparison with those of control animals (Fig. 2). Fig. 3However, significant changes in subunit composition of proteasomes and content of proteasome regulators were observed. Significant elevation of expression of immune subunit LMP7 (Fig. 2) and the regulator PA28 (Fig. 3) were revealed in the frontal cortex. This explains an increase in ChTL activity in accordance with modern concepts about the influence of immune subunit LMP7, which substitutes the corresponding constitutive proteolytic subunit, on ChTL activity. The same is true regarding the influence on activity of the regulator PA28 that facilitates hydrolysis of short oligopeptides including the substrates used in our work.
Fig. 2. Expression of subunits α1, 2, 3, 5, 6, 7, LMP7 and LMP2 in different brain compartments of C57BL/6 and B2m-knockout mice. a) Western blots of proteins in cleared homogenates performed using antibodies against subunits α1, 2, 3, 5, 6, 7, LMP7 and LMP2. b) Relative amount (represented by optical density of blots) of subunits α1, 2, 3, 5, 6, 7, LMP7 and LMP2 normalized against amount of β-actin. Amounts of α1, 2, 3, 5, 6, 7 in the brainstem of C57BL/6 mice and amounts of LMP7 and LMP2 in the brain cortex of B2m-knockout mice were considered as 100%. Mean values ± standard error are presented. 1) Cortex; 2) striatum; 3) cerebellum; 4) stem. * Significant difference from control at p < 0.05 (n ≥ 4).
Fig. 3. Expression of subunits PA28α and Rpt6 in different compartments of the brain of C57BL/6 and B2m-knockout mice. a) Western blots of proteins in cleared homogenates performed using antibodies against PA28α and Rpt6. b) Relative amount (optical density of blots) of subunits PA28α and Rpt6 normalized against amount of β-actin. Amount of PA28α in the brain cortex of B2m-knockout mice and of Rpt6 in cerebellum of C57BL/6 mice was as 100%. Mean values ± standard error are presented. 1) Cortex; 2) striatum; 3) cerebellum; 4) stem. * Significant difference from control at p < 0.05 (n ≥ 4).
Opposite changes to those in the cortex were revealed in the brainstem of B2m-knockout mice – decrease in expression of immune subunit LMP7 and regulator PA28 (Figs. 2 and 3), which caused, eventually, decrease in ChTL activity of proteasomes in comparison with that in control animals.
Striatum, like the brainstem, is characterized by decrease in expression of immune subunit LMP7 as well as the regulator PA28 in B2m-knockout mice in comparison with that in control animals (Figs. 2 and 3). Notably, the extent of decrease in PA28 expression both in striatum and in the brainstem is strongly correlated with decrease in ChTL activity in these brain compartments in B2m-knockout mice in comparison with that in the control group. This may indicate that the regulator PA28 makes a greater contribution to alteration of ChTL activity in these compartments than subunit LMP7.
More significant changes in the proteasome pool were revealed in the cerebellum of B2m-knockout mice in comparison with those in other compartments. Although the ChTL activity was not reliably changed in cerebellum of B2m-knockout mice, expression of immune subunit LMP7 and regulator PA28 was increased in this brain compartment to a greater extent than in the cortex (Figs. 2 and 3). Moreover, expression of another immune subunit, LMP2, was reliably increased, while expression of regulator PA700 was decreased in cerebellum (Figs. 2 and 3). Obviously, these two factors compensated stimulating effects of immune subunit LMP7 and regulator PA28 on ChTL activity. Perhaps the ChTL activity of proteasomes in cerebellum is regulated by other, unstudied factors.
It should be noted that changes in expression of immune subunit LMP2 proceeded in the same direction as changes in expression of immune subunit LMP7 in all brain compartments of B2m-knockout animals in comparison with those in the control groups (Fig. 2). However, changes in expression of subunit LMP2 were either less remarkable (in cerebellum) or have only a tendency (in cortex, striatum, and brainstem). This result indicates that immune subunits LMP7 and LMP2 are constituents of different forms of immune proteasomes in the brain of B2m-knockout mice. In other words, in all brain compartments of B2m-knockout mice three forms of immune subunits can exist: one form that contain subunit LMP7, another containing subunit LMP2, and a third that can contain both types of subunits.
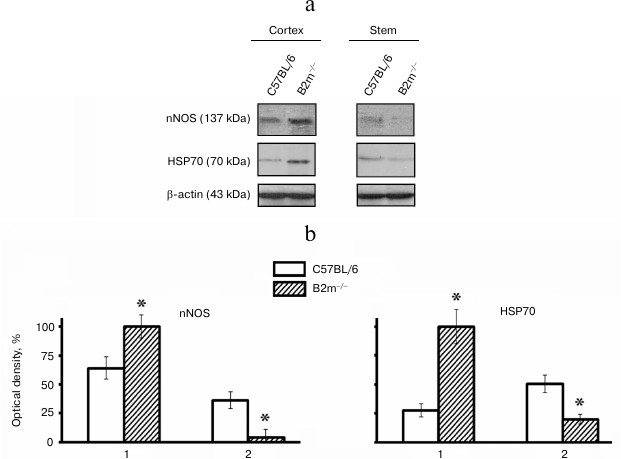
Expression of nNOS and HSP70 in brain of B2m-knockout mice. Expression of signaling molecules nNOS and HSP70 in the frontal cortex and the brainstem, compartments with different ratios of neurons to glial cells, was studied. It was found that expression of nNOS as well as HSP70, like expression of immune proteasomes and regulator PA28, was increased in the cortex and decreased in the stem of the brain in B2m-knockout mice in comparison with that in control animals (Fig. 4).
Fig. 4. Expression of nNOS and HSP70 proteins in frontal cortex and in the brainstem of C57BL/6 and B2m-knockout mice. a) Western blots of proteins in cleared homogenates using antibodies against nNOS and HSP70. b) Relative amount (optical density of blots) of nNOS and HSP70 proteins normalized against amount of β-actin. Amount of nNOS and HSP70 in the cortex in B2m-knockout mice was considered as 100%. Mean values ± standard error are presented. 1) Cortex; 2) stem. * Significant difference from control at p < 0.05 (n ≥ 4).
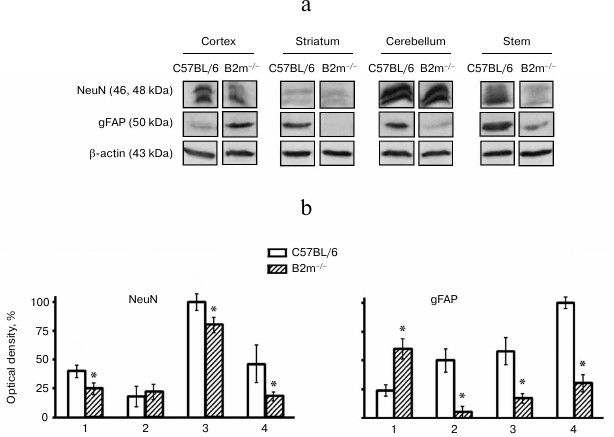
Cell types in brain compartments of B2m-knockout mice. Changes in cell type composition in the brain compartments of B2m-knockout mice were analyzed using data on expression of NeuN, a marker for neurons, and gFAP, a marker for astroglia. Decrease in NeuN amount and, subsequently, in number of neurons in the cortex, cerebellum, and brainstem was revealed (Fig. 5). In all the studied brain compartments of B2m-knockout mice, with the exception of the frontal cortex, expression of gFAP was decreased and, consequently, level of astrocytes is also decreased (Fig. 5). The number of astrocytes is increased in the frontal cortex. Seemingly, decrease in neurons in the cortex was compensated by increase in astroglia cells.
Fig. 5. Expression of NeuN and gFAP proteins in different compartments of the brain of C57BL/6 mice and B2m-knockout mice. a) Western blots of proteins in cleared homogenates using antibodies against NeuN and gFAP. b) Relative amount (optical density of blots) of proteins NeuN and gFAP normalized against amount of β-actin. Amount of NeuN in cerebellum and of gFAP in the brainstem in C57BL/6 mice was considered as 100%. Mean values ± standard errors are presented. 1) Cortex; 2) striatum; 3) cerebellum; 4) stem. * Significant difference from control at p < 0.05 (n ≥ 4).
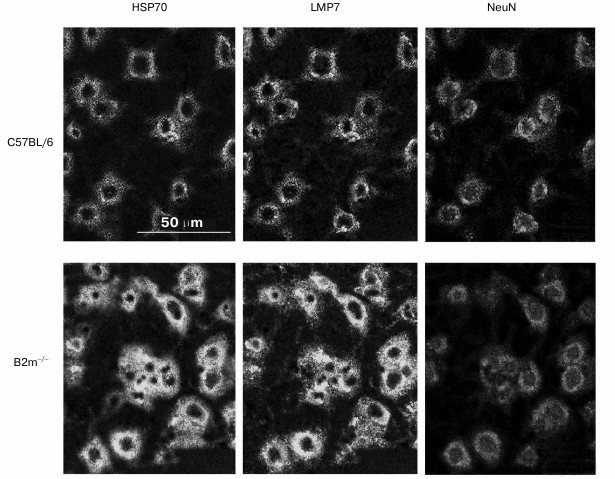
The question arises whether the significant changes in expression of immune subunit LMP7 and signaling protein HSP70 revealed in our work in the frontal cortex have linkage to neurons. To answer to this question, a triple immunofluorescent labeling of cells in cryostat sections by antibodies against subunit LMP7, and HSP70 and NeuN proteins was performed (Fig. 6). Processing of the obtained image using the standard computer program ImageJ revealed almost 2-fold increase in content of LMP7 subunit and HSP70 protein in neurons.
Fig. 6. Confocal microscopy of the brain frontal cortex cells of C57BL/6 and B2m-knockout mice. Immunohistochemical labeling of cells on sections using antibodies against subunit LMP7 and NeuN and HSP70 proteins.
DISCUSSION
The brain compartments of B2m-knockout mice studied in this work can be divided into two groups on the basis of changes detected in the pool of proteasomes. The first group consists of frontal cortex and cerebellum, which are characterized by elevated expression of immune proteasomes and by increase in ratio of regulator PA28 to PA700, at least due to increase in expression of regulator PA28. The second group includes striatum and the brainstem where, on the contrary, expression of immune proteasomes and the ratio of regulator PA28 to PA700 are decreased. There are data that show increase in content of immune proteasomes and regulator PA28 in cells under oxidative stress. They may demonstrate anti-stress and adaptive functions under these conditions [18, 27]. Most likely, immune proteasomes and the regulator PA28 play an adaptive role in maintaining plasticity of the central nervous system in the absence of MHC class I molecules in the frontal cortex and cerebellum, structures that provide sensory perception, execution of motor commands, coordination of movement activity, and other very important functions. Protein metabolism in the striatum and in the brainstem of B2m-knockout mice is maintained predominantly by constitutive proteolytic subunits under constant level of the entire pool of proteasomes. Obviously, such distribution of roles between proteasomes in different brain compartments of B2m-knockout mice provides integrity of central nervous system functioning in the absence of MHC class I molecules.
Immune proteasomes as well as the regulator PA28 were first described as factors induced by the action of γ-interferon [25]. The only role of these factors was suggested to be an increase in antigenic epitope production from foreign proteins for MHC class I molecules and participation in development of T-cell immune response. Later it was shown that induction of expression of immune proteasomes that participate in adaptation to oxidative stress takes place through various signaling cascades, in particular with the participation of nitrous oxide and transcription factor CREB or heat shock proteins [18, 19]. Moreover, HSP70 was shown to promote 26S-proteasome dissociation into 20S core subunit and regulatory subcomplex PA700 [17]. In this case, the amount of proteasomes that contain PA28 regulator, which replaces subcomplex PA700 and increases proteolytic activity of proteasomes in relation to oxidatively damaged proteins, was increased. Our findings show a correlation between expression of immune proteasomes, activator PA28, and signaling proteins nNOS and HSP70 in the frontal cortex and the brainstem of B2m-knockout mice and suggest the participation of these signaling proteins in regulation of expression of immune proteasomes and activator PA28 in the absence of MHC class I molecules.
According to our data, the brain compartments of B2m-knockout mice differ from those in control C57BL/6 mice in cell type composition: in most studied brain compartments of knockout mice the number of neurons and astrocytes decrease. This result most likely reflects consequences of disruption in migration of neuronal precursors from the brain ventricle lining in the absence of MHC class I molecules. Changes in cellular composition in nervous tissue of the telencephalon indicate complicated and specific disorders in processes of development of the frontal cortex and striatum during ontogenesis of B2m-knockout animals. Reduction in memorizing ability in adult B2m-knockout animals might be due to changes in structure of neuronal networks in cortical parts of the telencephalon, and, as a consequence, due to decrease in neocortex plasticity.
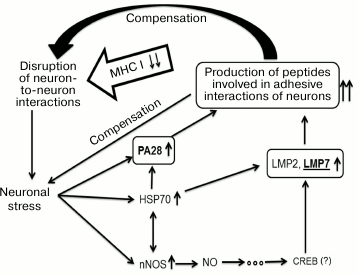
Results obtained in this work suggest a scheme of compensatory mechanisms that develop in the brain cortex and maintain synaptic plasticity in mice in the absence of MHC class I molecules (Fig. 7). The absence of MHC class I molecules leads to disruption of neuron-to-neuron interactions that, in turn, causes neuronal stress, and this is confirmed by elevation in amount of nNOS and HSP70. The latter molecules participate in signaling pathways that increase expression of immune proteasomes and regulator PA28. Probably, immune proteasomes together with regulator PA28 represent a definite, specific for each neuron, “molecular passport” by establishing a specific set of oligopeptides in neurons of the brain cortex of B2m-knockout mice. The oligopeptides, being presented on the cell surface by a specific mode, provide adhesive interactions with other neurons and compensate disruptions in these interactions created by MHC class I molecules in control animals.
Fig. 7. Hypothetical scheme of compensatory mechanisms of synaptic plasticity developing in the brain frontal cortex in mice in the absence of MHC class I molecules.
Further studies of brain development peculiarities in B2m-knockout mice will allow significant improvement in our knowledge of the mechanisms underlying central nervous system plasticity.
The present work was performed with financial support from the Russian Foundation for Basic Research (grants No. 12-04-00072-a and No. 11-04-00700-a).
REFERENCES
1.Segal, M. (2005) Nat. Rev. Neurosci.,
6, 277-284.
2.Popov, S. V. (2003) Ros. Vestn. Perinatol.
Pediatr., 2, 51-52.
3.Arendt, T. (2004) Int. J. Dev. Neurosci.,
22, 507-514.
4.Tai, H. C., Besche, H., Goldberg, A. L., and
Schuman, E. M. (2010) Front. Mol. Neurosci., 3; doi:
10.3389/fnmol.2010.00012.
5.Demartino, G. N., and Gillette, T. G. (2007)
Cell, 129, 659-662.
6.Strehl, B., Seifent, U., Kruger, E., Heniks, S.,
Kuckelkorn, U., and Kloetzel, P.-M. (2005) Immunol. Rev.,
207, 19-30.
7.Groettrup, M., Standera, S., Stohwasser, R., and
Kloetzel, P.-M. (1997) Proc. Natl. Acad. Sci. USA, 94,
8970-8975.
8.Sharova, N., and Zakharova, L. (2008) Recent
Pat. Endocr. Metab. Immune Drug Discov., 2, 152-161.
9.Cascio, P., Hilton, C., Kisselev, A. F., Rock, K.
L., and Goldberg, A. L. (2001) EMBO J., 20,
2357-2366.
10.Boulanger, L. M., and Shatz, C. J. (2004) Nat.
Rev. Neurosci., 5, 521-531.
11.Shatz, C. J. (2009) Neuron, 64,
40-45.
12.Garay, P. A., and McAlister, A. K. (2010)
Front. Synapt. Neurosci., 2; doi:
10.3389/fnsyn.2010.00136.
13.Coux, O., Tanaka, K., and Goldberg, A. L. (1996)
Ann. Rev. Biochem., 65, 801-847.
14.Lupas, A., Flanagan, J. M., Tamura, T., and
Baumeister, W. (1997) Trends Biochem. Sci., 22,
399-404.
15.Rechsteiner, M., and Hill, C. P. (2005) Trends
Cell Biol., 15, 27-33.
16.Yenari, M. A., Giffard, R. G., Sapolsky, R. M.,
and Steinberg, G. K. (1999) Mol. Med. Today, 5,
525-531.
17.Grune, T., Catalgol, B., Licht, A., Ermak, G.,
Pickering, A. M., Ngo, J. K., and Davies, K. J. A. (2011) Free Rad.
Biol. Med., 51, 1355-1364.
18.Kotamraju, S., Matalon, S., Matsunaga, T., Shang,
T., Hickman-Davis, J.-M., and Kalyanaraman, B. (2006) Free Rad.
Biol. Med., 40, 1034-1044.
19.Callahan, M. K., Wohlfert, E. A., Menoret, A.,
and Srivastava, P. K. (2006) J. Immunol., 177,
8393-8399.
20.Goddard, A., Butts, D. A., and Shatz, C. J.
(2007) Proc. Natl. Acad. Sci. USA, 104, 6828-6833.
21.Tolkunov, B. F. (1978) Striatum and Sensor
Specialization of Neuronal Network [in Russian], Nauka,
Leningrad.
22.Goldman-Rakic, P. S., and Selemon, L. D. (1990)
Trends Neurosci., 13, 241-244.
23.Shapovalova, K. B., and Pominova, E. V. (1993)
Fiziol. Zh. im. I. M. Sechenova, 79, 29-40.
24.Lowry, O. H., Rosebrough, N. J., Farr, A. L., and
Randall, R. J. (1951) J. Biol. Chem., 193,
265-275.
25.Tanaka, K. (2009) Proc. Jpn. Acad. Ser.
B, 85, 12-36.
26.Boes, B., Hengel, H., Ruppert, T., Multhaup, G.,
Koszinowski, U. H., and Kloetzel, P. M. (1994) J. Exp.
Med., 179, 901-909.
27.Pickering, A. M., Koop, A. L., Teoh, C. Y.,
Ermak, G., Grune, T., and Davies, K. J. A. (2010) Biochem. J.,
432, 585-594.