REVIEW: Long-Distance Signal Transmission and Regulation of Photosynthesis in Characean Cells
A. A. Bulychev* and A. V. Komarova
Department of Biophysics, Faculty of Biology, Lomonosov Moscow State University, 119992 Moscow, Russia; E-mail: bulychev@biophys.msu.ru* To whom correspondence should be addressed.
Received November 12, 2013
Photosynthetic electron transport in an intact cell is finely regulated by the structural flexibility of thylakoid membranes, existence of alternative electron-transport pathways, generation of electrochemical proton gradient, and continuous exchange of ions and metabolites between cell organelles and the cytoplasm. Long-distance interactions underlying reversible transitions of photosynthetic activity between uniform and spatially heterogeneous distributions are of particular interest. Microfluorometric studies of characean cells with the use of saturating light pulses and in combination with electrode micromethods revealed three mechanisms of distant regulation ensuring functional coordination of cell domains and signal transmission over long distances. These include: (1) circulation of electric currents between functionally distinct cell domains, (2) propagation of action potential along the cell length, and (3) continuous cyclical cytoplasmic streaming. This review considers how photosynthetic activity depends on membrane transport of protons and cytoplasmic pH, on ion fluxes associated with the electrical excitation of the plasmalemma, and on the transmission of photoinduced signals with streaming cytoplasm. Because of signal transmission with cytoplasmic flow, dynamic changes in photosynthetic activity can develop far from the point of photostimulus application and with a long delay (up to 100 s) after a light pulse stimulus is extinguished.
KEY WORDS: Chara corallina, transmembrane H+ fluxes, chlorophyll fluorescence, photosynthetic electron transport, intracellular diffusion, action potential, cytoplasmic streamingDOI: 10.1134/S0006297914030134
Abbreviations: AP, action potential; NPQ, non-photochemical quenching; pHc, cytoplasmic pH; pHo, pH on the outer cell surface; PSII, photosystem II.
Academician Alexander Abramovich Krasnovsky paid much attention in his
research, lectures, and seminars to the processes of light energy
transduction in model systems and living organisms. His seminars raised
deep interest in students for many forthcoming years in topics of
photosynthesis and the role of membranes in photobiological processes.
Unlike photoreactions in reaction mixtures, photosynthetic electron
transport in the intact cell is subject to fine regulation owing to the
labile structure of thylakoid membranes, existence of alternative
electron-transport pathways, effects of electrochemical proton
gradient, and continuous exchange of ions and metabolites between
chloroplasts and the cytoplasm [1-4].
One spectacular manifestation of regulation of photosynthesis in vivo is the reversible transitions between spatially uniform and heterogeneous distributions of photosynthetic activity in plant leaves and characean algae upon dark–light transitions. When leaves are illuminated after dark adaptation, homogeneous distributions of fluorescence parameters and photosynthetic rate are replaced with mosaic images showing damped oscillations [5-7]. The alternation of cell domains differing in fluorescence and quantum efficiency of photosystem II (PSII) occurs also in illuminated internodes of characean algae [8, 9], a suitable model for the photosynthesizing cell. The mechanisms underlying light-induced formation of functionally heterogeneous domains in an initially uniform system are not yet fully understood. However, it is known that spatial self-organization of photosynthesis in characean algae is closely related to the formation of spatial patterns of electrochemical H+ gradient at the thylakoid and plasma membranes [9, 10]. The cell regions with high and low photosynthetic activities are distributed over the charophyte internode in a quasi-periodic order with separation distances of 5-10 mm [11]. The emergence and maintenance of such shifts in photosynthetic activity implies the concerted operation of dissimilar cell domains and the occurrence of long-range interactions between these domains.
Diffusion is known to ensure fast interaction of reagents at lengths up to 10-50 µm, but it is ineffective for the transfer of substances over long distances. The transfer of low molecular weight substances by diffusion with coefficient D ~ 10–5 cm2/s over a distance of 1 cm would take about 14 h, which is much longer than the characteristic time for the formation of the heterogeneous distribution of photosynthesis (~10 min). Apparently, diffusion per se cannot account for spatial integration of metabolism in giant cells, whose length can be as long as 10 cm and even more. For example, the transfer of small molecules over the distance of the cell length would require a period of about three months [12]. It should be obvious that life functions in cells of such dimensions would rely on intracellular communications that extend over much longer distances compared to diffusion.
Microfluorometry of characean cells using saturating light pulses and in combination with electrode micromethods unveiled three factors in long-range regulation that ensure functional coordination of cell domains and signal transmission over large distances. These are: (1) passage of circulating electric currents between functionally distinct cell areas, (2) propagation of action potential along the cell, and (3) continuous circulatory streaming of cytoplasm at velocity up to 100 µm/s.
ROLE OF CIRCULATING CURRENTS IN SPATIAL COORDINATION OF
PHOTOSYNTHETIC ACTIVITY
Circulation of electric currents coupled with H+ transfer across functionally different regions of the plasmalemma is analogous to proton circulation in energy-transducing membranes of chloroplasts, mitochondria, and bacterial cells. The essential difference is that the area of circulation in giant cells is extended from micrometer and submicrometer levels to macroscopic (millimeter) range. The circulation of electric currents between cell regions separated by distances of a few millimeters is detected by means of vibrating microelectrodes. The currents crossing the external medium near the cell surface create a voltage drop in solution layers about 30 µm thick; the sign and amplitude of this voltage drop are determined by the direction and density of the current at each point of the examined space [13, 14]. The pathways of circulating currents mapped in this way were found to produce an intricate network reminiscent of a spider web, with nodes and arcs around individual cell segments. The condition of current continuity implies that the local disorders in some segments of the network might influence the activity associated with this current at a substantial distance from the point of impact.
Circulating current is generated by the plasma membrane H+-ATPase that extrudes protons from the cytoplasm to the external medium [15]. The extrusion of H+ in cell regions with active H+-ATPase lowers the pH near the cell surface (pHo) by 0.5-0.6 units with respect to pH 7.0-7.2 in the bulk medium. The involvement of H+-ATPase in acidification of the external medium is evidenced by colocalization of immunofluorescence label of the plasma-membrane H+-ATPase and of fluorescent probes detecting acidic regions in the cortical cytoplasm (plasmalemmal invaginations, charasomes) [16]. Additional evidence is that the pHo in areas of external acidification drops substantially upon activation of H+-ATPase by fusicoccin [17]. The electric current in the bulk medium is carried by Na+, Cl–, and other ions, the concentrations of which are several orders of magnitude higher than H+ concentration.
The circulating currents converge at narrow zones with high external pH (pH 9.5-10.0) where the density of the inward current is ~50 µA/cm2 [14]. It is known that the increase in pH of the medium to values typical of the alkaline zones elevates the plasma membrane conductance by 5-8-fold due to the increase in passive conductance for H+ or OH–, and the membrane potential approaches the equilibrium H+ potential defined by the Nernst equation [18, 19]. Circulation of current comprising the stages of active extrusion and passive influx of H+ in different cell parts indicates that the energy of the electrochemical proton gradient created by the plasma-membrane H+-ATPase is used not only for accumulation of nutrient elements and for removal of Na+ excess from the cytoplasm, but also for other purposes, including the supply of the cell with the permeant substrate of photosynthesis (CO2) and regulation of cytoplasmic pH.
The high plasmalemmal conductance restricted to areas of passive H+ influx from the medium into the cytoplasm (or OH– efflux from the cytoplasm to the outer solution) depolarizes the membrane, thereby stimulating the operation of the H+-pump. In the areas with high H+-pump activity, the surface pH is lowered to ~pK1 of carbonic acid (6.35), which converts the impermeant charged form of inorganic carbon (HCO3–), abundant in the slightly alkaline environment of natural habitats, to the membrane-permeant neutral form (CO2). Thus, one important function of circulating currents consists of providing the cell with the permeant substrate of photosynthesis. The sufficiency of the substrate for CO2 fixation in the areas of external acidity accounts for the high rate of linear electron flow in chloroplasts underlying these zones [8].
The chloroplasts underlying external alkaline zones experience CO2 deficiency. The resulting imbalance between the amount of light energy absorbed and the limited availability of CO2 activates a protective mechanism (non-photochemical quenching) dissipating the excess of chlorophyll excitations to heat. This mechanism involves an increase in thylakoid pH gradient and inhibition of photophosphorylation under diminished ATP consumption in the reactions of the Benson–Calvin cycle. The increase in thermal losses in chloroplasts underlying the external alkaline zones is manifested in lowering of maximal chlorophyll fluorescence Fm′, as measured by the method of saturating light pulses [9, 10].
The ATP requirements for the operation of the plasma-membrane H+-pump, intensified in the light, are partly satisfied by mitochondria of the cortical cytoplasm, which accumulate upon dark–light transition in cell regions with active photosynthesis by leaving from the photosynthetically inactive regions underlying the alkaline zones [20].
In studies of directional H+ transport across bilayer lipid membranes [21] and growing pollen tubes [22], the surface pH was found to shift in opposite directions on different sides of the membrane. In pollen tubes the cytoplasmic pH (pHc) was lowered to pH ~ 6.5 in regions of inward H+ flow and was elevated to pH 7.5-7.7 in the areas with active H+-ATPase expelling protons from the cytoplasm to the outer medium. A similar situation is expected to occur during H+ transport across the plasmalemma in Chara cells. The transmembrane H+ efflux should elevate the cytoplasmic pH in cell regions underlying external acidic zones, whereas the H+ influx should lower pHc in locations of external alkaline zones. Because of the presence of cytoplasmic buffers, the extent of pHc shifts is considerably lower than the pH shifts in the outer medium. However, the cytoplasmic buffer capacity in Chara (14.2 mM/pH) is at its minimum at physiological pHc (pH ~ 7.2), which might facilitate dynamic changes and regulatory function of pHc [23]. There is ample data indicating that pHc undergoes comparatively fast changes under various treatments. For example, increase in pHc by 0.3-0.5 units within ~2.5 min was observed in gravistimulated root cells [24] and upon illumination of green plant cells [25].
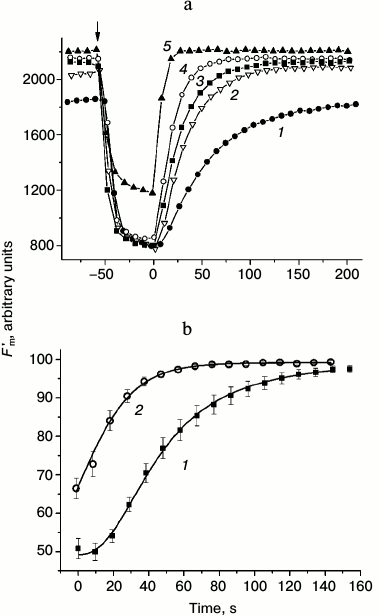
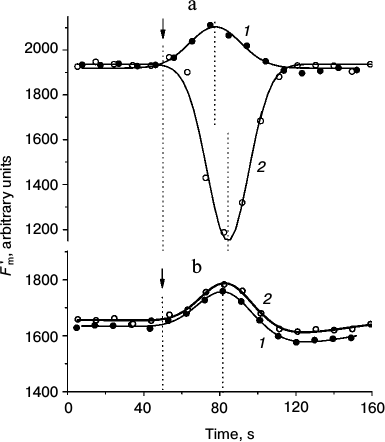
Presently, the differences in pHc between cell regions with active H+ efflux and passive H+ influx are not shown directly because of methodical obstacles including, in particular, strong binding of the fluorescent pH probe BCECF with chloroplasts. Nevertheless, there is indirect evidence supporting the notion that pHc in the photosynthetically active regions is higher than in the cell regions with low activity. For example, when the internodes were perfused internally with EGTA-containing media to destroy the tonoplast and control pHc, the increase in pHc diminished the non-photochemical quenching (decrease in NPQ, increase in Fm′) [26] and accelerated the dark relaxation of Fm′ (Fig. 1a). In accordance with observations on perfused cells and in conformity with the expected variations of pHc in intact cells, the light-induced decrease in Fm′ was stronger and its dark relaxation was slower in chloroplasts underlying the external alkaline zones than in chloroplasts located under external acidic zones (Fig. 1b).
Fig. 1. Dark relaxation kinetics of Fm′ chlorophyll fluorescence as affected by different cytoplasmic pH (pHc) in (a) internally perfused cells and (b) H+-extruding and H+-absorbing regions of intact Chara corallina internodes. a) Changes in Fm′ induced by illumination and darkening of the perfused cell after preliminary removal of the tonoplast and replacement of the cytoplasm with buffered sucrose solutions at various pH values (3 mM EGTA, 280 mM sucrose, and 20 mM Mes, Hepes, or Tris): 1) pH 6.5; 2) 7.0; 3) 7.5; 4) 8.0; 5) 8.5. The arrow marks the beginning of illumination (photon flux density 28 µmol·m–2·s–1); time t = 0 corresponds to the moment of switching off the actinic light. The results show a representative experiment obtained with one cell. It is seen that increase in pHc is accompanied by the release of Fm′ quenching and by acceleration of Fm′ dark relaxation (1-5). b) Kinetics of Fm′ dark relaxation in vivo in cell regions featuring (1) downhill H+ influx and (2) active H+ extrusion to the external medium. The time t = 0 corresponds to the moment of switching off the actinic light (100 µmol·m–2·s–1). Data represent mean values of Fm′ normalized to the Fm′ dark level and standard errors of the means obtained in experiments with different cells (n = 16 and 11 for curves 1 and 2, respectively).
Dissimilar kinetics of Fm′ (NPQ) relaxation in chloroplasts of different cell regions are probably caused by differences in H+-conductance of the coupling factor CFo in thylakoids with high and low rates of photophosphorylation and, consequently, faster or slower recovery of the lumenal proton concentration determining the extent of non-photochemical quenching [27].
Disturbances of circular electric currents may exert remote control over the plasmalemmal H+-pump activity. By analogy with H+-pump stimulation upon local increase in H+(OH–) conductance in remote plasma-membrane regions of illuminated cells, local perturbations of the membrane conductance by other treatments may also modulate the H+-pump operation. Disturbance at any segment in the system of circular currents should affect the functional state of the current source. Further studies in this area will expand our knowledge on the functional role of circulating electric currents occurring not only in characean algae but also in plant roots, growing pollen tubes, and fungal hyphae.
REGULATION OF PHOTOSYNTHETIC ACTIVITY BY PROPAGATION OF AN ACTION
POTENTIAL
Propagation of an action potential (AP) is one of the most rapid means of intracellular signal transmission. Modulation of circulating current transmits the signal over comparatively short distances determined by the cable properties of the cell (cable length 10-15 mm in the area of acidic zones [19]). In contrast, the action potential propagates over the whole internode at velocities ranging from 0.2 to 5 cm/s depending on the conductance of the outer medium [28]. Propagation of an AP ensures almost synchronous changes in photosynthetic activity and membrane H+ transport in the entire internodal cell.
Under natural environmental conditions, an AP arises in response to mechanical treatments or changes in ionic composition of the medium. In experiments, an AP can be induced by chemical or mechanical stimulation (application of 50 mM KCl or NH4Cl, insertion of a glass micropipette) or, most conveniently, by passing a short pulse of electric current across the plasma membrane. Irrespective of the origin of the stimulus, the AP propagation produces characteristic changes in pHo and fluorescence of chloroplasts.
The influence of an AP is particularly evident in the areas of external alkaline zones, where high pHo is established in the light and the photosynthetic activity is low [29]. In these areas the plasmalemmal conductance gm increases at the moment of AP generation but drops in the subsequent 30-60 s by almost an order of magnitude with respect to the initial (resting) level [30]. The decrease in gm is accompanied by a decrease in pHo near the cell surface by 0.8-3.0 units for 3-15 min depending on the incident light intensity [31]; it is also followed by prolonged hyperpolarization of the cell [29]. These changes provide evidence for long-lasting (up to 15 min) blockage of plasma-membrane H+(OH–) conductance as a result of cytoplasmic Ca2+ increase from 0.1 to 10-40 µM during the AP [32, 33].
In the areas with low pHo (acidic zones), the AP generation produces a comparatively small (with amplitude up to 0.4 unit) increase in pH due to inactivation of the plasma-membrane H+-pump for a period of up to 15 min [34]. The membrane conductance in these regions of the cell does not show significant changes after the AP. A likely cause of H+-pump inactivation is an almost 100-fold increase in cytoplasmic Ca2+ level during the AP, since it is known that Ca2+ inhibits the activity of plasma-membrane H+-ATPase [35-37]. Furthermore, the blockage of passive H+-conductance disturbs the concerted functioning of the “pump–leak” system, thus enhancing the H+-pump inhibition.
Propagation of an AP over an internode exerts a differential influence on photosynthesis of chloroplasts underlying the acidic and alkaline zones. In chloroplasts residing under acidic zones, the peak fluorescence Fm′ and the quantum yield of electron transport in PSII (ΔF/Fm′) are subject to minor changes during transmission of the excitation wave, whereas in chloroplasts underlying alkaline zones the parameters Fm′ and ΔF/Fm′ decrease substantially in parallel with the increase in NPQ. As a result of AP transmission along an internode, the longitudinal profiles of pHo become flattened, whereas the heterogeneity in the profiles of Fm′, ΔF/Fm′, and NPQ increases [9]. Inhibition in photosynthetic electron transport and quenching of excitations in the light-harvesting antenna by a propagating AP are presumably due to the combined impact of cytoplasmic Ca2+ increase [32, 33] and pHc changes induced by Ca2+-dependent cessation of H+ influx and H+ efflux in zones with high and low external pH, respectively.
The increasing contrast of photosynthetic patterns after AP propagation was explained by the notion that the cessation of transmembrane H+ fluxes in the areas of proton extrusion and passive leakage gives rise to oppositely directed pH shifts in the cytoplasm [9], so that alkaline shift of pHc promotes while acidic shift diminishes light-dependent Ca2+ uptake by chloroplasts [38]. It is known that increase in stromal Ca2+ concentration in chloroplasts inhibits the enzymes of the CO2 fixation cycle (reviewed in [39]). The resulting disproportion between light sufficiency and limited possibility of CO2 assimilation increases the energization of thylakoid membranes (buildup of ΔpH and lumen acidification with low ATP consumption) and enhances non-photochemical quenching. The uneven influence of AP on photosynthesis in different cell regions may also depend on competition of Ca2+ and H+ for binding with ionized acidic groups of the cytoplasmic Ca2+-H+ buffer [40]. In regions with elevated pHc (under external acidic zones), the buffer groups are ionized and readily bind Ca2+ released in the cytoplasm during AP generation, whereas in regions with lowered pHc (under external alkaline zones) the buffer groups are protonated, which hinders their capacity to bind Ca2+. In this case the recovery of cytoplasmic Ca2+ after the AP may be accomplished predominantly through Ca2+ removal into the chloroplasts or other organelles.
The inhibition of photosynthetic activity after triggering the AP is presumably associated with redirection of linear electron flow from the CO2-dependent pathway (assimilatory flow mediated by NADP reduction) to the alternative route with O2 as an electron acceptor (the Mehler reaction) [9, 41]. This is manifested in the enhanced non-photochemical quenching at the locations of alkaline zones and in decrease in O2 concentration at the outer cell surface after the AP generation [29]. The transfer of electrons to O2, unlike the reactions of CO2 fixation, does not involve ATP consumption, but it results in buildup of the proton gradient. Therefore, the redirection of electron flow toward oxygen as a terminal acceptor enhances the thylakoid energization and, accordingly, the energy-dependent non-photochemical quenching. Thus, the electrical pulse transmission increases heat losses, which lowers the photochemical activity of PSII and alleviates the stress produced by mechanical or chemical impacts.
TRANSMISSION OF PHOTOINDUCED SIGNALS WITH THE CYTOPLASMIC
FLOW
Circulating cytoplasmic streaming is another important mechanism of long-distance regulation of cell metabolism. Cytoplasmic streaming occurs in many plant cells, but it is most spectacular in the internodal cells of Characeae [12]. The immobile chloroplasts in internodal cells are densely packed over the periphery of a thin cytoplasmic layer. Subcortical actin filaments are attached at the inward-facing side of the chloroplasts. The cytoplasmic movement arises owing to ATP-dependent sliding of myosin molecules along the immobile actin bundles [42]. The myosin molecules are linked with intracellular organelles. Therefore, the movement of myosin with attached vesicles entrains the flow of the endoplasm, while a thin layer of ectoplasm remains immobile. The velocity of cytoplasmic streaming can be as high as 100 µm/s, which is the highest record observed with various organisms [43].
The counter-directed cytoplasmic flows are separated by a narrow neutral zone devoid of chloroplasts; no movement occurs in this zone. The cytoplasm flows along a spiral trajectory that makes a complete turn at a distance 10-20 mm. In the case of unilateral illumination, the chloroplast layers located on opposite cell sides are exposed to different irradiances. The chloroplast layer positioned closer to the light source attenuates the transmitted light at the peak of chlorophyll absorption approximately 3-fold. Under stationary conditions, the cytoplasm from the brightly illuminated cell side is carried to areas of dim illumination, while the cytoplasm from shaded regions moves to the fully illuminated side. Different metabolism in light-exposed and shaded cell parts, the ongoing exchange of solutes between chloroplasts and the cytoplasm, and the transfer of fluid between illuminated and shaded regions ensure mutual influence on the activities of organelles located in these regions. In addition to distribution of substances over the cell and smoothing the intracellular gradients, the liquid flow performs other important functions. The liquid flow accelerates the exchange of metabolites between the cytoplasm and immobile organelles and facilitates formation of the polarity stabilized in the nonuniform pHo profile of illuminated cells [26].
Although the transfer of metabolites and messengers with the cytoplasmic flow is generally recognized [44], there is only scarce information on specific manifestations of such transport and on the transported components. The long-distance interactions of chloroplasts, mediated by lateral transport of molecules with the streaming cytoplasm, as well as the role of these interactions in regulation of photosynthesis and plasmalemmal transport systems remained hidden for a long time. However, the long-distance intracellular interactions mediated by the fluid flow became apparent when localized, precisely positioned illumination was combined with micromethods for in vivo assessment of chlorophyll fluorescence and H+ fluxes across the plasma membrane at a distance of a few millimeters from the site of the photostimulus [26, 45].
When pHo was measured on a preselected cell region, the localized illumination of the neighboring area at a distance of 1-3 mm upstream from the analyzed region resulted in alkaline zone formation in the analyzed area, whereas identical illumination of the area positioned downstream at an equal distance did not produce an alkaline patch [45, 46]. It appears that the cytoplasm arriving from a brightly illuminated region to a shaded area contains a physiologically active intermediate that opens the plasmalemmal H+(OH–) channels in this area, giving rise to the alkaline shift of pHo at the cell surface. The channel opening is likely mediated by OH– ions transported with the cytoplasmic flow [15]. Clearly, the increase in OH– concentration in the “irradiated” cytoplasm is identical to the depletion of protons as a signaling factor, which may also cause physiological effects.
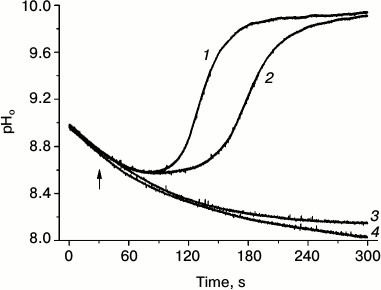
Closer studies of asymmetric formation of alkaline patches at the borders of a locally applied light beam have shown that the opening of H+ channels depends not only on cytoplasm modification in the brightly illuminated cell region, but also needs the operation of chloroplasts positioned downstream in the shaded area, i.e. at the site where the alkaline zone is being formed [47]. Figure 2 shows changes in pHo in the shaded cell region that were caused by localized illumination of the area on the upstream side with respect to the direction of cytoplasmic streaming. The initial part of the records represents the dark decay of pHo in the analyzed area after the end of pre-illumination of the whole cell. When the pH decreased to a certain level, a dim background illumination of the whole cell was switched on, and then a bright narrow beam (500 µmol·m–2·s–1, 400 µm diameter) was applied at a separation distance of 1.5-2 mm on the upstream side of the analyzed area. The combination of localized lighting with dim background illumination resulted in a fast restoration of the alkaline patch in the shaded analyzed area; the recovery of high pHo started earlier at the background intensity of 12.6 rather than 9.0 µmol·m–2·s–1 (curves 1 and 2). The localized lighting in the absence of background light, as well as background illumination alone, did not result in opening of H+-channels and alkaline zone formation (curves 3 and 4). This means that slight functional activity of chloroplasts under weak background light is needed for the perception of the signal transmitted with the cytoplasmic flow and for subsequent formation of the response (opening of H+ channels).
Fig. 2. Localized illumination (white light, 500 µmol·m–2·s–1, beam diameter 400 µm) of a cell region at 2-mm distance on the upstream side from the point of pHo measurement results in opening of H+-channels and generation of an alkaline zone in the apoplast under weak background illumination of the whole cell at photon flux densities of 12.6 (1) and 8.9 (2) µmol·m–2·s–1 but had no effect in the absence of background illumination (4). Background illumination (12.6 µmol·m–2·s–1) in the absence of localized illumination (3) caused no opening of H+ channels (no increase in pHo). The arrow marks the moment of switching on the localized light.
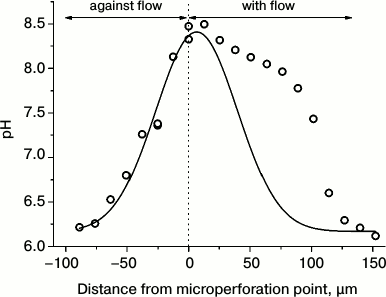
In contrast, other researchers observed light-dependent formation of alkaline zones in injured transparent cell regions from which the chloroplasts were mechanically displaced [48]. Comparison of the data for cells with different degree of injury indicates that the mechanisms of alkaline zone formation may differ in intact and damaged cells. This notion is substantiated by experiments with pinpoint injury of the Chara cell wall. These experiments revealed that microperforation of the cell wall with the tip of a glass micropipette is followed by a fast generation of the alkaline zone with pHo shifts as large as 2-2.5 units [49]. This process depends on illumination, even though its complete elimination is attained after 60-90-min darkness. The alkaline shifts of pHo in response to cell wall incision are highly sensitive to the increase in osmotic pressure of the medium, to inhibitors of Ca2+-channels, and to the cytoskeletal inhibitors, indicating that these pHo shifts are involved in the mechanism of mechanoreception. The alkaline zones induced by micropuncture are shaped asymmetrically around the point of cell wall incision. The border of the high pHo region extends to appreciably longer distance in the direction of cytoplasmic flow than in the direction opposite to the flow (Fig. 3), providing evidence that the activator of H+-channels is carried with the cytoplasmic flow for a distance of several hundred micrometers.
Fig. 3. Asymmetric axial profile of pHo in C. corallina internode around the site of cell wall microperforation. Experimental data (symbols) display the profile of steady-state pH distribution in comparison with the Gaussian curve of normal distribution (line) calculated from the assumed symmetrical shape of pH distribution in the directions of cytoplasmic flow and against the flow.
The influence of localized illumination on photosynthesis of remote cell regions, mediated by cytoplasmic streaming, extends to much longer distances. The wave of photosynthetic response to the signal generated at the point of photostimulus application propagates downstream the cytoplasmic flow for distances up to 6 mm [50] and perhaps even more. The signal arriving with the fluid flow from brightly illuminated cell areas suppresses photosynthesis in moderately lit cell regions. Conversely, when the cytoplasm was brought by flow from shaded regions to the area of measurements, the quantum efficiency of PSII was higher than that under identical irradiance of the whole cell [50, 51].
The influence of localized lighting on fluorescence of chloroplasts in the remote cell parts is clearly evident at photostimulus durations of ~30 s. Hence, the intermediate substance is subject to comparatively fast light-dependent translocation across the chloroplast envelope membranes. These properties might be attributed to protons, Ca2+, and reactive oxygen species, because the photosynthetic electron transport is coupled with translocation of H+ and counterions between the thylakoids and stroma and is closely associated with the production of superoxide and hydrogen peroxide on the acceptor side of PSI. Photoinduced changes in pH and Ca2+ in the surroundings of isolated chloroplasts and the release of photoproduced H2O2 from chloroplasts into the streaming cytoplasm have been shown by a variety of methods [38, 52, 53].
The lateral transport of several intermediates whose cytoplasmic levels undergo temporary changes points to the possibility that the chloroplast responses recorded as fluorescence transients are not monotonic and might comprise several kinetic components. This was confirmed by measuring fluorescence with the saturation pulse method on cell regions situated at various distances from the point of localized illumination. In general, changes in Fm′ fluorescence caused by illumination of the remote cell region are satisfactorily described with a sum of two Gaussian curves whose relative contributions depend on the intensity of background illumination in the area of measurements [26]. The Gaussian shape of the curves reflects their similarity to chromatographic peaks, because the components released from chloroplasts in the region of intense local illumination move with the cytoplasmic flow, passing by the area of measurements.
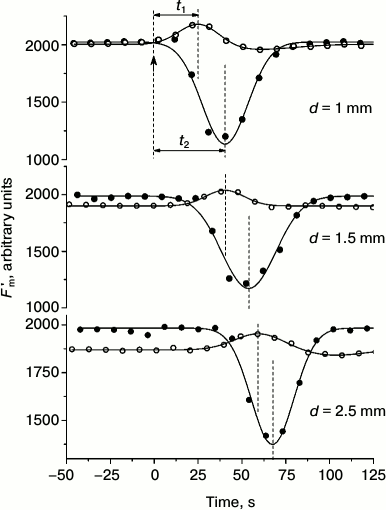
Under weak (“subthreshold”) background illumination, localized photostimulus of constant intensity induced only a wave of Fm′ elevation, whereas at high (“suprathreshold”) background intensity it induced a strong decrease in Fm′ (Fig. 4). As the distance between the centers of localized illumination and the analyzed area was extended, the appearance of extreme Fm′ values was proportionally delayed for the responses observed at background intensities both below and above the threshold levels. The plots for the delay time in Fm′ transients (at low and elevated intensities of background illumination) as a function of distance between the point of photostimulation and the area of measurements were fit to straight lines that had nearly identical slopes and were shifted along the ordinate axis [26]. These results point to the existence of two intermediates, one of which—with rapid equilibration between chloroplasts and the cytoplasm—is responsible for the increase in Fm′, and the other—with slower exchange between chloroplasts and cytosol—accounts for the strong quenching of Fm′ fluorescence. Nearly equal slopes of the straight lines indicate that both intermediates are laterally transported at equal velocities of ~70 µm/s [26]. These values correspond to the velocity of cytoplasmic streaming as measured on the same cells.
Fig. 4. Cyclosis-mediated Fm′ changes under weak (“subthreshold”, open symbols) and moderate (“suprathreshold”, filled circles) intensities of background light at various distances d between the site of localized photostimulation (beam diameter 400 µm, photon flux density 500 µmol·m–2·s–1, light pulse duration 30 s) and the point of fluorescence measurements. Arrow at moment t = 0 marks the onset of the localized light pulse. Designations t1 and t2 indicate the periods from the onset of localized illumination to the peaks and dips of Fm′ at low and elevated intensities of background illumination of the whole internode. Dashed lines mark the moments of switching on the localized light pulse and the positions of Fm′ maxima and minima.
In the presence of cytochalasin B, which inhibits cytoplasmic streaming, the time t from the onset of localized illumination to the Fm′ peak or Fm′ minimum under fixed distance between the fiber optic light guide and the microfluorometer objective lens changed in inverse relation to the rate of cytoplasmic streaming. The dependence was described by an equation t = c + d/(x + a), where с is time required for the production and processing of the intermediate when no time is needed for its lateral transport, x is the velocity of fluid movement, a ≈ 0 is a minor fitting parameter, and d is the distance between the centers of localized illumination and the area of fluorescence measurements [26].
Oppositely directed Fm′ changes arising in response to bright illumination of a remote cell region under low and moderate intensities of overall background illumination were differentially sensitive to treatments with ionophores. Increase in the membrane K+-conductance by the addition of valinomycin is known to diminish the electric potential difference in thylakoids and, probably, across the inner membrane of the chloroplast envelope. This ionophore inhibits the photoinduced electrogenic uptake of Ca2+ by intact chloroplasts, which is presumably due to elimination of the negative potential in the plastid stroma [54]. As shown in Fig. 5, treatment of C. corallina cells with valinomycin abolished the quenching of Fm′ fluorescence but had no inhibitory action on the response observed under weak background light (increase in Fm′). These observations present evidence that the two components in the Fm′ responses to the transmission of irradiated cytoplasm reflect cytoplasmic changes of various factors, e.g. pH and Ca2+, responsible for the increase and the decrease in Fm′, respectively.
Fig. 5. Changes in Fm′ fluorescence on a microscopic region of a C. corallina cell mediated by cytoplasm relocation from the area of localized illumination (beam diameter 400 µm, photon flux density 500 µmol·m–2·s–1, light pulse duration 30 s) over a distance d = 1 mm to the point of measurement at different intensities of overall background illumination under (a) control conditions and (b) after 10-min incubation of the cell in the presence 3.75 μM valinomycin: 1) at background light intensity of 40 µmol·m–2·s–1; 2) at 63 µmol·m–2·s–1. Arrows mark the moments of switching on the localized photostimulus; dashed lines show positions of maxima and minima of Fm′ with respect to the onset of localized lighting.
Thus, the cytoplasmic streaming appears to be an important participant in regulation and generation of spatial heterogeneity of photosynthesis. Such regulatory mechanisms operate in algae under natural conditions of unilateral illumination, under which cytoplasmic streaming along helical tracks relocates the substrates or signaling substances between irradiated and shaded cell sides. The occurrence of transparent spiraling neutral zones in the chloroplast layer enhances the nonuniform irradiance of the shaded cell side, thus promoting the formation of spatial patterns of photosynthesis and H+ transport. Similar mechanisms are mobilized under rapidly changing distribution of sunflecks along the internode in natural aquatic environments. The signals generated in the cytoplasmic flow during short incidence of direct sunlight on a small cell area continue traveling with the liquid flow even after cessation of the light pulse. These signals may cause dynamic photosynthetic responses at locations positioned far away from the point of sunfleck incidence and may develop with a long lapse (up to 100 s) after the excitation light was extinguished. Operation of such regulatory mechanisms, supported by cytoplasmic streaming in plant cells, remains underappreciated. Nevertheless, the transmission of signaling substances with the fluid stream represents a specific class of intracellular interactions that warrant further close examination.
This work was supported by the Russian Foundation for Basic Research, project No. 13-04-00158-а.
REFERENCES
1.Allen, J. F., and Forsberg, J. (2001) Trends
Plant Sci., 6, 317-326.
2.Bulychev, A. A., and Vredenberg, W. J. (1999)
Physiol. Plant., 105, 577-584.
3.Kramer, D. M., Avenson, T. J., Kanazawa, A., Cruz,
J. A., Ivanov, B., and Edwards, G. E. (2004) in Chlorophyll a
Fluorescence: A Signature of Photosynthesis (Papageorgiou, G. C.,
and Govindjee, eds.) Springer, Dordrecht, pp. 251-278.
4.Flugge, U.-N., and Heldt, H. W. (1991) Annu.
Rev. Plant Physiol. Plant Mol. Biol., 42, 129-144.
5.Siebke, K., and Weis, E. (1995) Photosynth.
Res., 45, 225-237.
6.Schurr, U., Walter, A., and Rascher, U. (2006)
Plant Cell Environ., 29, 340-352.
7.Baker, N. R. (2008) Annu. Rev. Plant Biol.,
59, 89-113.
8.Bulychev, A. A., Cherkashin, A. A., Rubin, A. B.,
Vredenberg, W. J., Zykov, V. S., and Muller, S. C. (2001)
Bioelectrochemistry, 53, 225-232.
9.Krupenina, N. A., Bulychev, A. A., Roelfsema, M. B.
G., and Schreiber, U. (2008) Photochem. Photobiol. Sci.,
7, 681-688.
10.Krupenina, N. A., and Bulychev, A. A. (2007)
Biochim. Biophys. Acta, 1767, 781-788.
11.Bulychev, A. A., Polezhaev, A. A., Zykov, S. V.,
Pljusnina, T. Y., Riznichenko, G. Y., Rubin, A. B., Jantoss, W., Zykov,
V. S., and Muller, S. C. (2001) J. Theor. Biol., 212,
275-294.
12.Verchot-Lubicz, J., and Goldstein, R. E. (2010)
Protoplasma, 240, 99-107.
13.Lucas, W. J., Keifer, D. W., and Sanders, D.
(1983) J. Membr. Biol., 73, 263-274.
14.Lucas, W. J., and Nuccitelli, R. (1980)
Planta, 150, 120-131.
15.Beilby, M. J., and Bisson, M. A. (2012) in
Plant Electrophysiology: Methods and Cell Electrophysiology
(Volkov, A. G., ed.) Springer, Berlin, pp. 247-271.
16.Schmolzer, P. M., Hoftberger, M., and Foissner,
I. (2011) Plant Cell Physiol., 52, 1274-1288.
17.Bulychev, A. A., Wijngaard, P. W. J., and de
Boer, A. H. (2005) Biochemistry (Moscow), 70,
55-61.
18.Bisson, M. A., and Walker, N. A. (1980) J.
Membr. Biol., 56, 1-7.
19.Smith, J. R., and Walker, N. A. (1983) J.
Membr. Biol., 73, 193-202.
20.Foissner, I. (2004) Protoplasma,
224, 145-157.
21.Antonenko, Y. N., and Bulychev, A. A. (1991)
Biochim. Biophys. Acta, 1070, 279-282.
22.Feijo, J. A., Sainhas, J., Hackett, G. R.,
Kunkel, J. G., and Hepler, P. K. (1999) J. Cell Biol.,
144, 483-496.
23.Takeshige, K., and Tazawa, M. (1989) Plant
Physiol., 89, 1049-1052.
24.Hou, G., Kramer, V. L., Wang, Y.-S., Chen, R.,
Perbal, G., Gilroy, S., and Blancaflor, E. B. (2004) Plant J.,
39, 113-125.
25.Felle, H., and Bertl, A. (1986) Biochim.
Biophys. Acta, 848, 176-182.
26.Bulychev, A. A., Alova, A. V., and Rubin, A. B.
(2013) Eur. Biophys. J., 42, 441-453.
27.Johnson, M. P., Zia, A., and Ruban, A. V. (2012)
Planta, 235, 193-204.
28.Beilby, M. J. (2007) Int. Rev. Cytol.,
257, 43-82.
29.Bulychev, A. A., and Kamzolkina, N. A. (2006)
Bioelectrochemistry, 69, 209-215.
30.Bulychev, A. A., and Krupenina, N. A. (2009)
Plant Signal. Behav., 4, 24-31.
31.Eremin, A., Bulychev, A. A., Krupenina, N. A.,
Mair, T., Hauser, M. J. B., Stannarius, R., Muller, S. C., and Rubin,
A. B. (2007) Photochem. Photobiol. Sci., 6, 103-109.
32.Berestovsky, G. N., and Kataev, A. A. (2005)
Eur. Biophys. J., 34, 973-986.
33.Hepler, P. K. (2005) Plant Cell,
17, 2142-2155.
34.Dodonova, S. O., Krupenina, N. A., and Bulychev,
A. A. (2010) Biochemistry (Moscow), Suppl. Series A: Membr.
Cell Biol., 4, 389-396.
35.De Nisi, P., Dell’Orto, M., Pirovano, L.,
and Zocchi, G. (1999) Planta, 209, 187-194.
36.Kinoshita, T., Nishimura, M., and Shimazaki, K.
(1995) Plant Cell, 7, 1333-1342.
37.Lino, B., Baizabal-Aguirre, V. M., and De la
Vara, L. E. G. (1998) Planta, 204, 352-359.
38.Muto, S., Izawa, S., and Miyachi, S. (1982)
FEBS Lett., 139, 250-254.
39.Johnson, C. H., Shingles, R., and Ettinger, W. F.
(2006) in The Structure and Function of Plastids (Wise, R. R.,
and Hoober, J. K., eds.) Springer, Dordrecht, The Netherlands, pp.
403-416.
40.Plieth, C., Sattelmacher, B., and Hansen, U.-P.
(1997) Protoplasma, 198, 107-124.
41.Krupenina, N. A., Bulychev, A. A., and Schreiber,
U. (2011) Protoplasma, 248, 513-522.
42.Shimmen, T., and Yokota, E. (2004) Curr. Opin.
Cell Biol., 16, 68-72.
43.Kimura, Y., Toyoshima, N., Hirakawa, N., Okamoto,
K., and Ishijima, A. (2003) J. Mol. Biol., 328,
939-950.
44.Pickard, W. F. (2003) Plant Cell
Environ., 26, 1-15.
45.Bulychev, A. A., and Dodonova, S. O. (2011)
Russ. J. Plant Physiol., 58, 233-237.
46.Dodonova, S. O., and Bulychev, A. A. (2011)
Protoplasma, 248, 737-749.
47.Bulychev, A. A. (2012) in Plant
Electrophysiology: Methods and Cell Electrophysiology (Volkov, A.
G., ed.) Springer, Berlin, pp. 273-300.
48.Shimmen, T., and Yamamoto, A. (2002) Plant
Cell Physiol., 43, 980-983.
49.Bulychev, A. A., Alova, A. V., and Bibikova, T.
N. (2013) Biochim. Biophys. Acta, 1828,
2359-2369.
50.Bulychev, A. A., and Dodonova, S. O. (2011)
Biochim. Biophys. Acta, 1807, 1221-1230.
51.Dodonova, S. O., and Bulychev, A. A. (2012)
Russ. J. Plant Physiol., 59, 35-41.
52.Remis, D., Bulychev, A. A., and Kurella, G. A.
(1988) J. Exp. Bot., 39, 633-640.
53.Eremin, A., Bulychev, A. A., and Hauser, M. J. B.
(2013) Protoplasma, 250, 1339-1349.
54.Kreimer, G., Melkonian, M., and Latzko, E. (1985)
FEBS Lett., 180, 253-258.