Ozone Ameliorates Age-Related Oxidative Stress Changes in Rat Liver and Kidney: Effects of Pre- and Post-ageing Administration
M. H. Safwat1*, M. M. El-Sawalhi1, M. N. Mausouf2, and A. A. Shaheen1
1Biochemistry Department, Faculty of Pharmacy, Cairo University, Kasr Al-Aini Street, Cairo, 11562, Egypt; fax: +202-2363-5140; E-mail: maheerasafwat@yahoo.com; maheerahsafwat@gmail.com; mahaelsawalhi@yahoo.com2Ozone Therapy Unit, National Cancer Institute, Cairo University, Cairo, Egypt; E-mail: nabil_mawsouf@yahoo.co.uk
* To whom correspondence should be addressed.
Received December 28, 2013
The ageing process is known to be accompanied by increased oxidative stress and compromised antioxidant defenses. Controlled ozone administration has been shown to be effective in various pathophysiological conditions with an underlying oxidative burden. However, its effect on the biochemical alterations associated with the ageing process has been rarely studied. Therefore, the present work was carried out to study the role of ozone in counteracting the state of oxidative stress associated with ageing in rat liver and kidneys using two experimental models. In the pre-ageing model, ozone was administered prior to the onset of ageing at adulthood and continued after the start of the ageing process (3-month-old rats until the age of 15 months). While in the post-ageing model, ozone was administered after ageing has begun and lasted for one month (14-month-old rats until the age of 15 months). The pre-ageing ozone administration effectively reduced lipid and protein oxidation markers, namely, malondialdehyde and protein carbonyl levels and decreased lipofuscin pigment deposition in rat liver and kidneys. Moreover, it significantly restored hepatic and renal reduced glutathione (GSH) contents and normalized cytosolic hepatic glutathione peroxidase activity. Similar but less pronounced effects were observed in the post-ageing ozone-treated group. Nevertheless, in the latter model ozone administration failed to significantly affect liver and kidney lipofuscin levels, as well as kidney GSH contents. These data provide evidences for potentially positive effects of pre-ageing ozone therapy in neutralizing chronic oxidative stress associated with ageing in rat liver and kidneys.
KEY WORDS: ozone, oxidative stress, liver, kidney, pre-ageing, post-ageingDOI: 10.1134/S0006297914050095
Abbreviations: GPx, glutathione peroxidase; GSH, reduced glutathione; MDA, malondialdehyde; ROS, reactive oxygen species.
Ageing is a normal complex physiological process. It is characterized by
a variety of morphological and biochemical changes that occur from
maturity to senescence. These changes trigger a progressive decline in
multiple organ systems, thus rendering the organism more vulnerable to
disease and toxicity, eventually leading to death [1, 2]. The process of ageing still
remains an unresolved biological problem; it cannot be clarified by a
single gene or the decline of a key body system [3,
4].
Many theories have been proposed to explain the phenomenon of ageing based on somatic mutations, accumulation of aberrant proteins, genetic programming, or changes in neural and endocrine functions. Currently, one of the most plausible and acceptable explanations for the mechanistic basis of ageing is the “free radical theory of ageing”. This theory states that free radicals elicit cumulative damage to cellular macromolecules (proteins, lipids, DNA), which in the absence of strong endogenous antioxidant defenses leads to ageing and its related diseases [5, 6]. The “oxidative stress theory” postulates that reactive oxygen species (ROS), rather than free radicals, are responsible for the functional changes that accompany ageing [7]. Oxidative stress may also provide a mechanism upon which other “damage” theories of ageing are based, such as the genomic instability as a result of DNA damage, and the accumulation of glycated crosslinks during protein damage that can result in the pathogenesis associated with cardiovascular and neurodegenerative disease [8, 9].
In the past it was proposed that the liver does not undergo significant ageing changes [10]; however, it has become clear that the liver undergoes substantial alterations in structure and function in old age. The senescent liver exhibits a number of characteristics consistent with oxidative injury, and many studies have shown that ROS tissue level impacts liver functions and is intimately linked to most age-associated diseases [11, 12].
One of the organs with high tendency to development of age-dependent tissue injury is the kidney [13, 14]. Excessive oxidative stress has been correlated with many age-dependent changes in kidney such as excessive fibrosis, a general lack of regenerative ability, and an increase in apoptosis [15]. A dramatic increase in the rate of H2O2 production in the kidneys of old rats has been reported by Gomes et al. [16], which indicates a significant increase in oxidative stress in this tissue. Moreover, the ageing kidney is highly subjected to increased lipid peroxidation, enhanced deposition of lipofuscin and advanced glycation end-products (AGEs), and increased apoptosis [17], which might contribute to the pathogenesis and progression of renal disease [18].
Improving the ageing process to achieve healthy ageing and thereby delay the onset and progression of multiple age-related diseases is one of the major challenges in the 21st century [3].
One possible intervention to achieve healthy ageing is ozone therapy, with its versatile uses and routes of administration. For several decades ozone therapy has been known to complement conventional medicine in many conditions such as resistant infections, orthopedic pathologies, as well as vascular, neurodegenerative, and inflammatory disorders. Judicious ozone doses are capable of counteracting oxidative stress by inducing cellular adaptation to it. This phenomenon is known as “oxidative preconditioning” and occurs via certain physiological messengers that are created by ozone and that act to activate numerous biological pathways [19]. The ability of ozone oxidative preconditioning to improve the redox status has been observed in a wide range of pathologies in different animal models such as cisplatin-induced nephrotoxicity [20], hepatic ischemia-reperfusion injury [21], renal ischemia reperfusion injury [22], diabetic nephropathy [23], and coronary artery disease [24].
However, very little is known about the effect of ozone on age-associated changes in various tissues. Two very recent studies conducted in our laboratory have investigated the effect of ozone on reversing certain biochemical alterations associated with ageing in rat cerebral cortex [25] and rat hippocampus and heart [26]. Nevertheless, the effect of ozone on biochemical changes related to ageing in rat liver and kidneys, to the best of our knowledge, has not been studied. Consequently, the present study was carried out to explore the possible anti-ageing effect of ozone administration in neutralizing chronic oxidative stress that accompanies ageing in rat liver and kidneys using two experimental models. In the pre-ageing model, ozone was administered prior to the onset of ageing at adulthood and continued after the start of the ageing process (3-month-old rats until the age of 15 months). In the post-ageing model, ozone was administered after ageing has begun and lasted for one month (14-month-old rats until the age of 15 months). While designing this work, we tried to avoid age-related extremes via comparing between 3- and 15-month-old rat groups. These selected ages exemplify the transition from full maturity to early ageing as it has been proposed that the differences, which emerge during this period, may create the grounds for future senescence [25, 27].
MATERIALS AND METHODS
Ozone generation and administration. Ozone was generated from medical-grade oxygen using an ozone generator system (EXT 120-T; Longevity Resources Inc., Canada). It is a high-quality oxygen-fed ozone generator for ultra-pure medical applications. The ozone concentration is precisely measured by using a built-in UV spectrophotometer set at 254 nm. The ozone obtained by this generator was administered to rats, immediately as generated, by rectal insufflation, performed with a suitable polyethylene cannula connected to a syringe. This route of ozone administration is considered as the most useful and the easiest procedure in rats [28]. The selected ozone dose in the present study was 0.6 mg/kg body weight [29]. In order to produce this concentration, the oxygen flow rate was adjusted to 125 ml/min and the voltage of the ozone generator was adjusted to 2 V.
Experimental animals. Adult male albino rats of Wistar strain (age – 3 months and weight – 180-220 g) were obtained from the animal facility of the National Institute for Vaccination, Helwan, Egypt. The animals were housed under controlled environmental conditions at constant temperature (25 ± 2°C) and a 12/12 h light/dark cycle. The rats were acclimatized to the facility for one week before any experimental procedures and were allowed standard rat chow diet and water ad libitum throughout the experimental period. Animal care was supervised and approved by the Ethical Committee for Animal Experimentation at the Faculty of Pharmacy, Cairo University.
Experimental design. Sixty rats were randomly divided into five experimental groups (n = 12, each). Group 1: aged control group was kept without any treatment until the age of 15 months. Group 2: pre-ageing ozone-treated group in which rats were treated with ozone/oxygen mixture at an ozone dose of 0.6 mg/kg body weight twice weekly for the first three months, then once per week till the age of 15 months. The volume of insufflated mixture was approximately 5 ml. Since the ozone/oxygen mixture consists of 5% ozone and 95% oxygen, it was necessary to test the effect of the oxygen vehicle on the experimental animals. Group 3: pre-ageing oxygen control group received only oxygen in the same manner as in group 2. Group 4: post-ageing ozone-treated group, animals were kept untreated until the age of 14 months, and then they were treated with ozone/oxygen mixture at an ozone dose of 0.6 mg/kg body weight three times weekly for four weeks. Group 5: post-ageing oxygen control group rats were subjected to the same treatment as in group 4, but using oxygen only instead of ozone/oxygen mixture. Additionally, group 6: adult control group consisted of a group of normal rats (n = 12, age – 3 months, average weight – 180-220 g) that were recruited one week before sacrifice and served as a control group for the aforementioned aged groups.
At the end of the experimental period, the animals were sacrificed under ether anesthesia. The liver and both kidneys were rapidly isolated, washed with ice-cold saline, then blotted between filter papers, weighed, and homogenized in ice-cold saline to make 20% and 10% homogenates for liver and kidney, respectively. The resultant homogenates were appropriately prepared and used for determination of hepatic and renal oxidative stress markers: malondialdehyde (MDA), protein carbonyls (pCO), lipofuscin, and glutathione (GSH) levels as well as glutathione peroxidase (GPx) activity.
Determination of lipid peroxidation and protein oxidation markers. Aliquots of liver and kidney homogenates were mixed with ice-cold 2.3% KCl (1 : 1), centrifuged at 600g at 4°C for 15 min, and the supernatants were used for estimation of MDA levels formed as an end product of the peroxidation of lipids according to the method of Mihara and Uchiyama [30]. The method is based on the measurement of the pink-colored complex produced by the reaction of MDA with thiobarbituric acid in acidic medium.
Another portion of each homogenate was mixed with an equal volume of ice-cold 100 mM phosphate buffer, pH 7.4, and centrifuged at 11,000g at 4ºC for 20 min using a DuPont Sorvall Combi Plus ultracentrifuge (DuPont Company, USA). Protein carbonyls (markers of protein oxidation) were measured in the resultant supernatants using the 2,4-dinitrophenylhydrazine (DNPH) method of Reznick and Packer [31] as modified by Liu et al. [32]. This method depends on the interaction of carbonyl groups of proteins with DNPH to form the corresponding protein hydrazones, which are measured spectrophotometrically at 375 nm. The protein carbonyl content was expressed as nmol/mg of protein. Since about 10-15% of proteins are lost in the reaction procedure, the protein levels were quantified in the final pellets by reading the absorption at 280 nm. The amount of proteins was calculated from a bovine serum albumin standard treated in the same way [33].
Assessment of lipofuscin pigment. Lipofuscin pigment was determined according to the method of Tappel et al. [34]. This assay is based on the spectrofluorometric measurement of lipofuscin in the organic phase of chloroform–methanol extracts of crude liver and kidney homogenates at maximum excitation and emission wavelengths of 350 and 435 nm, respectively, using a Shimadzu spectroflourometer and quinine sulfate as a standard solution (1 µg quinine sulfate/ml 0.1 N H2SO4). The results were expressed as relative fluorescence units (RFU).
Estimation of GSH contents and cytosolic GPx activity. Suitable portions of liver and kidney homogenates were mixed with ice-cold 5% sulfosalicylic acid (1 : 2) and centrifuged at 1800g at 4°C for 15 min. The glutathione content was measured using 5,5′-dithiobis-(2-nitrobenzoic acid) (Ellman’s reagent), which produces a stable yellow color that can be measured colorimetrically [35].
For preparation of cytosolic fractions, an aliquot of each homogenate was mixed with an equal volume of an ice-cold Tris-EDTA buffer (100 mM Tris and 0.2 mM EDTA, pH 7.6) and ultracentrifuged at 105,000g at 4°C for 20 min. Cytosolic GPx (EC 1.11.1.9) activity was determined according to the method of Paglia and Valentine [36] by following the rate of NADPH oxidation in the presence of hydrogen peroxide, GSH, and glutathione reductase as a decrease in absorbance at 340 nm. Enzyme activity was expressed as units per milligram protein, where one unit is defined as the amount of enzyme that oxidizes 1 µmol NADPH per minute at 25°C.
Assay of protein in cytosolic fractions. The protein content of the cytosolic fractions used for GPx assay was determined according to the method of Lowry et al. [37] using Folin–Ciocalteu reagent with bovine serum albumin as a standard.
Statistical analysis. The results were expressed as mean ± S.E. Statistical analysis was performed with INSTAT ΙΙ statistical software (Instat, USA). The minimal level of statistical significance was taken at p < 0.05 using one-way analysis of variance (ANOVA) followed by Tukey–Kramer multiple comparison test to judge the difference between various groups.
RESULTS
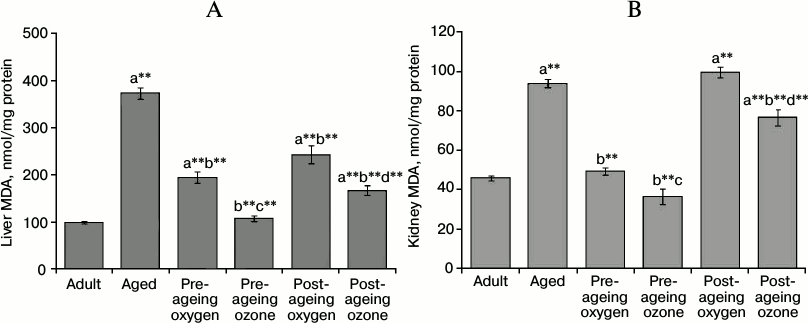
Effect of ozone administration on lipid peroxidation and protein oxidation products. The effect of oxidative stress burden on lipids and proteins was evaluated by determining their oxidation products – MDA and protein carbonyls. Ageing caused a substantial increase (p < 0.001) in liver MDA (about 4-fold) and kidney MDA (2-fold) as compared to the adult control group. Pre-ageing administration of ozone efficiently decreased the level of hepatic MDA by 71% and renal MDA by 61% (p < 0.001) as compared to the aged control group, thereby restoring their levels to the adult control values. On the other hand, the post-ageing use of ozone significantly reduced (p < 0.001) the level of MDA in the liver and kidney by 55 and 18%, respectively, relative to the aged control group. It is worth mentioning that the pre-ageing use of oxygen as a vehicle for ozone caused a significant decline (p < 0.001) in liver and kidney MDA levels relative to the aged control values, while the post-ageing use of oxygen significantly lowered the level (p < 0.001) of MDA in the liver only. However, the reductions of hepatic and renal MDA caused by both pre- and post-ageing ozone interventions were significantly higher than the effects of their respective control pre- and post-ageing oxygen vehicle administration (Fig. 1, A and B).
Fig. 1. Effects of pre- and post-ageing ozone administration on the level of MDA in liver (A) and kidney (B) of 15-month-old rats. Each value represents the mean of 8-10 experiments ± S.E.; a p < 0.001(**) vs adult control, b p < 0.001(**) vs aged control; c p < 0.001(**), 0.05 vs pre-ageing oxygen control; d p < 0.001(**) vs post-ageing oxygen control.
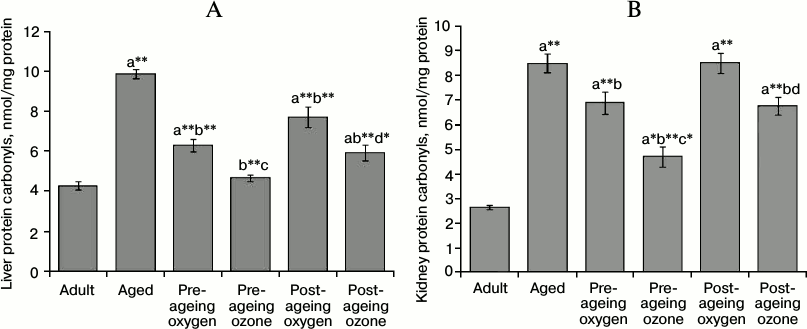
The protein carbonyls content was remarkably elevated by ageing (p < 0.001) reaching 231% in the liver and 321% in the kidney compared with the adult control values. The pre-ageing administration of ozone was effective in ameliorating the elevated level of protein carbonyls (p < 0.001) reaching 47% in the liver and 55% in the kidney of the aged control values. Similarly, post-ageing ozone administration caused a significant reduction (p < 0.001) in the level of protein carbonyls mounting to 40 and 21% in the liver and kidney, respectively, in comparison to the aged group. On the other hand, the pre-ageing administration of oxygen vehicle evoked a significant but milder decrease in carbonyl level reaching 36% in the liver (p < 0.001) and 19% in the kidney (p < 0.05) as compared to the aged group. Post-ageing oxygen administration reduced significantly (p < 0.001) the level of liver protein carbonyls by 22% relative to aged control rats. Both the pre- and post-ageing effects of ozone administration on hepatic and renal protein carbonyls content exceed significantly the effects of the oxygen vehicle (Fig. 2, A and B).
Fig. 2. Effects of pre- and post-ageing ozone administration on the level of protein carbonyls in the liver (A) and kidney (B) of 15-month-old rats. Each value represents the mean of 8-10 experiments ± S.E.; a p < 0.001(**), 0.01(*), 0.05 vs adult control; b p < 0.001(**), 0.05 vs aged control; c p < 0.01(*), 0.05 vs pre-ageing oxygen control; d p < 0.01(*), 0.05 vs post-ageing oxygen control.
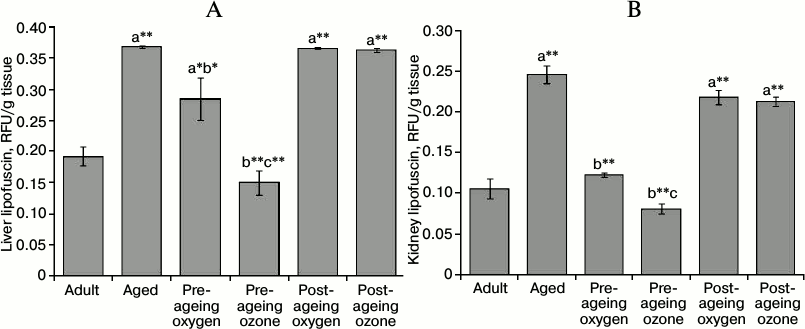
Effect of ozone administration on the ageing pigment lipofuscin. Ageing induced a marked increment (p < 0.001) in the content of lipofuscin pigment in the liver and kidney of aged rats to reach 191 and 233% of the adult control values, respectively. The pre-ageing administration of ozone successfully maintained the liver and kidney lipofuscin levels at the corresponding adult control values (p < 0.001). On the contrary, the post-ageing ozone administration failed to exert any significant effect on the level of liver and kidney lipofuscin. Furthermore, the pre-ageing oxygen administration exhibited a decrease in lipofuscin level by 23% (p < 0.01) and 50% (p < 0.001) in the liver and kidney, respectively, as compared to the aged control group. Nonetheless, the pre-ageing ozone administration exhibited a greater reduction of lipofuscin content than the oxygen control in both the liver and kidney (Fig. 3, A and B).
Fig. 3. Effects of pre- and post-ageing ozone administration on the level of lipofuscin in the liver (A) and kidney (B) of 15-month-old rats. Each value represents the mean of 8-10 experiments ± S.E.; a p < 0.001(**), 0.01(*) vs adult control; b p < 0.001(**), 0.01(*) vs aged control; c p < 0.001(**), 0.05 vs pre-ageing oxygen control.
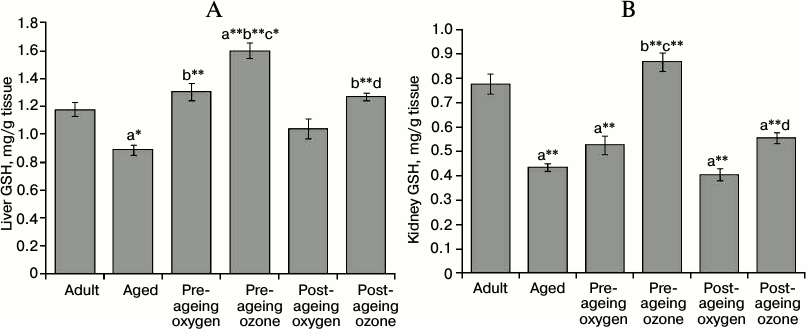
Effect of ozone administration on GSH contents and cytosolic GPx activity. Ageing elicited a significant decrease in liver and kidney GSH contents amounting to 75% (p < 0.01) and 56% (p < 0.001) of the adult control values, respectively. Pre-ageing use of ozone markedly elevated (p < 0.001) the level of GSH in both tissues reaching about 179 and 200% of the aged control values in the liver and kidney, respectively. The post-ageing administration of ozone induced a significant elevation (p < 0.001) of hepatic GSH; about 143% of the aged control group, while it failed to counteract significantly the age-associated GSH depletion in the kidney. On the other hand, the pre-ageing use of oxygen was efficient only in increasing liver GSH content (p < 0.001) by 146%, as compared to the aged group. Both pre- and post-ageing ozone administration induced a greater augmentation of liver GSH level than the oxygen control (Fig. 4, A and B).
Fig. 4. Effects of pre- and post-ageing ozone administration on the level of GSH in the liver (A) and kidney (B) of 15-month-old rats. Each value represents the mean of 8-10 experiments ± S.E.; a p < 0.001(**), 0.01(*) vs adult control; b p < 0.001(**) vs aged control; c p < 0.001(**), 0.01(*) vs pre-ageing oxygen control; d p < 0.05 vs post-ageing oxygen control.
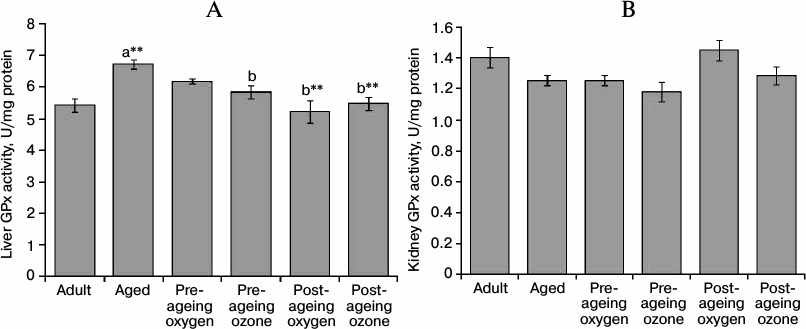
The activity of GPx enzyme in rat liver was significantly elevated (p < 0.001) in the aged group, while kidney GPx activity remained unaffected by ageing. Moreover, the pre-ageing use of ozone significantly reduced (p < 0.05) the activity of GPx enzyme in the liver by 13% of the aged group, thereby restoring its activity to the value of the adult control group. Meanwhile the pre-ageing use of ozone did not alter the renal GPx activity, which was already unchanged by ageing. Post-ageing administration of both ozone and oxygen significantly restored (p < 0.001) the activity of liver GPx to the value of the adult control (Fig. 5, A and B).
Fig. 5. Effects of pre- and post-ageing ozone administration on the activity of cytosolic GPx enzyme in the liver (A) and kidney (B) of 15-month-old rats. Each value represents the mean of 8-10 experiments ± S.E.; a p < 0.001(**) vs adult control; b p < 0.001(**), 0.05 vs aged control.
DISCUSSION
The present study may be the first to demonstrate significant effects of ozone administration in neutralizing chronic oxidative stress associated with ageing in rat liver and kidneys using two experimental models. The pre-ageing ozone administration effectively reduced lipid and protein oxidation products and decreased lipofuscin deposition in both tissues. Moreover, it was efficient in elevating the reduced hepatic and renal GSH contents as well as in normalizing hepatic GPx activity of aged rats. On the other hand, the post-ageing ozone administration succeeded in attenuating the elevated hepatic and renal MDA and protein carbonyls levels, in addition to raising the hepatic GSH contents.
The ageing process is undoubtedly accompanied by an increase in the production of free radicals and other reactive species resulting in a chronic state of oxidative stress in different organs [38]. Oxidative stress is responsible for cumulative tissue damage paralleled by alterations in the function of the genetic apparatus and modulation of various signal transduction pathways, which ultimately leads to cell death [39, 40]. Polyunsaturated fatty acids of the cell membrane are most likely to be oxidized by ROS, thus bringing about the accumulation of MDA and other lipid peroxidation products [41, 42]. In the current study, age-related changes in the oxidant status were evidenced by the substantial increase in the levels of lipid peroxidation and protein oxidation markers in the liver and kidneys of aged rats. The elevation in hepatic and renal protein carbonyl contents may be in part a consequence of increased lipid peroxidation, since molecules like MDA and 4-HNE can react with proteins and introduce carbonyl groups to their structures by non-oxidative mechanisms [43-45]. Another factor that might lead to age-associated accumulation of oxidatively modified proteins is the impairment of the proteasomal system with ageing [46, 47].
Oxidatively modified protein and lipid peroxidation products constitute the bulk of lipofuscin granules. In the present study, the age-associated increase in liver and kidney lipofuscin is in line with the results of Kumar et al. [48] and Uribarri et al. [49], respectively. Such an increase could be the long term result of a decreased degradation of oxidized proteins, increased deposition of lipid peroxidation products, and an increase in intracellular free radical formation [50, 51].
Likewise, the significant reduction of hepatic and renal GSH levels in our study might be ascribed to increased consumption of GSH and its conversion into GSSG due to enhanced oxidative burden [52]. Furthermore, the ageing process is known to compromise GSH biosynthesis by affecting the catalytic activity of the rate-limiting enzyme – γ-glutamylcysteine synthetase (γ-GCS) [53].
In conformity with the presence of oxidative events, our results showed an enhancement of hepatic GPx activity in aged rats, which could be regarded as an adaptive response to counterbalance the increased oxidative stress [54, 55]. Renal GPx enzyme activity was not affected by ageing in the current study, which is in agreement with the results of Tian et al. [56]. On the contrary, Alabarse et al. [57] reported an enhancement in renal GPx activity with ageing, while Ramesh et al. [58] documented a decline in its activity. Such discrepancy might be explained by the variation in experimental conditions such as the duration of the study, the animal species, and the environmental conditions.
The widespread use of medical ozone at well-defined and safe protocols proved to be efficient in preventing the damage caused by ROS generated in various animal models. This was supported by the reported beneficial effects of ozone on the parameters of oxidative stress [21-24, 59].
In the current study, the favorable effects of ozone administration in counteracting the state of chronic oxidative stress associated with ageing in rat liver and kidneys could be ascribed to the phenomenon of “ozone oxidative preconditioning”. It is a state obtained through judicious and controlled use of ozone that could trigger an acute and precisely calculated oxidative stress able to activate several biological processes leading to therapeutic benefits [60]. After its administration, ozone dissolves immediately in the plasma and reacts with biomolecules producing ROS and lipid peroxidation products (LOPs) [61]. ROS are the early and short-acting messengers, while LOPs are late and long-lasting messengers. LOPs diffuse into all cells and inform them of a minimal oxidative stress, thus activating a number of redox-sensitive signal transduction cascades that lead to the increased expression of antioxidant enzymes [19]. The repeated exposure to this minimal oxidative stress is the critical stimulus for inducing the adaptive response against a prolonged and severe stress [60]. Indeed this was reflected in the present study as a marked reduction in hepatic and renal MDA levels. Moreover, ozone-induced decrement in lipid peroxidation might have contributed to the decreased protein carbonyl levels, since the latter may be produced by reactions of proteins with aldehydes such as MDA or 4-HNE [62]. A further explanation for the observed effect of ozone on elevated protein carbonyl levels could be via the upregulation of heat shock protein 90 (HSP90), with its ability to activate the proteasome degradation pathway, thus reducing the accumulation of oxidized proteins [63]. Furthermore, the decreased accumulation of lipofuscin induced by ozone may well be a consequence of its ability to protect proteins and lipids against oxidative alterations [26].
In the present work, the preserving effect of pre-ageing ozone administration on hepatic and renal GSH contents could be attributed to the fact that ROS, produced by ozone therapy, are capable of activating the cysteine transport system [64] and inducing the expression of γ-GCS [65]. In addition, the preserved ATP/ADP ratio by ozone therapy might enhance the energy-requiring de novo GSH synthesis [25, 26]. Meanwhile, the restoration of hepatic GPx activity by pre-ageing ozone administration is in harmony with the results of El-Sawalhi et al. [26] on GPx activity in aged rat heart. It seems that ozone oxidative preconditioning was capable of improving the antioxidant/prooxidant balance and consequently sparing the compensatory increase in this vital antioxidant enzyme.
In the current study, post-ageing ozone administration exerted less pronounced effects on the elevated levels of liver and kidney MDA and protein carbonyls in comparison to the pre-ageing ozone-treated rats. Furthermore, the post-ageing ozone administration failed to counteract the age-associated increase in hepatic and renal lipofuscin levels and was incapable of elevating renal GSH contents. More so, the elevation of liver GSH content by post-ageing ozone administration was found to be lower than the pre-ageing one.
The inability of post-ageing ozone administration to reduce liver and kidney lipofuscin levels might be ascribed to the nature of lipofuscin formation and accumulation. Initial aggregates are formed from unfolded proteins, interweaved but potentially still degradable [66]. Over time the single peptide chains are more and more covalently cross-linked [67], resulting in an intracellular cluster that can be neither exocytosed nor degraded [66]. Hence, it might be concluded that early and pre-ageing intervention would be more effective in decreasing lipofuscin levels.
Finally, it is worth mentioning that certain favorable effects were observed in the oxygen vehicle-treated group, which might be attributed to the role of oxygen in enhancing organ oxygenation and regeneration, as well as the reduction of organ injury and apoptosis. Ijichi et al. [68] and He et al. [69] have demonstrated the beneficial effect of oxygen on lipid peroxidation and the antioxidant system.
In conclusion, the present study provides evidences for the promising effects of ozone therapy in neutralizing chronic oxidative stress associated with ageing in rat liver and kidneys. It also indicates that the pre-ageing ozone administration is more efficient than the post-ageing administration of ozone to aged rats. Consequently, the study warrants further studies and clinical trials to support the use of ozone as a new strategy that can delay or even reverse age-related impairments.
REFERENCES
1.Subathra, M., Shila, S., Devi, M. A., and
Panneerselvam, C. (2005) Exp. Gerontol., 40, 707-715.
2.Fontana, L., and Klein, S. (2007) JAMA,
297, 986-994.
3.Kirkwood, T. (2008) Nature, 451,
644-647.
4.Hayflick, L. (2007) Ann. N. Y. Acad. Sci.,
1100, 1-13.
5.Gil, L. (2011) Biomed. Ageing Pathol.,
1, 1-7.
6.Gemma, J., Bachstetter, A., and Bickford, P. (2007)
in Brain Ageing: Models, Methods, and Mechanisms
(Riddle, D., ed.) 1st Edn., CRC Press, London-New York, pp.
353-374.
7.Hagen, T. M. (2003) Antioxid. Redox Signal.,
5, 503-506.
8.Barja, G., Cadenas, S., Rojas, C., Lopez-Torres,
M., and Perez-Campo, R. (1994) Comp. Biochem. Physiol. Biochem. Mol.
Biol., 108, 501-512.
9.Droge, W., and Schipper, H. M. (2007) Ageing
Cell, 6, 361-370.
10.Schmucker, D. L. (1998) J. Gerontol.,
53, 315-320.
11.Jung, K., and Henke, W. (1996) Free Radic.
Biol. Med., 20, 613-617.
12.Lebel, M., de Souza-Pinto, N. C., and Bohr, V. A.
(2011) Curr. Gerontol. Geriatr. Res., 2011,
859415-859429.
13.Clark, B. (2000) Adv. Ren. Replace Ther.,
7, 11-21.
14.Percy, C. J., Brown, L., Power, D. A., Johnson,
D. W., and Gobe, G. C. (2009) Mech. Ageing Dev., 130,
129-138.
15.Uribarri, J., Peppa, M., Cai, W., Goldberg, T.,
Lu, M., He, C., and Vlassara, H. (2003) J. Am. Soc. Nephrol.,
14, 728-731.
16.Gomes, P., Simao, S., Silva, E., Pinto, V.,
Amaral, J. S., Afonso, J., Serrao, M. P., Pinho, M. J., and
Soares-da-Silva, P. (2009) Oxid. Med. Cell Longev., 2,
138-145.
17.Uribarri, J., Stirban, A., Sander, D., Cai, W.,
Negrean, M., Buenting, C. E., Koschinsky, T., and Vlassara, H. (2007)
Diabetes Care, 30, 2579-2582.
18.Negre-Salvayre, A., Auge, N., Ayala, V., Basaga,
H., Boada, J., Brenke, R., Chapple, S., Cohen, G., Feher, J., Grune,
T., Lengyel, G., Mann, G. E., Pamplona, R., Poli, G., Portero-Otin, M.,
Riahi, Y., Salvayre, R., Sasson, S., Serrano, J., Shamni, D., Siems,
W., Siow, R. C., Wiswedel, I., Zarkovic, K., and Zarkovic, N. (2010)
Free Radic. Res., 44, 1125-1171.
19.Bocci, V., Borrelli, E., Travagli, V., and
Zanardi, I. (2009) Med. Res. Rev., 29, 646-682.
20.Borrego, A., Zamora, Z. B., Gonzalez, R., Romay,
C., Menendez, S., Hernandez, F., Montero, T., and Rojas, E. (2004)
Mediators Inflamm., 13, 13-19.
21.Ajamieh, H. H., Menendez, S., Martinez-Sanchez,
G., Candelario-Jalil, E., Re, L., Giuliani, A., and Fernandez, O. S.
(2004) Liver Int., 24, 55-62.
22.Chen, H., Xing, B., Liu, X., Zhan, B., Zhou, J.,
Zhu, H., and Chen, Z. (2008) Arch. Med. Res., 39,
169-178.
23.Morsy, M. D., Hassan, W. N., and Zalat, S. I.
(2010) Diabetol. Metab. Syndr., 2, 29-35.
24.Martinez-Sanchez, G., Delgado-Roche, L.,
Diaz-Batista, A., Perez-Davison, G., and Re, L. (2012) Eur. J.
Pharmacol., 691, 156-162.
25.Shehata, N. I., Abd-Elgawad, H. M., Mawsouf, M.
N., and Shaheen, A. A. (2012) Biogerontology, 13,
565-581.
26.El-Sawalhi, M. M., Darwish, H. A., Mausouf, M.
N., and Shaheen, A. A. (2013) Cell Biochem. Funct., 31,
518-525.
27.Sharman, E. H., and Bondy, S. C. (2001)
Neurobiol. Ageing, 22, 629-634.
28.Gonzalez, R., Borrego, A., Zamora, Z., Romay, C.,
Hernandez, F., Menendez, S., Montero, T., and Rojas, E. (2004)
Mediators Inflamm., 13, 307-312.
29.Re, L., Mawsouf, M. N., Menendez, S., Leon, O.
S., Sanchez, G. M., and Hernandez, F. (2008) Arch. Med. Res.,
39, 17-26.
30.Mihara, M., and Uchiyama, M. (1978) Anal.
Biochem., 86, 271-278.
31.Reznick, A. Z., and Packer, L. (1994) Methods
Enzymol., 233, 357-363.
32.Liu, R., Liu, I. Y., Bi, X., Thompson, R. F.,
Doctrow, S. R., Malfroy, B., and Baudry, M. (2003) Proc. Natl. Acad.
Sci. USA, 100, 8526-8531.
33.Abd El Mohsen, M. M., Iravani, M. M., Spencer, J.
P., Rose, S., Fahim, A. T., Motawi, T. M., Ismail, N. A., and Jenner,
P. (2005) Biochem. Biophys. Res. Commun., 336,
386-391.
34.Tappel, A., Fletcher, B., and Deamer, D. (1973)
J. Gerontol., 28, 415-424.
35.Beutler, E., Duron, O., and Kelly, B. M. (1963)
J. Lab. Clin. Med., 61, 882-888.
36.Paglia, D. E., and Valentine, W. N. (1967) J.
Lab. Clin. Med., 70, 158-169.
37.Lowry, O. H., Rosebrough, N. J., Farr, A. L., and
Randall, R. J. (1951) J. Biol. Chem., 193, 265-275.
38.Ramesh, T., Yoo, S. K., Kim, S. W., Hwang, S. Y.,
Sohn, S. H., Kim, I. W., and Kim, S. K. (2012) Exp. Gerontol.,
47, 979-987.
39.Harman, D. (1994) Ann. N. Y. Acad. Sci.,
717, 1-15.
40.Pandey, K. B., and Rizvi, S. I. (2010) Oxid.
Med. Cell Longev., 3, 2-12.
41.Tamburini, I., Quartacci, M. F., Izzo, R., and
Bergamini, E. (2004) Ageing Clin. Exp. Res., 16,
425-431.
42.Berrougui, H., and Khalil, A. (2009)
Rejuvenation Res., 12, 117-126.
43.Goto, S., Hasegawa, A., Nakamoto, H., Nakamura,
A., Takahashi, R., and Kurochkin, I. V. (1995) in Oxidative Stress
and Ageing (Cutler, R. C., et al., ed.) 1st Edn., Birkhauser,
Basel, Switzerland, pp. 151-158.
44.Sohal, R. S. (2002) Free Radic. Biol.
Med., 33, 37-44.
45.Gruber, J., Ng, L. F., Fong, S., Wong, Y. T.,
Koh, S. A., Chen, C. B., Shui, G., Cheong, W. F., Schaffer, S., Wenk,
M. R., and Halliwell, B. (2011) PLoS One, 6,
e19444-19459.
46.Petropoulos, I., Conconi, M., Wang, X., Hoenel,
B., Bregegere, F., Milner, Y., and Friguet, B. (2000) J.
Gerontol., 55, 220-227.
47.Farout, L., and Friguet, B. (2006) Antioxid.
Redox Signal., 8, 205-216.
48.Kumar, P., Kale, R. K., and Baquer, N. Z. (2011)
J. Ageing Res., 2011, 580245-580252.
49.Uribarri, J., Cai, W., Peppa, M., Goodman, S.,
Ferrucci, L., Striker, G., and Vlassara, H. (2007) J. Gerontol. A
Biol. Sci. Med. Sci., 62, 427-433.
50.Hohn, A., Jung, T., Grimm, S., and Grune, T.
(2010) Free Radic. Biol. Med., 48, 1100-1108.
51.Shimizu, I., and Ito, S. (2007) Hepatol.
Res., 37, 239-247.
52.Rebrin, I., and Sohal, R. S. (2008) Adv. Drug
Deliv. Rev., 60, 1545-1552.
53.Toroser, D., and Sohal, R. S. (2007) Biochem.
J., 405, 583-589.
54.Sanz, N., Diez-Fernandez, C., Alvarez, A., and
Cascales, M. (1997) J. Hepatol., 27, 525-534.
55.Zubkova, E. V., and Robaire, B. (2004) Biol.
Reprod., 71, 1002-1008.
56.Tian, L., Cai, Q., and Wei, H. (1998) Free
Radic. Biol. Med., 24, 1477-1484.
57.Alabarse, P. V., Salomon, T. B., Medeiros, T. M.,
Hackenhaar, F. S., Schuller, A. K., Ehrenbrink, G., and Benfato, M. S.
(2011) Exp. Gerontol., 46, 773-780.
58.Ramesh, T., Kim, S. W., Sung, J. H., Hwang, S.
Y., Sohn, S. H., Yoo, S. K., and Kim, S. K. (2012) Exp.
Gerontol., 47, 77-84.
59.Candelario-Jalil, E., Mohammed-Al-Dalain, S.,
Fernandez, O. S., Menendez, S., Perez-Davison, G., Merino, N., Sam, S.,
and Ajamieh, H. H. (2001) J. Appl. Toxicol., 21,
297-301.
60.Bocci, V. (2007) Arch. Med. Res.,
38, 265-267.
61.Bocci, V., Borrelli, E., Corradeschi, F., and
Valacchi, G. (2000) Int. J. Med. Biol. Environ., 28,
109-113.
62.Berlett, B. S., and Stadtman, E. R. (1997) J.
Biol. Chem., 272, 20313-20316.
63.Bocci, V., Aldinucci, C., Mosci, F., Carraro, F.,
and Valacchi, G. (2007) Mediators Inflamm., 16,
26785-26790.
64.Ajamieh, H., Berlanga, J., Merino, N., Sanchez,
G. M., Carmona, A. M., Cepero, S. M., Giuliani, A., Re, L., and Leon,
O. S. (2005) Transpl. Int., 18, 604-612.
65.Iles, K. E., and Liu, R. M. (2005) Free Radic.
Biol. Med., 38, 547-556.
66.Hohn, A., Jung, T., Grimm, S., Catalgol, B.,
Weber, D., and Grune, T. (2011) Free Radic. Biol. Med.,
50, 585-591.
67.Shringarpure, R., Grune, T., Sitte, N., and
Davies, K. J. (2000) Cell Mol. Life Sci., 57,
1802-1809.
68.Ijichi, H., Taketomi, A., Yoshizumi, T.,
Uchiyama, H., Yonemura, Y., Soejima, Y., Shimada, M., and Maehara, Y.
(2006) J. Hepatol., 45, 28-34.
69.He, X., Xu, X., Fan, M., Chen, X., Sun, X., Luo,
G., Chen, L., Mu, Q., Feng, Y., Mao, Q., and Chao, Z. (2011) J.
Surg. Res., 170, 271-277.