Mitochondrial Energy-Dissipating Systems (Alternative Oxidase, Uncoupling Proteins, and External NADH Dehydrogenase) Are Involved in Development of Frost-Resistance of Winter Wheat Seedlings
O. I. Grabelnych1*, O. A. Borovik1, E. L. Tauson1, T. P. Pobezhimova1, A. I. Katyshev1, N. S. Pavlovskaya1, N. A. Koroleva1, I. V. Lyubushkina1, V. Yu. Bashmakov2, V. N. Popov2, G. B. Borovskii1, and V. K. Voinikov1
1Siberian Institute of Plant Physiology and Biochemistry, Siberian Division of the Russian Academy of Sciences, ul. Lermontova 132, 664033 Irkutsk, Russia; fax: (3952) 517-054; E-mail: grolga@sifibr.irk.ru2Voronezh State University, Universitetskaya pl. 1, 394006 Voronezh, Russia; fax: (4732) 208-755; E-mail: popov@vsu.ru
* To whom correspondence should be addressed.
Received August 9, 2013; Revision received December 9, 2013
Gene expression, protein synthesis, and activities of alternative oxidase (AOX), uncoupling proteins (UCP), adenine nucleotide translocator (ANT), and non-coupled NAD(P)H dehydrogenases (NDex, NDPex, and NDin) were studied in shoots of etiolated winter wheat (Triticum aestivum L.) seedlings after exposure to hardening low positive (2°C for 7 days) and freezing (-2°C for 2 days) temperatures. The cold hardening efficiently increased frost-resistance of the seedlings and decreased the generation of reactive oxygen species (ROS) during further cold shock. Functioning of mitochondrial energy-dissipating systems can represent a mechanism responsible for the decrease in ROS under these conditions. These systems are different in their response to the action of the hardening low positive and freezing temperatures. The functioning of the first system causes induction of AOX and UCP synthesis associated with an increase in electron transfer via AOX in the mitochondrial respiratory chain and also with an increase in the sensitivity of mitochondrial non-phosphorylating respiration to linoleic and palmitic acids. The increase in electron transfer via AOX upon exposure of seedlings to hardening freezing temperature is associated with retention of a high activity of NDex. It seems that NDex but not the NDPex and NDin can play an important role in maintaining the functional state of mitochondria in heterotrophic tissues of plants under the influence of freezing temperatures. The involvement of the mitochondrial energy-dissipating systems and their possible physiological role in the adaptation of winter crops to cold and frost are discussed.
KEY WORDS: Triticum aestivum L., cold hardening, reactive oxygen species, gene expression, energy-dissipating systems of mitochondriaDOI: 10.1134/S0006297914060030
Abbreviations: ANT, adenine nucleotide translocator; AOX, alternative oxidase; AP, alternative pathway; CP, cytochrome pathway; DTT, dithiothreitol; FFA, free fatty acids; NDex (NDB2), “external” NADH:quinone oxidoreductase; NDin (NDA2), “internal” non-coupled NADH:quinone oxidoreductase; NDPex (NDB1), “external” NADPH:quinone oxidoreductase; PCR, polymerase chain reaction; ROS, reactive oxygen species; UCP, uncoupling protein.
Oxidative reactions in the mitochondrial electron transfer chain (ETC)
result in generation of electrochemical proton gradient
(ΔmH+), which is used by ATP synthase for ADP
phosphorylation to ATP. However, oxidation is not always coupled with
phosphorylation. In mitochondria, so-called energy-dissipating systems
decrease the energetic efficiency of oxidative phosphorylation due to a
partial dissipation as heat of the energy generated by the
mitochondrial ETC both before and after its conversion into the
transmembrane gradient energy [1]. In plants such
energy-dissipating systems include: the alternative antimycin A- and
cyanide-resistant oxidase (AOX) [2-4], “external” and “internal”
non-coupled NAD(P)H-dehydrogenases (ND(P)ex and ND(P)in, respectively)
[5], free fatty acids (FFA) [6,
7] and FFA-bound uncoupling proteins (UCP) similar
to those of animals [8, 9], and
adenine nucleotide translocator (ANT) [6]. A
currently increased interest in these systems is explained by the
importance of their functions, which include thermogenesis, regulation
of production of reactive oxygen species (ROS) and active nitrogen
species, and the regulation of energetic and metabolic balance [3, 6, 10-12]. The possible role of the free oxidation systems
in the regulation of ROS production has been considered in a review [13]. The decreased ability to prevent ROS production
in mitochondria or to neutralize them causes programmed cell death in
plants [14].
Cold hardening gives winter cultures the ability to endure unfavorable freezing temperatures [15, 16], which is a specific feature of the biology of such plants. Numerous works have shown an increase in the level of transcripts, protein content, and AOX activity under the influence of low positive temperatures in different plants [17-28]. However, there are also data on the absence of positive correlation between AOX content, its activity, and cold resistance of plants [17, 18]. In wheat two genes encoding AOX have been identified, Waox1a and Waox1c, and the content of their transcripts increases during cold hardening (4°C) [19, 24]. Along with the accumulation of transcripts, the electron transfer via AOX increases, this being more pronounced in the more frost-resistant winter wheat than in the less resistant spring wheat [24]. Enhanced cold- and oxidative stress resistance of Arabidopsis thaliana cells transformed with Waox1a serves as a confirmation of the hypothesis about the antioxidant function of AOX at low temperature [21]. The antioxidant function of AOX and an increase in the contribution of the alternative pathway (AP) to respiration have been detected in mitochondria of etiolated seedlings of winter wheat during cold hardening [28]. The uncoupling protein UCP1 is encoded in wheat by the genes WhUCP1a and WhUCP1b, and, although their induction under the influence of low temperature was not observed [29], an abscisic acid (ABA)-related element (ABRE) and four G-box elements were identified in the promoter region of AtUCP1 encoding UCP1 in Arabidopsis [30]. The presence of such regulatory elements is consistent with the induction of UCP1 synthesis in Arabidopsis at low temperatures [10, 23]. The action of cold, in addition to induction of genes encoding AOX and UCP, can lead to co-expression of the gene encoding NDex (NDB2) [27, 31]. The functional state of the mitochondrial ETC complexes is known to influence the induction of cold-regulated (COR) genes and the frost-resistance of plants [32]. However, in the literature there are no data on the influence of freezing temperature that is specific for the cold hardening second stage on expression of the genes encoding components of ATP synthase and energy-dissipating systems of mitochondria and the contents and activities of proteins. There are also no data concerning gene expression and functioning of non-coupled NAD(P)H dehydrogenases in mitochondria of winter crops during cold hardening.
Thus, the purpose of this work was to comparatively analyze the influence of hardening at low positive and freezing temperatures on gene expression, synthesis of proteins, and activity of alternative oxidase, uncoupling proteins, and non-coupled NAD(P)H dehydrogenases in winter wheat.
MATERIALS AND METHODS
Plant material. The study was performed on shoots of winter wheat (Triticum aestivum L., Irkutskaya cultivar) seedlings grown during three days on humid filter paper at 25 ± 1°C (control). For the first stage of the cold hardening, the seedlings were kept for seven days in the dark at the temperature of 2°C (cold hardening stage one), and then for the second stage for two days at the temperature of –2°C (cold hardening stage two).
Determination of frost-resistance of seedlings. The frost-resistance of the control and cold-hardened seedlings of winter wheat was determined by their growth after their exposure at temperatures of –4, –6, –8, –10, –12, –14, and –16°C in a BINDER MKT 240 chamber (Germany) at the Fitotron experimental station (Siberian Institute of Plant Physiology and Biochemistry, Siberian Division of the Russian Academy of Sciences). The temperature was lowered once a day with the rate of 1°C/h. After the exposure, the seedlings were thawed for two days at 2°C and then left to grow at the control temperature. The survival of the seedlings was evaluated seven days after the freezing exposure.
Determination of carbohydrate contents. Contents of water-soluble carbohydrates were determined using anthrone reagent [33]. The carbohydrate contents were calculated with a sucrose-based calibration curve in percent of the absolute dry weight.
Isolation of RNA and preparation of cDNA. The total cellular RNA was isolated using an SV Total RNA Isolation System kit (Promega, USA). The RNA concentration was determined spectrophotometrically at 260 nm, and the preparation purity was assessed by the A260/A280 ratio (1.8-2.1).
The first chain was synthesized using a REVERTA kit (AmpliSense, Institute of Epidemiology, Russia) according to recommendations of the producer with some modifications. For reverse transcription, 2 µg total cellular RNA and 40 µM of oligo-(dT)18 primer were used. To prevent degradation of RNA templates, the reaction mixture was supplemented with an inhibitor of RNases (RNAsine; ThermoScientific, Lithuania) at the concentration of 1 U/µl of the reaction mixture.
Real time polymerase chain reaction (RT PCR). RT PCR was performed with a CFX96 device (Bio-Rad, USA) using SYBR Green I (Sigma, USA) as a dye and a kit of reagents from Fermentas (Lithuania). The total volume of the PCR reaction was 25 µl including 2 U of Taq DNA polymerase and 1 µl of cDNA. Gene-specific primers for COR14, NAD7, COB, COX2, ATP6, UCP1a, UCP1b, AOX1a, AOX1c, ANT1, NDA2, NDB2, Ta2291, and Ta2776 genes were used for amplification.
The sequences of primers to the genes NAD7, COB, COX, and ATP6 are taken from the work of Naydenov et al. [34] and to the genes COR14, Ta2291, and Ta2776 from the work of Paolacci et al. [35]. As referent genes, we used Ta2291 (ADP ribosylation factor) and Ta2776 (a protein similar to the ribonuclease L inhibitor) [35]. The forward primer for the UCP1a gene was 5′-CGAGTTGGGTCATGGAATGTG-3′, the reverse one was 5′-ATGAAACCTTGCAGCGCAGAT-3′. The forward primer for the UCP1b gene was 5′-GGCATCATCCCTGGCTTTCAC-3′, the reverse one was 5′-GCTCCAGAATAGTGCCTCTTGACA-3′. The forward primer for the AOX1a gene was 5′-CGATCTGACCAAGCACCACG-3′, the reverse one was 5′-CGGCACGGCGGCAACA-3′. The forward primer for the AOX1c gene was 5′-CGTCCTCCTCCGCCACCTG-3′, the reverse one was 5′-CCTCCTCCCTCGCCGCCT-3′. In the electronic databases there were no full-size sequences of mRNA genes; therefore, primers for wheat genes homologous in sequences to the ANT1, NDA2, and NDB2 genes of arabidopsis were chosen on the basis of transcript assembly (TA) from the database of The Institute for Genomic Research (TIGR, USA) [36]. The primers were selected using a Vector NTI v.5.1 program (InforMaxTMInvitrogenTMLife Science Software, USA). For the ANT1 (Ta59780) gene the primers were as follows: the forward one was 5′-AAGAGCCACCACCCCATCCCA-3′, the reverse one was 5′-ATGACAGACGGGTGGTTAGCT-3′. For the NDA2 (Ta68409_4565) gene the forward primer was 5′-ACAAAGGACAGGGAAATCGTGA-3′ and the reverse primer was 5′-GAAAGGATGGATTAGGATGTCTGAA-3′. For the NDB2 (Ta56397) gene the forward primer was 5′-TGTACGCAAGCAAGCAGGTG-3′ and the reverse one was 5′-AGTCCCTCCCGAATATGAACCT-3′. The amplification parameters were as follows: the preliminary denaturation: 95°C, 5 min; then 40 cycles 95°C, 40 s; 58°C, 40 s; 72°C, 40 s. The PCR amplification efficiency was determined for each pair of primers by the slope of the standard curve of five points using the fivefold dilution of the mixture of all cDNA specimens (dilutions from 1 : 1 to 1 : 625). The standard curve was plotted automatically using the Bio-Rad CFX Manager v.1.6.541.1028 program (Bio-Rad). The PCR efficiencies varied from 1.80 to 2.00.
The absence of nonspecific products of the amplification was determined by reading the melting curves and using electrophoresis in 2% (w/v) agarose gel in the presence of 0.1% ethidium bromide. The size of expected products was determined by comparing to DNA markers with the known length (ThermoScientific). All components of the reaction except for the cDNA were used as a negative control. The threshold cycle (Ct) value (the value of the threshold reaction cycle) – the intersection point of the plot of DNA accumulation and the threshold line – was determined automatically using the Bio-Rad CFX Manager v.1.6.541.1028 program. The relative expression level of the studied genes was determined using a 2–ΔΔCt-method with the same program. The data obtained for each sample were normalized to the referent genes and presented as QN – normalized level of the gene expression in the experimental specimen of the tissue relatively to the control sample (before the hardening). The gene expression level in the control sample is taken as 1.0.
Isolation of mitochondria. Mitochondria were isolated from shoots of seedlings by differential centrifugation and purified in a stepwise gradient of Percoll density (25 and 8 ml of 23% and 35% Percoll solutions (v/v)) as described earlier [28]. The isolated mitochondria were resuspended in medium containing 300 mM sucrose, 40 mM MOPS-KOH (pH 7.4), 10 mM KCl, 2 mM EDTA, and 1 mM MgCl2. The intactness of the external mitochondrial membrane measured as KCN-sensitive ascorbate-cytochrome c-dependent oxygen uptake in the absence and presence of 0.04% Triton X-100 was 91.7 ± 3.4% (n = 4), 91.6 ± 2.8% (n = 4), and 93.0 ± 1.9% (n = 4) for preparations of mitochondria from the control seedlings and from the variants “cold hardening stage one” and “cold hardening stage two”, respectively.
Polarographic analysis. The respiration intensity of the shoots and the respiration rate of the isolated mitochondria were determined with a Clark oxygen electrode using an OH-105 polarograph (Hungary) at 26°C. The medium for determination of the respiration intensity of the shoot tissues contained 100 mM Tris-HCl (pH 7.5) and 0.5 mM phenylmethylsulfonyl fluoride supplemented or not supplemented with respiration inhibitors (0.8 mM KCN and 2 mM benzhydroxamic acid (BHA) in final concentrations). The oxygen uptake remaining upon the addition of KCN and BHA was not taken into account on determination of respiration intensity. The electron transfer via AOX (the respiration inhibited by BHA in the presence of KCN) is designated in the text as the alternative pathway (AP) of respiration. The cytochrome pathway (CP) of respiration was calculated as the respiration part inhibited by KCN.
The reaction mixture for mitochondria contained 300 mM sucrose, 10 mM KCl, 18 mM KH2PO4, 1 mM MgCl2, 5 mM EDTA, and 0.3% BSA (pH 7.4). As oxidation substrates, we used malate (10 mM) in the presence of 10 mM glutamate and 1 mM NADH. Glutamate was included into the medium to prevent inhibition by oxaloacetate. Using NADH as oxidation substrate, EDTA was removed from the medium, and for activation of NDex 0.06 mM CaCl2 was added [37]. The maximal rate of substrate oxidation was measured in the presence of 100-200 µM ADP (state 3 according to Chance) or 0.5 µM carbonyl cyanide m-chlorophenylhydrazone (CCCP). Complex I of the ETC was inhibited with 3 µM rotenone, cytochrome oxidase was inhibited with 0.4 mM KCN, and AOX was inhibited with 1 mM BHA. The potential activity of AOX was assessed as the part of respiration in state 4 according to Chance that was inhibited by BHA in the presence of KCN (in the text designated as AP). The maximal activity of AOX was assessed in the presence of activators of this enzyme – 1 mM sodium pyruvate and 5 mM dithiothreitol (DTT). For determination of the UCP and ANT activities, winter wheat mitochondria in state 4 were supplemented with 10 µM linoleic (C18:2(Δ9,12)) or 10 µM palmitic (C16:0) acid; then an ANT inhibitor, carboxyatractyloside (Catr), in final concentration of 2 µM and an inhibitor of UCP-like uncoupling proteins, GDP, in final concentration of 2 mM were added. To prevent the induction of ATP hydrolysis–resynthesis cycles under the influence of FFA [38], the reaction medium contained oligomycin (2.5 µg/mg protein). The mitochondrial protein concentration was determined by the Lowry method [39] with BSA (Sigma, Germany) as a standard.
Determination of activities of non-coupled NAD(P)H dehydrogenases. Activities of non-coupled ND(P)ex and NDin in the mitochondrial suspension were measured at 26°C with an S100 spectrophotometer (Analytik Jena, Germany) at 340 nm. The millimolar absorption coefficient ε340 = 6.22 mM–1×cm–1 was used. The ND(P)ex activity was determined in the medium containing 300 mM sucrose, 10 mM MOPS-KOH (pH 7.4), 0.1 mM EGTA, 2.5 mM MgCl2, 0.5 µM CCCP, 0.1 mM NADH (or NADPH), and 1 mM CaCl2 [40]. To determine the NDin activity, the internal membrane of the mitochondria was permeabilized at room temperature upon preincubation of the mitochondrial suspension in buffer with a low osmolality (1 mM MOPS-KOH, 0.1 mM EGTA (pH 7.4)) for 6 min [40]. Upon the permeabilization, the NDin activity was determined in the medium containing 10 mM MOPS-KOH (pH 7.4), 0.1 mM EGTA, 2.5 mM MgCl2, 0.5 µM CCCP, 3 µM rotenone, and 0.1 mM NADH.
Determination of ROS content. The total content of ROS in the isolated mitochondria was determined using 2′,7′-dichlorofluorescein diacetate (H2DCF-DA) [28] with an RF-5301 PC spectrofluorimeter (Shimadzu, Japan). To determine the ROS content in intact seedlings, a sample of plant tissue was infiltrated with H2DCF-DA solution in the final concentration of 1 µM and incubated for 30 min in the dark at 26°C, and after the incubation the dichlorofluorescein (DCF) fluorescence was measured in the supernatant. The fluorescence of DCF was excited at 480 nm and the emission was recorded at 524 nm. The ROS content in the mitochondrial suspension was calculated in arbitrary units/mg protein and in the intact seedlings in arbitrary units/g wet weight.
Preparation of total mitochondrial protein and Western-blotting. The suspension of mitochondria was incubated for 30 min on ice with DTT in the final concentration of 50 mM, then the mitochondria were precipitated at 15,000g for 15 min, suspended in 62.5 mM Tris-HCl buffer (pH 6.8) containing 1 mM EDTA, 1% SDS (w/v), 20% glycerol (v/v), 5% b-mercaptoethanol (v/v), and 0.001% Bromophenol Blue, and incubated for 5 min at 97ºC. After centrifugation (10,000g, 15 min), the supernatant was subjected to SDS-PAGE in 12.5-15% polyacrylamide gel and subsequent Western-blotting. The Western-blotting was performed with anti-porin antibodies diluted 1 : 100 (kindly presented by Prof. T. Elthon, University of Nebraska, USA), α- and β-subunits of ATP synthase diluted 1 : 1000 [41] (kindly presented by Prof. T. Elthon), NDA and NDB diluted 1 : 1000 [40] (kindly presented by Prof. A. Rasmusson, Lund University, Sweden), UCP1/2 diluted 1 : 1000 (AS12 1850; Agrisera, Sweden), and AOX diluted 1 : 100 (AS10 699; Agrisera). Secondary antibodies conjugated with alkaline phosphatase were used, and the proteins were detected with 5-bromo-4-chloro-3-indolyl phosphate (Sigma) and Nitrotetrazolium Blue (Sigma). Using the Gel Doc Analysis program (Bio-Rad), the relative staining intensity of protein bands was determined and expressed relative to porin as the internal control [23], and then presented in percent with 100% being the protein/porin ratio in the control sample.
Statistical processing of the data. At least three independent experiments were performed with two-five repeats in every experiment. The data were processed statistically: mean arithmetic values and their standard deviations were determined.
RESULTS
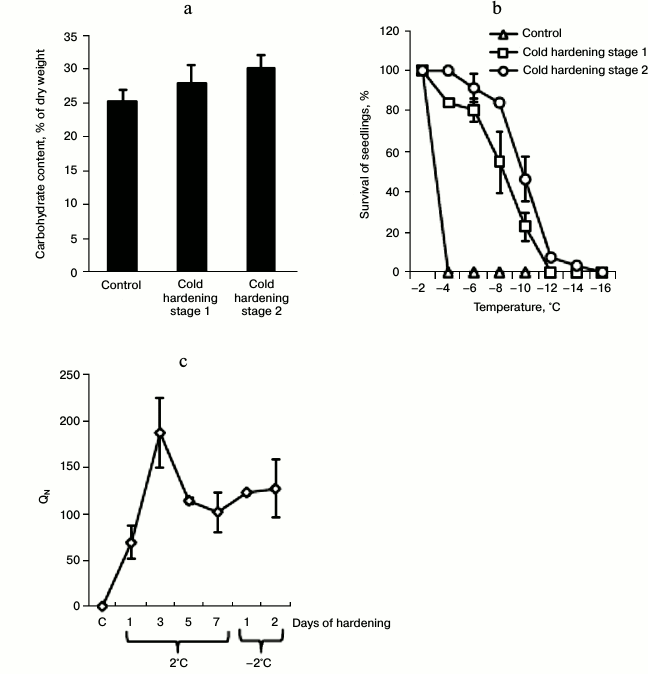
Efficiency parameters of cold hardening of etiolated winter wheat seedlings. The etiolated seedlings of winter wheat were characterized by a high level of water-soluble carbohydrates, and their level was increased significantly (by 19%) on the second stage of the hardening (Fig. 1a). The hardening temperatures (2 and —2°C) increased the frost-resistance of the winter wheat seedlings (Fig. 1b) accompanied by accumulation of transcripts of the COR14 gene encoding a cold-regulated protein of winter wheat (Fig. 1c). The accumulation of the COR14 transcripts was significant even on the first day of the hardening and reached the maximum on the third day (Fig. 1c). The increase in frost resistance was accompanied by inhibition of growth of the seedlings by 93.96 ± 2.48% (n = 5) (the lengths of the shoots grown after the hardening were compared with those of the control shoots).
Fig. 1. Content of water-soluble carbohydrates (a), frost-resistance (b), and the expression level of COR14 (c) in shoots of control and hardened winter wheat seedlings. The designations and conditions of QN calculations are described in “Materials and Methods”. For (b) no less than 150 seedlings (n = 3) were analyzed.
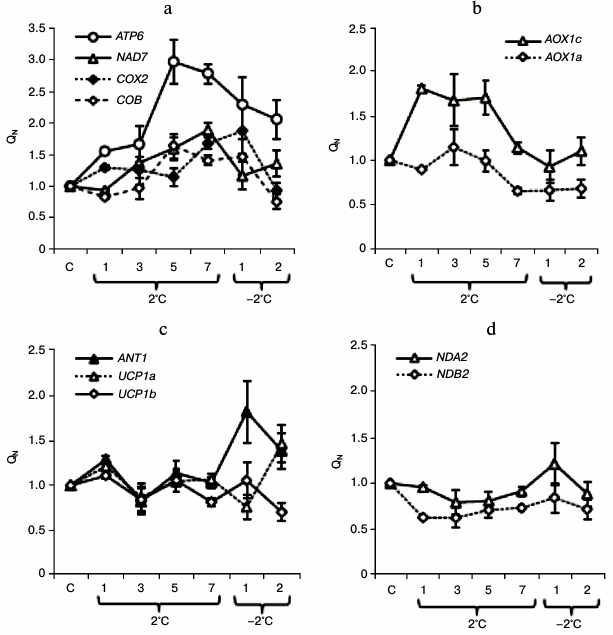
Influence of cold hardening on relative level of expression of genes encoding mitochondrial proteins in shoots of winter wheat seedlings. We studied the expression of genes encoding some components of the cytochrome pathway (CP) (subunit 7 of complex I – NAD7, apocytochrome b of complex III – COB, subunit II of complex IV — COX2), ATP-synthase (subunit a of factor Fo – ATP6), alternative oxidase (AOX1a and AOX1c), the “external” Ca2+-dependent NADH dehydrogenase (NDB2) and the “internal” Ca2+-independent NADH dehydrogenase (NDA2), ANT (ANT1), and UCP1 (UCP1a and UCP1b).
Assessment of changes in the expression of the studied genes during the cold hardening of the winter wheat seedlings revealed an increase in the expression of genes encoding components of CP and also of AP and other energy-dissipating systems. The expression of ATP6 was increased by 55% already on the next day after the cooling, and the highest expression level was observed on the fifth and seventh days of the exposure (expression level increased 3-fold) and the high expression was retained at the freezing temperatures (Fig. 2a). The NAD7 transcripts were accumulated on the third, fifth, and seventh days of cooling (by 37, 60, and 89%, respectively), and a slight increase in the expression level was maintained under the subsequent exposure to freezing temperatures (at the level of 35% increase as on the second day of the exposure) (Fig. 2a). The accumulation of the COB gene transcripts was expressed on the fifth and seventh days of the exposure to the low positive temperature and on the first day of the subsequent exposure to freezing temperature (transcript accumulation was increased by 64, 41, and 46%, respectively) (Fig. 2a). The increase in the accumulation of the COX2 gene transcripts was especially noticeable on the seventh day of the exposure to the low positive temperature (by 68%) and on the first day of the subsequent exposure to the freezing temperature (by 88%) (Fig. 2a). A slight increase in COX2 expression was also observed during the initial period of cooling – on the first and third days of the exposure to the low positive temperature (Fig. 2a).
Fig. 2. Changes in gene expression in shoots of etiolated winter wheat seedlings during cold hardening. Conditions of QN calculation are described in “Materials and Methods”. C, control (before hardening). The abscissa axis indicates days of the hardening.
The low positive temperature activated the expression of AOX1c and only slightly activated the expression of AOX1a (Fig. 2b). The expression of AOX1c was most significantly increased on the first, third, and fifth days of the exposure to the temperature of 2°C (by 81, 67, and 70%, respectively) (Fig. 2b). A significant increase (by 21%) in the expression of genes encoding UCP1 was observed on the first day of the low positive temperature and on the second day of the freezing temperature for UCP1a (by 46%), and only on the first day of the cooling for UCP1b (by 11%) (Fig. 2c). Beginning from the seventh day of the hardening, the expression of genes encoding UCP1 in winter wheat was differently directed (Fig. 2c). The ANT1 transcripts were accumulated on the first day of the temperature of 2°C (by 29%) and on the first and second days of the subsequent exposure to the temperature of –2°C (by 82 and 39%, respectively) (Fig. 2c). In the cases of NDA2 and NDB2 a slight decrease was observed in the expression of these genes under the influence of the low positive temperature and a tendency for the recovery of the expression level up to the control value under the influence of the freezing temperature (Fig. 2d).
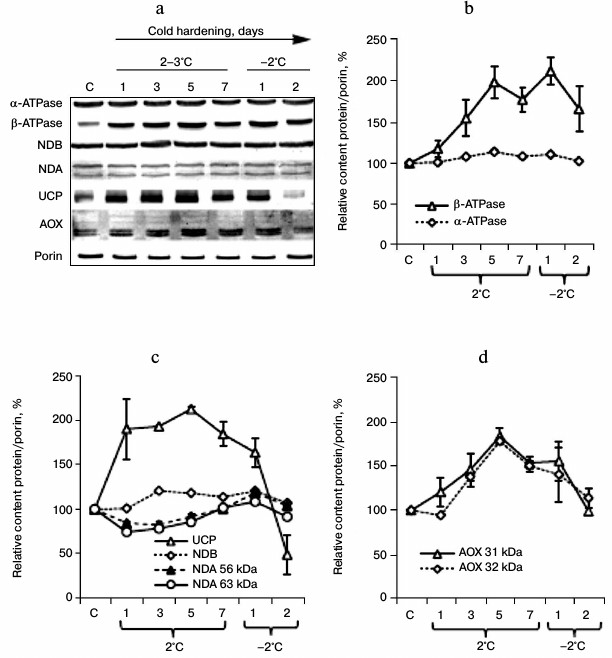
Contents of some mitochondrial proteins in shoots of winter wheat seedlings during cold hardening. During the cold hardening of the winter wheat seedlings, we observed significant changes in the contents of β-subunit of ATP synthase, AOX, and UCP. Figure 3 (a and b) shows that after three days of hardening the content of β-subunit of ATP synthase was increased. A similar tendency for increase in the protein content was observed for AOX represented in the winter wheat mitochondria by 31- and 32-kDa proteins (Fig. 3, a and c). The increase in the contents of these proteins was especially pronounced on the fifth day of the exposure to the low positive temperature (Fig. 3c). On the further influence of the hardening temperatures, the contents of these polypeptides decreased, especially on the second day of the exposure to the freezing temperature. However, the contents of 33- and 35-kDa proteins detectable with anti-AOX antibodies in immunoblots of mitochondrial proteins during the cold hardening (Fig. 3a) were not significant.
Fig. 3. Changes in the contents of some mitochondrial proteins during cold hardening of winter wheat seedlings. C, control (before hardening); a) immunoblot; b-d) calculations from immunoblotting data (n = 3). The calculations are described in “Materials and Methods”; b-d) hardening days are indicated along the abscissa axis.
Using anti-NDB antibodies, we revealed in the winter wheat mitochondria a 64-kDa protein whose content was slightly increased on the third day of the exposure to the low positive temperature, and this elevated level was retained during the subsequent hardening temperature exposure and decreased on the second day of exposure to the freezing temperature (Fig. 3d). With anti-NDA antibodies we also revealed in the winter wheat mitochondria 63- and 56-kDa proteins whose contents decreased similarly on the first, third, and fifth days of the exposure to the low positive temperature and then recovered to the control level during the exposure to the freezing temperature (Fig. 3d). Using antibodies against a peptide conserved in UCP1 and UCP2 of A. thaliana, we found a significant increase in the UCP level even upon one day of the low positive temperature exposure, the retention of this elevated level until the second stage of the hardening, and a sharp decrease in the UCP content by the end of the second stage of hardening (Fig. 3d).
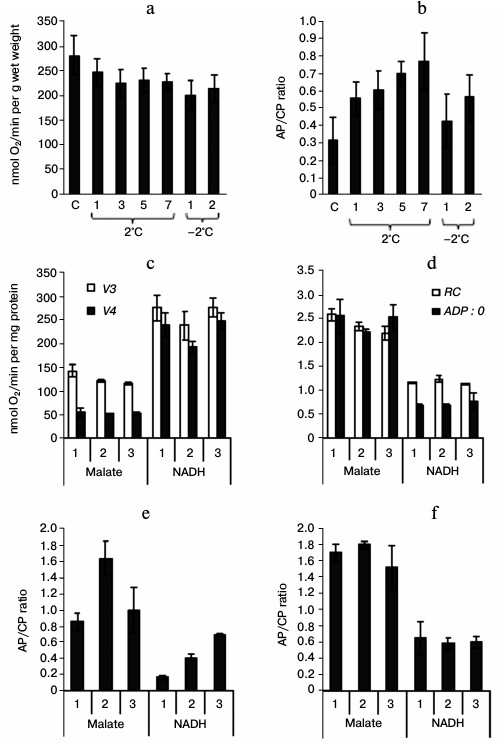
Changes in respiration intensity of shoots and in oxidation rate of different substrates by isolated mitochondria of winter wheat during cold hardening and in contributions of CP and AP to respiration. The hardening resulted in a slight decrease in the respiration intensity in tissues of shoots and in changes in the contributions of AP and CP to respiration (Fig. 4a). Prolongation of the cooling was associated with an increase in the contribution of AP to the respiration (Fig. 4b). After the freezing temperatures, the contribution of AP to respiration decreased (Fig. 4b).
Fig. 4. Respiration intensity of shoots (a) and mitochondria isolated from them (c), coupling parameters of the isolated mitochondria (d), and the ratio of the respiration alternative pathway (AP) activity to cytochrome pathway (CP) in the shoots (b) and mitochondria isolated from them (e, f) during cold hardening of winter wheat seedlings. a, b: C, control (hardening days are indicated on the abscissa axis); c-f: 1) control; 2) cold hardening stage 1; 3) cold hardening stage 2; V3, respiration rate of mitochondria during phosphorylation of ADP (according to Chance); V4, respiration rate of mitochondria after phosphorylation of ADP (according to Chance); RC, respiration control coefficient according to Chance–Williams, ADP : O, the ratio of ADP moles phosphorylated to the number of oxygen atoms taken up; Malate, 10 mM malate in the presence of 10 mM glutamate; NADH, 1 mM NADH; in (e) AP/CP ratio in the absence of sodium pyruvate and DTT; in (f) AP/CP ratio in the presence of 1 mM sodium pyruvate and 5 mM DTT.
Mitochondria isolated from the shoots oxidized exogenous NADH most intensively and malate with a lower rate (Fig. 4c). After the exposure to hardening temperatures, the respiration rate in state 4 and the ADP : O ratio in malate-oxidizing mitochondria remained stable, whereas the respiration rate in state 3 and the respiration control (RC) coefficient were slightly decreased (Fig. 4, c and d). The oxidation of NADH by the mitochondria was associated with a slight decrease in the respiration rate in state 4 after the low positive temperature exposure, accompanied by an increase in RC, whereas the respiration rate in state 3 and the ADP : O ratio were unchanged (Fig. 4, c and d). The electron transfer via AOX depended inversely on the substrate oxidation rate: it was the highest on the oxidation of malate and the lowest on the oxidation of NADH (Fig. 4e). The activity of AOX in malate-oxidizing mitochondria increased after the exposure of the seedlings to low positive temperature and decreased after the subsequent exposure to the freezing temperature, which was evidenced by the corresponding changes in the AP/CP ratio (Fig. 4e). The electron transfer via AOX was increased in the NADH-oxidizing mitochondria along with the hardening of the seedlings (Fig. 4e). However, we did not reveal differences in the maximal activity of AOX measured in the presence of its activators (pyruvate and DTT) in the mitochondria from the control seedlings and from the seedlings after the first and second phases of cold hardening on oxidation of both malate and NADH (Fig. 4f).
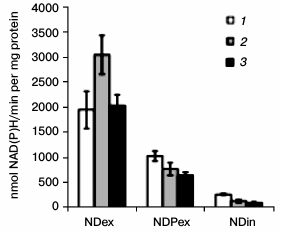
Changes in activities of “external” and “internal” non-coupled NAD(P)H-dehydrogenases in winter wheat mitochondria during cold hardening. Spectrophotometric analysis revealed differences in the ND(P)ex and NDin activities after exposure to low hardening temperatures. The NDex activity in seedlings increased after exposure to low positive temperature and was maintained on the level of the control in mitochondria after exposure to freezing hardening temperature (Fig. 5). The NDPex activity was lower than the activity of NDex and decreased after exposure to the low hardening temperatures similarly to the activity of NDin (Fig. 5).
Fig. 5. Changes in activities of non-coupled “external” NAD(P)H dehydrogenases (NDex and NDPex) and of “internal” NADH dehydrogenase (NDin) in winter wheat mitochondria after cold hardening of seedlings: 1) control; 2) cold hardening stage 1; 3) cold hardening stage 2. The conditions of determination of the NDex, NDPex, and NDin activities are described in “Materials and Methods”.
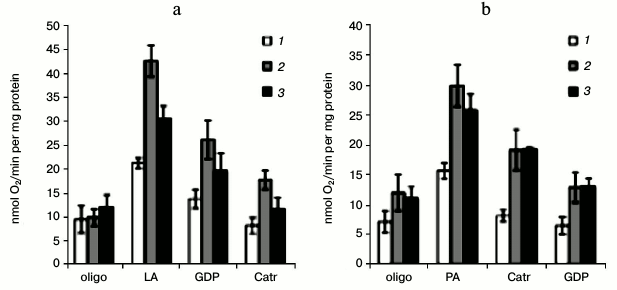
Changes in UCP and ANT activities in winter wheat mitochondria during cold hardening. We showed earlier that the uncoupling effect of polyunsaturated linoleic acid and of saturated palmitic acid in winter wheat mitochondria oxidizing malate is associated with the activities of UCP and ANT [6]. According to the inhibitory analysis data, the increase in non-phosphorylating respiration rate induced by linoleic acid is mainly due to UCP (Fig. 6a), whereas the increase induced by palmitic acid is mainly due to ANT (Fig. 6b). After the exposure of seedlings to low positive temperature, the non-phosphorylating respiration rate in the mitochondria isolated from them became more sensitive to both linoleic and palmitic acids (Fig. 6a). However, the linoleate-induced respiration in the mitochondria isolated from the seedlings was decreased after exposure to the freezing hardening temperature, whereas the palmitate-induced respiration remained elevated (Fig. 6).
Fig. 6. Non-phosphorylating respiration rate induced by linoleic (a) and palmitic (b) acids and contribution of UCP and ANT in winter wheat mitochondria during cold hardening of seedlings. The oxidation substrate was 10 mM malate + 10 mM glutamate; 1-3) as in Fig. 5; oligo, respiration rate in the presence of oligomycin; LA, respiration rate in the presence of linoleic acid; PA, respiration rate in the presence of palmitic acid; Catr, FFA-induced respiration rate upon addition of carboxyatractyloside; GDP, FFA-induced respiration rate upon addition of GDP. The succession of the addition of the substances is indicated from left to right.
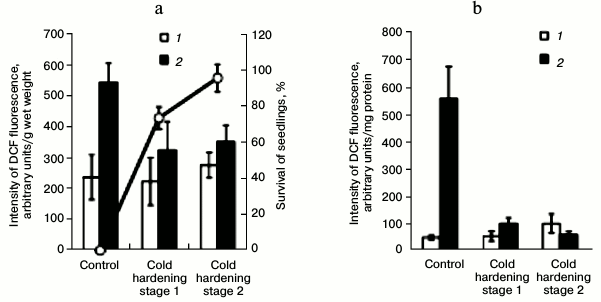
Effect of hardening temperatures on ROS content in winter wheat shoots and in mitochondria isolated from them after cold shock. Cold shock (–8°C for 6 h) led to development of oxidative stress and to death of the unhardened winter wheat seedlings, whereas preliminary hardening of the seedlings prevented the development of oxidative stress and significantly increased survival of the seedlings (Fig. 7a). The cold shock also caused a pronounced generation of ROS in mitochondria of the unhardened seedlings but had virtually no influence on ROS production in mitochondria from the cold-hardened seedlings (Fig. 7b).
Fig. 7. Effect of cold shock on ROS generation in control and hardened shoots of etiolated winter wheat seedlings, their survival (a) and ROS generation in mitochondria isolated from the shoots (b): 1) without cold shock; 2) after cold shock at –8°C for 6 h. In Fig. 7a the ROS content in tissues of the shoots is shown by columns and the survival of the seedlings is presented by the line.
DISCUSSION
The ability of plants to withstand exposure to temperatures lower than 0°C without disorders in their ontogenetic development (frost-resistance) is developing during cold hardening (cold acclimation) and is due to different changes in the transcriptome, metabolome, and proteome [42-45]. The hardening of winter cereals is a biphasic process, and the development of frost-resistance during its first stage is promoted by low positive temperatures and non-damaging freezing temperatures during the second stage [15]. The increase in frost-resistance (Figs. 1b and 7a), expression of the gene of a COR-protein in the hardened seedlings (Fig. 1c), and the decreased level of ROS in tissues of shoots of the hardened seedlings and in mitochondria isolated from them on subsequent cold shock (Fig. 7) suggest efficiency of the cold hardening and the ability of hardening temperatures to prevent the development of oxidative stress under conditions of cold shock. The activation of antioxidant systems at freezing temperatures was shown to be an effective mechanism for preventing disorders in pro/antioxidant balance and of increasing survival of winter wheat [45]. The feature of COR14 expression in shoots of etiolated winter wheat seedlings is in accordance with data of other authors [46], and it is known from the literature that the induction of COR genes (in particular, of RD29A, KIN1, COR15a, and COR47) and the ability of plants for cold acclimation depend on the functional state of the mitochondrial ETC [32]. In wheat, the mitochondria can be the major site of damage under conditions of oxidative stress [47]. Moreover, plant mitochondria act as the main source of ROS in non-photosynthesizing cells [12]. AOX, UCP and, possibly, non-coupled NAD(P)H dehydrogenases control the levels of O2·— and NO in mitochondria of plants [10, 12, 13]. Therefore, an increase in the contents and activities of these proteins seems to be a possible pathway for decreasing the development of oxidative stress in plants under conditions of cold shock. Functioning of AOX in plants after exposure to low temperatures has been studied in numerous works [17-28], but all of them were aimed to elucidate the influence of low positive temperatures. The same also concerns UCP [48-50], ANT [51-53], and non-coupled NAD(P)H dehydrogenases [54]. Taking into account the complete absence of data on functioning of the system of uncoupled respiration under conditions of freezing temperatures, we have analyzed gene expression, protein synthesis, and activities of AOX, UCP, and non-coupled NAD(P)H dehydrogenases in winter wheat seedlings after their exposure to low positive temperature (2°C for 7 days) and to the freezing temperature (–2°C for 2 days).
We found that depending on the hardening duration, in winter wheat seedling shoots the expression of genes cor14, nad7, cob, cox2, atp6, aox1a, aox1c, ant1, ucp1a, and ucp1b increased (Fig. 2), the contents of AOX, UCP, and the β-subunit of ATPase increased (Fig. 3), the electron transfer via AP in both intact shoots and in mitochondria isolated from them increased (Fig. 4, b and e), the high rate of oxidation of exogenous NADH was retained (Fig. 4c), and the rate of non-phosphorylating oxidation by free fatty acids was stimulated (Fig. 6). The hardening at freezing temperature was characterized by its inhibitory action on synthesis of UCP1/2 (Fig. 3c) and by retention of the high rate of oxidation of exogenous NADH (Fig. 4c) with involvement of AOX (Fig. 4e).
Using malate and exogenous NADH, we have shown different contributions of AP to the respiration of the winter wheat mitochondria (Fig. 4, e and f). Malate seems to be the major final product of glycolysis in plants and is oxidized under the influence of NAD+-dependent malic enzyme and malate dehydrogenase [4]. If conversions of malate occur with involvement of malate dehydrogenase, the final products of the reaction are oxaloacetate and NADH, whereas with the involvement of malic enzyme the final products are pyruvate, NADH, and CO2. The production of pyruvate by mitochondria of the etiolated wheat seedlings on oxidation of malate [55] indicates the leading role of malic enzyme in malate metabolism in etiolated wheat tissues. And on malate oxidation the maximal activity of AOX is reached (Fig. 4, e and f), which seems to be associated with functioning of malic enzyme under these conditions, because the activation by organic α-keto acids (in particular by pyruvate) is known to be a mechanism of the posttranslational regulation of AOX activity [2-4]. Therefore, on malate oxidation the contribution of AP to respiration is high (Fig. 4, e and f). During the first stage of the cold hardening on the background of a general decrease in the respiration rates on malate oxidation by mitochondria, the contribution of AP to respiration increased, and during this period concurrently with the induction of AOX1c gene expression during the hardening an increase was observed in expression of the ATP6 and NAD7 genes, whose products play a leading role in the functioning of ATP synthase [56] and complex I [57], respectively. A high degree of oxidation and phosphorylation coupling was characteristic for mitochondria from shoots of hardened winter wheat seedlings isolated in the presence of BSA during all stages of the mitochondria preparation, the RC coefficient being 3.55 ± 0.16 (n = 4), 4.17 ± 0.34 (n = 4), and 3.85 ± 0.42 (n = 3), and the ADP : O ratio being 2.91 ± 0.16 (n = 4), 2.70 ± 0.16 (n = 4), and 3.07 ± 0.08 (n = 3) in the mitochondria from the control seedlings and seedlings hardened by exposure to low positive and freezing temperatures, respectively. In the absence of BSA in the resuspending medium, the RC coefficient in the mitochondria from the hardened seedlings was slightly decreased (Fig. 4d). We think that the energetic activity of mitochondria during the hardening of the etiolated winter wheat seedlings can be retained concurrently with the induction of ATP6 and AOX1c expression and of expression of the NAD7 gene encoding subunit 7 of ETC complex I. The involvement of AOX1c or other genes of the AOX family in low-temperature adaptation has been shown in arabidopsis and tomato [22, 27]. The functioning of AOX during malate oxidation by mitochondria of the etiolated wheat seedlings can maintain Δψ on the internal mitochondrial membrane due to the coupling point in ETC complex I [58, 59].
Expression of the NDA2 and NDB2 genes encoding non-coupled “internal” NADH dehydrogenase and “external” NADH dehydrogenase was higher in heterotrophic tissues [60]. In bursting pea seeds, the ability of mitochondria to oxidize exogenous NADH was retained under the influence of freezing temperatures [61]. We have shown also a high rate of Ca2+-dependent oxidation of exogenous NADH after exposure to freezing temperature associated with an increase in electron transfer via AOX (Fig. 4b). The oxidation of exogenous NADPH associated with the functioning of NDPex in the mitochondria decreased under the influence of low hardening temperatures (Fig. 5), and this indicated that on the outer side of the internal mitochondrial membrane of winter wheat at least two “external” NAD(P)H dehydrogenases are located, one which is specific for NADH and the other for NADPH. Similarly to oxidation of exogenous NADPH, the activity of NDin decreased in the winter wheat mitochondria during the hardening (Fig. 5), which was associated with a decrease in NDA2 expression (Fig. 2d) and the NDA protein content (Fig. 3c), which is in agreement with data of other authors [31, 54].
The UCP-like proteins and ANT catalyze proton conductance across mitochondrial membranes and dissipate the proton gradient [1]. A slight increase in proton conductance of the mitochondrial internal membrane is a mechanism of a “soft” uncoupling responsible in mammals for the important physiological function of heat production for maintaining the necessary body temperature under conditions of low environmental temperature and for decrease in O2·— generation by the respiratory chain [1, 13]. Gene expression and UCP activation occur in plants under the influence of low positive temperature [48-50]. We observed a significant increase in UCP1/2 protein in mitochondria from winter wheat seedlings shoots exposed to low positive temperature (Fig. 3c), as well as the stimulation of GDP-sensitive linoleate-induced respiration (Fig. 6a). However, after the second hardening stage the UCP1/2 content in the seedlings decreased, which was associated with stabilization of the oxidative phosphorylation coupling mechanism in the mitochondria [62]. The gene expression and activities of ANT and UCP significantly increased in plants after cooling [51-53]. The ANT1 gene expression significantly increased in winter wheat shoots on the first day of exposure to low positive and freezing temperatures, and the tendency for increase in ANT1 expression was similar to changes in the contents of UCP1b transcripts (Fig. 2c). The Catr-sensitive palmitate-dependent increase in the rate of non-phosphorylating respiration was higher in winter wheat mitochondria isolated from the hardened seedlings (Fig. 6b). These findings indicate the importance of UCP and ANT in cold hardening of winter wheat seedlings.
Thus, the comparative analysis of gene expression, protein synthesis, and activities of alternative oxidase, uncoupling proteins, and non-coupled NAD(P)H dehydrogenases performed in this work has revealed that the mitochondrial energy-dissipating systems are involved in the increase in frost-resistance of winter cereals, possibly by providing stable functioning of the mitochondrial ETC. These systems function differently during the first and second stages of cold hardening. The effect of low positive temperature (first stage of hardening) leads to induction of AOX and UCP syntheses associated with an increase in electron transfer via AOX in the mitochondrial respiratory chain and an increase in the sensitivity of the non-phosphorylating respiration of mitochondria to linoleic and palmitic acids. The increase in the electron transfer via AOX after the exposure of seedlings to freezing hardening temperature is associated with the retention of the high activity of NDex, which seems to play an important role in maintaining the functional state of mitochondria in heterotrophic tissues of plants after exposure to freezing temperatures.
The authors are grateful to D. N. Fedorin and T. E. Putilina for their help in the work. The RT PCR was performed using the equipment of the Baikal Analytic Center (Research Center, Siberian Division, Russian Academy of Sciences).
This work was supported by the Russian Federation Ministry of Education and Science (the agreement 8266 and VSU State assignment 1035).
REFERENCES
1.Skulachev, V. P., Bogachev, A. V., and Kasparinsky,
F. O. (2010) Membrane Bioenergetics [in Russian], Moscow State
University Publishers, Moscow.
2.Millenaar, F. F., and Lambers, H. (2003) Plant
Biol., 5, 2-15.
3.Vanlerberghe, G. C. (2013) Int. J. Mol.
Sci., 14, 6805-6847.
4.Lambers, H., Robinson, A., and Ribas-Carbo, M.
(2005) in Plant Respiration: From Cell to Ecosystem (Lambers,
H., and Ribas-Carbo, M., eds.) Springer, Hamburg, pp. 1-15.
5.Rasmusson, A. G., Geisler, D. A., and Moller, I. M.
(2008) Mitochondrion, 8, 47-60.
6.Grabelnych, O. I., Pivovarova, N. Y., Pobezhimova,
T. P., Kolesnichenko, A. V., and Voinikov, V. K. (2009) Russ. J.
Plant Physiol., 56, 332-342.
7.Voinikov, V. K. (2011) Plant Mitochondria on the
Temperature Stress [in Russian], Akademicheskoe Izdatelstvo Geo,
Novosibirsk.
8.Hourton-Cabassa, C., Matos, A. R., Zachowski, A.,
and Moreau, F. (2004) Plant Physiol. Biochem., 42,
283-290.
9.Vercesi, A. E., Borecky, J., Maia, I. G., Arruda,
P., Cuccovia, I. M., and Chaimovich, H. (2006) Annu. Rev. Plant
Biol., 57, 383-404.
10.Moller, M., and Kristensen, B. K. (2004)
Photochem. Photobiol. Sci., 3, 730-735.
11.Grabelnych, O. I., Kolesnichenko, A. V.,
Pobezhimova, T. P., Zykova, V. V., and Voinikov, V. K. (2006) Russ.
J. Plant Physiol., 53, 418-429.
12.Blokhina, O., and Fagerstedt, K. V. (2010)
Physiol. Plant., 138, 447-462.
13.Popov, V. N. (2003) Biochem. Soc. Trans.,
31, 1316-1317.
14.Vanyushin, B. F. (2001) Usp. Biol. Khim.,
41, 3-38.
15.Tumanov, I. I. (1979) Physiology of Hardening
and Cold Resistance of Plants [in Russian], Nauka, Moscow.
16.Trunova, T. I. (2007) Plant and
Low-Temperature Stress [in Russian], Nauka, Moscow.
17.Stewart, C. R., Martin, B. A., Reding, L., and
Cerwick, S. (1990) Plant Physiol., 92, 761-766.
18.Ribas-Carbo, M., Aroca, R., Conzalez-Meler, M.
A., Irigoyen, J. J., and Sanchezdiaz, M. (2000) Plant Physiol.,
122, 199-204.
19.Takumi, S., Tomioka, M., Eto, K., Naydenov, N.,
and Nakamura, C. (2002) Gen. Cenet. Syst., 77, 81-88.
20.Kurimoto, K., Millar, A. H., Lambers, H., Day, D.
A., and Noguchi, K. (2004) Plant Cell Physiol., 45,
1015-1022.
21.Sugie, A., Naydenov, N., Mizuno, N., Nakamura,
C., and Takumi, S. (2006) Gen. Genet. Syst., 81,
349-354.
22.Matos, A. R., Hourton-Cabassa, C., Cicek, D.,
Reze, N., Arrabaca, J. D., Zachowski, A., and Moreau, F. (2007)
Plant Cell Physiol., 48, 856-865.
23.Armstrong, A. F., Murray, R. B., Day, D. A.,
Barthet, M. M., Smith, P. M. C., Millar, A. H., Whelan, J., and Atkin,
O. K. (2008) Plant Cell Environ., 31, 1156-1169.
24.Mizuno, N., Sugie, A., Kobayashi, F., and Takumi,
S. (2008) Plant Physiol., 165, 462-467.
25.Wang, J., Rajakulendran, N., Amirsadeghi, S., and
Vanlerberghe, C. (2011) Physiol. Plant., 142,
339-351.
26.Li, C.-R., Liang, D.-D., Li, J., Duan, Y.-B., Li,
H., Yang, Y.-C., Qin, R.-Y., Li, L., Wei, P.-C., and Yang, J.-B. (2013)
Plant Cell Environ., 36, 775-788.
27.Shi, K., Fu, L.-J., Zhang, S., Li, X., Liao,
Y.-W.-K., Xia, X.-J., Zhou, Y.-H., Wang, R.-Q., Chen, Z.-X., and Yu,
J.-Q. (2013) Planta, 237, 589-601.
28.Grabelnych, O. I., Pobezhimova, T. P.,
Pavlovskaya, N. S., Koroleva, N. A., Borovik, O. A., Lyubushkina, I.
V., and Voinikov, V. K. (2011) Biochemistry (Moscow), Suppl. Ser. A:
Membrane Cell Biol., 5, 249-257.
29.Murayama, S., and Handa, H. (2000) Mol. Gen.
Genet., 264, 112-118.
30.Nogueira, F. T. S., Sassaki, F. T., and Maia, I.
G. (2011) J. Bioenerg. Biomembr., 43, 71-79.
31.Elhavez, D., Mureha, M. W., Clifton, R., Soole,
K. L., Day, D. A., and Whelan, J. (2006) Plant Cell Physiol.,
47, 43-54.
32.Lee, B., Lee, H., Xiong, L., and Zhu, J.-K.
(2002) Plant Cell, 14, 1235-1251.
33.Kochetkov, N. K. (ed.) (1967) Methods in
Carbohydrate Chemistry [Russian translation], Mir, Moscow, pp.
21-24.
34.Naydenov, N. G., Khanam, S. M., Atanassov, A.,
and Nakamura, G. (2008) Gen. Genet. Syst., 83, 31-41.
35.Paolacci, A. R., Tanzarella, O. A., Porceddu, E.,
and Ciaffi, M. (2009) BMC Mol. Biol., 10, 11.
36.Childs, K. L., Hamilton, J. P., Zhu, W., Ly, E.,
Cheung, F., Wu, H., Rabinowicz, P. D., Town, C. D., Buell, C. R., and
Chan, A. P. (2007) Nucleic Acids Res., 35 (Database
issue), D846-851.
37.Douce, R. (1985) Mitochondria in Higher
Plants: Structure, Function and Biogenesis, Academic Press,
London.
38.Wojtczak, L., and Schonfeld, P. (1993)
Biochim. Biophys. Acta, 1183, 41-57.
39.Lowry, O. H., Rosebrough, N. J., Farr, A. L., and
Randall, R. J. (1957) J. Biol. Chem., 193, 265-275.
40.Svensson, A. S., and Rasmusson, A. G. (2001)
Plant J., 28, 73-82.
41.Luethy, M. H., Horak, A., and Elthon, T. E.
(1993) Plant Physiol., 101, 931-937.
42.Gulick, P. J., Drouin, S., Yu, Z., Danyluk, J.,
Poisson, G., Monroy, A. F., and Sarhan, F. (2005) Genome,
48, 913-923.
43.Herman, E. M., Rotter, K., Premakumar, R.,
Elwinger, G., Bae, R., Ehler-King, L., Chen, S., and Livingston, D. P.
(2006) J. Exp. Botany, 57, 3601-3618.
44.Vítamvas, P., Prasil, I. T., Kosova, K.,
Planchon, S., and Renaut, J. (2012) Proteomics, 12,
68-85.
45.Xu, J., Li, Y., Sun, J., Du, L., Zhang, Y., Yu,
Q., and Liu, X. (2013) Plant Biol., 15, 292-303.
46.Tsvetanov, S., Ohno, R., Tsuda, K., Takumi, S.,
Mori, N., Atanassov, A., and Nakamura, C. (2000) Gen. Genet.
Syst., 75, 49-57.
47.Bartoli, C. G., Gomez, F., Martinez, D. E., and
Guiamet, J. J. (2004) J. Exp. Botany, 55, 1663-1669.
48.Nantes, I. L., Fagian, M. M., Catisti, R.,
Arruda, P., Maia, I. G., and Vercesi, A. E. (1999) FEBS Lett.,
457, 103-106.
49.Calegario, F. F., Cosso, R. G., Fagian, M. M.,
Almeida, F. V., Jardim, W. F., Jezek, P., Arruda, P., and Vercesi, A.
E. (2003) J. Bioenerg. Biomembr., 35, 211-220.
50.Valente, C., Pasqualim, P., Jacomasso, T.,
Maurer, J. B. B., de Souza, E. M., Martinez, G. R., Rocha, M. E. M.,
Carnieri, E. G. S., and Cadena, S. M. S. C. (2012) Plant Sci.,
197, 84-91.
51.Hashimoto, H., Nishi, R., Umeda, M., Ichimiya,
H., and Kato, A. (1993) Plant Mol. Biol., 22,
163-164.
52.De Santis, A., Landi, P., and Genchi, G. (1999)
Plant Physiol., 119, 743-754.
53.Popov, V. N., Markova, O. V., Mokhova, E. N., and
Skulachev, V. P. (2002) Biochim. Biophys. Acta, 1553,
232-237.
54.Svensson, A. S., Johansson, F. I., Moller, I. M.,
and Rasmusson, A. G. (2002) FEBS Lett., 517, 79-82.
55.Brunton, C. J., and Palmer, J. M. (1973) Eur.
J. Biochem., 39, 283-291.
56.Moghadam, A. A., Taghavi, S. M., Niazi, A.,
Djavaheri, M., and Ebrahimie, E. (2012) Genet. Mol. Res.,
11, 3547-3567.
57.Pineau, B., Mathieu, C., Gerard-Hirne, C., De
Paepe, R., and Chetrit, P. (2005) J. Biol. Chem., 280,
25994-26001.
58.Abdrakhimova, I. P., Andreev, I. M., and Shugaev,
A. G. (2011) Russ. J. Plant Physiol., 58, 567-574.
59.Pastore, D., Trono, D., Laus, M. N., Di Fonzo,
N., and Passarella, S. (2001) Plant Cell Physiol., 42,
1373-1382.
60.Michalecka, A. M., Svensson, A. S., Johansson, F.
I., Agius, S. C., Johanson, U., Brennicke, A., Binder, S., and
Rasmusson, A. G. (2003) Plant Physiol., 133, 642-652.
61.Stupnikova, I., Benamar, A., Tolleter, D.,
Grelet, J., Borovskii, G., Dorne, A. J., and Macherel, D. (2006)
Plant Physiol., 140, 326-335.
62.Nariichuk, F. D., and Babenko, V. I. (1981)
Fiziol. Biokhim. Kul’t. Rast., 13, 582-586.