REVIEW: Interaction of Early Secretory Pathway and Golgi Membranes with Microtubules and Microtubule Motors
A. I. Fokin1, I. B. Brodsky1, A. V. Burakov1, and E. S. Nadezhdina1,2*
1Lomonosov Moscow State University, Belozersky Institute of Physico-Chemical Biology, 119991 Moscow, Russia; fax: (495) 939-31812Institute of Protein Research, Russian Academy of Sciences, 119334 Moscow, Russia; fax: (499) 135-2147; E-mail: elena.nadezhdina@gmail.com
* To whom correspondence should be addressed.
Received June 9, 2014
This review summarizes the data describing the role of cellular microtubules in transportation of membrane vesicles – transport containers for secreted proteins or lipids. Most events of early vesicular transport in animal cells (from the endoplasmic reticulum to the Golgi apparatus and in the opposite recycling direction) are mediated by microtubules and microtubule motor proteins. Data on the role of dynein and kinesin in early vesicle transport remain controversial, probably because of the differentiated role of these proteins in the movements of vesicles or membrane tubules with various cargos and at different stages of secretion and retrograde transport. Microtubules and dynein motor protein are essential for maintaining a compact structure of the Golgi apparatus; moreover, there is a set of proteins that are essential for Golgi compactness. Dispersion of ribbon-like Golgi often occurs under physiological conditions in interphase cells. Golgi is localized in the leading part of crawling cultured fibroblasts, which also depends on microtubules and dynein. The Golgi apparatus creates its own system of microtubules by attracting γ-tubulin and some microtubule-associated proteins to membranes. Molecular mechanisms of binding microtubule-associated and motor proteins to membranes are very diverse, suggesting the possibility of regulation of Golgi interaction with microtubules during cell differentiation. To illustrate some statements, we present our own data showing that the cluster of vesicles induced by expression of constitutively active GTPase Sar1a[H79G] in cells is dispersed throughout the cell after microtubule disruption. Movement of vesicles in cells containing the intermediate compartment protein ERGIC53/LMANI was inhibited by inhibiting dynein. Inhibiting protein kinase LOSK/SLK prevented orientation of Golgi to the leading part of crawling cells, but the activity of dynein was not inhibited according to data on the movement of ERGIC53/LMANI-marked vesicles.
KEY WORDS: dynein, kinesin, endoplasmic reticulum, ERGIC, ERES, fibroblasts, protein kinaseDOI: 10.1134/S0006297914090053
Abbreviations: ER, endoplasmic reticulum; ERES, endoplasmic reticulum exit sites; ERGIC, endoplasmic reticulum–Golgi intermediate compartment; GFP, green fluorescent protein; γTuRC, ring complexes of γ-tubulin; VSV-G, vesicular stomatitis virus G protein; VTC, vesicular-tubular cluster.
Components secreted by cells into the external environment or to
plasmalemma (proteins, lipids, etc.) travel a multistage route through
the cellular compartments, which is accompanied by physical movement of
membrane containers – vesicles – in the
cytoplasm. During the anterograde route the vesicles bud from the
endoplasmic reticulum (ER), move into the intermediate compartment
ERGIC (endoplasmic reticulum–Golgi intermediate compartment),
then to the cis-Golgi cisternae. After that transfer of vesicles
occurs between cis-cisternae, intermediate cisternae,
trans-cisternae, and the trans-Golgi network; from there
the vesicles move to the plasmalemma or to lysosomes. Inverse, or
retrograde, flow of vesicles occurs at all stages; it carries recycling
components of different compartments. Vesicles can be moved through the
cytoplasm in different ways: by diffusion, by forces generated by
polymerization of actin filaments (see review of S. Yu. Khaitlina in
this issue), as well as with active transport, i.e. transport driven by
motor proteins along actin filaments or microtubules. The choice
between the types of transport depends on the distance of movement of
the traveling vesicle: transfer by diffusion can provide just a short
distance, but longer displacement requires active transport.
Microtubule motors, dynein and the kinesins, are able to organize fast
and directed movement along relatively long microtubule tracks, and
such movement of vesicles significantly increases the average speed of
movement compared to simple diffusion in the cytoplasm [1]. This should facilitate the delivery of shipping
containers to a destination point and accelerate the cell reaction to
various stimuli.
In fact, the whole picture is somewhat more complicated. Experimental disruption of cell microtubules (e.g. treatment of cells with nocodazole or colchicine) affects secretion in an ambiguous manner: disassembly of microtubules can inhibit secretion or not influence it or even increase its level depending on the cell type [2, 3]. During disassembly of microtubules collagen secretion, in particular, is not disturbed, despite the fact that collagen protofibrils are packaged in large 300-nm membrane containers [4]. This means that even such large structures can be moved in the cell without the assistance of microtubules. We summarize in this review the available data on the specific role of microtubules and microtubule motors in the individual events of vesicular transport. It has recently become evident that different vesicles can use different motor proteins, various methods for their regulation, etc. In this review we will not discuss the myosin-dependent transport along actin filaments and, basically, we will not discuss secretion itself – the movement of vesicles from the trans-Golgi network to the plasmalemma.
Analysis of the movement of particles in the cell requires live imaging of cells. A very convenient model system for detailed description of the kinetics of the secretory pathway is based on use of the protein of vesicular stomatitis virus VSV-G. A peak of works using this protein came in the 1990s. Thermo-sensitive mutant ts045-VSV-G at 40ºC does not leave the ER [5], which enables the synchronization of secretion of VSV-G. Cells are incubated 9-16 h at 40°C, and then secretion is induced by decreasing the temperature to 32°C [5, 6] or even to 15ºC [7]. At 32°C VSV-G passes the entire secretory pathway and remains on the plasma membrane, but at 15°C it is delayed in ERGIC [7]. Lifetime observations of GFP-labeled VSV-G showed that temperature decrease from 40 to 32°C leads to fluorescent vesicle (shipping containers) formation. They arise throughout the cytoplasm and move along linear tracks to the central region of the cell and further to the plasma membrane. The tracks of container movement usually coincide with microtubules [8, 9].
Observations of secreted proteins with fluorescent label are severely impeded by the fact that a significant amount of protein usually remains in the ER, creating an intense fluorescence background in cells. For better synchronization and decrease in the background, inhibition of protein synthesis is usually used, and this can introduce artifacts. Now, when the main service proteins of transport containers (e.g. proteins of vesicles coat) have become known, researchers prefer to use it for marking secretory pathways. The problem remains about the contents of shipping containers and homogeneity of these vesicles. It has been shown that various cargos can use one and the same [10] or various [11] vesicles for moving through the secretory pathway, wherein some cargo coincides with VSV-G, and some not. For secretion of large proteins that do not fit into COPII-vesicles (e.g. for procollagen or chylomicrons) the cell forms special, larger containers [12].
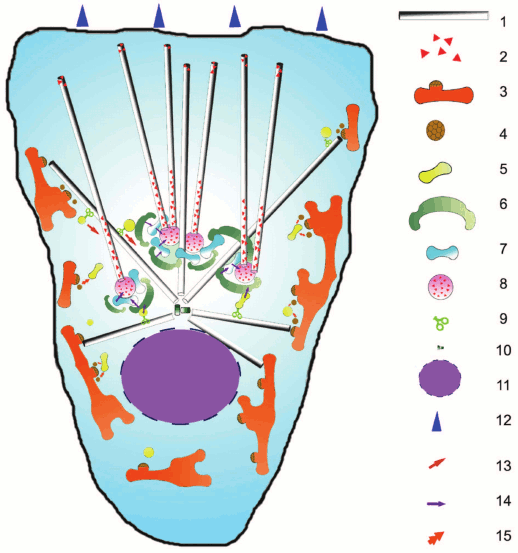
Disruption of microtubules does not significantly slow the passage of VSV-G through the secretory pathway, although, as already mentioned, the tracks of VSV-G vesicle movement coincide with microtubules [8, 9]. It can be assumed that if there are microtubules in the cell, transport proceeds along them; if not, the cell rearranges its secretory system in a different way, to remove the necessity of transport over long distances. This problem is discussed further. The type of motor proteins that can be used for transportation depends on localization of plus and minus ends of microtubules between the ER and Golgi apparatus. The generally accepted view is that the ER is placed on the cell periphery, and the Golgi apparatus is placed in its center, at the centrosome, and cis-cisternae are located near the centrosome. The centrosome organizes microtubules in such a way that microtubule minus ends are located near it, and the plus ends are located in the cell periphery. Consequently, vesicles during their anterograde movement must move from the ER to Golgi from the plus ends of microtubules to their minus ends, i.e. by a dynein-dependent pathway [13]. However, this picture is too simplistic and sometimes incorrect. In fact, endoplasmic reticulum exit sites (ERES) are scattered across the entire volume of the cell (Fig. 1); often the route of vesicles from ERES to cis-Golgi cisternae looks very complicated; sometimes the transported vesicles must pass through the trans-Golgi compartments. Another problem is that microtubules in the cytoplasm are often arranged randomly, i.e. some of them can be located in ERES region with their minus end turned to ER and plus end to the Golgi. This arrangement expects the use of kinesins, i.e. motor proteins carrying cargo from minus to plus ends of microtubules.
Fig. 1. Scheme of secretory pathways in the cell and their relationship to microtubules: 1) microtubules; 2) CLASP protein; 3) ER and ERES, exit sites for vesicles; 4) COPII-coated vesicle; 5) ERGIC/VTC (intermediate compartment between ER and Golgi); 6) cis-Golgi cistern; 7) intermediate cistern of Golgi; 8) trans-Golgi cistern; 9) motor protein; 10) centrosome; 11) nucleus; 12) direction of cell locomotion; 13) active transport along microtubules; 14) transport within the Golgi; 15) fusion of coated vesicles.
Vesicles formed in ERES possess a COPII-coat that assembles on the surface of the membrane after activation of small GTPase Sar1a. After detaching from ER, the vesicles move to ERGIC, also called VTC (vesicle tubule clusters) [14, 15], which is also scattered around the cell, although part of it is concentrated around the centrosome [13, 16]. It is also believed that VTC can be formed by merging of COPII-coated vesicles with each other. Moving of vesicles from ERES to ERGIC usually occurs over a very short distance. Molecular mechanisms of this movement are not clear; there have been attempts to study it by lifetime observations of cells synthesizing COPII-coating proteins fused with fluorescent proteins. The yeast S. cerevisiae lacks the ERGIC compartment; vesicles enter immediately into the cis-Golgi from ERES. Using high-resolution lifetime microscopy, it was demonstrated that the cis-Golgi swims to ERES, contacts for a short time, and the cargo at the time of contact moves from ERES to cis-Golgi, whereupon the contact is interrupted. That is, in this case transport does not occur in terms of physical movement of vesicle, and ERES are stationary [17].
In animal cells, a component of COPII-coat – Sec23 protein – can bind to dynactin, a complex protein cofactor of the motor protein dynein. Namely, Sec23 binds to the C-terminal domain of the large subunit of dynactin p150Glued, thereby potentially associating the ERES with microtubules [18]. p150Glued is a large fibrillar protein that has binding domains with microtubules, dynein, transported cargos, as well as with other subunits of the dynactin complex. The second essential component of the dynactin complex is a short filament consisting of actin-like protein Arp1 and capping proteins attached to p150Glued by additional subunits [19, 20]. The association of dynein with ERES has not been detected experimentally. Many authors believe that the presence of dynactin suggests the presence of dynein, but in this case this is probably an overstatement. The transport of ERES over long distances is also not shown. In animal cells ERES undergoes short fluctuating movements with amplitude of about 200 nm and slowly diffuses through the cytoplasm [21]. If the microtubules are disrupted, these movements are inhibited, and the diffusion slows by several-fold. It is difficult to show whether the slowing depends on loss of link with microtubules or, for example, with increasing viscosity of the cytoplasm. ERES distribution in the cytoplasm depends not only on microtubules, but also on the molecular motors – dynein and kinesin-1 [22]. When kinesin-1 (KIF5b) is knocked down (depleted by RNA interference) in HeLa cells, clustering of ERES is observed – it moves into the central area of the cell. Knockdown of kinesin-2 (KIF3a) does not affect the distribution of ERES. When kinesin-1 is knocked down, in most cases ERES become practically immobile, undergoing short (about 100 nm) bipolar displacements. When the dynein heavy chain is depleted, in contrast, the average displacement of fluctuating ERES significantly increases (more than 1000 nm). However, when both kinesin-1 and dynein heavy chains are depleted, ERES becomes a stationary structure in most observed cases [22]. The conclusion that follows from this is that kinesin-1 determines the fluctuating movement of ERES and dynein breaks them. Such fluctuating movements may be, for example, the result of one-dimensional diffusion along microtubules due to dynactin or some coating proteins. Interestingly, the overall structure of ER does not change in this experimental system, confirming that the force of molecular motors is applied directly to ERES. Perhaps such force promotes the elongation of membrane protrusions and budding of vesicles (see below).
Gemmations from ER vesicles fuse to form the intermediate compartment ERGIC or VTC. There have been long-lasting debates whether ERGIC and VTC are two different compartments or they are just variants of one compartment, as well as whether ERGIC/VTC arises only at the fusion of COPII-vesicles or they constantly present and accept these vesicles. Definitive answers to these questions have not been found yet. A compromise point of view is that both static ERGIC and dynamic VTC coexist in cells [23], with different dynamics but with similar functions, and the COPI-coating vesicles are separated both from ERGIC and VTC. They must find the cis-Golgi for fusion with it or go back to the ER dependently on their cargo. The cargo is sorted inside the compartment, and VTC often carries both COPI- and COPII-coating; it is usually differentially located on different sides of the compartment (facing to the ER retrograde side and facing to the cis-Golgi anterograde side) [23, 24]. That is why researchers have to be very careful to distinguish ERES from ERGIC/VTC during time-lapse observations. ERGIC/VTC are mobile, and they are transported in the anterograde direction [24]. Some transport vesicles, such as containers with procollagen, move directly from ER to cis-Golgi, bypassing ERGIC/VTC [25].
A vesicle transported from ER to Golgi changes COPII- and COPI-coating [26, 27]; and the adapters for the COPI assembly are already attracted during assembling of COPII-coating in ERES [26]. The protein complex TRAPPI on the ERGIC membrane performs anchoring (tethering) function for COPII-coated vesicles. When a COPII-coated vesicle comes to ERGIC, the TRAPPC9 subunit of TRAPPI competes with the coating protein Sec23 for binding with p150Glued: dynactin transfers to TRAPPI and preserves the binding of the newly formed structure with microtubules [28, 29]. Probably dynactin in this case also serves as a tethering factor, contributing to fusion of COPII-coated vesicles with ERGIC. TRAPPI-membrane complex is attracts to the membrane the small GTPase Rab1, which activates small GTPase Arf1 through GBF protein, initiating the formation of COPI-coating. COPI-coated vesicles and tubules gradually acquire the distinctive features of the cis-Golgi and move to the central region of the cell, indicating the activity of dynein motor [30], possibly interacting with dynactin bound through TRAPPI. Interestingly, GFP-VSV-G containing vesicles are static in the cytoplasm until they are touched by the growing microtubule plus end: then rapid vesicle movement starts [31]. It is believed that the microtubule plus end brings dynactin, helping membrane-bound dynein to start movement along microtubules [32]. Meanwhile, if dynactin is associated with the membrane, the microtubules need to bring dynein. The problem still exists how dynein and dynactin are located on coated vesicles and along microtubules; solving it requires a more precise identification and separation of vesicles and studying of their composition.
Other COPI-coated vesicles move in the opposite direction and deliver back to the ER recycling resident proteins. When a non-motor chain of kinesin-2 KAP3 is depleted, the anterograde transport ERES – ERGIC – cis-Golgi is not affected, whereas the reverse COPI-dependent return of KDEL-R marker protein to ER is inhibited [33]. Detailed analysis of movements of COPI-coated ts045-VSV-G-containing vesicles in the cell showed that the depletion of dynein, as expected, leads to dramatically decreased number of moving particles [6]. The effect of depletion of kinesin-1 was unexpected: the number of moving particles was also reduced, but the remaining moving vesicles strongly increased the length of tracks. Depletion of kinesin-2 did not affect the number of moving vesicles, but also increased the length of the tracks [6]. From these data it can be concluded that kinesins inhibit dynein-dependent anterograde transport from the ER to Golgi, and kinesin-1 is involved in retrograde transport [6].
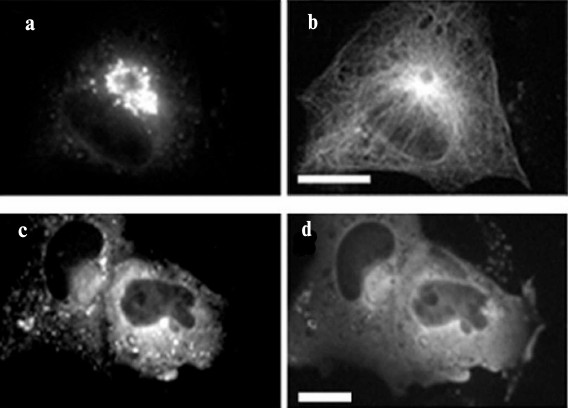
When the constitutively-active form of the GTPase Sar1a (Sar1a[H79G]) is expressed in cells, a violation of the assembly and disassembly of the COPI- and COPII-coating, respectively, occurs, and vesicles having protein composition of both ERES and ERGIC are clustered in the center of the cell [34, 35] (Fig. 2). Cluster formation depends on microtubules: when microtubules are disrupted, the cluster splits and its vesicles are dispersed in the cell (Fig. 2). It is believed, however, that only transport containers containing large proteins such as procollagen are involved in the formation of the cluster, and “normal” vesicles can move through the secretory pathway despite the defects of coating. Probably the containers, which form a cluster, are permanently associated with dynein, but this conjecture still requires experimental evidence.
Fig. 2. Density of cluster of ERES/ERGIC vesicles depends on microtubules. HeLa cells were transfected with DNA construct encoding Sar1a[H79G] and GFP-Sec23; immunostaining of microtubules with antibodies to tubulin DM-1A and mouse immunoglobulins conjugated with TRITC. Scale bar, 10 µm. a, b) Control cells; c, d) cells treated with nocodazole. a, c) GFP-Sec23; b, d) microtubules. In cells treated with nocodazole, microtubules are disrupted and the cluster of GFP-Sec23 is dispersed.
The dependence of ERGIC movements on dynein was also shown in our own experiments. GFP-fused lectin ERGIC53/LMANI, a marker for the ERGIC, was expressed in cultured Vero cells (green monkey fibroblast-like cells) using transient transfection, and then a live imaging of fluorescent vesicles was performed using an Axiovert 200M fluorescent microscope (Carl Zeiss, Germany) with Tempcontrol 37-2 Digital heating system (PeCon GmbH, Germany) at 37ºC, digital camera AxiocamHRm, and controlling software Axiovision (v.4.6). Interval between frames was 1 s. The movement of vesicles was evaluated using the Manual Tracking plug-in of the ImageJ program. The displacement of all visible vesicles (100-200 per cell) during 1 s was estimated at three time intervals for three cells (totally about 1500 vesicles per experimental condition were counted). The histograms of vesicle velocity were then constructed, and the average speed was calculated using Microsoft Excel.
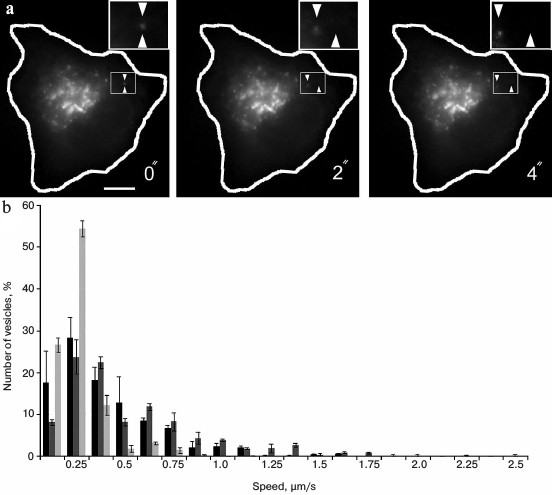
The analysis of the video shows that the movement of vesicles is rather chaotic and occurs at different rates (Fig. 3). The average speed was 0.39 ± 0.06 µm/s, but some individual particles could move with speed more that 1 µm/s (Fig. 3). This is evidence of their active transport in cells. For inhibition of dynein, the cells were co-transfected with a construct encoding a CC1-fragment of p150Glued fused with a red fluorescent protein (dsRed). The inhibitory effect of this fragment of p150Glued on dynein activity has been shown previously in many studies [36, 37]. In our experiments in the presence of dsRed-CC1-p150Glued the movement of GFP-ERGIC53/LMANI-containing vesicles was severely impaired. Their average speed was reduced to 0.19 ± 0.02 µm/s (p < 0.05), and there were no vesicles moving at a speed greater than about 0.75 µm/s (Fig. 3). This indicates that dynein is involved in the movements of ERGIC53/LMANI-containing vesicles in the cell, and it confirms previously published data about the movements of the ERGIC [9, 29], although we did not find published data about movement of ERGIC53/LMANI-containing vesicles.
Fig. 3. Analysis of vesicle movement in cells. a) Video shots. Expression of GFP-ERGIC53 in Vero cell. Scale bar, 10 µm. The cell contour is shown in white. The rectangle-marked region is shown enlarged, the starting position of a vesicle is shown by bottom triangle mark, in progress position – by top triangle mark. b) Histogram of speed distribution of vesicles labeled with GFP-ERGIC53. Black bars, control cells; light-gray bars, coexpression of dynein-inhibiting construct dsRed-CC1-p150Glued; dark-gray bars, coexpression of protein kinase LOSK/SLK-inhibiting construct dsRed-LOSK-K63R-ΔT.
The best-known and most easily detectable effect of disassembly or stabilization of microtubules in cells is the dispersion of the Golgi, when the ribbon-like Golgi is divided into separate small cisterna stacks scattered around the cell and located near ERES [38-40]. Inhibition of dynein in the cell by, e.g. microinjection of antibodies to an intermediate or heavy chain [41], or by obtaining mouse cells lacking dynein heavy chain [42], or by synthesis of the CC1 fragment of p150Glued as described above [36, 37] leads to the same effect as the disruption of microtubules: the Golgi apparatus is dispersed. Dispersing the Golgi after destruction of the microtubule is a complex process. It is believed that the original Golgi in the absence of dynamic microtubules fused with ER through the membrane tubules growing from the cisternae. For elongation of tubules under these conditions stable microtubules are needed, which are not disassembled under relatively mild disassembly conditions, as well as kinesin sliding on these microtubules and pulling the membrane. If kinesin activity is inhibited, and stable microtubules are also disrupted, the Golgi apparatus retains its original shape [40].
Membrane tubules usually occur in the Golgi under normal conditions [43], and often their appearance is enhanced by a variety of effects on cells. In particular, tubules are induced by brefeldin A (BFA), and for BFA-induced formation of tubules microtubules are required [44]. Tubules were also induced at low (15ºC) temperature, and under these conditions actin is required for the formation of the tubules [45]. Usually the microtubule motor proteins are sufficient to stretch the membrane to tubules [46]. Pulling of membrane tubules is an important function of motor proteins, somewhat less studied and less discussed in the literature than carrying of vesicles. In particular, motor protein-dependent pulling of tubules occurs during the formation and growth of the ER [47] or during the fusion of endosomes [48, 49], or in the ERGIC/VTC [50, 51], etc. Under BFA treatment COPI-vesicle formation is inhibited, leading to fusion of the Golgi and the ER because of developing of tubules fusing with the ER at the Golgi membrane [44]. Intracellular synthesis of a dominant-negative mutant of Sar1a GTPase, responsible for assembling of COPII-coat, causes very similar phenomena, although by a somewhat different molecular mechanism [35]. Golgi destruction is accompanied by the formation of small stacks of cisternae near ERES – the so-called mini-stacks, including all the elements of Golgi and enriched by trans-Golgi proteins. Thus, the proper motion during the dispersion of the Golgi does not happen – Golgi dissolves in one place in the cell and occurs in another places. During restoring of the system of microtubules after nocodazole washout or after washout of BFA, the ribbon-shaped Golgi apparatus is restored also if it existed in the cells beforehand. For its restoration the dynein-dependent transport of corresponding vesicles (ERGIC, cisternae themselves, etc.) is needed along microtubules [50, 51].
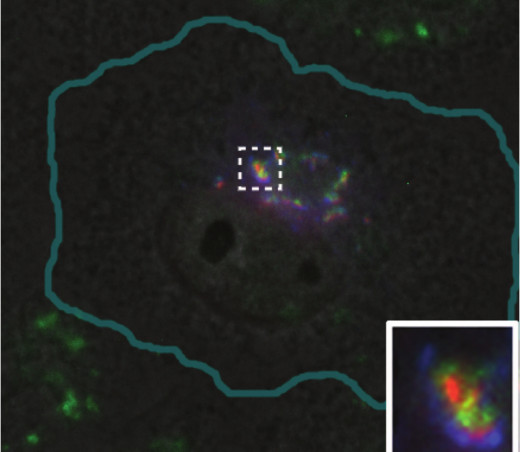
During the formation of ribbon-like Golgi, stacks of rather small cisternae are formed first, and then the lateral membrane tubules are pulled from them that connect the entire structure into a coherent whole wherein cis-cisternae are associated with cis-cisternae, intermediate – with intermediate, etc. [52, 53]. Lateral assembly of stacks, mediated by tubules, requires the dynein-dependent transport along microtubules and does not occur without it [51]. Ribbon-like Golgi is characteristic for vertebrate cells; invertebrates, plants, and fungi usually contain Golgi as many stacks of cisternae – dictyosomes. In vertebrates, the fragmentation of the Golgi (see also review of M. S. Vildanova et al. in this issue) is often observed under physiological conditions without any effects on microtubules. Undifferentiated epithelial cells of the bladder have ribbon-shaped Golgi, and in differentiated cells it splits into numerous mini-stacks scattered in the cytoplasm. This fragmentation provides uniform delivery of uroplakins to the apical membrane, which contributes to a powerful barrier between the bladder and blood vessels [54]. Fragmentation of the Golgi is also characteristic for differentiated muscle cells: when myoblasts fuse to form myotubules, the pericentrosomal Golgi is fragmented and numerous mini-stacks surrounding each nucleus arises [55]. Such movement of Golgi is an important part of muscle differentiation [56]. Fragmented Golgi can also occur in some diseases: it is observed in neurons of patients suffering neurodegenerative diseases such as Parkinson’s and Alzheimer’s diseases, as well as certain infections [57]. Typically, fragmentation of Golgi is accompanied by loss of its mobility in the cell. The intermediate states of Golgi, i.e. partial dispersion of a whole stack of cisternae or, for example, its cis-side, are also common. In Vero cells (cultured green monkey kidney cells) the Golgi apparatus is compact and placed in the perinuclear region, but it is composed of individual medium-sized stacks of cisternae, including ERGIC components, cis- and trans-Golgi (Fig. 4). Such a composition of Golgi is probably useful for delivery of vesicles from the ER to cis-cisternae and is quite typical for cultured cells.
Fig. 4. Golgi apparatus in Vero cell. ERGIC53-GFP (a marker of intermediate compartment of ERGIC and proximal part of Golgi) shown in blue; green, immunostaining of mannosidase-2 (cis-Golgi and intermediate cisterna); red, 1,4-galactosyltransferase–RFP (a marker of trans-Golgi).
Maintaining of a ribbon-shaped Golgi is related to the function of numerous proteins. Among these proteins the anchoring (tethering) proteins could be distinguished: golgin-84 [58], golgin-160 [59], GM130 [60], GRASP65 [60], GRASP55 [61], giantin [62], p115 [63], COG3 [64], COG6 [65], a regulatory GTPase Rab1 [66], and SNARE proteins syntaxin 5 and syntaxin 17 [67]. Inhibition (depletion) of any of these proteins leads to the dissociation of Golgi to mini-stacks. In contrast, expression of giantin in Drosophila cells S2, which have no such protein and possess dispersed Golgi, leads to the appearance of ribbon-like Golgi [62]. Giantin, a giant protein more than 3000 a.a. in size, forms a complex with p115, Rab1, GM130, and GRASP65 proteins. It is believed that this complex plays a major role in the formation of ribbon-like Golgi [62]. However, some authors pay specific attention to GRASP65 and GRASP55 proteins. These proteins are subjected to homodimerization and could form lateral crosslinks between the cisternae wherein GRASP65 is located at the cis-Golgi and GRASP55 at intermediate and trans-cisternae [52]. A mutant of CHO cells is described (ldlG) wherein protein GM130 synthesis does not occur despite the presence of mRNA encoding for this protein. CHO-ldlG cells have fragmented Golgi, and at an elevated temperature of 39.5ºC demonstrate a highly dispersed Golgi and the complete absence of secretion, leading to cell death [68].
Dispersion of the Golgi apparatus occurs in prophase of mitosis; in telophase Golgi regains its integrity. Fragmentation of the Golgi in early mitosis is accompanied by phosphorylation of GRASP65 and GRASP55 proteins by different protein kinases; in most cases when inhibition of Golgi fragmentation occurs, the progress of mitosis is also inhibited [69, 70]. Cleavage of membrane tubules between cisternae occurs with the participation of CtBP1-S/BARS protein (C-terminal binding protein/BFA-induced ADP-ribosylated substrate), which acetylates lysophosphatidic acid in membranes, contributing to its transformation into phosphatidic acid [71]. The same protein also contributes to budding and fission of COPI-coated and some other vesicles. In general, the details of the process of Golgi fragmentation still remain unknown. A detailed analysis of this process is beyond the scope of this review.
So we have established that microtubules are involved in the movements of early vesicular transport vesicles in the cell and in maintaining the integrity of the Golgi. Microtubules grow mainly from the centrosome. Relatively recently it was found that Golgi membranes are also directly involved in the nucleation and organization of cellular microtubules [72, 73]. By gliding along microtubules growing from them, Golgi membranes can concentrate in a sufficiently compact area within the cell even in the absence of a centrosome, e.g. in non-centrosomal cytoplasts [74] or in a cell with a centrosome ablated by a laser [75]. In muscle cells, where the centrosome is inactive and Golgi is dispersed, there is a network of dynamic microtubules that is organized into individual stacks of cisternae [76]. Now it is established that the ring complexes of γ-tubulin (γTuRC), which mainly determine the nucleation of microtubules, are associated with the Golgi membranes [77]. Microtubule nucleation at the Golgi membranes can be reproduced in vitro using isolated membranes and purified tubulin: under suitable conditions the tubulin is polymerized into microtubules extending from the membrane [72]. If the microtubule system in the cells is destroyed by nocodazole and cooling, then at early stages of recovery (nocodazole wash-out and warming) the microtubules can be seen growing directly from vesicles containing Golgi markers [73]. Several proteins are involved in the process of γ-tubulin recruitment to the Golgi: GMAP210 – golgin of cis-Golgi [78]; CG-NAP (AKAP450, yotiao) – a long fibrillar protein, acceptor of protein kinase A [79, 80]; p115 [81]; myomegalin – a protein similar to AKAP450 [82]; CDK5RAP2 protein [83]. All these major fibrillar proteins are difficult to study. Some of them (or perhaps their isoforms) are also involved in microtubule nucleation at the centrosome. The functional domains in γTuRC-binding proteins were recently identified, for example, the CM1 domain of myomegalin and CDK5RAP2 [82, 83]. These domains may regulate microtubule-nucleating activity of γTuRC [84].
Perhaps some proteins besides γTuRC can nucleate microtubules; in any case, they can participate in the stabilization of nucleated microtubules and promote their further growth. In particular, the multifunctional fibrillar proteins CLASP1/2 (Orbit in Drosophila), localized in the cell at Golgi and along microtubules, mainly at their plus ends, and also at kinetochores of chromosomes [85, 86] participate in microtubule growth. Dimers of CLASP1/2 proteins form a sort of tweezers that are able to catch tubulin dimers and to deliver them to the microtubule and assist its assembly [87]. CLASPs are associated with Golgi through golgin GCC185, with the participation of small GTPases Arl4a and Rab6 [88]. Unlike the centrosome, where the microtubules departs radially from it, the Golgi forms an asymmetric loose bundle of microtubules directed towards the cell edge, and these microtubules may be coated with excess of CLASP1/2 protein [89].
Ribbon-shaped Golgi is usually located in the center of the cell, tightly adherent to the centrosome, and is connected with it through CG-NAP (AKAP450, Yotiao) long fibrillar protein. The N-terminal domain of CG-NAP is associated with the Golgi, and the C-terminal with the centrosome [80, 90]. Most likely, the membranes of the Golgi “seek” the centrosome, moving toward the minus ends of microtubules, concentrate near the centrosome, and then establish a connection with it through specific proteins. In polarized cells, for example, in flat fibroblast-like cells moving along a substrate, the ribbon-like Golgi is localized in their front part [91]. One can assume that the Golgi provides the secretion of the necessary components of plasma membrane to the front edge of growing cells, probably along an asymmetric array of microtubules organized by the Golgi itself. Otherwise, Golgi can be a necessary platform for signal transduction from/to the front edge. Displacement of the Golgi to the front edge requires activity of small GTPase Cdc42, dynein, and microtubules [92]. Dynein participation in moving of membranes to the peripheral part of the cell seems to be a paradox, but it is confirmed in many studies. It still remains unclear whether the polarization includes the physical movement of the Golgi in the cytoplasm or its disassembly in the cell center and gradual assembly at the front edge. Detailed live imaging of reorientation of the Golgi has been done to the best of our knowledge. After depletion of either p160 or GMAP210, Golgi partially fragments and loses its directional orientation in cells. These changes infringe neither the cytoskeleton structures nor the overall level of secretion, but the cells lose their ability for directional movement in a wound in a monolayer [59]. Unfortunately, the agent that can influence orientation of the Golgi in cells without causing its fragmentation and without affecting the cytoskeleton is still not found.
It was shown previously that after inhibition of the activity of serine-threonine protein kinase LOSK/SLK the Golgi retains its integrity, but it does not move into the front part of the cell [93]. Probably, the reason is a disturbance of microtubules extending from the centrosome, whereas Golgi elements use these microtubules to move [93]. The substrate of LOSK/SLK is the p150Glued subunit of dynactin, and the site of phosphorylation is located in the p150Glued domain that is not involved in dynactin binding to dynein [94]. To check whether phosphorylation with kinase LOSK/SLK affects dynein-dependent motility, we used the same system (movement of GFP-ERGIC53-containing vesicles). For inhibition of LOSK/SLK activity, expression of a dominant-negative mutant of the kinase catalytic domain dsRed-LOSK-K63R-ΔT was used. We found that the average velocity of GFP-ERGIC53 vesicles in cells with inhibited kinase activity was 0.49 ± 0.04 µm/s and was not different from control cells (0.39 ± 0.06 µm/s). The velocity distribution of the vesicles also did not differ (Fig. 3), i.e. dynein activity was not repressed after inhibition of LOSK/SLK. This experiment showed that the orientation of the Golgi apparatus in polarized cells requires not only the activity of dynein, but also other factors. Perhaps such a factor is the presence of radially arranged microtubules extending from the centrosome, which could be directed to move elements of the Golgi apparatus. For complete assembly of compact Golgi after nocodazole washout or after mitosis, both microtubule systems are needed: extending from the centrosome [75], and organized on the membranes of the Golgi apparatus [36, 86]. In the absence of either the Golgi remains disordered [36, 86, 90].
The mechanism of the Golgi assembly after repolymerization of microtubules remains unclear. Obviously, the mini-stacks disappear at the cell periphery and new containers form in the more central area of the cell [75]. However, direct tracing of mini-stacks in the central region of the cell is difficult, and so far no such direct observation has been made. It is possible that the formation of a compact Golgi goes through the recycling of proteins through ER. If ts045-GFP-VSV-G protein in ERGIC is blocked using the transition of temperature from 40 to 15ºC and adding an inhibitor of protein synthesis, and then disassemble microtubules with nocodazole, ts045-GFP-VSV-G remains dispersed in ERGIC together with dispersed Golgi markers. If we now shift the temperature to 40ºC again but wash out nocodazole, the Golgi apparatus will be formed, but it will be deprived of ts045-GFP-VSV-G. The later is expected to recycle to the ER though it cannot leave it at an elevated temperature. This experiment proves the necessity of recycling through the ER for recovery of the Golgi after microtubule system restoration [95].
The Golgi is associated with the major cellular motor proteins: dynein [96], kinesin-1 (at least its light chain) [97], kinesin-2 (its structural chain KAP3) [33], and myosin [96]. Dynein and kinesins are associated mainly with the most mobile parts of the Golgi apparatus: the trans-Golgi network and the elements of early vesicular transport, though they are found on the cisterns also. The mechanism of motor protein binding to membranes at the molecular level is obscure. Dynein probably uses different adapter proteins in different cases. The main adapter for dynein in membranes is considered to be dynactin. Overexpression of dynamitin protein (p50), breaking dynactin complex causes Golgi fragmentation [98]. Also, as already mentioned, the expression of the CC1 fragment of p150Glued competitively disrupts association of dynein with dynactin and causes Golgi fragmentation too [36]. Perhaps the destruction of dynactin or CC1-p150Glued expression inhibits dynein activity, and this inhibition is the reason for the observed effects. Depletion of p150Glued by RNA interference does not lead to fragmentation of the Golgi, which indicates the presence of other mechanisms of binding dynein to membranes [99].
Dynactin, however, can play its own role, linking vesicles and cisterns with microtubules. The most likely way for dynactin binding to membranes is the interaction of Arp1-filament with spectrin. Golgi elements are entangled with an ankyrin–spectrin network consisting mainly of spectrin βIΣ* (beta I sigma 1 and beta I sigma 2) [100]. β-Spectrin can be partially co-fractionated with dynactin complex and βIΣ-spectrin and Arp1 filaments colocalize in cells overexpressing Arp1 [101]. In biochemical assays in vitro, incubation with high concentrations of salts and even in the presence of detergent the matrix of isolated Golgi membranes contains dynactin, and these extracted Golgi membranes retain the ability to bind to the cytoplasmic dynein from the cell extract. At alkaline pH both p150Glued and Arp1 dissociate from the membranes of the Golgi, and the ability of membranes to bind dynein is suppressed. Arp1 can be restored in membranes incubated in the cytosol, but p150Glued is not able to do so [102]. The existence of cytosolic Arp1, capable of binding to membranes in the absence of p150Glued, is surprising, but, in any case, Arp1 is not able to recruit dynein to membranes by itself: it requires p150Glued, which associates directly with an intermediate chain of dynein DIC [102].
The association of dynein with the Golgi apparatus is regulated by small GTPase Cdc42. It interacts with COPI and is located on the lateral parts of cis- and intermediate cisterns of Golgi [103], as well as COPI-coated transport vesicles [104]. Cdc42 plays a crucial role in the recruitment of dynein to COPI-vesicles [105], while it is involved in the reconstruction of the Golgi apparatus after restoring the microtubule network in cells [106]. Apparently, Cdc42 stimulates the activity of dynein only on its hydrolysis of its GTP, which occurs under the action of factor ARHGAP21 with participation of small GTPase Arf1 [106]. Data describing the effect of this protein on the activity of dynein are rather contradictory; perhaps the influence of Cdc42 is mediated by other proteins, in particular by the system of regulation of actin polymerization [106]. Most likely, Cdc42 is involved in destruction and reconstruction of the Golgi during mitosis.
It was shown that dynein can directly contact golgin GM160 through its intermediate chain DIC [107]. Depletion of GM160 leads to fragmentation of the Golgi apparatus. Another acceptor of dynein motor protein is bicaudal (BIC), which is similar to golgins though it does not have a characteristic membrane GRIP-domain. Bicaudal isoform D2 (BIC-D2) is associated with the membranes of the trans-Golgi cisterns and trans-Golgi network [108], and coat-lacking vesicles transporting cargo from the Golgi to the ER [109]. BIC-D2 is accepted there with the participation of the small GTPase Rab6 [110]. BIC-D2 can bind directly dynein and dynactin, forming a tertiary complex; moreover, the LIS1 protein can be involved in the complex as well [111]. Depletion of bicaudal also leads to fragmentation of the Golgi apparatus. Another protein involved in the regulation of the dynein–dynactin complex is ZW10. It is localized in the kinetochores of chromosomes during mitosis, in ER in interphase, and is involved in transport from the Golgi to the ER [112]. ZW10 binds dynactin component dynamitin, is associated with dynein, and regulates its activity [113].
Heterogeneity of acceptors of dynein on membranes may reflect the heterogeneity of the dynein itself. The molecule of cytoplasmic dynein-1 (basic dynein in cells) consists of two heavy chains carrying the motor domain, two intermediate chains DIC, with isoforms 1 and 2, two light intermediate chains LDIC, having also isoforms, and a variety light chains [114]. For a more detailed analysis of the role of dynein motor in vesicle transport, its individual subunits were depleted by RNA interference, and the distribution of organelles of vesicular transport in cells was described [115]. In this study it was found that the light intermediate chain 1 (LIC1) is necessary for the work of the dynein motor in ER–Golgi distance, whereas LIC2 works in recycling endosomes [115]. Dynein light chain Tctex is mainly localized in the Golgi apparatus, although its localization only partially overlaps with the localization of dynein heavy chain or dynactin [116].
Thus, membranes of the Golgi apparatus and early secretory vesicles are associated with many proteins that interact with microtubules, including γTuRC, fibrillar structural proteins, motor proteins, and their cofactors. These proteins organize a specific pool of microtubules extending from the Golgi, provide a compact Golgi structure, ensure its orientation in polarized cells, provide anterograde and retrograde transport of vesicles, and finally, are necessary for the formation of membrane tubules. Localization and activity of proteins that interact with microtubules are subject to regulation that is currently poorly understood. The regulatory mechanisms in different cell types will most likely be the subject of the experimental works in the near future.
This work was supported by the Russian Foundation for Basic Research (grants 11-04-01022-a, 12-04-33178-mol-a-ved and 14-04-01729-a).
REFERENCES
1.Kahana, A., Kenan, G., Feingold, M., Elbaum, M.,
and Granek, R. (2008) Active transport on disordered microtubule
networks: the generalized random velocity model, Phys. Rev.,
78, 051912.
2.Robin, P., Rossignol, B., and Raymond, M. N. (1995)
Effect of microtubule network disturbance by nocodazole and docetaxel
(taxotere) on protein secretion in rat extraorbital lacrimal and
parotid glands, Eur. J. Cell Biol., 67, 227-237.
3.Kockx, M., Guo, D. L., Huby, T., Lesnik, P., Kay,
J., Sabaretnam, T., Jary, E., Hill, M., Gaus, K., Chapman, J., Stow, J.
L., Jessup, W., and Kritharides, L. (2007) Secretion of apolipoprotein
E from macrophages occurs via a protein kinase A and calcium-dependent
pathway along the microtubule network, Circ. Res., 101,
607-616.
4.Bodo, M., Carinci, P., Baroni, T., Becchetti, E.,
Bellucci, C., Pezzetti, F., Giammarioli, M., Stabellini, G., and Arena,
N. (1996) Collagen synthesis and cell growth in chick embryo
fibroblasts: influence of colchicine, cytochalasin B and concanavalin
A, Cell Biol. Int., 20, 177-185.
5.Lodish, H. F., and Kong, N. (1983) Reversible block
in intracellular transport and budding of mutant vesicular stomatitis
virus glycoproteins, Virology, 125, 335-348.
6.Brown, A. K., Hunt, S. D., and Stephens, D. J.
(2014) Opposing microtubule motors control motility, morphology and
cargo segregation during ER-to-Golgi transport, Biol. Open,
3, 307-313.
7.Schweizer, A., Fransen, J. A. M., Matter, K.,
Kreis, T. E., Ginsel, L., and Hauri, H. P. (1990) Identification of an
intermediate compartment involved in protein transport from endoplasmic
reticulum to Golgi apparatus, Eur. J. Cell Biol., 53,
185-196.
8.Sciaky, N., Presley, J., Smith, C., Zaal, K., Cole,
N., Moreira, J., Terasaki, M., Siggia, E., and Lippincott-Schwartz, J.
(1997) Golgi tubule traffic and the effects of brefeldin A visualized
in living cells, J. Cell Biol., 139, 1137-1155.
9.Presley, J. F., Cole, N. B., Schroer, T. A.,
Hirschberg, K., Zaal, K. J., and Lippincott-Schwartz, J. (1997)
ER-to-Golgi transport visualized in living cells, Nature,
389, 81-85.
10.Strous, G. J., Willemsen, R., van Kerkhof, P.,
Slot, J. W., Geuze, H. J., and Lodish, H. F. (1983) Vesicular
stomatitis virus glycoprotein, albumin, and transferrin are transported
to the cell surface via the same Golgi vesicles, J. Cell Biol.,
97, 1815-1822.
11.Hormia, M., Lehto, V. P., and Virtanen, I. (1984)
Intracellular localization of factor VIII-related antigen and
fibronectin in cultured human endothelial cells: evidence for divergent
routes of intracellular translocation, Eur. J. Cell Biol.,
33, 217-228.
12.Malhotra, V., and Erlmann, P. (2011) Protein
export at the ER: loading big collagens into COPII carriers, EMBO
J., 30, 3475-3480.
13.Sutterlin, C., and Colanzi, A. (2010) The Golgi
and the centrosome: building a functional partnership, J. Cell
Biol., 188, 621-628.
14.Hauri, H. P., Kappelel, F., Andersson, H., and
Appenzeller, C. (2000) ERGIC-53 and traffic in the secretory pathway,
J. Cell Sci., 113, 587-596.
15.Appenzeller-Herzog, C., and Hauri, H. P. (2006)
The ER–Golgi intermediate compartment [ERGIC]: in search of its
identity and function, J. Cell Sci., 119, 2173-2183.
16.Marie, M., Dale, H. A., Sannerud, R., and
Saraste, J. (2009) The function of the intermediate compartment in
pre-Golgi trafficking involves its stable connection with the
centrosome, Mol. Biol. Cell, 20, 4458-4470.
17.Kurokawa, K., Okamoto, M., and Nakano, A. (2014)
Contact of cis-Golgi with ER exit sites executes cargo capture
and delivery from the ER, Nature Commun., 5, 3653.
18.Watson, P., Forster, R., Palmer, K. J.,
Pepperkok, R., and Stephens, D. J. (2005) Coupling of ER exit to
microtubules through direct interaction of COPII with dynactin,
Nature Cell Biol., 7, 48-55.
19.Schroer, T. A. (2004) Dynactin, Ann. Rev. Cell
Dev. Biol., 20, 759-779.
20.Kardon, J. R., and Vale, R. D. (2009) Regulators
of the cytoplasmic dynein motor, Nature Rev. Mol. Cell Biol.,
10, 854-865.
21.Hammond, A. T., and Glick, B. S. (2000) Dynamics
of transitional endoplasmic reticulum sites in vertebrate cells,
Mol. Biol. Cell, 11, 3013-3030.
22.Gupta, V., Palmer, K., Spence, P., Hudson, A.,
and Stephens, D. J. (2008) Kinesin-1 [uKHC/KIF5B] is required for
bidirectional motility of ER exit sites and efficient ER-to-Golgi
transport, Traffic, 9, 1850-1866.
23.Verissimo, F., and Pepperkok, R. (2013) Imaging
ER-to-Golgi transport: towards a systems view, J. Cell Sci.,
126, 5091-5100.
24.Stephens, D. J., and Pepperkok, R. (2001)
Illuminating the secretory pathway: when do we need vesicles, J.
Cell Sci., 114, 1053-1059.
25.Stephens, D. J., and Pepperkok, R. (2002) Imaging
of procollagen transport reveals COPI-dependent cargo sorting during
ER-to-Golgi transport in mammalian cells, J. Cell Sci.,
115, 1149-1160.
26.Altan-Bonnet, N., Sougrat, R., and
Lippincott-Schwartz, J. (2004) Molecular basis for Golgi maintenance
and biogenesis, Curr. Opin. Cell Biol., 16, 364-372.
27.Hughes, H., and Stephens, D. J. (2008) Assembly,
organization, and function of the COPII coat, Histochem. Cell
Biol., 129, 129-151.
28.Cai, H., Yu, S., Menon, S., Cai, Y., Lazarova,
D., Fu, C., Reinisch, K., Hay, C., and Ferro-Novick, S. (2007) TRAPPI
tethers COPII vesicles by binding the coat subunit Sec23,
Nature, 445, 941-944.
29.Zong, M., Satoh, A., Yu, M. K., Siu, K. Y., Ng,
W. Y., Chan, H. C., Tanner, J. A., and Yu, S. (2012) TRAPPC9 mediates
the interaction between p150 and COPII vesicles at the target membrane,
PLoS One, 7, e29995.
30.Tomas, M., Martinez-Alonso, E., Ballesta, J., and
Martinez-Menarguez, J. A. (2010) Regulation of ER–Golgi
intermediate compartment tubulation and mobility by COPI coats, motor
proteins and microtubules, Traffic, 11, 616-625.
31.Vaughan, P. S., Miura, P., Henderson, M., Byrne,
B., and Vaughan, K. T. (2002) A role for regulated binding of
p150(Glued) to microtubule plus ends in organelle transport, J. Cell
Biol., 158, 305-319.
32.Lomakin, A. J., Semenova, I., Zaliapin, I.,
Kraikivski, P., Nadezhdina, E., Slepchenko, B. M., Akhmanova, A., and
Rodionov, V. (2011) CLIP-170-dependent capture of membrane organelles
by microtubules initiates minus-end directed transport, Mol. Biol.
Cell, 22, 4029-4037.
33.Stauber, T., Simpson, J. C., Pepperkok, R., and
Vernos, I. (2006) A role for kinesin-2 in COPI-dependent recycling
between the ER and the Golgi complex, Curr. Biol., 16,
2245-2251.
34.Seemann, J., Jokitalo, E., Pypaert, M., and
Warren, G. (2000) Matrix proteins can generate the higher order
architecture of the Golgi apparatus, Nature, 407,
1022-1026.
35.Ward, T. H., Polishchuk, R. S., Caplan, S.,
Hirschberg, K., and Lippincott-Schwartz, J. (2001) Maintenance of Golgi
structure and function depends on the integrity of ER export, J.
Cell Biol., 155, 557-570.
36.Quintyne, N. J., Gill, S. R., Eckley, D. M.,
Crego, C. L., Compton, D. A., and Schroer, T. A. (1999) Dynactin is
required for microtubule anchoring at centrosomes, J. Cell
Biol., 147, 321-334.
37.Zhapparova, O. N., Burakov, A. V., and
Nadezhdina, E. S. (2007) The centrosome keeps nucleating microtubules
but looses the ability to anchor them after the inhibition of
dynein–dynactin complex, Biochemistry (Moscow), 72,
1233-1240.
38.Sandoval, I. V., Bonifacino, J. S., Klausner, R.
D., Henkart, M., and Wehland, J. (1984) Role of microtubules in the
organization and localization of the Golgi apparatus, J. Cell
Biol., 99, 113-118.
39.Cole, N. B., Sciaky, N., Marotta, A., Song, J.,
and Lippincott-Schwartz, J. (1996) Golgi dispersal during microtubule
disruption: regeneration of Golgi stacks at peripheral endoplasmic
reticulum exit sites, Mol. Biol. Cell, 7, 631-650.
40.Minin, A. A. (1997) Dispersal of Golgi apparatus
in nocodazole-treated fibroblasts is a kinesin-driven process, J.
Cell Sci., 110, 2495-2505.
41.Thyberg, J., and Moskalewski, S. (1999) Role of
microtubules in the organization of the Golgi complex, Exp. Cell
Res., 246, 263-279.
42.Harada, A., Takei, Y., Kanai, Y., Tanaka, Y.,
Nonaka, S., and Hirokawa, N. (1998) Golgi vesiculation and lysosome
dispersion in cells lacking cytoplasmic dynein, J. Cell Biol.,
141, 51-59.
43.Trucco, A., Polishchuk, R. S., Martella, O., Di
Pentima, A., Fusella, A., Di Giandomenico, D., San Pietro, E.,
Beznoussenko, G. V., Polishchuk, E. V., Baldassarre, M., Buccione, R.,
Geerts, W. J., Koster, A. J., Burger, K. N., Mironov, A. A., and Luini,
A. (2004) Secretory traffic triggers the formation of tubular
continuities across Golgi sub-compartments, Nature Cell Biol.,
6, 1071-1081.
44.Lippincott-Schwartz, J., Donaldson, J. G.,
Schweizer, A., Berger, E. G., Hauri, H. P., Yuan, L. C., and Klausner,
R. D. (1990) Microtubule-dependent retrograde transport of proteins
into the ER in the presence of brefeldin A suggests an ER recycling
pathway, Cell, 60, 821-836.
45.Martinez-Alonso, E., Egea, G., Ballesta, J., and
Martinez-Menarguez, J. A. (2005) Structure and dynamics of the Golgi
complex at 15°C: low temperature induces the formation of
Golgi-derived tubules, Traffic, 6, 32-44.
46.Roux, A., Cappello, G., Cartaud, J., Prost, J.,
Goud, B., and Bassereau, P. (2002) A minimal system allowing tubulation
with molecular motors pulling on giant liposomes, Proc. Natl. Acad.
Sci. USA, 99, 5394-5399.
47.Vale, R. D., and Hotani, H. (1988) Formation of
membrane networks in vitro by kinesin-driven microtubule
movement, J. Cell Biol., 107, 2233-2241.
48.Skjeldal, F. M., Strunze, S., Bergeland, T.,
Walseng, E., Gregers, T. F., and Bakke, O. (2012) The fusion of early
endosomes induces molecular-motor-driven tubule formation and fission,
J. Cell Sci., 125, 1910-1919.
49.Delevoye, C., Miserey-Lenkei, S., Montagnac, G.,
Gilles-Marsens, F., Paul-Gilloteaux, P., Giordano, F., Waharte, F.,
Marks, M. S., Goud, B., and Raposo, G. (2014) Recycling endosome tubule
morphogenesis from sorting endosomes requires the kinesin motor KIF13A,
Cell Rep., 6, 445-454.
50.Judson, B. L., and Brown, W. J. (2009) Assembly
of an intact Golgi complex requires phospholipase A2 (PLA2) activity,
membrane tubules, and dynein-mediated microtubule transport,
Biochem. Biophys. Res. Commun., 389, 473-477.
51.Robertson, A. M., and Allan, V. J. (2000)
Brefeldin A-dependent membrane tubule formation reconstituted in
vitro is driven by a cell cycle-regulated microtubule motor,
Mol. Biol. Cell, 11, 941-955.
52.Jarvela, T., and Linstedt, A. D. (2014)
Isoform-specific tethering links the Golgi ribbon to maintain
compartmentalization, Mol. Biol. Cell, 25, 133-144.
53.Nakamura, N., Wei, J. H., and Seemann, J. (2012)
Modular organization of the mammalian Golgi apparatus, Curr. Opin.
Cell Biol., 24, 467-474.
54.Kreft, M. E., Di Giandomenico, D., Beznoussenko,
G. V., Resnik, N., Mironov, A. A., and Jezernik, K. (2010) Golgi
apparatus fragmentation as a mechanism responsible for uniform delivery
of uroplakins to the apical plasma membrane of uroepithelial cells,
Biol. Cell, 102, 593-607.
55.Ralston, E. (1993) Changes in architecture of the
Golgi complex and other subcellular organelles during myogenesis, J.
Cell Biol., 120, 399-409.
56.Lu, Z., Joseph, D., Bugnard, E., Zaal, K. J., and
Ralston, E. (2001) Golgi complex reorganization during muscle
differentiation: visualization in living cells and mechanism, Mol.
Biol. Cell, 12, 795-808.
57.Martinez-Alonso, E., Tomas, M., and
Martinez-Menarguez, J. A. (2013) Golgi tubules: their structure,
formation and role in intra-Golgi transport, Histochem. Cell
Biol., 140, 327-339.
58.Diao, A., Rahman, D., Pappin, D. J., Lucocq, J.,
and Lowe, M. (2003) The coiled-coil membrane protein golgin-84 is a
novel rab effector required for Golgi ribbon formation, J. Cell
Biol., 160, 201-212.
59.Yadav, S., Puri, S., and Linstedt, A. D. (2009) A
primary role for Golgi positioning in directed secretion, cell
polarity, and wound healing, Mol. Biol. Cell, 20,
1728-1736.
60.Puthenveedu, M. A., Bachert, C., Puri, S., Lanni,
F., and Linstedt, A. D. (2006) GM130 and GRASP65-dependent lateral
cisternal fusion allows uniform Golgi-enzyme distribution,
Nature Cell Biol., 8, 238-248.
61.Feng, Y., Yu, W., Li, X., Lin, S., Zhou, Y., Hu,
J., and Liu, X. (2013) Structural insight into Golgi membrane stacking
by GRASP65 and GRASP55 proteins, J. Biol. Chem., 288,
28418-28427.
62.Koreishi, M., Gniadek, T. J., Yu, S., Masuda, J.,
Honjo, Y., and Satoh, A. (2013) The golgin tether giantin regulates the
secretory pathway by controlling stack organization within Golgi
apparatus, PLoS One, 8, e59821.
63.Puthenveedu, M. A., and Linstedt, A. D. (2004)
Gene replacement reveals that p115/SNARE interactions are essential for
Golgi biogenesis, Proc. Natl. Acad. Sci. USA, 101,
1253-1256.
64.Zolov, S. N., and Lupashin, V. V. (2005) Cog3p
depletion blocks vesicle-mediated Golgi retrograde trafficking in HeLa
cells, J. Cell Biol., 168, 747-759.
65.Kudlyk, T., Willett, R., Pokrovskaya, I. D., and
Lupashin, V. (2013) COG6 interacts with a subset of the Golgi SNAREs
and is important for the Golgi complex integrity, Traffic,
14, 194-204.
66.Tomas, M., Marin, M. P., Martinez-Alonso, E.,
Esteban-Pretel, G., Diaz-Ruiz, A., Vazquez-Martinez, R., Malagon, M.
M., Renau-Piqueras, J., and Martinez-Menarguez, J. A. (2012) Alcohol
induces Golgi fragmentation in differentiated PC12 cells by
deregulating Rab1-dependent ER-to-Golgi transport, Histochem. Cell
Biol., 138, 489-501.
67.Muppirala, M., Gupta, V., and Swarup, G. (2011)
Syntaxin 17 cycles between the ER and ERGIC and is required to maintain
the architecture of ERGIC and Golgi, Biol. Cell, 103,
333-350.
68.Vasile, E., Perez, T., Nakamura, N., and Krieger,
M. (2003) Structural integrity of the Golgi is temperature sensitive in
conditional-lethal mutants with no detectable GM130, Traffic,
4, 254-272.
69.Colanzi, A., and Sutterlin, C. (2013) Signaling
at the Golgi during mitosis, Methods Cell Biol., 118,
383-400.
70.Tang, D., Yuan, H., Vielemeyer, O., Perez, F.,
and Wang, Y. (2012) Sequential phosphorylation of GRASP65 during
mitotic Golgi disassembly, Biol. Open, 1, 1204-1214.
71.Valente, C., Luini, A., and Corda, D. (2013)
Components of the CtBP1/BARS-dependent fission machinery, Histochem.
Cell Biol., 140, 407-421.
72.Chabin-Brion, K., Marceiller, J., Perez, F.,
Settegrana, C., Drechou, A., Durand, G., and Pous, C. (2001) The Golgi
complex is a microtubule-organizing organelle, Mol. Biol. Cell,
12, 2047-2060.
73.Grimaldi, A. D., Fomicheva, M., and Kaverina, I.
(2013) Ice recovery assay for detection of Golgi-derived microtubules,
Methods Cell Biol., 118, 401-415.
74.Malikov, V., Cytrynbaum, E., Kashina, A.,
Mogilner, A., and Rodionov, V. (2005) Centering of a radial microtubule
array by translocation along microtubules spontaneously nucleated in
the cytoplasm, Nature Cell Biol., 7, 1213-1218.
75.Vinogradova, T., Paul, R., Grimaldi, A. D.,
Loncarek, J., Miller, P. M., Yampolsky, D., Magidson, V., Khodjakov,
A., Mogilner, A., and Kaverina, I. (2012) Concerted effort of
centrosomal and Golgi-derived microtubules is required for proper Golgi
complex assembly but not for maintenance, Mol. Biol. Cell,
23, 820-833.
76.Oddoux, S., Zaal, K. J., Tate, V., Kenea, A.,
Nandkeolyar, S. A., Reid, E., Liu, W., and Ralston, E. (2013)
Microtubules that form the stationary lattice of muscle fibers are
dynamic and nucleated at Golgi elements, J. Cell Biol.,
203, 205-213.
77.Erlemann, S., Neuner, A., Gombos, L., Gibeaux,
R., Antony, C., and Schiebel, E. (2012) An extended γ-tubulin
ring functions as a stable platform in microtubule nucleation, J.
Cell Biol., 197, 59-74.
78.Rios, R. M., Sanchis, A., Tassin, A. M.,
Fedriani, C., and Bornens, M. (2004) GMAP-210 recruits gamma-tubulin
complexes to cis-Golgi membranes and is required for Golgi
ribbon formation, Cell, 118, 323-335.
79.Takahashi, M., Yamagiwa, A., Nishimura, T.,
Mukai, H., and Ono, Y. (2002) Centrosomal proteins CG-NAP and kendrin
provide microtubule nucleation sites by anchoring gamma-tubulin ring
complex, Mol. Biol. Cell, 13, 3235-3245.
80.Rivero, S., Cardenas, J., Bornens, M., and Rios,
R. M. (2009) Microtubule nucleation at the cis-side of the Golgi
apparatus requires AKAP450 and GM130, EMBO J., 28,
1016-1028.
81.Radulescu, A. E., Mukherjee, S., and Shields, D.
(2011) The Golgi protein p115 associates with gamma-tubulin and plays a
role in Golgi structure and mitosis progression, J. Biol. Chem.,
286, 21915-21926.
82.Roubin, R., Acquaviva, C., Chevrier, V., Sedjai,
F., Zyss, D., Birnbaum, D., and Rosnet, O. (2013) Myomegalin is
necessary for the formation of centrosomal and Golgi-derived
microtubules, Biol. Open, 2, 238-250.
83.Wang, Z., Wu, T., Shi, L., Zhang, L., Zheng, W.,
Qu, J. Y., Niu, R., and Qi, R. Z. (2010) Conserved motif of CDK5RAP2
mediates its localization to centrosomes and the Golgi complex, J.
Biol. Chem., 285, 22658-22665.
84.Lin, T. C., Neuner, A., Schlosser, Y. T., Scharf,
A. N., Weber, L., and Schiebel, E. (2014) Cell-cycle dependent
phosphorylation of yeast pericentrin regulates γ-TuSC-mediated
microtubule nucleation, Elife, 3, e02208.
85.Lansbergen, G., Grigoriev, I., Mimori-Kiyosue,
Y., Ohtsuka, T., Higa, S., Kitajima, I., Demmers, J., Galjart, N.,
Houtsmuller, A. B., Grosveld, F., and Akhmanova, A. (2006) CLASPs
attach microtubule plus ends to the cell cortex through a complex with
LL5beta, Dev. Cell, 11, 21-32.
86.Miller, P. M., Folkmann, A. W., Maia, A. R.,
Efimova, N., Efimov, A., and Kaverina, I. (2009) Golgi-derived
CLASP-dependent microtubules control Golgi organization and polarized
trafficking in motile cells, Nature Cell Biol., 11,
1069-1080.
87.Al-Bassam, J., Kim, H., Brouhard, G., van Oijen,
A., Harrison, S. C., and Chang, F. (2010) CLASP promotes microtubule
rescue by recruiting tubulin dimers to the microtubule, Dev.
Cell, 19, 245-258.
88.Lin, Y. C., Chiang, T. C., Liu, Y. T., Tsai, Y.
T., Jang, L. T., and Lee, F. J. (2011) ARL4A acts with GCC185 to
modulate Golgi complex organization, J. Cell Sci., 124,
4014-4026.
89.Efimov, A., Kharitonov, A., Efimova, N.,
Loncarek, J., Miller, P. M., Andreyeva, N., Gleeson, P., Galjart, N.,
Maia, A. R., McLeod, I. X., Yates, J. R., 3rd, Maiato, H., Khodjakov,
A., Akhmanova, A., and Kaverina, I. (2007) Asymmetric CLASP-dependent
nucleation of noncentrosomal microtubules at the trans-Golgi
network, Dev. Cell, 12, 917-930.
90.Hurtado, L., Caballero, C., Gavilan, M. P.,
Cardenas, J., Bornens, M., and Rios, R. M. (2011) Disconnecting the
Golgi ribbon from the centrosome prevents directional cell migration
and ciliogenesis, J. Cell Biol., 193, 917-933.
91.Vinogradova, T., Miller, P. M., and Kaverina, I.
(2009) Microtubule network asymmetry in motile cells: role of
Golgi-derived array, Cell Cycle, 8, 2168-2174.
92.Hehnly, H., Xu, W., Chen, J. L., and Stamnes, M.
(2010) Cdc42 regulates microtubule-dependent Golgi positioning,
Traffic, 11, 1067-1078.
93.Burakov, A. V., Zhapparova, O. N., Kovalenko, O.
V., Zinovkina, L. A., Potekhina, E. S., Shanina, N. A., Weiss, D. G.,
Kuznetsov, S. A., and Nadezhdina, E. S. (2008) Ste20-related protein
kinase LOSK (SLK) controls microtubule radial array in interphase,
Mol. Biol. Cell, 19, 1952-1961.
94.Zhapparova, O. N., Fokin, A. I., Vorobyeva, N.
E., Bryantseva, S. A., and Nadezhdina, E. S. (2013) Ste20-like protein
kinase SLK (LOSK) regulates microtubule organization by targeting
dynactin to the centrosome, Mol. Biol. Cell, 24,
3205-3214.
95.Storrie, B., White, J., Rottger, S., Stelzer, E.
H., Suganuma, T., and Nilsson, T. (1998) Recycling of Golgi-resident
glycosyltransferases through the ER reveals a novel pathway and
provides an explanation for nocodazole-induced Golgi scattering, J.
Cell Biol., 143, 1505-1521.
96.Fath, K. R., Trimbur, G. M., and Burgess, D. R.
(1994) Molecular motors are differentially distributed on Golgi
membranes from polarized epithelial cells, J. Cell Biol.,
126, 661-675.
97.Gyoeva, F. K., Bybikova, E. M., and Minin, A. A.
(2000) An isoform of kinesin light chain specific for the Golgi
complex, J. Cell Sci., 113, 2047-2054.
98.Burkhardt, J. K., Echeverri, C. J., Nilsson, T.,
and Vallee, R. B. (1997) Overexpression of the dynamitin (p50) subunit
of the dynactin complex disrupts dynein-dependent maintenance of
membrane organelle distribution, J. Cell Biol., 139,
469-484.
99.Haghnia, M., Cavalli, V., Shah, S. B.,
Schimmelpfeng, K., Brusch, R., Yang, G., Herrera, C., Pilling, A., and
Goldstein, L. S. (2007) Dynactin is required for coordinated
bidirectional motility, but not for dynein membrane attachment, Mol.
Biol. Cell, 18, 2081-2089.
100.Devarajan, P., Stabach, P. R., Mann, A. S.,
Ardito, T., Kashgarian, M., and Morrow, J. S. (1996) Identification of
a small cytoplasmic ankyrin (AnkG119) in the kidney and muscle that
binds beta I sigma spectrin and associates with the Golgi apparatus,
J. Cell Biol., 133, 819-830.
101.Holleran, E. A., Tokito, M. K., Karki, S., and
Holzbaur, E. L. (1996) Centractin (ARP1) associates with spectrin
revealing a potential mechanism to link dynactin to intracellular
organelles, J. Cell Biol., 135, 1815-1829.
102.Fath, K. R., Trimbur, G. M., and Burgess, D. R.
(1997) Molecular motors and a spectrin matrix associate with Golgi
membranes in vitro, J. Cell Biol., 139,
1169-1181.
103.Matas, O. B., Martinez-Menarguez, J. A., and
Egea, G. (2004) Association of Cdc42/NWASP/Arp2/3 signaling pathway
with Golgi membranes, Traffic, 5, 838-846.
104.Luna, A., Matas, O. B., Martinez-Menarguez, J.
A., Mato, E., Duran, J. M., Ballesta, J., Way, M., and Egea, G. (2002)
Regulation of protein transport from the Golgi complex to the
endoplasmic reticulum by CDC42 and N-WASP, Mol. Biol. Cell,
13, 866-879.
105.Chen, J. L., Fucini, R. V., Lacomis, L.,
Erdjument-Bromage, H., Tempst, P., and Stamnes, M. (2005)
Coatomer-bound Cdc42 regulates dynein recruitment to COPI vesicles,
J. Cell Biol., 169, 383-389.
106.Hehnly, H., Xu, W., Chen, J. L., and Stamnes,
M. (2010) Cdc42 regulates microtubule dependent Golgi positioning,
Traffic, 11, 1067-1078.
107.Yadav, S., Puthenveedu, M. A., and Linstedt, A.
D. (2012) Golgin 160 recruits the dynein motor to position the Golgi
apparatus, Dev. Cell, 23, 153-165.
108.Hoogenraad, C. C., Akhmanova, A., Howell, S.
A., Dortland, B. R., De Zeeuw, C. I., Willemsen, R., Visser, P.,
Grosveld, F., and Galjart, N. (2001) Mammalian Golgi-associated
Bicaudal-D2 functions in the dynein–dynactin pathway by
interacting with these complexes, EMBO J., 20,
4041-4054.
109.Matanis, T., Akhmanova, A., Wulf, P., Del Nery,
E., Weide, T., Stepanova, T., Galjart, N., Grosveld, F., Goud, B., De
Zeeuw, C. I., Barnekow, A., and Hoogenraad, C. C. (2002) Bicaudal-D
regulates COPI-independent Golgi–ER transport by recruiting the
dynein–dynactin motor complex, Nature Cell Biol.,
4, 986-992.
110.Wanschers, B. F., van de Vorstenbosch, R.,
Schlager, M. A., Splinter, D., Akhmanova, A., Hoogenraad, C. C.,
Wieringa, B., and Fransen, J. A. (2007) A role for the Rab6B
Bicaudal-D1 interaction in retrograde transport in neuronal cells,
Exp. Cell Res., 313, 3408-3420.
111.Splinter, D., Razafsky, D. S., Schlager, M. A.,
Serra-Marques, A., Grigoriev, I., Demmers, J., Keijzer, N., Jiang, K.,
Poser, I., Hyman, A. A., Hoogenraad, C. C., King, S. J., and Akhmanova,
A. (2012) BICD2, dynactin, and LIS1 cooperate in regulating dynein
recruitment to cellular structures, Mol. Biol. Cell, 23,
4226-4241.
112.Hirose, H., Arasaki, K., Dohmae, N., Takio, K.,
Hatsuzawa, K., Nagahama, M., Tani, K., Yamamoto, A., Tohyama, M., and
Tagaya, M. (2004) Implication of ZW10 in membrane trafficking between
the endoplasmic reticulum and Golgi, EMBO J., 23,
1267-1278.
113.Inoue, M., Arasaki, K., Ueda, A., Aoki, T., and
Tagaya, M. (2008) N-terminal region of ZW10 serves not only as a
determinant for localization but also as a link with dynein function,
Genes Cells, 13, 905-914.
114.Allan, V. J. (2011) Cytoplasmic dynein,
Biochem. Soc. Trans., 39, 1169-1178.
115.Palmer, K. J., Hughes, H., and Stephens, D. J.
(2009) Specificity of cytoplasmic dynein subunits in discrete
membrane-trafficking steps, Mol. Biol. Cell, 20,
2885-2899.
116.Tai, A. W., Chuang, J. Z., and Sung, C. H.
(1998) Localization of Tctex-1, a cytoplasmic dynein light chain, to
the Golgi apparatus and evidence for dynein complex heterogeneity,
J. Biol. Chem., 273, 19639-19649.