REVIEW: Intracellular Transport Based on Actin Polymerization
S. Yu. Khaitlina
Institute of Cytology, Russian Academy of Sciences, Tikhoretsky pr. 4, 194064 St. Petersburg, Russia; fax: +7 (812) 297-0341; E-mail: skhspb@gmail.com
Received April 17, 2014
In addition to the intracellular transport of particles (cargo) along microtubules, there are in the cell two actin-based transport systems. In the actomyosin system the transport is driven by myosin, which moves the cargo along actin microfilaments. This transport requires the hydrolysis of ATP in the myosin molecule motor domain that induces conformational changes in the molecule resulting in the myosin movement along the actin filament. The other actin-based transport system of the cell does not involve myosin or other motor proteins. This system is based on a unidirectional actin polymerization, which depends on ATP hydrolysis in actin polymers and is initiated by proteins bound to the surface of transported particles. Obligatory components of the actin-based transport are proteins of the WASP/Scar family and a complex of Arp2/3 proteins. Moreover, the actin-based systems often contain dynamin and cortactin. It is known that a system of actin filaments formed on the surface of particles, the so-called “comet-like tail”, is responsible for intracellular movements of pathogenic bacteria, micropinocytotic vesicles, clathrin-coated vesicles, and phagosomes. This movement is reproduced in a cell-free system containing extract of Xenopus oocytes. The formation of a comet-like structure capable of transporting vesicles from the plasma membrane into the cell depth has been studied in detail by high performance electron microscopy combined with electron tomography. A similar mechanism provides the movement of vesicles containing membrane rafts enriched with sphingolipids and cholesterol, changes in position of the nuclear spindle at meiosis, and other processes. This review will consider current ideas about actin polymerization and its regulation by actin-binding proteins and show how these mechanisms are realized in the intracellular actin-based vesicular transport system.
KEY WORDS: cytoskeleton, actin, Arp2/3, dynamin, intracellular vesicles, “comet-like tails”DOI: 10.1134/S0006297914090089
To the memory of Georgii Petrovich Pinaev
It is thought that the intracellular transport is, first of all, associated with the movement of particles (cargo) along microtubules. In addition, there are two intracellular actin-based transport systems. In the first, the actomyosin system, the transport is realized by myosin, which interacts with actin microfilaments. Similarly to sliding of muscle myosin along actin filaments, non-muscle myosin isoforms are moved along the actin microfilaments due to the ATP-dependent interaction of the myosin motor domain (the head) with actin, hydrolysis of ATP bound in the myosin motor domain, and conformational changes in the neck part of the myosin molecule that are induced by ATP hydrolysis. However, as differentiated from muscle myosin, non-muscle myosins usually have short tail domains which do not produce filaments but contain structural motifs promoting the interaction of myosin with other proteins and lipids, i.e. fastening of the cargo [1-3]. Myosin V, the most studied motor protein of this transport system [3-6], is similar in structure and functions to the tubulin transport system motor proteins, kinesin and dynein [7, 8]. This similarity allows myosin V to move not only along actin filaments but also along microtubules [9-11]. It is supposed that, as differentiated from the transport system consisting of microtubules and kinesin/dynein moving the cargo over long distances, the transport along the actin filaments should be local [6].
Another actin-based transport system is not associated with myosin and other motor proteins. This system is based on moving a particle by means of a “comet-like tail” produced on the surface of the particle as a result of actin polymerization directed by ATP hydrolysis in the actin polymers and initiated by proteins bound to the surface of the transported particles. Proteins of the WASP/Scar family and a complex of the Arp2/3 proteins and/or dynamin and cortactin are obligatory components of the actin-based transport [12, 13]. The “comet-like tail” was shown to be responsible for translocation of micropinocytotic vesicles (pinosomes) [14, 15], clathrin-coated vesicles [16], endosomes, and lysosomes in oocytes of the clawed frog Xenopus and in the cell-free system containing an extract from oocytes of this frog [17]. A similar mechanism is involved in the movement of vesicles containing membrane rafts enriched with sphingolipids and cholesterol [18] and in changes in the nuclear spindle position at meiosis [19-21]. This review will consider current ideas about actin polymerization and its regulation by actin-binding proteins and show the action of these mechanisms in the intracellular actin-based transport system.
ACTIN POLYMERIZATION AND DYNAMICS OF ACTIN FILAMENTS
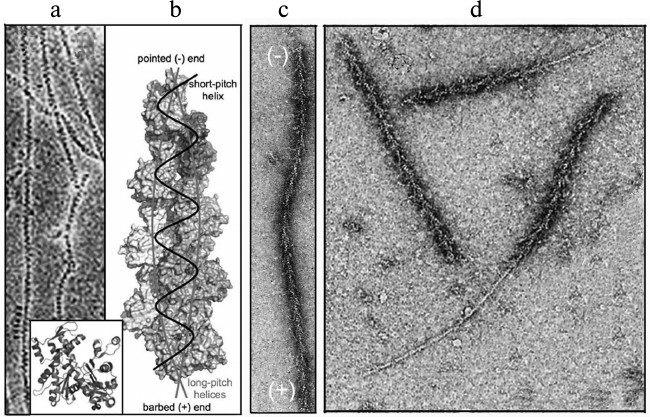
The dynamics of actin structures of the cell are based on the most important property of actin – its ability for reversible polymerization. Actin in solution is a globular protein (G-actin) with molecular weight of 42 kDa. The actin molecule consists of two domains separated by a deep cleft with a tightly bound cation, Ca2+ or Mg2+, and a nucleotide, ATP or ADP [22] (Fig. 1a, inset). Thus, actin is an ATP-binding protein, and nucleotide exchange and ATP hydrolysis play a functional role in it. Under physiological conditions, actin monomers associate with each other producing a double-helical polymer (actin filament, microfilament, F-actin) [23]. The structure of this polymer is described either as two long-pitch helixes noncovalently bound to each other, or as one short-pitch helix [24] (Fig. 1, a and b). Because all actin monomers are oriented in the same direction, actin filaments are polar. The filament polarity can be easily determined morphologically on the interaction of F-actin with myosin or its fragments, which results in so-called “arrow-head structures” with the direction determined by the barbed or (+)-end of the arrow and by the pointed or (–)-end of the filament that corresponds, respectively, to its rapid or slow elongation (Fig. 1c) [25].
Fig. 1. Structure of actin and polarity of filaments. a) Ultrastructure of actin filaments by electron microscopy data [23]; the inset presents a crystal structure of actin monomer [22]. b) Model of actin filament based on results of electron microscopy and data on the crystal structure of the monomer [24]. c, d) Ultrastructure of actin filaments decorated by a fragment of myosin molecule by data of electron microscopy [25]. Explanations in detail are in the text. The figures are printed by courtesy of the relevant publishers.
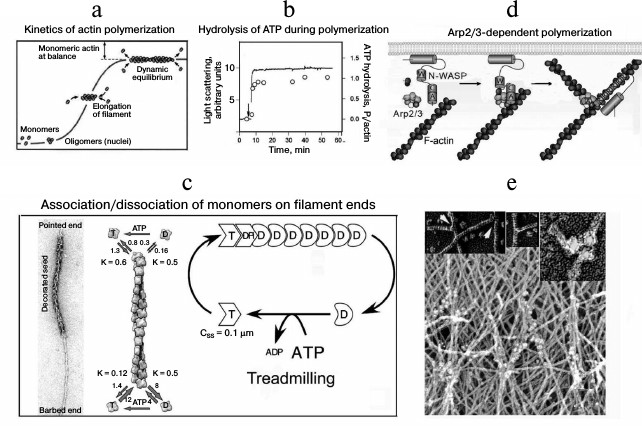
The actin polymerization occurs as a polycondensation with nucleation as its first stage. The nucleation is an interaction of monomers leading to appearance of polymerization nuclei. Stable polymerization nuclei consist of actin trimers, which elongate up to establishment of balance (Fig. 2a). The elongation of actin filaments is accompanied by an irreversible hydrolysis of bound ATP (Fig. 2b) and release of inorganic phosphate. The hydrolysis of ATP leads to changes in the conformation of actin subunits, and the bonds between them weaken [26]. Direct measurements of association and dissociation rates of the monomers on the filament ends have shown that ATP-G-actin is efficiently joined to the fast (+)-end of the filament, whereas ADP-G-actin dissociates from the slow (–)-end (Fig. 2c). This is associated with a slow “movement” of actin subunits from the fast to the slow end of the filament, so-called treadmilling [27].
Fig. 2. Actin polymerization and production of a “dendrite-like” network. a) Kinetics of the spontaneous polymerization of actin. b) Hydrolysis of actin-bound ATP during polymerization; solid line, polymerization kinetics; circles, amount of inorganic phosphate. c) Schemes illustrating the growth of actin filaments decorated with a myosin fragment (to the left), the rate of association/dissociation of monomers of the filament ends (center), and the dynamics of actin polymers (to the right) [27]. d) Scheme illustrating the nucleation of the actin polymerization by the N-WASP–Arp2/3 complex [44]. e) Ultrastructure of a submembrane network of actin filaments by data of immunoelectron microscopy with antibodies to Arp2/3 conjugated with colloidal gold; the insets show the network nodes containing Arp2/3 at different magnifications [32]. Detailed explanations are presented in the text. The figures are fragments of illustrations in the indicated papers printed by courtesy of the relevant publishers.
The intracellular polymerization of actin is actively regulated by a multiplicity of actin-binding proteins, which determine its localization and the length and lifetime of the filaments or interact with monomers maintaining the pool of globular actin and the bound nucleotide state [28-30]. For the actin-dependent transport based on actin polymerization protein-nucleators, in particular the complex Arp2/3 (actin-related proteins), are the most important. This complex consists of seven proteins including Arp2 and Arp3 with structures similar to that of actin and auxiliary proteins responsible for binding with activators (Fig. 2d). Model experiments revealed that the Arp2/3 complex should be activated for interaction with actin. In the cell this function is realized by proteins of the WASP/Scar family. The activated Arp2/3 initiates actin polymerization or interacts with an actin filament; and Arp2 and Arp3 are the first subunits of the new filament. When the Arp2/3 complex is bound with the actin filament, it acts as a nucleator of new filaments and blocks their slow end. This produces a network of actin filaments located with angle of 70° to one another and bound by Y-like contacts [31]. Thus, the Arp2/3 complex geometry determines the formation of branched (dendrite-like) structures, which occur to be like submembrane structures detected in the cortical cytoskeleton on the front edge of moving cells (Fig. 2e) [32]. These structures are also found to be able to provide for intracellular transport, which was for first shown on investigation of pathogenic bacteria moving in the cytoplasm of eukaryotic cells using a bundle of actin filaments reminiscent of the tail of a comet.
INTRACELLULAR MOVEMENT OF BACTERIA USING A “COMET-LIKE
TAIL”
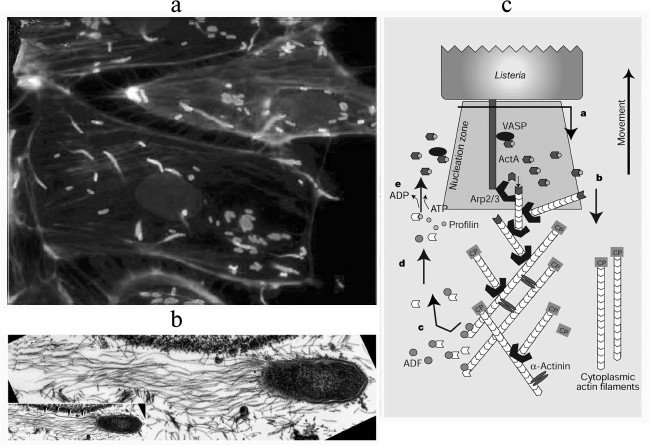
Invasion of eukaryotic cells by bacteria can be observed using fluorescence or electron microscopy (Fig. 3). Adhering to the surface of a non-phagocytic eukaryotic cell, the pathogenic bacteria Listeria monocytogenes and Shigella flexneri induce massive rearrangements of the cortical cytoskeleton and production of phagosomes to penetrate within them into the cell. Then the bacteria lyse the phagosomal membrane and enter the cytoplasm. The next stage is the nucleation of actin filaments on the surface of the bacteria and formation on the apical end of the bacteria of a “comet-like tail” – a bundle of actin filaments used by the bacteria for movement inside the cell [33]. The rate of this movement is equal to the rate of actin polymerization [34]. If this movement brings the bacteria near the cell membrane, they initiate formation of protrusions whose contacts with another cell results in repeating the process [35-38].
Fig. 3. Actin “comet-like tails” providing for intracellular movement of pathogenic bacteria. a) Cultured PtK2 cells infected with the bacterium Listeria monocytogenes. The bacteria are stained with FITC-antibodies (white “comet heads” in the figure), the “comet-like tails” are stained with rhodamine—phalloidin (gray in the figure) [38]. b) Electron microphotograph of a “comet-like tail” of the bacterium Rickettsia conorii. Actin filaments of the “tail” are decorated by S1 fragment of the myosin head [37]. c) Scheme illustrating the involvement of the bacterial protein ActA and actin-binding proteins in formation of the “comet-like tail” on the apical end of the bacterium L. monocytogenes. Results are presented of the reconstruction of the bacterial movement in vitro in the presence of purified actin-binding proteins [48, 49]. Detailed explanations are given in the text. The figures are fragments of illustrations in the indicated papers and are printed by courtesy of the relevant publishers.
The surface protein ActA is a bacterial factor initiating actin polymerization on the apical surface of L. monocytogenes [39, 40]. In the bacterium Sh. flexneri the protein IcsA plays a similar role [41]. In cells transfected with the protein ActA gene, the protein induced the assembly of actin filaments on mitochondria [40]. The assembly of actin filaments was also observed on granules of DEAE-cellulose bound with ActA [41]. However, neither mitochondria nor cellulose granules were moved, because for a directed movement an asymmetric distribution of the protein on the particle surface is required [42, 43].
Although the presence of ActA and IcsA proteins is necessary for the assembly of actin filaments, this process is not observed in solution of F-actin because either ActA or IcsA do not directly interact with actin. Model experiments have shown that the nucleation of actin filaments in solution can be successful in the presence of the protein complex Arp2/3 and proteins of the WASP family [44-46]. Thus, on the surface of the bacterium Sh. flexneri the polymerization of actin is initiated by interaction of IcsA with the protein N-WASP and components of the Arp2/3 complex. The interaction with IcsA activates N-WASP due to opening the actin-binding domain of this protein. After the nucleation the activating protein (WASP or ActA) dissociates from the Arp2/3 complex and can participate in a new cycle of nucleation. The Arp2/3 complex activated by the WASP family protein remains bound on the lateral side of actin filaments and serves as a nucleator for production of a dendrite-like network of filaments on the surface of the bacterium.
The existence of a firm network consisting of short segments is fundamentally important for the ability of actin filaments to push bacteria forward [47]. The model explaining the movement based on actin polymerization [47] considers actin filaments as elastic helical bands bending because of thermal fluctuations. This results in generation of a gap between the bacterial surface and the filament end where an actin monomer can be inserted. The backward movement of the elongated actin filament creates a force that pushes the bacterium. The rate of movement depends on the frequency of filament bending, i.e. on the flexibility and elasticity of the filaments.
The concentration of globular actin is another very important factor determining the rate of movement of bacteria. For functioning of the movement mechanism based on actin polymerization, the actin filament elongation due to addition of actin monomers on the fast end of the filaments (facing the bacterium) has to be caused by dissociation of actin monomers from the slow end. The in vitro reconstruction of bacterial movement in the presence of purified actin-binding proteins has shown [48, 49] that this balance is maintained by the combined action of two proteins, ADF/cofilin [50] and profilin [51]. The affinity of cofilin for ADP-actin is higher than for ATP-actin, and therefore cofilin significantly accelerates the dissociation of actin monomers from the slow end [28]. Profilin effectively binds actin monomers and thus prevents their rejoining the slow end of the filaments, and it transfers them to the fast end [28]. The effective depolymerization of many slow ends of the filaments is responsible for rapid elongation of the filaments on the opposite end and, consequently, promotes efficient movement of the bacterium.
Thus, the intracellular movement of a bacterium is maintained by a complex interaction of actin, the activated Arp2/3 complex, the depolarizing protein ADF/cofilin, and a capping protein. The efficiency of L. monocytogenes movement increases in the presence of profilin, VASP, and α-actinin (Fig. 3c). This movement is not associated with the presence of myosin or other motor proteins, i.e. it is a specific kind of motility based on the energy of ATP hydrolysis with the directed polymerization of actin. Note also that the majority of the components of this system are multidomain proteins or protein complexes, and their interactions leads to necessary conformational and structural rearrangements. Studies on these interactions resulted in elucidation of mechanisms of the movement based on actin polymerization in other processes, in particular, on the generation of lamellipodia and phyllopodia.
In the cell proteins of the WASP/Scar family are associated with the membrane and in turn are activated by proteins of signaling cascades. Enlargement of this process results in the generation of a network of actin filaments on the front edge of moving cells, and this becomes a basis for the hypothesis that actin polymerization should play a leading part in translocation of the cytoplasmic membrane on the generation of lamellipodia [52]. This hypothesis has been confirmed by subsequent studies [12, 53]. Moreover, studies on the comet-like movement of bacteria induced the question whether a similar mechanism could be used for the movement of intracellular organelles.
MOVEMENT OF CELLULAR VESICLES USING THE “COMET-LIKE
TAIL”
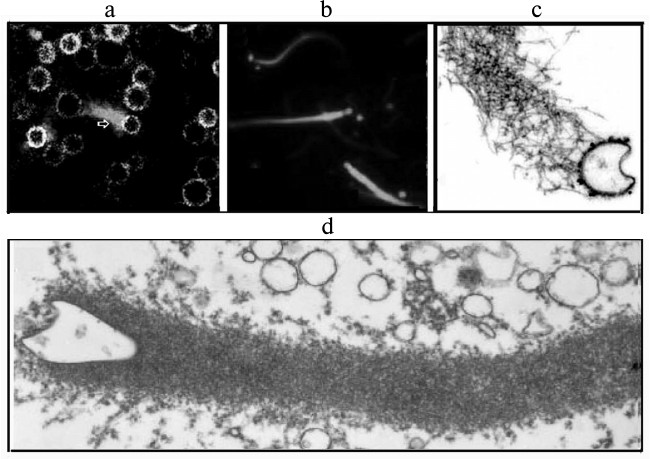
Pathogenic bacteria penetrating into the cells are similar in shape and size to intracellular organelles (endosomes, lysosomes, mitochondria, and endoplasmic reticulum and Golgi apparatus vesicles). This similarity suggests that for the movement within the cell bacteria can use mechanisms that are normally used by cellular organelles for assembly of actin structures [17]. This was confirmed by data on the existence in activated oocytes of Xenopus of vesicles that moved in the cytoplasm using an actin “comet-like tail” (Fig. 4). The ultrastructure and ability of these vesicles to accumulate Acridine Orange (a marker of endosomes) or Lamp 1 (a marker of late endosomes and lysosomes) indicated them to be endosomes and lysosomes. These vesicles were enriched with protein kinase C, their generation was stimulated by an activator of protein kinase C, and the process could be reconstructed in a cell-free system. On the surface of these vesicles the protein N-WASP was found [17, 54].
Fig. 4. Actin “comet-like tails” responsible for the intracellular movement of endosomes in the cytosol of activated oocytes of the clawed frog Xenopus (a) or in the cell-free extract from the frog oocytes (b-d). a) Movement of endogenous endosomes containing GFP-protein kinase using actin “comets” (arrow) in oocytes of Xenopus. Actin structures are stained with rhodamine—phalloidin. b) Movement of endosomes generated on the internalization of Texas-Red-transferrin in cell-free extract from Xenopus oocytes. The actin structures are stained with Alexa(488)–phalloidin. c) Detection of N-WASP, an activator of “dendrite-like” actin polymerization, in the actin tail of the surface of the endosome stained with antibodies to N-WASP conjugated with colloidal gold. d) Ultrastructure of the “comet-like tail” on the surface of a vesicle. The figures are fragments of illustrations in the work of Taunton et al. [17] printed by courtesy of the relevant publishers.
The “comet-like tails” moved not only clathrin-containing vesicles, but also pinocytotic vesicles and lysosomes in different cultured cells and cell extracts, including 3T3 fibroblasts, mast cells, and extracts from HeLa cells [14-16, 55]. It was also shown that phagocytosis induced by treatment of macrophages with zinc or lanthanum ions induced directed actin polymerization that moved these particles in the cytoplasm [56, 57]. Although the lifetime of actin “comets” moving different particles varied, the movement rate of these different vesicles was approximately the same and similar to the rate of the intracellular movement of bacteria that was earlier shown [34] to be equal to the rate of actin polymerization.
The transport of vesicles associated with the polymerization of actin has been found not only in clathrin-dependent endocytosis, but also in caveolin/raft-type endocytosis. A local polymerization of actin was observed on internalization of caveolae induced by SV40 virus [58]. Endogenous or exogenous vesicles containing phosphatidylinositol (PIP2) were moved in the cell-free system due to generation of actin “comets”; excess expression in the cells of the phosphatidylinositol-4-phosphate-5-kinase (PIP5KI) gene induced the generation of “comets” that could be inhibited by methyl-β-cyclodextrin selectively binding cholesterol [18]. Actin “comet-like tails” are generated with involvement of activators of the WASP–Arp2/3 complex: small GTPases Cdc42 [54] and ARF6 [59], tyrosine kinase, phospholipase D, D3-phosphoinositides [60], or phosphatidylinositol-4,5-bisphosphate (PIP2) [18] and other components of signaling systems of the cell. A similar Arp2/3-dependent involvement of actin polymerization is also suggested to contribute to the movement of mitochondria [60, 61]. Another nucleator of actin polymerization, formin, participates in spindle displacement during meiosis of mammalian oocytes: the actin polymerization under the influence of formin leads to production of a “cloud” of actin filaments around chromosomes that later becomes asymmetric and pushes the chromosomes and spindle to the oocyte cortex [19-21].
Specific features of the actin structures generated on a vesicle seem to be determined by duration of the signal initiating the polymerization. A short-term signal induces the generation of an actin “cloud” incapable of moving a vesicle or capable of supporting the movement over only a very small distance. Thus, the internalization of clathrin vesicles is accompanied by an “explosive” polymerization of actin and advancement only by 150-200 nm [16]. However, prolongation of the signal stimulating the polymerization of actin leads to generation of a “comet-like tail”. Thus, the internalization of caveolae induced by the SV40 virus is associated with a slow polymerization and generation of “tails” (i.e. vesicle translocation) with length up to 1300 nm [58]. Therefore, the actin polymerization can be responsible for both the separation of the vesicles from the plasma membrane and pushing away the generated vesicle from the membrane over a small distance to make the vesicle available for moving by another transport system [62].
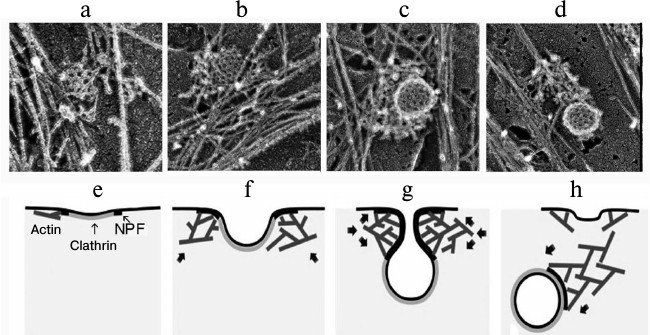
Experiments to discriminate these processes revealed that the inhibition of actin polymerization by latrunculin B blocked many stages in the generation of clathrin vesicles including the separation from the plasma membrane and pushing away the produced vesicle. These observations suggested that actin should be involved in these stages of the generation of endosomes and that actin filaments adjacent to the neck of the clathrin pit through their “fast” ends should initially promote vesicles closing and separation from the membrane and then produce a “comet-like tail” on the separated vesicle [63]. This model has been confirmed by data obtained by high-performance electron microscopy combined with electron tomography on the three-dimensional organization of the actin cytoskeleton during clathrin-mediated endocytosis [64]. This work showed that formation of the active “comet-like tail” is a terminal stage of the actin assembly on coated vesicles. The polymerization of actin induced by proteins of the WASP family and the Arp2/3 complex leads to formation of a network of filaments initially as small lateral islets on the periphery of the vesicle. Then the filaments encircle the partially invaginated vesicle and transform to a polarized “tail” when the vesicle is ready for separation or is separated from the plasma membrane. And “fast” ends of actin filaments push away the endocytotic vesicles from the plasma membrane. This means the conversion of the branched network of actin filaments formed around the coated pit into a comet-like structure capable of transporting the vesicle from the plasma membrane into the depth of the cell (Fig. 5) [64]. It seems that a similar mechanism can regulate the generation of “comet-like tails” and transport of other intracellular vesicles.
Fig. 5. Sequential stages of formation of a “comet-like tail” during clathrin-dependent endocytosis. a-d) Electron microphotographs of actin structures produced on the surface of endosomes during clathrin-dependent endocytosis. The figure is composed of fragments of illustrations presented in the work of Collins et al. [64]. e-h) Scheme illustrating the generation of actin “comet-like tails” on the surface of endosomes during clathrin-dependent endocytosis [64]. e) Initial stage of assembly of a dendrite-like actin network initiated on the periphery of clathrin structures by proteins stimulating the nucleation of actin (NPF, nucleation promoting factors). f) Enlargement of the actin network that accompanies the generation of the clathrin pit. g) Dendrite-like actin network encircles a bridge between the membrane and clathrin vesicle squeezing and elongating it. h) Reorganization of the actin network into a “comet-like tail” terminating the closing of the clathrin-coated vesicle and its separation from the cytoplasmic membrane. Arrows in (f-h) indicate the direction of the pushing force. The figure is printed by courtesy of the relevant publishers.
Thus, the movement of intracellular vesicles and organelles seems to be provided for by the same mechanisms as the movement of the front end of the cell and of intracellular pathogenic bacteria. These data indicate that mobility based on actin polymerization is the same widely distributed type of mobility as the myosin-dependent transport along actin filaments. Necessary components of this transport system first of all are proteins of the WASP/Scar family and the Arp2/3 complex. Moreover, other proteins of signaling systems and the cytoskeleton capable of initiating actin polymerization as a result of a subsequent activation reaction can also be involved in the generation of actin “comets”. Dynamin is one such protein.
DYNAMIN REGULATES ASSEMBLY OF A “COMET-LIKE TAIL” ON
INTRACELLULAR VESICLES
In addition to the proteins WASP/Scar and Arp2/3, the multidomain large GTPase dynamin is an integral component of all “comet-like tails” moving intracellular vesicles. Dynamin is known as one of major proteins of endocytosis involved in the separation of newly generated vesicles from the cellular membrane and Golgi apparatus. Moreover, dynamin participates in the production of caveolae, remodeling the membrane during fusion of vesicles and in other processes [13, 16, 65-67]. Dynamin is also involved in rearrangements of the cytoskeleton unassociated with membrane remodeling [13, 68, 69]. In particular, dynamin co-localization with actin was shown in the “comet-like tails” of moving vesicles, whereas expression of mutant dynamin decreased the generation of comets, their length, and the rate and regularity of their movement [13, 15, 70]. And the influence of the mutant dynamin was associated not with inhibition of the clathrin-mediated endocytosis, but with disorders in the interaction of dynamin with cortactin [70]. The multidomain structure of dynamin allows it to immediately interact with cortactin, profilin, syndapin, and the protein Abp1, which regulate the active cytoskeleton. Studies in vitro with purified proteins revealed that dynamin influences actin polymerization induced by Arp2/3 and cortactin. Moreover, dynamin promoted the Arp2/3- and cortactin-dependent association of actin with lipid vesicles containing PIP2 [70]. As cortactin binds Arp2/3 and co-localizes with it in actin “comets” [58, 71], the influence of dynamin seems to be determined by the sequential interaction of dynamin–cortactin–Arp2/3–actin.
Moreover, dynamin can promote actin polymerization through an indirect interaction with protein CIP4 from the BAR family that concurrently interacts with N-WASP [72]. Dynamin was also shown to interact with actin filaments, displacing the capping protein from the ends of the filaments that promotes their elongation [73], and it may be essential for the generation of actin “comets”.
Thus, dynamin structurally bound with vesicles at all stages of their generation can play a key role in the initiation of the assembly of “comet-like tails” on these vesicles. It is supposed that during early stages of vesicle generation dynamin should be involved in the formation and narrowing of the bridge between the plasma membrane and the vesicle [69, 74], and at a later stage of vesicle generation dynamin could promote the assembly of proteins initiating actin polymerization and generation of the “comet-like tail” [63, 69].
The movement of vesicles and organelles by means of directed polymerization of actin seems to be a widely distributed transport system of the cell similar to systems of intracellular transport along microtubules and microfilaments. It is known that the direction and rate of movement of vesicles in this system are determined by proteins of the Arp2/3 complex, which can be activated by proteins of the WASP/Scar family and by the system of proteins including dynamin and cortactin. Data now available describe the transformation stages of the symmetric network of actin filaments into an asymmetric structure more or less reminiscent of a “comet-like tail” and capable of moving vesicles and organelles in the cytoplasm. However, it is obvious that in some cases this transporter carries cargo over a small distance and works for a short time. Therefore, it cannot always be detected, and actin-based transport can appear to be myosin-based. These processes coincide in the cell, and this makes them to appear not as clear as in a cell-free system. Moreover, it is important that myosin is not only a motor protein, but it is also a nucleator of actin polymerization [75, 76]. Thus, the presence of myosin 1E during endocytosis coincides with active actin polymerization and is associated with attraction of proteins of the WASP family [77]. Such activity seems to be inherent also in other myosins 1, which suggests a possible role of myosin not only in transport but also in time and spatial regulation of the assembly of actin structures.
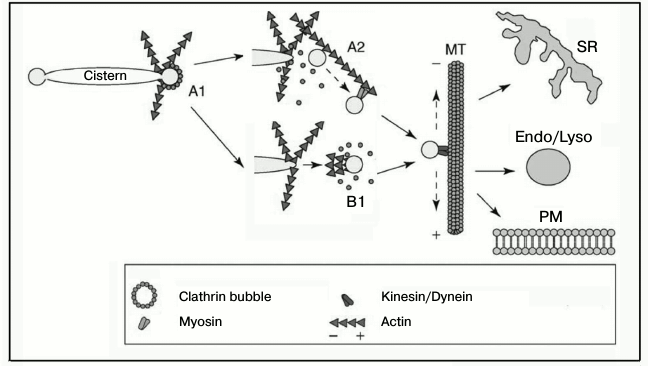
On the other hand, the movement of a vesicle can occur due to cooperation of different transport systems, as it seems to occur in the transport of Golgi apparatus vesicles (Fig. 6) [78]. It is reasonable to suppose that the movement of other vesicles should occur via a similar mechanism. During the initial stages of movement, vesicles are transported over a small distance by the actin-dependent polymerization or myosin-based transport along the actin filaments, whereas the movement over long distances occurs along microtubules under the influence of kinesin or dynein [78, 79]. Regulatory aspects of these interactions are insufficiently studied. For such studies it seems very promising to combine optical and molecular genetics methods, in particular, high-performance electron microscopy and electron tomography, which allows researchers to see the localization of individual proteins and their mutual positions.
Fig. 6. Scheme illustrating the cooperation of the actin-based transport system with other transport systems of the cell. A) Formation of a dendrite-like network of actin filaments as a result of sequential actions of inducers and nucleators (CDC42, WASP/Scar, Arp2/3, dynamin, BAR-proteins, cortactin) (A1). Actin filaments promote the closing of the membrane and separation of the vesicle and its transfer over a small distance using the myosin motor (A2). Concurrently with the vesicle separation or immediately upon its separation from the membrane, a “comet-like tail” is generated on the vesicle surface that transfers the vesicle to the microtubule (B1). In the next stage, vesicles are delivered along the microtubules to their localizations under the influence of kinesin or dynein. SR, sarcoplasmic reticulum; Endo/Lyso, endosomes/lysosomes; PM, plasma membrane; MT, microtubule. The figure is modified from the work of Egea et al. [78] and printed by courtesy of the relevant publishers.
I am grateful to Professor Georgii Petrovich Pinaev for fruitful discussions.
This work was supported by the Russian Foundation for Basic Research (project 14-04-00316) and the Program “Molecular and Cell Biology” (project No. 2211) of the Russian Academy of Sciences Presidium.
REFERENCES
1.Langford, G. M. (1995) Actin- and
microtubule-dependent organelle motors: interrelationships between the
two motility systems, Curr. Opin. Cell Biol., 7,
82-88.
2.Maravillas-Montero, J., and Santos-Argumedo, L.
(2013) The myosin family: unconventional roles of actin-dependent
molecular motors in immune cells, J. Leukocyte Biol., 91,
35-45.
3.Hammer, J. A., 3rd, and Sellers, J. R. (2012)
Walking to work: roles for class V myosins as cargo transporters,
Nat. Rev. Mol. Cell. Biol., 13, 13-26.
4.Mehta, A. D., Rock, R. S., Rief, M., Spudich,
J. A., Mooseker, M. S., and Cheney, R. E. (1999) Myosin-V is a
processive actin-based motor, Nature, 400, 590-593.
5.Wollert, T. D., Weiss, G., Gerdes, H.-H., and
Kuznetsov, S. A. (2002) Activation of myosin V-based motility and
F-actin-dependent network formation of endoplasmic reticulum during
mitosis, J. Cell Biol., 150, 571-577.
6.Kapitein, L. C., van Bergeijk, P., Lipka, J.,
Keijzer, N., Wulf, P. S., Katrukha, E. A., Akhmanova, A., and
Hoogenraad, C. C. (2013) Myosin-V opposes microtubule-based cargo
transport and drives directional motility on cortical actin, Curr.
Biol., 23, 828-834.
7.Von Delius, M., and Leigh, D. A. (2011)
Walking molecules, Chem. Soc. Rev., 40, 3656-3676.
8.Van den Berg, R., and Hoogenraad, C. C. (2012)
Molecular motors in cargo trafficking and synapse assembly, Adv.
Exp. Med. Biol., 970, 173-196.
9.Ali, M. Y., Lu, H., Bookwalter, C. S., Warshaw, D.
M., and Trybus, K. M. (2008) Myosin V and kinesin act as tethers
to enhance each others’ processivity, Proc. Natl. Acad. Sci.
USA, 105, 4691-4696.
10.Ross, J. L., Ali, M. Y., and Warshaw, D. M.
(2008) Cargo transport: molecular motors navigate a complex
cytoskeleton, Curr. Opin. Cell Biol., 20, 41-47.
11.Schroeder, H. W., III, Mitchell, C., Shuman, H.,
Holzbaur, E. L., and Goldman, Y. E. (2010) Motor number controls cargo
switching at actin–microtubule intersections in vitro,
Curr. Biol., 20, 687-696.
12.Le Clainche, C., and Carlier, M.-F. (2008)
Regulation of actin assembly associated with protrusion and adhesion in
cell migration, Physiol. Rev., 88, 489-513.
13.Schafer, D. A., Weed, S. A., Binns, D., Karginov,
A. V., Parsons, J. T., and Cooper, J. A. (2002) Dynamin 2 and cortactin
regulate actin assembly and filament organization, Curr. Biol.,
12, 1852-1857.
14.Merrifield, C. J., Moss, S. E., Ballestrem, C.,
Imhof, B. A., Giese, G., Wunderlich, I., and Almers, W. (1999)
Endocytic vesicles move at the tips of actin tails in cultured mast
cells, Nat. Cell Biol., 1, 72-74.
15.Orth, J. D., Krueger, E. W., Cao, H., and
McNiven, M. A. (2002) The large GTPase dynamin regulates actin comet
formation and movement in living cells, Proc. Natl. Acad. Sci.
USA, 99, 167-172.
16.Merrifield, C. J., Feldman, M. E., Wan, L., and
Almers, W. (2002) Imaging actin and dynamin recruitment during
invagination of single clathrin-coated pits, Nature Cell Biol.,
4, 691-698.
17.Taunton, J., Rowning, B. A., Coughlin, M. L., Wu,
M., Moon, R. T., Mitchison, T. J., and Larabell, C. A. (2000)
Actin-dependent propulsion of endosomes and lysosomes by recruitment of
N-WASP, J. Cell. Biol., 148, 519-530.
18.Rozelle, A. L., Machesky, L. M.,
Yamamoto, M., Driessens, M. H., Insall, R. H., Roth, M. G.,
Luby-Phelps, K., Marriott, G., Hall, A., and Yin, H. L. (2000)
Phosphatidylinositol 4,5-bisphosphate induces actin-based movement of
raft enriched vesicles through WASP-Arp2/3, Curr. Biol.,
10, 311-320.
19.Bezanilla, M., and Wadsworth, P. (2008) Spindle
positioning: actin mediates pushing and pulling, Curr. Biol.,
19, R168-R169.
20.Fabritius, A. S., Ellefson, M. L., and McNally,
J. (2011) Nuclear and spindle positioning during oocyte meiosis,
Curr. Opin. Cell Biol., 23, 78-84.
21.Li, R., and Albertini, D. F. (2013) The road to
maturation: somatic cell interaction and self-organization of the
mammalian oocyte, Nat. Rev. Mol. Cell. Biol., 14,
141-152.
22.Kabsch, W., Mannherz, H. G., Suck, D., Pai, E.
F., and Holmes, K. C. (1990) Atomic structure of the actin–DNase
I complex, Nature, 347, 37-44.
23.Hanson, J., and Lowy, J. (1963) The structure of
F-actin and of actin filaments isolated from muscle, J. Mol.
Biol., 6, 46-60.
24.Dominguez, R., and Holmes, K. C. (2011) Actin
structure and function, Annu. Rev. Biophys., 40,
169-186.
25.Woodrum, D. T., Rich, S. A., and Pollard, T. D.
(1975) Evidence for biased bidirectional polymerization of actin
filaments using heavy meromyosin prepared by an improved method, J.
Cell Biol., 67, 231-237.
26.Schuler, H. (2001) ATPase activity and
conformational changes in the regulation of actin, Biochim. Biophys.
Acta, 1549, 137-147.
27.Pollard, T. D., and Borisy, G. G. (2003) Cellular
motility driven by assembly and disassembly of actin filaments,
Cell, 112, 453-465.
28.Pantaloni, D., Le Clainche, C., and Carlier,
M.-F. (2001) Mechanism of actin-based motility, Science,
292, 1502-1506.
29.Chhabra, E. S., and Higgs, H. N. (2007) The many
faces of actin: matching assembly factors with cellular structures,
Nat. Cell Biol., 9, 1110-1121.
30.Domingues, R. (2009) Actin filament nucleation
and elongation factors – structure-function relationships,
Crit. Rev. Biochem. Mol. Biol., 44, 351-366.
31.Mullins, R. D., Heuser, J. A., and Pollard, T. D.
(1998) The interaction of Arp2/3 complex with actin: nucleation, high
affinity pointed end capping, and formation of branching networks of
filaments, Proc. Natl. Acad. Sci. USA, 95, 6181-6186.
32.Svitkina, T. M., Verkhovsky, A. B., and
Borisy, G. G. (1995) Improved procedures for electron microscopic
visualization of the cytoskeleton of cultured cells, J. Struct.
Biol., 115, 290-303.
33.Cossart, P., and Sansonetti, P. J. (2004)
Bacterial invasion: the paradigms of enteroinvasive pathogens,
Science, 304, 242-248.
34.Theriot, J. A., Mitchison, T. J., Tilney, L. G.,
and Portnoy, D. A. (1992) The rate of actin-based motility of
intracellular Listeria monocytogenes equals the rate of actin
polymerization, Nature, 357, 257-260.
35.Tilney, L. G., Connelly, P. S., and Portnoy, D.
A. (1990) Actin filament nucleation by the bacterial pathogen,
Listeria monocytogenes, J. Cell Biol., 111,
2979-2988.
36.Mounier, J., Ryter, A., Coquis-Rondon, M., and
Sansonetti, P. J. (1989) Intracellular and cell-to-cell spread of
Listeria monocytogenes involves interaction with F-actin in the
enterocyte-like cell line Caco-2, Infect. Immun., 58,
1048-1058.
37.Gouin, E., Gantelet, H., Egile, C., Lasa, I.,
Ohayon, H., Villiers, V., Gounon, P., Sansonetti, P. J., and Cossart,
P. (1999) A comparative study of the actin-based motilities of the
pathogenic bacteria Listeria monocytogenes, Shigella
flexneri and Rickettsia conorii, J. Cell Sci.,
112, 1697-1708.
38.Gouin, E., Welch, M. D., and Cossart, P. (2005)
Actin-based motility of intracellular pathogens, Curr. Opin.
Microbiol., 8, 35-45.
39.Pistor, S., Chakraborty, T., Niebuhr, K., Domann,
E., and Wehland, J. (1994) The ActA protein of Listeria
monocytogenes acts as a nucleator inducing reorganization of the
actin cytoskeleton, EMBO J., 13, 758-763.
40.Kocks, C., Marchand, J. B., Gouin, E.,
d’Hauteville, H., Sansonetti, P. J., Carlier, M. F., and Cossart,
P. (1995) The unrelated surface proteins ActA of Listeria
monocytogenes and IcsA of Shigella flexneri are sufficient
to confer actin-based motility on Listeria innocua and
Escherichia coli, respectively, Mol. Microbiol.,
18, 413-423.
41.Bernardini, M. L., Mounier, J., Hauteville, H.
L., Coquis-Ronton, M., and Sansonetti, P. J. (1989) Identification of
IcsA, a plasmid locus of Shigella flexneri that governs
bacterial intra- and intercellular spread through interaction with
F-actin, Proc. Nat. Acad. Sci. USA, 86, 3867-3871.
42.Smith, G. A., Portnoy, D. A., and Theriot, J. A.
(1995) Asymmetric distribution of the Listeria monocytogenes
ActA protein is required and sufficient to direct actin-based motility,
Mol. Microbiol., 17, 945-951.
43.Goldberg, M. B., Barzu, O., Parsot, C., and
Sansonetti, P. J. (1993) Unipolar localization and ATPase activity of
Ics A, a Shigella flexneri protein involved in intracellular
movement, J. Bacteriol., 175, 2189-2196.
44.Campellone, K. G., and Welch, M. D. (2010) A
nucleator arms race: cellular control of actin assembly, Nat. Rev.
Mol. Cell Biol., 11, 237-251.
45.Suzuki, T., Miki, H., Takenawa, T., and Sasakawa,
C. (1998) Neural Wiskott–Aldrich syndrome protein is implicated
in the actin-based motility of Shigella flexneri, EMBO
J., 17, 2767-2776.
46.Egile, C., Loisel, T. P., Laurent, V., Pantaloni,
D., Sansonetti, P. J., and Carlier, M.-F. (1999) Activation of the
CDC42 effector N-WASP by the Shigella flexneri IcsA protein
promotes actin nucleation by Arp2/3 complex and bacterial actin-based
motility, J. Cell Biol., 146, 1319-1322.
47.Mogilner, A., and Oster, G. (1996) Cell motility
driven by actin polymerization, Biophys. J., 71,
3030-3045.
48.Loisel, T. P., Boujemaa, R., Pantaloni, D., and
Carlier, M. F. (1999) Reconstitution of actin-based motility of
Listeria and Shigella using pure proteins, Nature,
401, 613-616.
49.Machesky, L. M., and Cooper, J. A. (1999) Bare
bones of the cytoskeleton, Nature, 401, 542-543.
50.Bravo-Cordero, J. J., Magalhaes, M. A., Eddy, R.
J., Hodgson, L., and Condeelis, J. (2013) Functions of cofilin in cell
locomotion and invasion, Nat. Rev. Mol. Cell. Biol.,
14, 405-415.
51.Krishnan, K., and Moens, P. D. J. (2009)
Structure and functions of profilins, Biophys. Rev., 1,
71-81.
52.Borisi, G. G., and Svitkina, T. M. (2000) Actin
machinery: pushing the envelope, Curr. Opin. Cell Biol.,
12, 104-112.
53.Svitkina, T. (2013) Ultrastructure of protrusive
actin filament arrays, Curr. Opin. Cell Biol., 25,
574-581.
54.Taunton, J. (2001) Actin filament nucleation by
endosomes, lysosomes and secretory vesicles, Curr. Opin. Cell
Biol., 13, 85-91.
55.Kaksonen, M., Peng, H. B., and Rauvala, H. (2000)
Association of cortactin with dynamic actin in lamellipodia and on
endosomal vesicles, J. Cell Sci., 113, 4421-4426.
56.Zhang, F., Southwick, F. S., and Purich, D. L.
(2002) Actin-based phagosome motility, Cell Motil. Cytoskeleton,
53, 81-88.
57.Southwick, F. S., Li, W., Zhang, F., Zeile, W.
L., and Purich, D. L. (2003) Actin-based endosome and phagosome
rocketing in macrophages: activation by the secretagogue antagonists
lanthanum and zinc, Cell Motil. Cytoskeleton, 54,
41-55.
58.Pelkmans, L., Puntener, D., and Helenius, A.
(2002) Local actin polymerization and dynamin recruitment in
SV40-induced internalization of caveolae, Science, 296,
535-539.
59.Schafer, D. A., D’Souza-Schorey, C., and
Cooper, J. A. (2000) Actin assembly at membranes controlled by ARF6,
Traffic, 1, 892-903.
60.Boldogh, I. R., Yang, H.-C., Nowakowski, W. D.,
Karmon, S. L., Hays, L. G., Yates III, J. R., and Pon, L. A. (2001)
Arp2/3 complex and actin dynamics are required for actin-based
mitochondrial motility in yeast, Proc. Natl. Acad. Sci. USA,
98, 63162-63167.
61.Bazinet, C., and Rollins, J. E. (2003)
Rickettsia-like mitochondrial motility in Drosophila
spermiogenesis, Evol. Dev., 5, 379-385.
62.Merrifield, C. J. (2004) Seeing is believing:
imaging actin dynamics at single sites of endocytosis, Trends Cell
Biol., 14, 352-358.
63.Merrifield, C. J., Perrais, D., and Zenisek, D.
(2005) Coupling between clathrin-coated-pit invagination, cortactin
recruitment, and membrane scission observed in live cells, Cell,
121, 593-606.
64.Collins, A., Warrington, A., Taylor, K. A., and
Svitkina, T. (2011) Structural organization of the actin cytoskeleton
at sites of clathrin-mediated endocytosis, Curr. Biol.,
21, 1167-1175.
65.Schmid, S. L., and Frolov, V. A. (2011) Dynamin:
functional design of a membrane fission catalyst, Annu. Rev. Cell.
Dev. Biol., 27, 79-105.
66.Ferguson, S. M., and De Camilli, P. (2012)
Dynamin, a membrane-remodeling GTPase, Nat. Rev. Mol. Cell
Biol., 13, 75-88.
67.Menon, M., and Schafer, D. A. (2013)
Dynamin: expanding its scope to the cytoskeleton, Int. Rev. Cell.
Mol. Biol., 302, 187-219.
68.Conner, S. D., and Schmid, S. L. (2003) Regulated
portals of entry into the cell, Nature, 422, 37-44.
69.Yarar, D., Waterman-Storer, C. M., and Schmid, S.
L. A. (2005) Dynamic actin cytoskeleton functions at multiple stages of
clathrin-mediated endocytosis, Mol. Biol. Cell, 16,
964-975.
70.Lee, E., and De Camilli, P. (2002) Dynamin at
actin tails, Proc. Natl. Acad. Sci. USA, 99, 161-166.
71.Daly, R. J. (2004) Cortactin signaling and
dynamic actin networks, Biochem. J., 382, 13-25.
72.Hartig, S. M., Ishikura, S., Hicklen, R. S.,
Feng, Y., Blanchard, E. G., Voelker, K. A., Pichot, C. S., Grange, R.
W., Raphael, R. M., Klip, A., and Corey, S. J. (2009) The F-BAR
protein CIP4 promotes GLUT4 endocytosis through bidirectional
interactions with N-WASp and Dynamin-2, J. Cell Sci.,
122, 2283-2291.
73.Gu, C., Yaddanapudi, S., Weins, A., Osborn, T.,
Reiser, J., Pollak, M., Hartwig, J., and Sever, S. (2010) Direct
dynamin–actin interactions regulate the actin cytoskeleton,
EMBO J., 29, 3593-3606.
74.Taylor, M. J., Lampe, M., and Merrifield, C. J.
(2012) A feedback loop between dynamin and actin recruitment during
clathrin-mediated endocytosis, PLoS Biol., 10,
e1001302.
75.Miller, L., Phillips, M., and Reisler, E. (1988)
Polymerization of G-actin by myosin subfragment 1, J. Biol.
Chem., 263, 1996-2002.
76.Wawro, B., Khaitlina, S. Yu., Galinska-Rakoczy,
A., and Strzelecka-Golaszewska, H. (2005) Role of DNase-I-binding loop
in myosin subfragment 1-induced actin polymerization. Implications to
the polymerization mechanism, Biophys. J., 88,
2883-2896.
77.Cheng, J., Grassart, A., and Drubin, D. G. (2012)
Myosin 1E coordinated actin assembly and cargo trafficking during
clathrin-mediated endocytosis, Mol. Biol. Cell, 23,
2891-2904.
78.Egea, G., Lazaro-Dieguez, F., and Vilella, M.
(2006) Actin dynamics at the Golgi complex in mammalian cells, Curr.
Opin. Cell Biol., 18, 168-178.
79.Mooren, O. L., Galletta, B. J., and Cooper, J. A.
(2012) Roles for actin assembly in endocytosis, Annu. Rev.
Biochem., 81, 661-686.