Prevention of Peroxidation of Cardiolipin Liposomes by Quinol-Based Antioxidants
A. V. Lokhmatikov1,2, N. E. Voskoboynikova1, D. A. Cherepanov3, N. V. Sumbatyan4, G. A. Korshunova5, M. V. Skulachev6, H.-J. Steinhoff1, V. P. Skulachev2,5, and A. Y. Mulkidjanian1,2,5*
1School of Physics, University of Osnabruck, D-49069 Osnabruck, Germany; E-mail: amulkid@uni-osnabrueck.de2School of Bioengineering and Bioinformatics, Lomonosov Moscow State University, 119991 Moscow, Russia
3Frumkin Institute of Physical Chemistry and Electrochemistry, Russian Academy of Sciences, Leninsky pr. 31, 119991 Moscow, Russia
4School of Chemistry, Lomonosov Moscow State University, 119991 Moscow, Russia
5Belozersky Institute of Physico-Chemical Biology, Lomonosov Moscow State University, 119991 Moscow, Russia
6Institute of Mitoengineering, Lomonosov Moscow State University, 119991 Moscow, Russia
* To whom correspondence should be addressed.
Received March 11, 2014; Revision received May 28, 2014
In mammalian mitochondria, cardiolipin molecules are the primary targets of oxidation by reactive oxygen species. The interaction of oxidized cardiolipin molecules with the constituents of the apoptotic cascade may lead to cell death. In the present study, we compared the effects of quinol-containing synthetic and natural amphiphilic antioxidants on cardiolipin peroxidation in a model system (liposomes of bovine cardiolipin). We found that both natural ubiquinol and synthetic antioxidants, even being introduced in micro- and submicromolar concentrations, fully protected the liposomal cardiolipin from peroxidation. The duration of their action, however, varied; it increased with the presence of either methoxy groups of ubiquinol or additional reduced redox groups (in the cases of rhodamine and berberine derivates). The concentration of ubiquinol in the mitochondrial membrane substantially exceeds the concentrations of antioxidants we used and would seem to fully prevent peroxidation of membrane cardiolipin. In fact, this does not happen: cardiolipin in mitochondria is oxidized, and this process can be blocked by amphiphilic cationic antioxidants (Y. N. Antonenko et al. (2008) Biochemistry (Moscow), 73, 1273-1287). We suppose that a fraction of mitochondrial cardiolipin could not be protected by natural ubiquinol; in vivo, peroxidation most likely threatens those cardiolipin molecules that, being bound within complexes of membrane proteins, are inaccessible to the bulky hydrophobic ubiquinol molecules diffusing in the lipid bilayer of the inner mitochondrial membrane. The ability to protect these occluded cardiolipin molecules from peroxidation may explain the beneficial therapeutic action of cationic antioxidants, which accumulate electrophoretically within mitochondria under the action of membrane potential.
KEY WORDS: apoptosis, respiratory supercomplexes, reactive oxygen species, penetrating cations, plastoquinol, SkQ1DOI: 10.1134/S0006297914100101
Abbreviations: AAPH, 2,2′-azobis(2-aminopropane)dihydrochloride; BHT, 2,6-di-tert-butyl-4-methylphenol; CL, cardiolipin; C11-BODIPY 581/591, 4,4-difluoro-5-(4-phenyl-1,3-butadienyl)-4-bora-3a,4a-diaza-s-indacene-3-undecanoic acid; decPQH2, decylplastoquinol; decUQH2, decylubiquinol; HPMC, 6-hydroxy-2,2,5,7,8-pentamethylbenzochroman; MeO-AMVN, 2,2′-azobis(4-methoxy-2,4-dimethylvaleronitryl); MitoQH2, 10-(2,3-dimethoxy-5-methyl-1,4-benzoquinonyl-6)decyltriphenylphosphonium; ML, methyl linoleate; POPC, 1-palmitoyl-2-oleoylphosphatidylcholine; POPG, 1-palmitoyl-2-oleoylphosphatidylglycerol; Q6H2, ubiquinol-6; Q10H2, ubiquinol-10; ROS, reactive oxygen species; SkQ, compounds composed of penetrating cation and quinone; SkQH2, reduced (quinol) forms of SkQ; SkQ1H2, 10-(plastoquinonyl-6)decyltriphenylphosphonium; SkQ3H2, 10-(methylplastoquinonyl-6)decyltriphenylphosphonium; SkQBerbH2, 13-[9-(6-plastoquinonyl)nonyloxycarbonylmethyl]dihydroberberine; SkQT1H2, 10-(p-toluquinonyl)decyltriphenylphosphonium; SkQR1H2, 10-(plastoquinonyl-6)decyldihydrorhodamine 19.
Organisms that dwell in oxic environments constantly encounter the
problem of neutralizing reactive oxygen species (ROS) that can form
upon metabolic reactions [1]. The ROS include such
products of partial reduction of oxygen as superoxide anion
(O2–•) and hydrogen peroxide
(H2O2) [2, 3]. The most devastating are hydroxyl radicals
(OH•) formed upon the metal-catalyzed cleavage of
hydrogen peroxide; the rate of oxidation of organic molecules by such
radicals is limited only by the diffusion rate. ROS may damage key
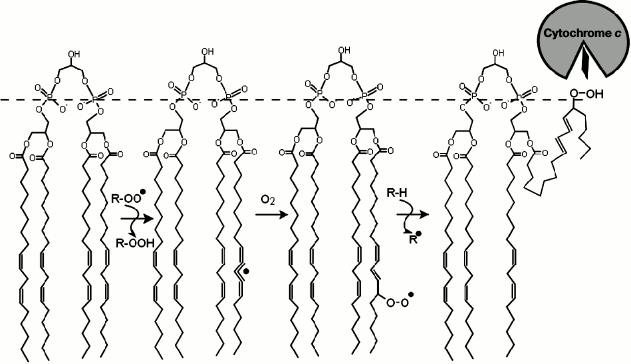
components of the cell including membrane lipids (Fig. 1) and DNA molecules [1] and lead
to cell death and tissue degradation [3-5]. Therefore, the increase in ROS level in the cell
serves as one of the main signals for apoptosis – programmed
cell death – aimed at eliminating cells incapable of normal
metabolism [6-8].
Mitochondria are the primary source of ROS in eukaryotic organisms [1]. About 2% of the oxygen molecules that are consumed by mitochondria under physiological conditions are converted to ROS as a result of one-electron reduction [9]. The main producers of mitochondrial ROS are the proton-pumping NADH:ubiquinol oxidoreductase (complex I) and the ubiquinol:cytochrome c oxidoreductase (cytochrome bc1 complex, complex III) [10-15]. However, complex I produces ROS under conditions of reverse electron flow (from ubiquinol to NAD+) [16-18]. In contrast, complex III generates ROS under conditions of “direct” electron flow, upon ubiquinol oxidation. This oxidation occurs in two steps and involves formation of an anionic ubisemiquinone radical, which is capable of one-electron reduction of oxygen to superoxide in a side reaction [19, 20].
Accumulation of ROS in the cell can be prevented by mitochondria-targeted antioxidants. Studies on such substances are being performed in several groups [21-29]. For example, at the Lomonosov Moscow State University antioxidants of this kind have been developed on the basis of penetrating cations [30]. Such cations, termed “Skulachev ions” by D. Green [31], penetrate through biological membranes in the charged state and distribute according to the transmembrane difference of electrical potentials [32, 33]. Due to the ability to selectively accumulate in energized mitochondria, which are charged negatively relatively to the cell cytoplasm, such compounds can be used as “locomotives” for delivery of drugs into mitochondria [34, 35]. So far, we have synthesized and tested a series of mitochondria-targeted antioxidants that feature constructs comprised of a penetrating cation and an antioxidant, usually a quinol group, separated by a C-10 linker, which is reflected in the title name of the whole lineage of preparations – SkQ ions, i.e. conjugates of penetrating Skulachev ions (Sk) and quinols (Q) [36-38]. Initially, a triphenylphosphonium group was used as the penetrating cation [32, 33]. Later, other compounds were also used as penetrating cations, both of synthetic (rhodamine 19 in the case of SkQR1) [36] and of natural (berberine in the case of SkQBerb) [39] origin.
The plastoquinol (2,3-dimethyl-1,4-benzoquinol) group or its analogs have been routinely used as antioxidants in SkQ ions. The reduced quinol form of an SkQ ion (SkQH2) is a typical chain-breaking antioxidant. The partition coefficient for SkQ1 in the “octanol–water” system is about 10,000 [40-42], so such ions specifically accumulate in the lipid bilayer, protecting its components from oxidation. In mitochondria, SkQH2 ions act as renewable antioxidants since they can be constantly “recharged” by the respiratory chain [36, 37, 40, 43].
The investigations of SkQ ions have shown their ability to protect cells in culture from ROS-induced apoptosis and, particularly, to prevent the fragmentation of mitochondria [36, 37, 39, 42, 44-51]. In vivo, several cases of prevention of the symptoms of cardiovascular [52], tumor [53], and ocular [54] diseases were reported; in addition, SkQ ions increased the life span of many organisms including mice [55].
The main targets for antioxidants in the inner mitochondrial membrane are seemingly cardiolipin molecules [40, 56], which are composed of two phosphatidylglycerol residues linked together by an additional glycerol molecule via the phosphate groups (Fig. 1). Cardiolipin that is derived from mammalian muscles contains mostly linoleic acid esters (18:2) (Fig. 1) with two unsaturated bonds [57]. In brain cells the composition is more diverse, with increasing ratio of polyunsaturated fatty acids such as arachidonic (20:4) and docosahexaenoic (22:6) acids [29, 58]. The presence of multiple unsaturated bonds makes cardiolipin molecules perfect targets for ROS. Due to their enhanced oxidizability, cardiolipin molecules are considered to be the key players during the initial phases of apoptosis [59, 60]. In particular, one of the important stages in the apoptotic mechanism is the release of cytochrome c molecules from mitochondria and their interaction with molecules of apoptotic protease-activating factor 1 (Apaf-1). The oxidation of cardiolipin was shown to precede the release of cytochrome c to the cytoplasm, and the interaction of oxidized cardiolipin with cytochrome c was shown to lead to partial denaturation of the latter and to activation of peroxidase activity of the heme of cytochrome c [56, 61, 62].
Fig. 1. Scheme of a chain peroxidation reaction of lipid polyunsaturated fatty acid on the example of tetralinoleylcardiolipin. Peroxyl radical (ROO.), which may belong to other lipid molecule or have another origin (for instance, hydrogen peroxide), attacks the hydrogen atom at the bis-allylic carbon atom of the fatty acid resulting in a formation of a conjugated radical π-system on the fatty acid comprised of five carbon atoms. Addition of an oxygen molecule yields a peroxyl radical, whereas the configuration of the double bonds rearranges into the form of a conjugated diene. The newly formed peroxyl radical is able to attack a new substrate (LH) via the chain mechanism. The hydrophilic peroxide group of cardiolipin can then move towards the membrane surface (dashed line), interact with the heme-binding cleft of cytochrome c and contribute to the partial denaturation of the latter and to activation of peroxidase activity of the heme of cytochrome c [56, 61].
Since cardiolipin is the first lipid that is oxidized under oxidative stress [29, 37, 40], protection of cardiolipin molecules from oxidation should protect also all the other compounds of the mitochondrial membrane from oxidation by ROS. Therefore, in parallel with studies on the influence of quinol-based mitochondria-targeted antioxidants on cardiolipin oxidation [30, 37], other approaches towards preventing the peroxidation of cardiolipin have been tested. Specifically, phenolic antioxidants, such as propofol [63] or nitroxyl radical-based antioxidants [24-26] were shown to prevent cardiolipin oxidation. Hemigramicidin S was used for directed delivery of antioxidants into mitochondria [27-29]. In addition, possibilities for blocking the interaction of the cytochrome c heme with cardiolipin molecules were exploited [64]. A review of different approaches can be found in [65].
In sum, both the mechanisms of cardiolipin peroxidation and its quenching by antioxidants are worth investigating. However, in comparison with the numerous studies of cardiolipin oxidation in isolated mitochondria or in vivo, there are only a few studies of cardiolipin oxidation in chemical model systems, and their results are contradictory. Particularly, the monitoring of Fe3+-induced peroxidation of asolectin liposomes by the fluorescent probe C11-BODIPY revealed that the addition of 0.5 µM SkQ1H2 almost completely quenched the fluorescence response of the probe, while introduction of oxidized SkQ1 had no effect on peroxidation [66]. However, when peroxidation within micelles that were built of Triton X-100 and methyl linoleate was induced by AAPH (2,2′-azobis(2-aminopropane)dihydrochloride) and was followed polarographically, addition of SkQ1H2 only partially prevented the consumption of oxygen; the reactivity of SkQ1H2 in these experiments exceeded the reactivity of the respective ubiquinol derivative MitoQH2 by a factor of four [66]. Similar partial inhibition of oxygen consumption by SkQ1H2 ions was observed in experiments with micelles that were built of Triton X-100 and cardiolipin [67].
Comparison of studies on inhibition of cardiolipin oxidation in micelles by SkQ1H2 ions and a water-soluble analog of vitamin E, HPMC (6-hydroxy-2,2,5,7,8-pentamethylbenzochroman), respectively, revealed that oxygen consumption was much higher in the presence of SkQH2 ions [67, 68]. This might indicate that SkQH2 ions, contrary to HPMC, were not fully inhibiting oxidation of cardiolipin in the Triton X-100 micelles. As the hydrophobic antioxidant BHT (2,6-di-tert-butyl-4-methylphenol), similarly to SkQ1H2, only partially suppressed oxygen consumption during AAPH-induced cardiolipin oxidation in Triton X-100 micelles [68], the molecules of water-soluble HPMC, most likely, prevented the initiation of peroxidation in micelles by “catching” radicals of the water-soluble initiator AAPH, as formed outside micelles, more efficiently than the more hydrophobic molecules of SkQH2 and BHT. Incomplete inhibition of peroxidation by SkQH2 ions in model micellar systems [66-68] is in a certain contradiction both with the C11-BODIPY fluorescence data and in vivo and in situ data on protection of cardiolipin from oxidation by SkQH2 ions [37, 40] and other mitochondria-targeted antioxidants [21, 24, 26, 69].
The above-mentioned discrepancies in the data obtained on model systems together with unique role of cardiolipin in mitochondria necessitated studying cardiolipin peroxidation and the effect of mitochondria-targeted antioxidants on this oxidation in a simple model system. Here we have monitored the peroxidation in liposomes made of pure cardiolipin and have studied the impact of quinol-containing antioxidants on this process. To be more accurate in analyzing the effect of the lipophilic ions, we followed the peroxidation reaction not by oxygen consumption, but by following conjugated dienes formed during the reaction of lipid peroxidation. We showed that quinol-based antioxidants, in micro- and even submicromolar concentrations, fully protected the liposomal cardiolipin from peroxidation by functioning as chain-breaking antioxidants. The duration of their action, differed however, increasing substantially in presence of a ubiquinol methoxy group, as well as additional reduced redox groups (in cases of rhodamine and berberine derivates). We propose that the therapeutic action of SkQ ions and, perhaps, other mitochondria-targeted antioxidants can be explained by their ability to specifically protect from oxidation the cardiolipin molecules bound within membrane protein complexes.
MATERIALS AND METHODS
Chemicals and equipment. Cardiolipin (CL) from bovine heart, 1-palmitoyl-2-oleoylphosphatidylcholine (POPC), 1-palmitoyl-2-oleoylphosphatidylglycerol (POPG), and yeast ubiquinone-6 (Q6) were obtained from Avanti Polar Lipids Inc. (USA). The phospholipids were purchased in the form of lyophilized powder. Chemicals for buffer solutions were from Sigma-Aldrich (USA) or Roth (Germany). Azo initiator 2,2′-azobis(4-methoxy-2,4-dimethylvaleronitryl) (MeO-AMVN) was from Wako Pure Chemical Industries (Japan). Decylplastoquinone (decPQ), decylubiquinone (decUQ), 5(6)-carboxyfluorescein, and Triton X-100 were from Sigma-Aldrich, and sodium borohydride from Roth. The substances from the “Skulachev ions” lineage were synthesized as described earlier [36, 39, 51].
Preparation of liposomes. Lipids in the form of lyophilized powder were taken as a starting material. The dry lipids were weighted and suspended in 50 mM sodium phosphate buffer (pH 7.4) containing 100 µM diethyltriaminopentaacetic acid, a chelator of heavy metals. The final concentration of lipids in the suspension was usually 3 mg/ml. After adding the pH buffer to the sample tube containing the lipid, the suspension was intensively mixed for 3-5 min until homogeneity. Liposomes were obtained with a mini-extruder equipped with two syringes (Avanti), each of 1 ml volume, and a membrane with pore diameter of 100 nm (Avanti). The suspension was passed through the membrane 19 times. The liposome samples were stored on ice until use within the same day.
Measuring particle size distribution. The size of the particles in a liposome suspension was measured by dynamic light scattering using a Zen 3600 Zetasizer device (Malvern Instruments Ltd, UK). The final concentration of cardiolipin during the measurement was 50-100 µM. Size distribution was obtained by averaging the results of three experiments.
Transmission electron microscopy. For visualization by transmission electron microscopy (TEM), 5-µl samples of liposomes containing 3 mg/ml of cardiolipin were placed for 2 min on copper grids with single mesh covered by formvar (Agar Scientific, UK). Excess surface water was removed from the grid with filter paper, and then the grid was air-dried. Electron micrographs were recorded with a Zeiss EM 902 A transmission electron microscope (Carl Zeiss, Germany) operating at 50 kV.
Checking of liposome integrity. Cardiolipin liposomes were investigated for integrity by the carboxyfluorescein release method [70]. Liposomes were prepared in a sodium phosphate buffer, pH 7.4, that contained 100 µM diethyltriaminopentaacetic acid and 100 mM 5(6)-carboxyfluorescein. After extrusion, the external carboxyfluorescein was removed by passage through a Sephadex G-25M PD-10 column (Pharmacia, Sweden). The liposomes were diluted to lipid concentration 1.5 mg/ml and placed into an LS 55 spectrofluorimeter (Perkin Elmer, USA). The measurement was performed at excitation and emission wavelengths of 430 and 520 nm, respectively. The release of carboxyfluorescein was induced by addition of 0.3% Triton X-100 to the liposome suspension.
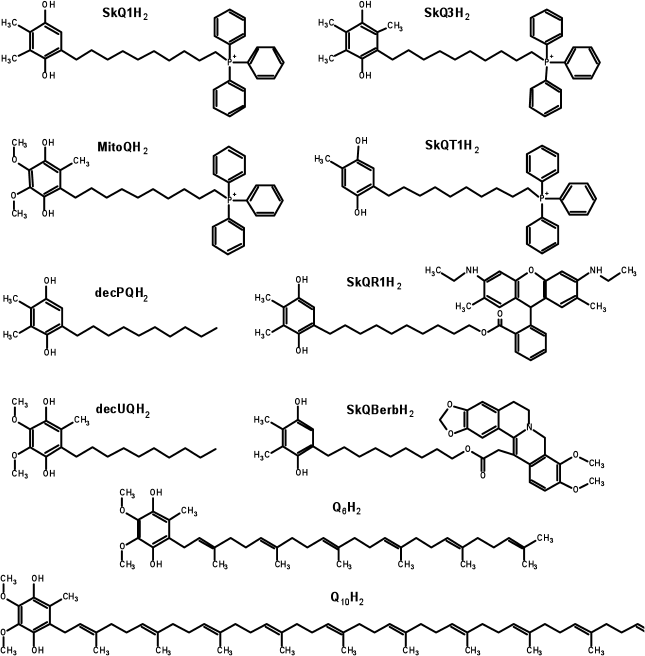
Preparation of reduced quinol-containing antioxidants. Before use, the quinol-based antioxidants were reduced by intensive mixing of the stock solution (usually at 1 mM) with 2-3 mg of dry sodium borohydride for 1 min. Then a small amount (5-10 µl) of fuming hydrochloric acid was added to the solution to eliminate the reductant. After the pH value of the solution was brought to approximately 3.0, sodium borate was removed by two consecutive centrifugations at 15,800g for 10 min each (at 4°C). This procedure leads to full reduction of quinone groups [67]. The reduced preparations were stored at –80°C. The concentration of the antioxidants was determined spectrophotometrically by absorbance at 290 nm. The following extinction coefficients were used: 4140 M–1·cm–1 for ubiquinol-containing substances, and 3540 M–1·cm–1 for antioxidants based on plastoquinol, methylplastoquinol, or toluquinol [71]. The structures of the reduced forms of antioxidants are shown in Fig. 2.
Fig. 2. Structures of the quinol forms of the antioxidants used in the study. In the cases of SkQBerbH2 and SkQR1H2, the structures show not only the reduced quinone moieties, but also reduced berberine and rhodamine groups, respectively. In the experiments on biological systems, the reduction of quinol groups is not accompanied by the reduction of the low-potential berberine or rhodamine moieties, so that the molecules remain positively charged independently of the redox state of the quinone group [44, 121].
Initiating oxidation of cardiolipin in liposomes and investigating effect of antioxidants. For initiation of the chain peroxidation reaction, the hydrophobic azo initiator MeO-AMVN was used [72]. The azo initiator in the form of an ethanol solution was added to a liposome suspension with the total amount of introduced ethanol not exceeding 1% of the sample volume. Quinols used as antioxidants were routinely added to the liposomes as ethanol solutions 30 min after starting the peroxidation by the azo initiator.
The UV- and visible spectra were measured with a UV-2450 spectrophotometer (Shimadzu, Japan). The investigated suspension was incubated in a 3-ml quartz cuvette in a thermostatted cell holder. The optical absorbance of the sample was measured in comparison to the reference cuvette, to which all the components were added successively, except for the azo initiator solution, which was replaced by a same volume of ethanol. Oxidation of cardiolipin in the liposomes was monitored by tracing the accumulation of conjugated dienes in the fatty acid “tails” of cardiolipin. The formation of conjugated dienes was followed by measuring the absorbance in the UV region where conjugated dienes show a broad peak with maximum at 234 nm (with molar extinction coefficient of 28,000 M–1·cm–1 [73]).
RESULTS
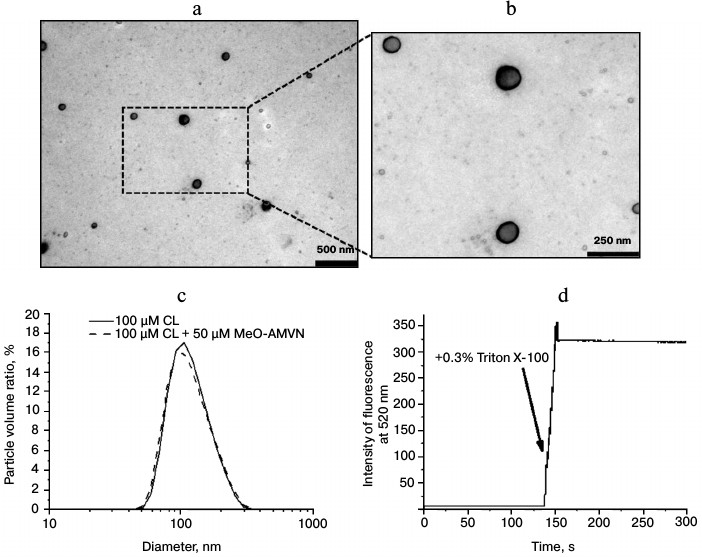
Characterization of cardiolipin liposomes. Suspensions of cardiolipin liposomes obtained by extrusion were investigated by electron microscopy, which demonstrated the presence of round particles with thin walls about 100 nm in diameter (Fig. 3, a and b). These images indicated that the extrusion performed on a cardiolipin suspension resulted in formation of liposomal structures.
Fig. 3. Characterization of particles obtained by extrusion of a cardiolipin (CL) suspension. a, b) Electron micrographs of liposomes. c) Distribution of particle volumes as a function of their sizes as measured by dynamic light scattering. d) Fluorescence of 100 mM of 5(6)-carboxyfluorescein restricted in liposomes of cardiolipin (100 µM) before and after addition of 0.3% Triton X-100. Excitation and emission wavelength were 430 and 520 nm, respectively.
The dynamic light scattering method, which allows recording particle size distribution in a suspension (Fig. 3c), showed that the diameter of the resulting liposomes, 90 nm on average, corresponded to the filters used during extrusion with a pore diameter of 100 nm. Upon addition of the azo initiator MeO-AMVN to the liposomes, the average liposome size remained unchanged, which indicated the absence of any influence of the initiator on the structure of the particles (Fig. 3c).
The standard carboxyfluorescein release test was used for checking liposome integrity [70, 74]. Liposomes were prepared in a buffer with high carboxyfluorescein concentration, and then the dye was removed from the external medium by gel chromatography. At carboxyfluorescein concentration of 100 mM, the fluorescence of the dye was low because of self-quenching. Fluorescence intensity was not increasing with time, which indicated that liposomes were impermeable for the dye. The addition of Triton X-100 detergent disrupted the membrane structures and led to the increase in fluorescence signal in the sample due to dilution of the carboxyfluorescein (Fig. 3d).
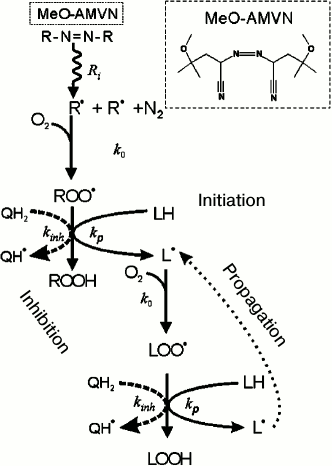
Monitoring of cardiolipin oxidation in liposomes. We followed the oxidation of cardiolipin in liposomes by measuring the formation of conjugated dienes according to the mechanism depicted in Fig. 1. The peroxidation of cardiolipin was initiated by the hydrophobic azo initiator MeO-AMVN [72]. As depicted in Fig. 4, the azo initiator decays spontaneously at a constant rate forming two carbon-centered radicals (R•) that further react with oxygen at a diffusion-limited rate to give peroxyl radicals (ROO•), which can then propagate in a chain reaction (Figs. 1 and 4). A formed ROO• radical attacks a hydrogen atom at the bis-allylic (i.e. adjacent to two double bonds) carbon atom (LH) yielding a lipid radical L•, as shown in Figs. 1 and 4. The lipid radical L• interacts with an oxygen molecule to form a peroxide radical LOO•, which could turn into a peroxide group LOOH after abstracting a hydrogen atom from another lipid molecule and producing thus a further radical L•; in this way the classical peroxidation chain reaction is launched [75, 76]. As a rule, after several dozens of oxidation cycles, a termination of the reaction occurs due to collision of two radical products. Figure 4 shows that the propagation of a chain reaction can be inhibited by an antioxidant present in the chemical system. In particular, antioxidants that possess quinol group(s) (QH2) interact with LOO• and ROO• radicals much faster than lipid molecules, breaking thus the chain reaction. For polyunsaturated linolenic acid, the rate constant of radical formation is 60 M–1·s–1, whereas the quinol groups interact with peroxides much faster, with a rate constant of about 105 M–1·s–1 [75-77]. One-electron oxidation of a quinol QH2 yields a semiquinone radical Q(H)• which, depending on pH value, can exist either as a protonated radical (QH•) or as an anion-radical (Q•–). A semiquinone radical can, in principle, quench another peroxide radical of lipid or of initiator, being oxidized to quinone Q, if it does not lose its electron beforehand as a result of interaction with an oxygen molecule [76]. The possible conversions of a semiquinone radical are described by the following reactions.
Quenching of a lipid radical:
Quenching of an initiator radical:
Disproportionation of semiquinones:
Interaction with oxygen:
Fig. 4. Scheme of chemical processes that occur upon azo compound-induced initiation of a chain radical reaction of lipid polyunsaturated fatty acid moieties in the presence of an inhibitor of quinol nature according to [75]. The main stages of the process are indicated by respective rate constants: Ri, rate constant of thermal decay of the initiator; k0, rate constant of interaction of a carbon-centered radical with oxygen; kp, chain propagation rate constant; kinh, rate constant of interaction of a peroxyl radical with a quinol.
It is noteworthy that in the studied model system the interactions between azo initiator radicals and molecules of amphiphilic quinols should have occurred both in the lipid and water phases of the sample. However, the molar concentrations of both quinols and azo initiator, due to their high affinity to the lipid phase, should have differed in water and membrane phases by several orders of magnitude.
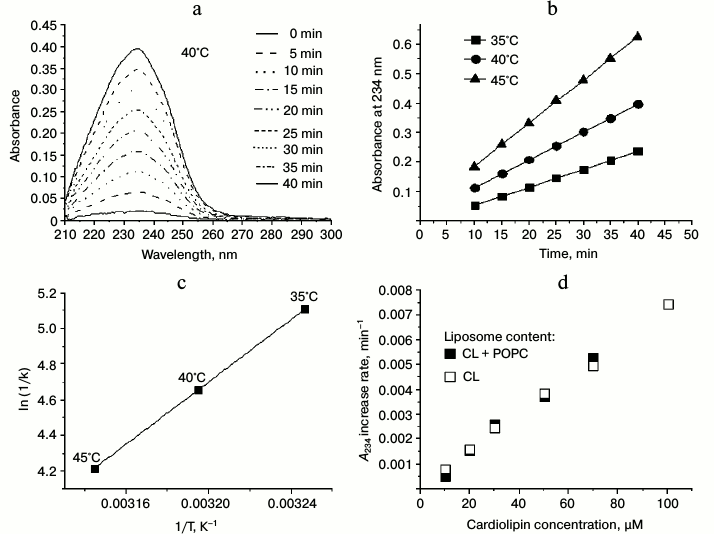
In Fig. 5a, the results of the control measurements are presented, where the UV-spectra of the liposome suspension of cardiolipin (100 µM) were recorded each 5 min after the addition of 50 µM MeO-AMVN at 40°C. The absorbance increase at 234 nm reflected the formation of conjugated dienes as primary products of peroxidation (Fig. 1). The chosen method of generation of peroxyl radicals, as follows from the presented data, led to a linear increase in absorbance at 234 nm, which reflected accumulation of conjugated dienes with a constant rate. For the experimental investigation of the properties of antioxidants, we first had to select an optimal reaction rate. Too slow oxidation would lead to a low signal-to-noise ratio upon measuring the absorbance of conjugated dienes, whereas too fast oxidation would quickly lead to large values of optical absorbance at 234 nm, exceeding the limit of linearity of the spectrophotometer. To choose the optimal conditions, the experiments were made at various temperatures and concentrations of MeO-AMVN. Based on the measured data at three different temperatures (Fig. 5b), the average rates of increase in absorbance at 234 nm (A234) were calculated and then plotted in Arrhenius coordinates 1/T – ln(1/k) (Fig. 5c). Using the Arrhenius equation, the effective activation energy of oxidation of liposomal cardiolipin in our model system was calculated as approximately 73 kJ/mol.
Fig. 5. Oxidation of cardiolipin in liposomes in response to the addition of MeO-AMVN. Determination of temperature and concentration dependences. a) Absorbance spectra as measured when the oxidation of 100 µM of liposomes made of pure cardiolipin was initiated by the addition of 50 µM of MeO-AMVN at 40°C. b) Changes in the absorbance at 234 nm with time on oxidation of liposomal cardiolipin (for conditions see Fig. 5a) as measured at temperatures of 35, 40, and 45°C. c) The rate of A234 increase (k) as function of temperature (T). The trend line was used to calculate the effective activation energy. d) Oxidation of cardiolipin in liposomes of mixed content (POPC + CL, filled squares) and in liposomes of pure CL (unfilled squares). The values of the A234 increase rate per minute are given as a function of the final concentration of CL in the sample.
The experimental conditions for the further experiments were chosen so that (1) the temperature would be close to physiological and (2) the concentration of the initiator would not be too high, so that the contribution of the absorption of the initiator in the UV range and its possible destabilizing effect on the liposomes would be avoided. After performing experiments under different conditions, we chose the following parameters as standard conditions: cardiolipin concentration of 100 µM, initiator concentration of 50 µM, and temperature of 40°C; the lipid oxidation experiments described below were usually performed under these conditions.
In the simplest case, the rate of product formation in the net reaction described by the equations above is proportional to the concentration of the substrate (hydrogen atom of the bis-allylic group) [75, 78]. We compared formation of conjugated dienes in liposomes made of pure cardiolipin and of a mixture of CL and POPC, respectively. The POPC contained no polyunsaturated fatty acid residues, and thus could not be a target for peroxidation.
To elucidate the dependence of the reaction rate on the cardiolipin concentration in the sample, we performed two series of measurements. In the first case, the liposomes were prepared of pure cardiolipin with various final lipid concentrations from 10 to 100 µM (or from 15 to 150 µg of cardiolipin per ml). In concurrent experiments, the same amount of cardiolipin was mixed with POPC at different ratios to yield the same resulting final concentrations of cardiolipin as in the first series of experiments. The total concentration of lipid in the mixture of CL and POPC was 150 µg/ml. With these samples, we measured the increase in absorbance at 234 nm (after the addition of MeO-AMVN) as a function of time; for the time interval from 5 to 30 min after the addition, we plotted a linear regression and determined the rate of A234 increase per minute. The comparison of the rates, which were proportional to the rates of conjugated dienes formation, is made in Fig. 5d. The two dependences nearly matched; apparently, the rate of conjugated diene formation in liposomes depended only on CL amount, and dilution of cardiolipin by the inert lipid up to 10-fold had no effect on the peroxidation rate within the membrane (Fig. 5d).
Testing effect of antioxidants on cardiolipin peroxidation in liposomes. After selecting the optimal temperature and concentration of initiator (40°C, 50 µM MeO-AMVN), we tested substances from the SkQ lineage for their ability to prevent oxidation of cardiolipin molecules in the liposomal membranes. The studied substances differed in the structures of the penetrating group (triphenylphosphonium, berberine, or rhodamine 19) and in the side groups of the quinol moiety (Fig. 2).
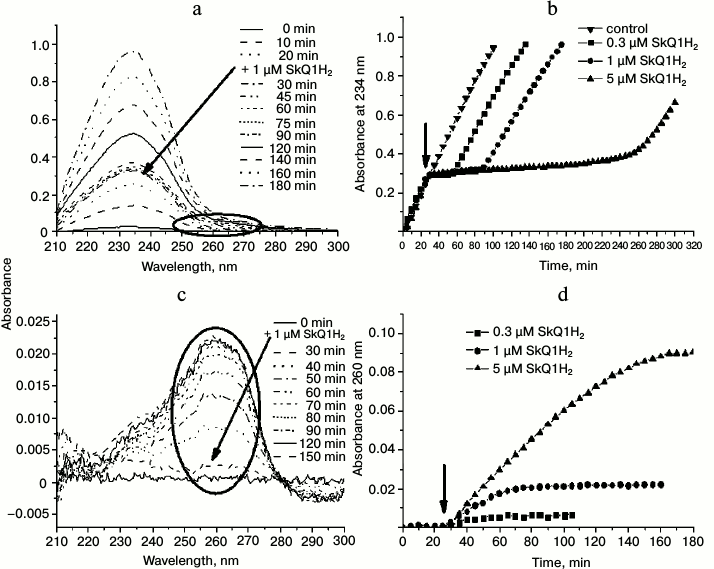
Figure 6a contains the absorption spectra in the UV region for a typical experiment where peroxidation was initiated by MeO-AMVN and an antioxidant (1 µM SkQ1H2) was added 30 min later. The addition of the initiator led to the appearance of the steadily growing characteristic absorbance peak with maximum at 234 nm. Immediately after the addition of SkQ1H2, the growth of the 234 nm signal stopped, but resumed after a certain time. The moment when the formation of conjugated dienes resumed probably represented the full oxidation of the quinol form of the antioxidant to quinone form (SkQ1). Thus, SkQ1H2 quenched peroxyl radicals in the system and protected cardiolipin from oxidation. Even at a concentration of 1 µM, SkQ1H2 completely prevented the formation of conjugated dienes for approximately 1 h (Fig. 6, a and b).
Fig. 6. Effect of SkQ1H2 on reactions in liposomes as induced by 50 µM MeO-AMVN at 40°C. In all experiments, the antioxidant was added 30 min after the start of the reaction (the time of addition is marked by an arrow). a) Absorbance changes in the suspension of cardiolipin liposomes (100 µM) in the course of peroxidation reaction and after the addition of 1 µM of SkQ1H2. b) Kinetic curves for absorbance change at 234 nm at different concentrations of added SkQ1H2. In the control experiment, pure ethanol treated with sodium borohydride was added to the liposomes. c) Changes in the absorbance of the suspension of liposomes made of the inert lipid POPG (200 µM) upon addition of the initiator. SkQ1H2 (1 µM) was added 30 min after the start of the experiment. The corresponding areas in the spectra of conjugated dienes (Fig. 6a) and of oxidizing quinol (Fig. 6c) are indicated by ovals. d) Kinetic curves for absorbance at 260 nm (maximum for SkQ1) in the experiment with the inert lipid (POPG) and various concentrations of SkQ1H2.
With SkQ1H2, we studied the dependence of the kinetics of cardiolipin oxidation on the amount of the antioxidant added. The results are shown in Fig. 6b. As follows from the kinetic curves, larger amount of antioxidant prolonged the lag phase that reflected the prevention of cardiolipin oxidation by quinol moieties. With increasing concentration of antioxidant, the difference between the rates of accumulation of conjugated dienes before the addition of the antioxidant and after the end of the lag phase, respectively, increased. Only in the case of 0.3 µM SkQ1H2, the rate of conjugated dienes formation after the lag phase was apparently the same as before the addition of the antioxidant (squares in Fig. 6b).
In a control experiment, an ethanol solution that was treated by sodium borohydride as in the procedure of quinone reduction (see “Materials and Methods”) but lacking antioxidants was added to the sample instead of the active substance. The introduction of this solution into the reaction mixture did not change the rate of conjugated diene formation (the “control” curve in Fig. 6b), which meant that the excess of sodium borohydride, also a potential antioxidant, was completely removed by the procedure used (see “Materials and Methods”), and that the prevention of cardiolipin peroxidation in our experiments was due exclusively to the action of the added quinols.
During the oxidation of quinols, their optical changes in the 200-300-nm range also occurred, but these were hardly resolvable on the background of the pronounced absorbance peak of conjugated dienes. To measure the oxidation kinetics of the quinol group, the measurements were performed with an inert lipid, POPG, to which SkQ1H2 was added 30 min after the azo initiator. Figure 6 shows the UV spectra of the suspension after addition of 1 µM of the antioxidant (Fig. 6c), as well as the kinetic curves of absorbance at 260 nm (absorbance maximum for oxidized SkQ1 [36]) for three different concentrations of the antioxidant (Fig. 6d). The time of complete oxidation of SkQ1H2 correlated with the end of the lag phase of conjugated diene formation during oxidation of cardiolipin (compare Figs. 6c and 6d with Figs. 6a and 6b), with the rate of SkQ1H2 oxidation decreasing with diminishing concentration of SkQ1H2 in the cuvette. Importantly, since the initial source of all the radicals is the azo compound (Fig. 4), the oxidation kinetics of the antioxidant in the system with an inert lipid has to be similar to the kinetics of its oxidation in the presence of lipid with polyunsaturated lipid “tails”, such as cardiolipin.
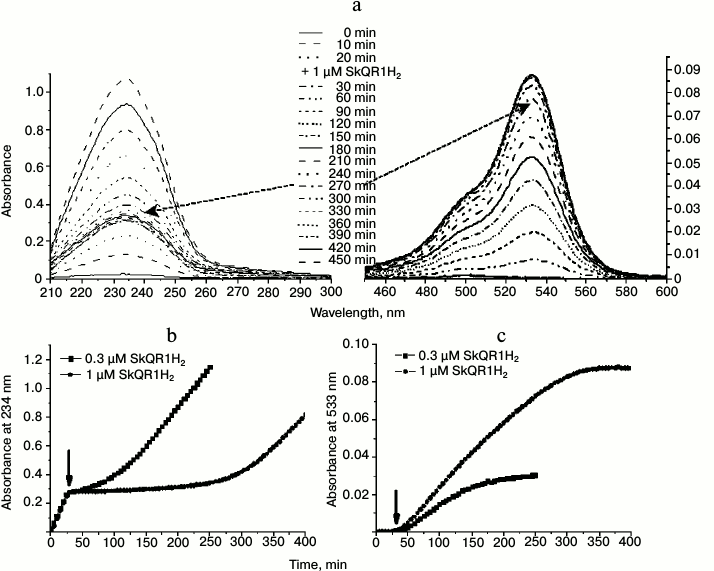
The studies of other plastoquinol-containing antioxidants, SkQR1H2 and SkQBerbH2 (Fig. 2), which contain alternative penetrating cations instead of triphenylphosphonium group, namely rhodamine 19 (SkQR1H2) and berberine group (SkQBerbH2), respectively (Fig. 2), showed that these substances also hindered the oxidation of cardiolipin in the liposomes (Figs. 7, 8a, and Fig. S1a (see Supplement to this paper on the website of the journal at http://protein.bio.msu.ru/biokhimiya)).
Fig. 7. Effect of SkQR1H2 on peroxidation of cardiolipin in liposomes induced by 50 µM MeO-AMVN at 40°C. SkQR1H2 was added 30 min after start of the experiment (as marked by arrow). a) Absorbance changes of the suspension of cardiolipin liposomes (100 µM) in the course of peroxidation reaction and in response to the addition of 1 µM of SkQR1H2. The UV spectra are shown on the left panel; the spectra in the region of rhodamine absorbance are shown on the right panel. The dashed arrows indicate that the resumption of the formation of conjugated dienes (~270 min after the beginning of the experiment) matches the end of oxidation of the rhodamine group of SkQR1. b) Kinetic curves for the absorbance at 234 nm at different concentrations of the introduced SkQR1H2. c) Changes in absorbance during the oxidation of the rhodamine group of SkQR1 (maximum at 533 nm) in the experiment. For conditions, see Fig. 7a.
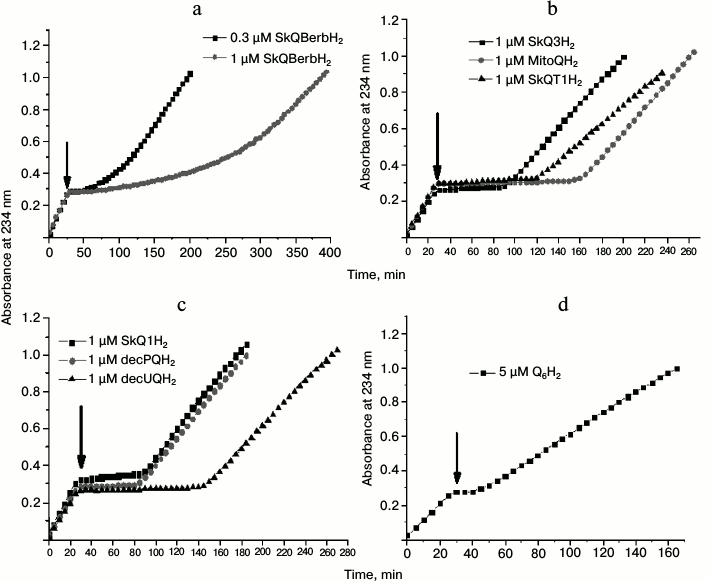
Fig. 8. Effect of various quinols on peroxidation of cardiolipin in liposomes induced by 50 µM MeO-AMVN at 40°C. The moments of addition of antioxidants are marked by arrows (30 min after the start of the experiment). Kinetic curves for the absorbance changes at 234 nm. The following antioxidants were tested: a) 0.3 and 1 µM SkQBerbH2; b) 1 µM SkQ3H2, 1 µM MitoQH2, 1 µM SkQT1H2; c) 1 µM SkQ1H2, 1 µM decPQH2, and 1 µM decUQH2; d) 5 µM Q6H2. For other experimental conditions see Fig. 6, a and b.
In contrast to SkQ1H2, the positively charged groups of both these compounds are redox-active and should have been reduced upon the reduction of the plastoquinol group by sodium borohydride. Addition of SkQR1H2 to the cardiolipin liposomes led to emergence of a lag phase in the formation of conjugated dienes that was distinctively longer than with SkQ1H2 (cf. Figs. 6 and 7); the antioxidative action was accompanied by the oxidation of the rhodamine group, which could be followed by appearance of the strong rhodamine absorption in the visible spectral range (absorbance maximum at 533 nm) (Fig. 7, a and c). In the case of the berberine-containing substance SkQBerbH2, the lag phase was also long; the transition at the end of the lag phase was smoother than when SkQ1H2 was added (cf. Figs. 6 and 7, see also Fig. S1a and “Discussion”).
The other group of substances that we tested for antioxidant properties differed from SkQ1H2 not by the penetrating cation, but by the type of the attached quinol. This group included MitoQH2 (ubiquinol), SkQ3H2 (quinol with three methyl substituting groups, or methylplastoquinol), and SkQT1H2 (toluquinol) (Fig. 2). In experiments where these substances were used at the concentration of 1 µM, the lag phase duration for SkQ3H2 and SkQT1H2 was comparable to that observed for SkQ1H2 (Fig. 8b and Fig. S2, a-c (see Supplement)). In the case of MitoQH2, the lag phase was approximately two times longer.
Since the membranes in our experiments were not energized, the influence of the penetrating cation on the rate of antioxidation reactions was expected to be negligible. To test this hypothesis, we used decylplastoquinol (decPQH2) and decylubiquinol (decUQH2), the synthetic analogs of the natural plastoquinol and ubiquinol. The kinetic curve for decPQH2 as an antioxidant almost completely matched the respective curve for SkQ1H2 (Fig. 8c and Fig. S1c (Supplement)). In the case of decUQH2, the lag period, as in the case of MitoQH2, was about two times longer than for decPQH2 and SkQ1H2 at the same concentration (Fig. 8c and Fig. S1d (Supplement)).
Thus, for the same 1-µM concentration of antioxidant, the duration of the lag phase in the reaction of cardiolipin peroxidation changes in the series MitoQH2 = decUQH2 > SkQT1H2 > SkQ3H2 = SkQ1H2 = decPQH2; the lag phase was much longer when the equivalent concentrations (1 µM) of SkQR1H2 and SkQBerbH2 were added.
As shown in Fig. 8d and Fig. S1b (Supplement), the natural yeast ubiquinol-6 (Q6H2) added at the concentration of 5 µM fully blocked the oxidation of cardiolipin. However, the duration of inhibition was even shorter than for the other antioxidants tested at 1-µM concentration.
DISCUSSION
In the present study, we established a novel method of testing cardiolipin peroxidation in liposomes. Bovine cardiolipin, which is analogous to human cardiolipin, was studied in the experiments. Characterization of the sample showed that the suspension, as obtained via extrusion, contained closed cardiolipin liposomes with average diameter of about 100 nm (Fig. 3).
In the system chosen, the azo compound MeO-AMVN was used so that the peroxidation proceeded according to the aforementioned mechanism (Figs. 1 and 4) yielding peroxides and conjugated dienes. This allowed quantitatively tracing the rate of accumulation of the primary product of the lipid peroxidation reaction by following the changes in absorbance of conjugated dienes in the UV region at 234 nm. The high efficiency of the hydrophobic initiator MeO-AMVN at low temperatures [72] allowed us to use low amounts of the initiator, which decreased the possible influence of the initiator on the structure of the liposomes (Fig. 3c). Given that the partition coefficient hydrophobic phase–water for MeO-AMVN is about 1500 [79], we estimate that, within the lipid bilayer, one molecule of initiator was present, on average, per each 50 linoleic acid “tails”; the majority of the initiator molecules (circa 90%) resided in the water phase. Addition of the azo initiator had no effect on the size of the liposomes (Fig. 3c). Upon the fission of the initiator molecules into two more polar radicals (Fig. 4), the concentration of the azo initiator within the membrane should have diminished.
The method we used is a direct method, and, along with polarography [75, 78, 80], can be used for continuous tracing of the peroxidation (see the present study and previous works [81, 82]). In some experimental systems, the mechanisms of reactions beyond the formation of conjugated dienes were described including the formation of oxodienes, cleavage of the part of fatty acid residue, and formation of covalent bonds between adjacent lipid “tails” [83-85]. Since oxygen is consumed in these secondary reactions and the quantity of the primary product of lipid oxidation decreases, several studies were aimed at estimating the relation between the observed quantities of consumed oxygen and formed conjugated dienes and determining the respective correcting coefficients. The difference in the measured rates of oxygen consumption and accumulation of conjugated dienes, respectively, did not exceed 10-20% [86, 87]. Since we followed the absorbance changes in the whole UV and visible range, we can assume that the formation of side products of degradation of lipid peroxides occurred far more slowly than the formation of dienes, and the percentage of conjugated dienes having undergone further oxidation should have been lower than the spread in the values of molar extinction coefficients of those dienes (the respective numbers from the literature vary between 25,000 [86] and 29,500 [82] M–1·cm–1). Hence, the spectrophotometric method can provide accuracy of the measurements of lipid oxidation that is comparable to the accuracy of the measurements of oxygen consumption. The advantage of the spectrophotometric approach is the ability to simultaneously trace both the accumulation of the reaction products and the depletion of the antioxidant, which is essential for kinetic analysis of peroxidation.
The temperature dependence of the chain reaction could be described by the Arrhenius equation, and the effective activation energy of formation of conjugated dienes was estimated to be about 70 kJ/mol (Fig. 5, b and c). The relatively prompt generation of peroxide radicals by MeO-AMVN already at physiological temperatures allowed us to use less initiator than in an earlier study [66], thereby diminishing the impact of the absorbance of the azo compound in the investigated UV range.
Using the developed experimental setup for tracing oxidation of cardiolipin molecules within the membrane bilayer, we tested mitochondria-targeted antioxidants from the SkQ ion lineage (Fig. 2). The dynamics of oxidation of the quinol group could be judged by the increase in absorbance at 260 nm in the case of SkQ1 (Fig. 6, b and d), and performing the experiment with the inert lipid POPG helped to discriminate the contribution of oxidizing SkQ1H2 molecules, which otherwise would be hardly distinguishable on the background of the broad absorbance band of conjugated dienes in case of cardiolipin liposomes. Immediately after the addition of SkQ ions, the rate of oxidation of the quinol group was the fastest and then it smoothly slowed. Despite this, the experimental data, as obtained with various quinol antioxidants, demonstrated that the majority of them, even if added at small concentrations (0.3 µM), were effective in protection of cardiolipin from oxidation until the full exhaustion of the antioxidant molecules.
While comparing the duration of the lag phase of inhibition for various concentrations of SkQ1H2, we noticed the absence of a clear linear dependence between the amount of introduced substance and duration of inhibition. We suppose that the mismatch might be caused by incomplete solubility of the antioxidants. The critical micelle concentration determined for SkQ1 is equal to 1.1 µM [36]; hence, at higher concentrations of SkQ1 a significant part of the antioxidant could have formed micelles after addition. Micelles could slowly exchange antioxidant molecules with lipid membranes. This slow exchange, most likely, accounted for the distinct decrease in the rate of formation of conjugated dienes after the end of the lag phase as compared with the rate of their formation before the addition of antioxidant. Only when SkQ1H2 was added at 0.3 µM, which is less than the critical micelle concentration, the rates of formation of conjugated dienes before addition of the antioxidant and after its oxidation were virtually the same (Fig. 6b, squares). The latter observation also indicates that the antioxidant activity of the oxidized SkQ1 molecules is below our detection limit, in good accordance with both the literature data that claim negligibly low antioxidative activity of quinones [88] and the absence of antioxidant activity of SkQ1, as observed in the experiments where the oxidation of asolectin liposomes was traced by C11-BODIPY fluorescence [66].
The substances with a triphenylphosphonium moiety as a penetrating cation, but different antioxidative groups, differed in the duration of the lag phase for the same amounts of the introduced quinol (Fig. 8 (b-d) and Figs. S1 (b-d) and S2). Particularly, ubiquinol-containing antioxidants MitoQH2 and decUQH2 provided longer lag phase than the other studied quinols. This could have several reasons. First, the longer action of ubiquinol-containing antioxidants, as compared to other antioxidants, may be caused by lower reactivity of the ubiquinol moiety towards peroxyl radicals (both of lipid and of initiator), which could have diminished the expenditure of antioxidant due to its non-productive interaction with radicals of the azo initiator outside of the membrane. The specific structural feature of ubiquinols is the presence of methoxy groups, the oxygen atoms of which are capable of forming intramolecular hydrogen bonds with phenolic groups. Kramer and colleagues showed that oxidation of deuterated analogs of ubiquinol was characterized by a lower activation energy as compared to protonated analogs, while the activation energies of oxidation of deuterated and protonated quinols lacking methoxy group showed no difference [89, 90]. This very unusual phenomenon was observed for oxidation of ubiquinol by cytochrome bc1 complex, as well as for oxidation of ubiquinol by ruthenium complex in a nonpolar solvent. As long as the rate of oxidation of a quinol group is determined by the rate of the loss of proton/hydrogen atom (depending on the polarity of the environment) [91-94], the peculiar dependence of activation energy on H/D replacement in the case of ubiquinols, as described by Kramer and colleagues [89, 90], might indicate that the rate of oxidation of ubiquinol analogs was determined by the disruption rate of intramolecular hydrogen bond [95, 96]. In the case of a D for H substitution, the length of the bond between the oxygen atom and proton in the phenolic group should decrease. Correspondingly, the length of the bond to the methoxy oxygen atom should increase, i.e. this bond should be weaker in the case of deuterated molecules, which could account for the experimental observations [89, 90]. No need to break the intramolecular hydrogen bond might explain the higher kinetic efficiency of SkQ1H2 as compared to MitoQH2 [66]. The higher reactivity should also manifest itself in faster depletion of the antioxidant upon non-productive reactions, e.g. those occurring outside the membrane, and, accordingly, in the shorter duration of the antioxidant action.
Second, the available data [97, 98] indicate that the radicals of ubisemiquinone are more resistant to oxidation by oxygen (see reaction (4) in the “Results” section) than the radicals of the quinones lacking methoxy groups. In the case of plastoquinone radical, this might be due to its relatively low redox potential [98]. More generally, the methyl of the o-methoxy group should stabilize the radical oxygen [95] and increase thus the effective redox potential of the semiquinone. In addition, steric constraints on binding of an oxygen molecule from the oxygen atoms of methoxy groups may serve as an additional retarding factor [99]. If less vulnerable to oxidation by oxygen, the ubisemiquinone radicals may contribute more to the quenching of peroxyl radicals according to reactions (1)-(3) than the radicals of the other studied quinones.
It is noteworthy that the ability to efficiently prevent the oxidation of cardiolipin molecules is a more important characteristics of an antioxidant in vivo than the duration of action, since the quinol-based antioxidants are regenerated by the mitochondrial respiratory chain [36, 37, 40].
In our experiments, the effectiveness of inhibition of peroxidation in the membrane by the molecules of SkQR1H2 and SkQBerbH2 was slightly lower than for the other tested antioxidants that contained triphenylphosphonium group as a penetrating cation. The slopes of the curves of absorbance increase at 234 nm, which reflected the relation between the rates of radical inhibition and chain propagation in the peroxidation reaction, respectively, were steeper for SkQR1H2 and SkQBerbH2 than for the other tested antioxidants. This observation contradicts the results obtained in vivo, where SkQR1 appeared to be the better antioxidant than SkQ1 [36, 52]. However, this contradiction may be accounted for by the particular procedure that we used to reduce antioxidants. Sodium borohydride, a strong reductant, should have reduced both the rhodamine and berberine groups, in addition to the quinone group, which was reflected by bleaching of the antioxidant solution. In the case of SkQR1H2, the reduction should provide at least two additional electrons on the rhodamine group [100], whereas berberine could be, in principle, reduced either to a dihydroberberine form (see the SkQBerbH2 structure in the Fig. 2), or to a tetrahydroberberine/canadine [101, 102]. Thus, upon the reduction of SkQR1 and SkQBerb, the cation moiety was reduced together with the quinol moiety resulting in the loss of the electrical charge by the cation moiety and its transition to a neutral form. Supposedly, loss of the positive charge and relatively high hydrophilicity of SkQR1H2 and SkQBerbH2 could decrease their affinity to the negatively charged cardiolipin liposomes and could diminish thus the kinetic efficiency of inhibition. In this case, the fraction of the bound SkQR1H2 and SkQBerbH2 molecules would then account for the strong inhibiting effect at the beginning of the lag phase, while the molecules not bound to liposomes could account for a less efficient, but prolonged subsequent inhibition of cardiolipin oxidation (Figs. 7 and 8a and Fig. S1a).
The action of SkQR1H2 and SkQBerbH2 (Figs. 7 and 8a) also differed from the effects of the other SkQ ions containing the triphenylphosphonium group (Figs. 6 and 8b and Fig. S2, a-c) by the length of the lag phase. For instance, the lag phase for 1 µM of SkQR1H2 was twice as long as the lag phase for 1 µM MitoQH2, and almost four times longer than the lag phase that was measured with 1 µM of SkQ1H2. This was most likely due to the presence of additional reducing equivalents, so that in the cases of SkQR1H2 and SkQBerbH2 both the quinol group and the rhodamine/berberine groups served as antioxidants.
It is noteworthy that the deviating kinetic characteristics of SkQR1H2 and SkQBerbH2 are interesting mostly from the chemical point of view. In in vivo experiments, both berberine and rhodamine groups carried no additional reducing equivalents, remained positively charged, and could specifically bind to cardiolipin molecules [36, 52].
In the case of ubiquinol Q6H2, which possesses a hydrophobic “tail” of 30 carbon atoms, the lag after the addition of 5 µM of antioxidant (Fig. 8d) was shorter than the duration of inhibition as caused by 1 µM of more hydrophilic compounds (Figs. 6-8). As the solubility of Q6H2 in water calculated by ALOGPS server (http://www.vcclab.org/lab/alogps/) is about 0.5 µM, the majority of hydrophobic molecules of Q6H2 should have formed micelles where non-productive oxidation of ubiquinol molecules by radicals of azo initiator could occur, thereby reducing the effective concentration of the antioxidant.
In sum, the tested cationic mitochondrial-targeted antioxidants could effectively, i.e. fully block peroxidation of cardiolipin molecules in the membrane bilayer, confirming thus the results that had been obtained earlier by tracing the changes in C11-BODIPY fluorescence in response to SkQ1H2 addition. Only partial inhibition of peroxidation by SkQ1H2 upon earlier polarographic measurements [66, 67] could be most likely attributed to the use of Triton X-100 micelles instead of phospholipid membranes in those experiments. It could be envisioned that amphiphilic SkQ ions, being bound at the lipid/water interface, on one hand could not fully “quench” the radicals of water-soluble azo initiator AAPH in the water phase and, on the other hand, could not impede the peroxidation of molecules sequestered in the depth of micelles and unreachable for positively charged SkQ ions.
Our data suggest that natural ubiquinols and SkQH2 ions equally successfully protect cardiolipin molecules from oxidation. Although the ubiquinol-based antioxidants are apparently oxidized somewhat more slowly than the quinol antioxidants lacking methoxy groups, like SkQ ions [66], the observed minor differences are insufficient to explain the strong antioxidative action of SkQ ions, as earlier described in in vivo systems, on the background of large amounts of ubiquinol molecules constantly present in mitochondrial membranes, namely ubiquinol-6 in yeast or ubiquinol-10 in mammals; at the molecular level, this effect was manifested in preventing the oxidation of CL molecules [36, 37, 39, 42, 44-55].
To understand the putative action mechanism of mitochondria-targeted antioxidants, the specific role of cardiolipin in the mitochondrial membrane should be considered. Cardiolipin molecules are remarkable for the presence of four fatty acid “tails” and two neighboring phosphate groups. Since the four fatty acid “tails” of cardiolipin may bind to different proteins, cardiolipin molecules were suggested to link functionally coupled membrane enzymes together [103]. Indeed, cardiolipin molecules were shown to be present in large amounts within the energy-converting supercomplexes that are formed by the NADH:ubiquinone oxidoreductase, cytochrome bc1 complex, and cytochrome c oxidase (mitochondrial complex I, complex III, and complex IV, respectively), as shown for mitochondria of yeast [104] and beef heart mitochondria [105]. As demonstrated in vitro, in the absence of cardiolipin mitochondrial complexes III and IV dissociated during non-denaturing electrophoresis [106].
Owing to the electrostatic interaction of the two phosphate groups, one of them is titrated in the range of pH values from 7.0 to 9.0, i.e. this group is characterized by a pKa value that is close to the physiological pH value [107]. Cardiolipin is supposed to participate in the transfer of protons between the enzymes of the respiratory chain and the ATP-synthases [108-110] and to act as a proton trap; these putative functions might justify the presence of cardiolipin molecules inside some energy-converting membrane enzyme complexes [111-114], particularly within the cytochrome bc1 complex [115]. All these features necessitate the presence of cardiolipin within the coupling membranes of bacteria and eukaryotes.
It was recently shown that distortions in the structure of the cytochrome bc1 complex induced by different factors, such as digestion with proteinase K or exposing to elevated temperatures, enhanced the generation of superoxide anions [14]. It has been speculated that the increase in the level of ROS in response to an in vivo damage to the cytochrome bc1 complex may result in oxidation of cardiolipin molecules that are “trapped” within this enzyme complex and, accordingly, in triggering the chain peroxidation reaction [116]. In view of these considerations, the reported pronounced impact of the SkQ ions in vivo might be due to their greater mobility as compared to natural ubiquinols. Indeed, bulky ubiquinol-10 molecules bear long hydrophobic “tails” which “anchor” them in the middle of the lipid [117]. In contrast, small amphiphilic SkQ ions, as suggested earlier [116, 118], should be capable of “sampling” all regions of the mitochondrial membrane and, particularly, reaching those molecules of cardiolipin that are tightly bound within the membrane proteins [114, 115] and respiratory supercomplexes [104, 105, 119, 120]. Such an ability to protect mitochondrial cardiolipin from oxidation, as already mentioned, is not restricted to SkQ ions, but is also inherent in other low molecular weight amphiphilic antioxidants of various chemical nature [24-29, 63].
We propose that the therapeutic effect of SkQ ions should be most apparent in the cases of acute, but temporal and reversible distortion of mitochondrial redox balance, which could happen in response to traumatic injuries, heart attack, or stroke. In such cases, the mobile antioxidants, including the SkQ ions, may “attenuate” the moment of activation of the apoptotic cascade by preventing oxidation of the cardiolipin molecules trapped within protein complexes until the redox balance within the cell/tissue is restored. Conversely, in cases of long-lasting and irreversible breakdowns in the electron transport chain, primarily in the cytochrome bc1 complex [14, 116], causing irreversible enzyme distortions, the ROS production would eventually exceed the threshold values even in the presence of antioxidative defense, and thus the cells that bear such defects would be removed by apoptosis.
The authors are grateful to Drs. V. A. Roginsky, M. Y. Vyssokikh, and R. A. Simonyan for valuable advice and interest in the present study, as well as to Drs. L. S. Yaguzhinsky and Y. N. Antonenko for useful critical remarks.
This work was supported by the Institute of Mitoengineering of Moscow State University, Ministry of Education of Russian Federation (grant No. 02.740.11.5228), Russian Scientific Foundation, Deutsche Forschungsgemeinschaft (DFG-Mu-1285/1-10, DFG-436-RUS 113/963/0-1), Deutscher Akademischer Austausch Dienst (Ostpartnerschaften-Program), and COST Action CM0902.
REFERENCES
1.Skulachev, V. P. (1997) Membrane-linked systems
preventing superoxide formation, Biosci. Rep., 17,
347-366.
2.Ames, B. N., Shigenaga, M. K., and Hagen, T. M.
(1993) Oxidants, antioxidants, and the degenerative diseases of aging,
Proc. Natl. Acad. Sci. USA, 90, 7915-7922.
3.Halliwell, B., and Gutteridge, J. M. (1990) Role of
free radicals and catalytic metal ions in human disease: an overview,
Methods Enzymol., 186, 1-85.
4.Halliwell, B., and Gutteridge, J. M. (1985) The
importance of free radicals and catalytic metal ions in human diseases,
Mol. Aspects Med., 8, 89-193.
5.Kujoth, G. C., Hiona, A., Pugh, T. D., Someya, S.,
Panzer, K., Wohlgemuth, S. E., Hofer, T., Seo, A. Y., Sullivan, R.,
Jobling, W. A., Morrow, J. D., Van Remmen, H., Sedivy, J. M., Yamasoba,
T., Tanokura, M., Weindruch, R., Leeuwenburgh, C., and Prolla, T. A.
(2005) Mitochondrial DNA mutations, oxidative stress, and apoptosis in
mammalian aging, Science, 309, 481-484.
6.Wang, C., and Youle, R. J. (2009) The role of
mitochondria in apoptosis, Annu. Rev. Genet., 43,
95-118.
7.Skulachev, V. P. (1996) Why are mitochondria
involved in apoptosis? Permeability transition pores and apoptosis as
selective mechanisms to eliminate superoxide-producing mitochondria and
cell, FEBS Lett., 397, 7-10.
8.Skulachev, V. P. (2002) Programmed death phenomena:
from organelle to organism, Ann. N. Y. Acad. Sci., 959,
214-237.
9.Chance, B., Sies, H., and Boveris, A. (1979)
Hydroperoxide metabolism in mammalian organs, Physiol. Rev.,
59, 527-605.
10.Andreyev, A. Y., Kushnareva, Y. E., and Starkov,
A. A. (2005) Mitochondrial metabolism of reactive oxygen species,
Biochemistry (Moscow), 70, 200-214.
11.Kushnareva, Y., Murphy, A. N., and Andreyev, A.
(2002) Complex I-mediated reactive oxygen species generation:
modulation by cytochrome c and NAD(P)+
oxidation-reduction state, Biochem. J., 368,
545-553.
12.Esterhazy, D., King, M. S., Yakovlev, G., and
Hirst, J. (2008) Production of reactive oxygen species by complex I
(NADH:ubiquinone oxidoreductase) from Escherichia coli and
comparison to the enzyme from mitochondria, Biochemistry,
47, 3964-3971.
13.Drose, S., and Brandt, U. (2008) The mechanism of
mitochondrial superoxide production by the cytochrome
bc1 complex, J. Biol. Chem., 283,
21649-21654.
14.Yin, Y., Yang, S., Yu, L., and Yu, C. A. (2010)
Reaction mechanism of superoxide generation during ubiquinol oxidation
by the cytochrome bc1 complex, J. Biol. Chem.,
285, 17038-17045.
15.Malinska, D., Kulawiak, B., Kudin, A. P., Kovacs,
R., Huchzermeyer, C., Kann, O., Szewczyk, A., and Kunz, W. S. (2010)
Complex III-dependent superoxide production of brain mitochondria
contributes to seizure-related ROS formation, Biochim. Biophys.
Acta, 1797, 1163-1170.
16.Boveris, A., and Chance, B. (1973) The
mitochondrial generation of hydrogen peroxide. General properties and
effect of hyperbaric oxygen, Biochem. J., 134,
707-716.
17.Turrens, J. F., and Boveris, A. (1980) Generation
of superoxide anion by the NADH dehydrogenase of bovine heart
mitochondria, Biochem. J., 191, 421-427.
18.Korshunov, S. S., Skulachev, V. P., and Starkov,
A. A. (1997) High protonic potential actuates a mechanism of production
of reactive oxygen species in mitochondria, FEBS Lett.,
416, 15-18.
19.Ksenzenko, M., Konstantinov, A. A., Khomutov, G.
B., Tikhonov, A. N., and Ruuge, E. K. (1983) Effect of electron
transfer inhibitors on superoxide generation in the cytochrome
bc1 site of the mitochondrial respiratory chain,
FEBS Lett., 155, 19-24.
20.Han, D., Williams, E., and Cadenas, E. (2001)
Mitochondrial respiratory chain-dependent generation of superoxide
anion and its release into the intermembrane space, Biochem. J.,
353, 411-416.
21.Kelso, G. F., Porteous, C. M., Coulter, C. V.,
Hughes, G., Porteous, W. K., Ledgerwood, E. C., Smith, R. A., and
Murphy, M. P. (2001) Selective targeting of a redox-active ubiquinone
to mitochondria within cells: antioxidant and antiapoptotic properties,
J. Biol. Chem., 276, 4588-4596.
22.Asin-Cayuela, J., Manas, A. R., James, A. M.,
Smith, R. A., and Murphy, M. P. (2004) Fine-tuning the hydrophobicity
of a mitochondria-targeted antioxidant, FEBS Lett., 571,
9-16.
23.James, A. M., Cocheme, H. M., Smith, R. A., and
Murphy, M. P. (2005) Interactions of mitochondria-targeted and
untargeted ubiquinones with the mitochondrial respiratory chain and
reactive oxygen species. Implications for the use of exogenous
ubiquinones as therapies and experimental tools, J. Biol. Chem.,
280, 21295-21312.
24.Jiang, J., Stoyanovsky, D. A., Belikova, N. A.,
Tyurina, Y. Y., Zhao, Q., Tungekar, M. A., Kapralova, V., Huang, Z.,
Mintz, A. H., Greenberger, J. S., and Kagan, V. E. (2009) A
mitochondria-targeted triphenylphosphonium-conjugated nitroxide
functions as a radioprotector/mitigator, Radiat. Res.,
172, 706-717.
25.Jiang, J., Kurnikov, I., Belikova, N. A., Xiao,
J., Zhao, Q., Amoscato, A. A., Braslau, R., Studer, A., Fink, M. P.,
Greenberger, J. S., Wipf, P., and Kagan, V. E. (2007) Structural
requirements for optimized delivery, inhibition of oxidative stress,
and antiapoptotic activity of targeted nitroxides, J. Pharmacol.
Exp. Ther., 320, 1050-1060.
26.Huang, Z., Jiang, J., Belikova, N. A.,
Stoyanovsky, D. A., Kagan, V. E., and Mintz, A. H. (2010) Protection of
normal brain cells from gamma-irradiation-induced apoptosis by a
mitochondria-targeted triphenyl-phosphonium-nitroxide: a possible
utility in glioblastoma therapy, J. Neurooncol., 100,
1-8.
27.Fink, M. P., Macias, C. A., Xiao, J., Tyurina, Y.
Y., Jiang, J., Belikova, N., Delude, R. L., Greenberger, J. S., Kagan,
V. E., and Wipf, P. (2007) Hemigramicidin-TEMPO conjugates: novel
mitochondria-targeted antioxidants, Biochem. Pharmacol.,
74, 801-809.
28.Jiang, J., Belikova, N. A., Hoye, A. T., Zhao,
Q., Epperly, M. W., Greenberger, J. S., Wipf, P., and Kagan, V. E.
(2008) A mitochondria-targeted nitroxide/hemigramicidin S conjugate
protects mouse embryonic cells against gamma irradiation, Int. J.
Radiat. Oncol. Biol. Phys., 70, 816-825.
29.Ji, J., Kline, A. E., Amoscato, A., Samhan-Arias,
A. K., Sparvero, L. J., Tyurin, V. A., Tyurina, Y. Y., Fink, B.,
Manole, M. D., Puccio, A. M., Okonkwo, D. O., Cheng, J. P., Alexander,
H., Clark, R. S., Kochanek, P. M., Wipf, P., Kagan, V. E., and Bayir,
H. (2012) Lipidomics identifies cardiolipin oxidation as a
mitochondrial target for redox therapy of brain injury, Nat.
Neurosci., 15, 1407-1413.
30.Skulachev, V. P. (2007) A biochemical approach to
the problem of aging: “megaproject” on membrane-penetrating
ions. The first results and prospects, Biochemistry (Moscow),
72, 1385-1396.
31.Green, D. E. (1974) The electromechanochemical
model for energy coupling in mitochondria, Biochim. Biophys.
Acta, 346, 27-78.
32.Liberman, E. A., Topaly, V. P., Tsofina, L. M.,
Jasaitis, A. A., and Skulachev, V. P. (1969) Mechanism of coupling of
oxidative phosphorylation and the membrane potential of mitochondria,
Nature, 222, 1076-1078.
33.Liberman, E. A., and Skulachev, V. P. (1970)
Conversion of biomembrane-produced energy into electric form. IV.
General discussion, Biochim. Biophys. Acta, 216,
30-42.
34.Severin, S. E., Skulachev, V. P., and
Yaguzhinsky, L. S. (1970) Possible role of carnitine in transport of
fatty acids through mitochondrial membranes, Biochemistry
(Moscow), 35, 1250-1253.
35.Levitsky, D. O., and Skulachev, V. P. (1972)
Carnitine: the carrier transporting fatty acyls into mitochondria by
means of an electrochemical gradient of H+, Biochim.
Biophys. Acta, 275, 33-50.
36.Antonenko, Y. N., Avetisyan, A. V., Bakeeva, L.
E., Chernyak, B. V., Chertkov, V. A., Domnina, L. V., Ivanova, O. Y.,
Izyumov, D. S., Khailova, L. S., Klishin, S. S., Korshunova, G. A.,
Lyamzaev, K. G., Muntyan, M. S., Nepryakhina, O. K., Pashkovskaya, A.
A., Pletjushkina, O. Y., Pustovidko, A. V., Roginsky, V. A.,
Rokitskaya, T. I., Ruuge, E. K., Saprunova, V. B., Severina, I. I.,
Simonyan, R. A., Skulachev, I. V., Skulachev, M. V., Sumbatyan, N. V.,
Sviryaeva, I. V., Tashlitsky, V. N., Vassiliev, J. M., Vyssokikh, M.
Y., Yaguzhinsky, L. S., Zamyatnin, A. A., Jr., and Skulachev, V. P.
(2008) Mitochondria-targeted plastoquinone derivatives as tools to
interrupt execution of the aging program. 1. Cationic plastoquinone
derivatives: synthesis and in vitro studies, Biochemistry
(Moscow), 73, 1273-1287.
37.Skulachev, V. P., Anisimov, V. N., Antonenko, Y.
N., Bakeeva, L. E., Chernyak, B. V., Erichev, V. P., Filenko, O. F.,
Kalinina, N. I., Kapelko, V. I., Kolosova, N. G., Kopnin, B. P.,
Korshunova, G. A., Lichinitser, M. R., Obukhova, L. A., Pasyukova, E.
G., Pisarenko, O. I., Roginsky, V. A., Ruuge, E. K., Senin, I. I.,
Severina, I. I., Skulachev, M. V., Spivak, I. M., Tashlitsky, V. N.,
Tkachuk, V. A., Vyssokikh, M. Y., Yaguzhinsky, L. S., and Zorov, D. B.
(2009) An attempt to prevent senescence: a mitochondrial approach,
Biochim. Biophys. Acta, 1787, 437-461.
38.Rokitskaya, T. I., Klishin, S. S., Severina, I.
I., Skulachev, V. P., and Antonenko, Y. N. (2008) Kinetic analysis of
permeation of mitochondria-targeted antioxidants across bilayer lipid
membranes, J. Membr. Biol., 224, 9-19.
39.Lyamzaev, K. G., Pustovidko, A. V., Simonyan, R.
A., Rokitskaya, T. I., Domnina, L. V., Ivanova, O. Y., Severina, I. I.,
Sumbatyan, N. V., Korshunova, G. A., Tashlitsky, V. N., Roginsky, V.
A., Antonenko, Y. N., Skulachev, M. V., Chernyak, B. V., and Skulachev,
V. P. (2011) Novel mitochondria-targeted antioxidants: plastoquinone
conjugated with cationic plant alkaloids berberine and palmatine,
Pharm. Res., 28, 2883-2895.
40.Skulachev, V. P., Antonenko, Y. N., Cherepanov,
D. A., Chernyak, B. V., Izyumov, D. S., Khailova, L. S., Klishin, S.
S., Korshunova, G. A., Lyamzaev, K. G., Pletjushkina, O. Y., Roginsky,
V. A., Rokitskaya, T. I., Severin, F. F., Severina, I. I., Simonyan, R.
A., Skulachev, M. V., Sumbatyan, N. V., Sukhanova, E. I., Tashlitsky,
V. N., Trendeleva, T. A., Vyssokikh, M. Y., and Zvyagilskaya, R. A.
(2010) Prevention of cardiolipin oxidation and fatty acid cycling as
two antioxidant mechanisms of cationic derivatives of plastoquinone
(SkQs), Biochim. Biophys. Acta, 1797, 878-889.
41.Skulachev, V. P. (2013) Cationic antioxidants as
a powerful tool against mitochondrial oxidative stress, Biochem.
Biophys. Res. Commun., 441, 275-279.
42.Skulachev, M. V., Antonenko, Y. N., Anisimov, V.
N., Chernyak, B. V., Cherepanov, D. A., Chistyakov, V. A., Egorov, M.
V., Kolosova, N. G., Korshunova, G. A., Lyamzaev, K. G., Plotnikov, E.
Y., Roginsky, V. A., Savchenko, A. Y., Severina, I. I., Severin, F. F.,
Shkurat, T. P., Tashlitsky, V. N., Shidlovsky, K. M., Vyssokikh, M. Y.,
Zamyatnin, A. A., Jr., Zorov, D. B., and Skulachev, V. P. (2011)
Mitochondrial-targeted plastoquinone derivatives. Effect on senescence
and acute age-related pathologies, Curr. Drug Targets,
12, 800-826.
43.Gu, L. Q., Yu, L., and Yu, C. A. (1990) Effect of
substituents of the benzoquinone ring on electron-transfer activities
of ubiquinone derivatives, Biochim. Biophys. Acta, 1015,
482-492.
44.Antonenko, Y. N., Avetisyan, A. V., Cherepanov,
D. A., Knorre, D. A., Korshunova, G. A., Markova, O. V., Ojovan, S. M.,
Perevoshchikova, I. V., Pustovidko, A. V., Rokitskaya, T. I., Severina,
I. I., Simonyan, R. A., Smirnova, E. A., Sobko, A. A., Sumbatyan, N.
V., Severin, F. F., and Skulachev, V. P. (2011) Derivatives of
rhodamine 19 as mild mitochondria-targeted cationic uncouplers, J.
Biol. Chem., 286, 17831-17840.
45.Plotnikov, E. Y., Chupyrkina, A. A., Jankauskas,
S. S., Pevzner, I. B., Silachev, D. N., Skulachev, V. P., and Zorov, D.
B. (2011) Mechanisms of nephroprotective effect of
mitochondria-targeted antioxidants under rhabdomyolysis and
ischemia/reperfusion, Biochim. Biophys. Acta, 1812,
77-86.
46.Skulachev, V. P. (2012) Mitochondria-targeted
antioxidants as promising drugs for treatment of age-related brain
diseases, J. Alzheimers Dis., 28, 283-289.
47.Jankauskas, S. S., Plotnikov, E. Y., Morosanova,
M. A., Pevzner, I. B., Zorova, L. D., Skulachev, V. P., and Zorov, D.
B. (2012) Mitochondria-targeted antioxidant SkQR1 ameliorates
gentamycin-induced renal failure and hearing loss, Biochemistry
(Moscow), 77, 666-670.
48.Kolosova, N. G., Stefanova, N. A., Muraleva, N.
A., and Skulachev, V. P. (2012) The mitochondria-targeted antioxidant
SkQ1 but not N-acetylcysteine reverses aging-related biomarkers in
rats, Aging (Albany NY), 4, 686-694.
49.Silachev, D. N., Isaev, N. K., Pevzner, I. B.,
Zorova, L. D., Stelmashook, E. V., Novikova, S. V., Plotnikov, E. Y.,
Skulachev, V. P., and Zorov, D. B. (2012) The mitochondria-targeted
antioxidants and remote kidney preconditioning ameliorate brain damage
through kidney-to-brain cross-talk, PLoS One, 7,
e51553.
50.Kapay, N. A., Popova, O. V., Isaev, N. K.,
Stelmashook, E. V., Kondratenko, R. V., Zorov, D. B., Skrebitsky, V.
G., and Skulachev, V. P. (2013) Mitochondria-targeted plastoquinone
antioxidant SkQ1 prevents amyloid-beta-induced impairment of long-term
potentiation in rat hippocampal slices, J. Alzheimers Dis.,
36, 377-383.
51.Severina, I. I., Severin, F. F., Korshunova, G.
A., Sumbatyan, N. V., Ilyasova, T. M., Simonyan, R. A., Rogov, A. G.,
Trendeleva, T. A., Zvyagilskaya, R. A., Dugina, V. B., Domnina, L. V.,
Fetisova, E. K., Lyamzaev, K. G., Vyssokikh, M. Y., Chernyak, B. V.,
Skulachev, M. V., Skulachev, V. P., and Sadovnichii, V. A. (2013) In
search of novel highly active mitochondria-targeted antioxidants:
thymoquinone and its cationic derivatives, FEBS Lett.,
587, 2018-2024.
52.Bakeeva, L. E., Barskov, I. V., Egorov, M. V.,
Isaev, N. K., Kapelko, V. I., Kazachenko, A. V., Kirpatovsky, V. I.,
Kozlovsky, S. V., Lakomkin, V. L., Levina, S. B., Pisarenko, O. I.,
Plotnikov, E. Y., Saprunova, V. B., Serebryakova, L. I., Skulachev, M.
V., Stelmashook, E. V., Studneva, I. M., Tskitishvili, O. V.,
Vasilyeva, A. K., Victorov, I. V., Zorov, D. B., and Skulachev, V. P.
(2008) Mitochondria-targeted plastoquinone derivatives as tools to
interrupt execution of the aging program. 2. Treatment of some ROS- and
age-related diseases (heart arrhythmia, heart infarctions, kidney
ischemia, and stroke), Biochemistry (Moscow), 73,
1288-1299.
53.Agapova, L. S., Chernyak, B. V., Domnina, L. V.,
Dugina, V. B., Efimenko, A. Y., Fetisova, E. K., Ivanova, O. Y.,
Kalinina, N. I., Khromova, N. V., Kopnin, B. P., Kopnin, P. B.,
Korotetskaya, M. V., Lichinitser, M. R., Lukashev, A. L., Pletjushkina,
O. Y., Popova, E. N., Skulachev, M. V., Shagieva, G. S., Stepanova, E.
V., Titova, E. V., Tkachuk, V. A., Vasiliev, J. M., and Skulachev, V.
P. (2008) Mitochondria-targeted plastoquinone derivatives as tools to
interrupt execution of the aging program. 3. Inhibitory effect of SkQ1
on tumor development from p53-deficient cells, Biochemistry
(Moscow), 73, 1300-1316.
54.Neroev, V. V., Archipova, M. M., Bakeeva, L. E.,
Fursova, A. Z., Grigorian, E. N., Grishanova, A. Y., Iomdina, E. N.,
Ivashchenko, Z. N., Katargina, L. A., Khoroshilova-Maslova, I. P.,
Kilina, O. V., Kolosova, N. G., Kopenkin, E. P., Korshunov, S. S.,
Kovaleva, N. A., Novikova, Y. P., Philippov, P. P., Pilipenko, D. I.,
Robustova, O. V., Saprunova, V. B., Senin, I. I., Skulachev, M. V.,
Sotnikova, L. F., Stefanova, N. A., Tikhomirova, N. K., Tsapenko, I.
V., Shchipanova, A. I., Zinovkin, R. A., and Skulachev, V. P. (2008)
Mitochondria-targeted plastoquinone derivatives as tools to interrupt
execution of the aging program. 4. Age-related eye disease. SkQ1
returns vision to blind animals, Biochemistry (Moscow),
73, 1317-1328.
55.Anisimov, V. N., Bakeeva, L. E., Egormin, P. A.,
Filenko, O. F., Isakova, E. F., Manskikh, V. N., Mikhelson, V. M.,
Panteleeva, A. A., Pasyukova, E. G., Pilipenko, D. I., Piskunova, T.
S., Popovich, I. G., Roshchina, N. V., Rybina, O. Y., Saprunova, V. B.,
Samoylova, T. A., Semenchenko, A. V., Skulachev, M. V., Spivak, I. M.,
Tsybul’ko, E. A., Tyndyk, M. L., Vyssokikh, M. Y., Yurova, M. N.,
Zabezhinsky, M. A., and Skulachev, V. P. (2008) Mitochondria-targeted
plastoquinone derivatives as tools to interrupt execution of the aging
program. 5. SkQ1 prolongs lifespan and prevents development of traits
of senescence, Biochemistry (Moscow), 73, 1329-1342.
56.Kagan, V. E., Tyurin, V. A., Jiang, J., Tyurina,
Y. Y., Ritov, V. B., Amoscato, A. A., Osipov, A. N., Belikova, N. A.,
Kapralov, A. A., Kini, V., Vlasova, I. I., Zhao, Q., Zou, M., Di, P.,
Svistunenko, D. A., Kurnikov, I. V., and Borisenko, G. G. (2005)
Cytochrome c acts as a cardiolipin oxygenase required for
release of proapoptotic factors, Nat. Chem. Biol., 1,
223-232.
57.Wang, H. Y., Jackson, S. N., and Woods, A. S.
(2007) Direct MALDI-MS analysis of cardiolipin from rat organs
sections, J. Am. Soc. Mass. Spectrom., 18, 567-577.
58.Kiebish, M. A., Han, X., Cheng, H., Lunceford,
A., Clarke, C. F., Moon, H., Chuang, J. H., and Seyfried, T. N. (2008)
Lipidomic analysis and electron transport chain activities in C57BL/6J
mouse brain mitochondria, J. Neurochem., 106,
299-312.
59.Haines, T. H. (2009) A new look at cardiolipin,
Biochim. Biophys. Acta, 1788, 1997-2002.
60.Gonzalvez, F., and Gottlieb, E. (2007)
Cardiolipin: setting the beat of apoptosis, Apoptosis,
12, 877-885.
61.Kapralov, A. A., Kurnikov, I. V., Vlasova, I. I.,
Belikova, N. A., Tyurin, V. A., Basova, L. V., Zhao, Q., Tyurina, Y.
Y., Jiang, J., Bayir, H., Vladimirov, Y. A., and Kagan, V. E. (2007)
The hierarchy of structural transitions induced in cytochrome c
by anionic phospholipids determines its peroxidase activation and
selective peroxidation during apoptosis in cells, Biochemistry,
46, 14232-14244.
62.Vladimirov, Y. A., Proskurnina, E. V., Izmailov,
D. Y., Novikov, A. A., Brusnichkin, A. V., Osipov, A. N., and Kagan, V.
E. (2006) Cardiolipin activates cytochrome c peroxidase activity
since it facilitates H2O2 access to heme,
Biochemistry (Moscow), 71, 998-1005.
63.Shao, H., Li, J., Zhou, Y., Ge, Z., Fan, J.,
Shao, Z., and Zeng, Y. (2008) Dose-dependent protective effect of
propofol against mitochondrial dysfunction in ischemic/reperfused rat
heart: role of cardiolipin, Br. J. Pharmacol., 153,
1641-1649.
64.Atkinson, J., Kapralov, A. A., Yanamala, N.,
Tyurina, Y. Y., Amoscato, A. A., Pearce, L., Peterson, J., Huang, Z.,
Jiang, J., Samhan-Arias, A. K., Maeda, A., Feng, W., Wasserloos, K.,
Belikova, N. A., Tyurin, V. A., Wang, H., Fletcher, J., Wang, Y.,
Vlasova, I. I., Klein-Seetharaman, J., Stoyanovsky, D. A., Bayir, H.,
Pitt, B. R., Epperly, M. W., Greenberger, J. S., and Kagan, V. E.
(2011) A mitochondria-targeted inhibitor of cytochrome c
peroxidase mitigates radiation-induced death, Nat. Commun.,
2, 497.
65.Kagan, V. E., Wipf, P., Stoyanovsky, D.,
Greenberger, J. S., Borisenko, G., Belikova, N. A., Yanamala, N.,
Samhan Arias, A. K., Tungekar, M. A., Jiang, J., Tyurina, Y. Y., Ji,
J., Klein-Seetharaman, J., Pitt, B. R., Shvedova, A. A., and Bayir, H.
(2009) Mitochondrial targeting of electron scavenging antioxidants:
regulation of selective oxidation vs random chain reactions, Adv.
Drug Deliv. Rev., 61, 1375-1385.
66.Antonenko, Y. N., Roginsky, V. A., Pashkovskaya,
A. A., Rokitskaya, T. I., Kotova, E. A., Zaspa, A. A., Chernyak, B. V.,
and Skulachev, V. P. (2008) Protective effects of mitochondria-targeted
antioxidant SkQ in aqueous and lipid membrane environments, J.
Membr. Biol., 222, 141-149.
67.Roginsky, V. A., Tashlitsky, V. N., and
Skulachev, V. P. (2009) Chain-breaking antioxidant activity of reduced
forms of mitochondria-targeted quinones, a novel type of
geroprotectors, Aging (Albany NY), 1, 481-489.
68.Roginsky, V. A. (2010) Oxidizability of cardiac
cardiolipin in Triton X-100 micelles as determined by using a Clark
electrode, Chem. Phys. Lipids, 163, 127-130.
69.Murphy, M. P., and Smith, R. A. (2007) Targeting
antioxidants to mitochondria by conjugation to lipophilic cations,
Annu. Rev. Pharmacol. Toxicol., 47, 629-656.
70.Goessens, W. H., Driessen, A. J., Wilschut, J.,
and van Duin, J. (1988) A synthetic peptide corresponding to the
C-terminal 25 residues of phage MS2 coded lysis protein dissipates the
proton-motive force in Escherichia coli membrane vesicles by
generating hydrophilic pores, EMBO J., 7, 867-873.
71.Rich, P. R. (1984) Electron and proton transfers
through quinones and cytochrome bc complexes, Biochim.
Biophys. Acta, 768, 53-79.
72.Noguchi, N., Yamashita, H., Gotoh, N., Yamamoto,
Y., Numano, R., and Niki, E. (1998)
2,2′-Azobis(4-methoxy-2,4-dimethylvaleronitrile), a new
lipid-soluble azo initiator: application to oxidations of lipids and
low-density lipoprotein in solution and in aqueous dispersions, Free
Radic. Biol. Med., 24, 259-268.
73.Yamamoto, Y., Niki, E., Kamiya, Y., and
Shimasaki, H. (1984) Oxidation of lipids. 7. Oxidation of
phosphatidylcholines in homogeneous solution and in water dispersion,
Biochim. Biophys. Acta, 795, 332-340.
74.Elferink, M. G., de Wit, J. G., Driessen, A. J.,
and Konings, W. N. (1994) Stability and proton-permeability of
liposomes composed of archaeal tetraether lipids, Biochim. Biophys.
Acta, 1193, 247-254.
75.Barclay, L. R. C., and Vinqvist, M. R. (2003)
Phenols as antioxidants, in The Chemistry of Phenols, John Wiley
& Sons, Ltd., pp. 839-908.
76.Loshadkin, D., Roginsky, V., and Pliss, E. (2002)
Substituted p-hydroquinones as a chain-breaking antioxidant
during the oxidation of styrene, Int. J. Chem. Kinet.,
34, 162-171.
77.Pratt, D. A., Tallman, K. A., and Porter, N. A.
(2011) Free radical oxidation of polyunsaturated lipids: new
mechanistic insights and the development of peroxyl radical clocks,
Acc. Chem. Res., 44, 458-467.
78.Barclay, L. R. C., and Ingold, K. U. (1981)
Autooxidation of biological molecules. 2. The autooxidation of a model
membrane – a comparison of the autooxidation of egg lecithin
phosphatidylcholine in water and in chlorobenzene, J. Am. Chem.
Soc., 103, 6478-6485.
79.Culbertson, S. M., Vinqvist, M. R., Barclay, L.
R., and Porter, N. A. (2001) Minimizing tocopherol-mediated radical
phase transfer in low-density lipoprotein oxidation with an amphiphilic
unsymmetrical azo initiator, J. Am. Chem. Soc., 123,
8951-8960.
80.Roginsky, V. A. (2003) Chain-breaking antioxidant
activity of natural polyphenols as determined during the chain
oxidation of methyl linoleate in Triton X-100 micelles, Arch.
Biochem. Biophys., 414, 261-270.
81.Vossen, R. C., van Dam-Mieras, M. C., Hornstra,
G., and Zwaal, R. F. (1993) Continuous monitoring of lipid peroxidation
by measuring conjugated diene formation in an aqueous liposome
suspension, Lipids, 28, 857-861.
82.Esterbauer, H., Striegl, G., Puhl, H., and
Rotheneder, M. (1989) Continuous monitoring of in vitro
oxidation of human low density lipoprotein, Free Radic. Res.
Commun., 6, 67-75.
83.Liu, W., Porter, N. A., Schneider, C., Brash, A.
R., and Yin, H. (2011) Formation of 4-hydroxynonenal from cardiolipin
oxidation: intramolecular peroxyl radical addition and decomposition,
Free Radic. Biol. Med., 50, 166-178.
84.Kim, J., Minkler, P. E., Salomon, R. G.,
Anderson, V. E., and Hoppel, C. L. (2011) Cardiolipin: characterization
of distinct oxidized molecular species, J. Lipid Res.,
52, 125-135.
85.Yin, H., and Zhu, M. (2012) Free radical
oxidation of cardiolipin: chemical mechanisms, detection and
implication in apoptosis, mitochondrial dysfunction and human diseases,
Free Radic. Res., 46, 959-974.
86.Kuhn, H., Salzmann-Reinhardt, U., Ludwig, P.,
Ponicke, K., Schewe, T., and Rapoport, S. (1986) The stoichiometry of
oxygen uptake and conjugated diene formation during the dioxygenation
of linoleic acid by the pure reticulocyte lipoxygenase. Evidence for
aerobic hydroperoxidase activity, Biochim. Biophys. Acta,
876, 187-193.
87.Pryor, W. A., Cornicelli, J. A., Devall, L. J.,
Tait, B., Trivedi, B. K., Witiak, D. T., and Wu, M. D. (1993) A rapid
screening-test to determine the antioxidant potencies of natural and
synthetic antioxidants, J. Org. Chem., 58, 3521-3532.
88.Landi, L., Fiorentini, D., Stefanelli, C.,
Pasquali, P., and Pedulli, G. F. (1990) Inhibition of autooxidation of
egg yolk phosphatidylcholine in homogenous solution and in liposomes by
oxidized ubiquinone, Biochim. Biophys. Acta, 1028,
223-228.
89.Cape, J. L., Bowman, M. K., and Kramer, D. M.
(2005) Reaction intermediates of quinol oxidation in a photoactivatable
system that mimics electron transfer in the cytochrome
bc1 complex, J. Am. Chem. Soc., 127,
4208-4215.
90.Forquer, I., Covian, R., Bowman, M. K.,
Trumpower, B. L., and Kramer, D. M. (2006) Similar transition states
mediate the Q-cycle and superoxide production by the cytochrome
bc1 complex, J. Biol. Chem., 281,
38459-38465.
91.O’Malley, P. J. (2002) The reaction profile
for hydrogen atom transfer from phenol to peroxyl free radicals,
Chem. Phys. Lett., 364, 318-322.
92.Singh, N., O’Malley, P. J., and Popelier,
P. L. (2005) Mechanistic aspects of hydrogen abstraction for phenolic
antioxidants. Electronic structure and topological electron density
analysis, Phys. Chem. Chem. Phys., 7, 614-619.
93.Espinosa-Garcia, J., and Gutierrez-Merino, C.
(2003) The trapping of the OH radical by coenzyme Q. A theoretical and
experimental study, J. Phys. Chem., 107, 9712-9723.
94.Musialik, M., and Litwinienko, G. (2005)
Scavenging of dpph* radicals by vitamin E is accelerated by its partial
ionization: the role of sequential proton loss electron transfer,
Org. Lett., 7, 4951-4954.
95.De Heer, M. I., Korth, H. G., and Mulder, P.
(1999) Poly methoxy phenols in solution: O–H bond dissociation
enthalpies, structures, and hydrogen bonding, J. Org. Chem.,
64, 6969-6975.
96.De Heer, M. I., Mulder, P., Korth, H. G., Ingold,
K. U., and Lusztyk, J. (2000) Hydrogen atom abstraction kinetics from
intramolecularly hydrogen bonded ubiquinol-0 and other (poly)methoxy
phenols, J. Am. Chem. Soc., 122, 2355-2360.
97.Pethig, R., Gascoyne, P. R., McLaughlin, J. A.,
and Szent-Gyorgyi, A. (1983) Ascorbate-quinone interactions:
electrochemical, free radical, and cytotoxic properties, Proc. Natl.
Acad. Sci. USA, 80, 129-132.
98.Roginsky, V. (2001) Superoxide dismutase enhances
chain-breaking antioxidant capability of hydroquinones, Free Radic.
Res., 35, 55-62.
99.Valgimigli, L., Amorati, R., Fumo, M. G.,
DiLabio, G. A., Pedulli, G. F., Ingold, K. U., and Pratt, D. A. (2008)
The unusual reaction of semiquinone radicals with molecular oxygen,
J. Org. Chem., 73, 1830-1841.
100.Toader, V., Xu, X., Nicolescu, A., Yu, L.,
Bolton, J. L., and Thatcher, G. R. (2003) Nitrosation, nitration, and
autooxidation of the selective estrogen receptor modulator raloxifene
by nitric oxide, peroxynitrite, and reactive nitrogen/oxygen species,
Chem. Res. Toxicol., 16, 1264-1276.
101.Monkovic, I., and Spenser, I. D. (1965)
Biosynthesis of berberine and berberastine – incorporation of
catecholamines, Can. J. Chem., 43, 2017-2026.
102.Elliott, I. W. (1967) Conversion of berberinium
chloride to stereoisomeric ophiocarpines, J. Heteroc. Chem.,
4, 639-640.
103.Mikel’saar, K., Severina, I. I., and
Skulachev, V. P. (1974) Phospholipids and oxidative phosphorylation,
Usp. Sovrem. Biol., 78, 348-370.
104.Mileykovskaya, E., Penczek, P. A., Fang, J.,
Mallampalli, V. K., Sparagna, G. C., and Dowhan, W. (2012) Arrangement
of the respiratory chain complexes in Saccharomyces cerevisiae
supercomplex III2IV2 revealed by single
particle cryoelectron microscopy, J. Biol. Chem., 287,
23095-23103.
105.Althoff, T., Mills, D. J., Popot, J. L., and
Kuhlbrandt, W. (2011) Arrangement of electron transport chain
components in bovine mitochondrial supercomplex
I1III2IV1, EMBO J., 30,
4652-4664.
106.Zhang, M., Mileykovskaya, E., and Dowhan, W.
(2002) Gluing the respiratory chain together. Cardiolipin is required
for supercomplex formation in the inner mitochondrial membrane, J.
Biol. Chem., 277, 43553-43556.
107.Kates, M., Syz, J. Y., Gosser, D., and Haines,
T. H. (1993) pH-dissociation characteristics of cardiolipin and its
2′-deoxy analogue, Lipids, 28, 877-882.
108.Haines, T. H. (1983) Anionic lipid head groups
as a proton-conducting pathway along the surface of membranes: a
hypothesis, Proc. Natl. Acad. Sci. USA, 80, 160-164.
109.Haines, T. H., and Dencher, N. A. (2002)
Cardiolipin: a proton trap for oxidative phosphorylation, FEBS
Lett., 528, 35-39.
110.Mulkidjanian, A. Y., Heberle, J., and
Cherepanov, D. A. (2006) Protons @ interfaces: implications for
biological energy conversion, Biochim. Biophys. Acta,
1757, 913-930.
111.Lange, C., Nett, J. H., Trumpower, B. L., and
Hunte, C. (2001) Specific roles of protein–phospholipid
interactions in the yeast cytochrome bc1 complex
structure, EMBO J., 20, 6591-6600.
112.Hielscher, R., Wenz, T., Hunte, C., and
Hellwig, P. (2009) Monitoring the redox and protonation dependent
contributions of cardiolipin in electrochemically induced FTIR
difference spectra of the cytochrome bc1 complex from
yeast, Biochim. Biophys. Acta, 1787, 617-625.
113.Hielscher, R., Wenz, T., Stolpe, S., Hunte, C.,
Friedrich, T., and Hellwig, P. (2006) Monitoring redox-dependent
contribution of lipids in Fourier transform infrared difference spectra
of complex I from Escherichia coli, Biopolymers,
82, 291-294.
114.Arias-Cartin, R., Grimaldi, S., Arnoux, P.,
Guigliarelli, B., and Magalon, A. (2012) Cardiolipin binding in
bacterial respiratory complexes: structural and functional
implications, Biochim. Biophys. Acta, 1817,
1937-1949.
115.Huang, L. S., Cobessi, D., Tung, E. Y., and
Berry, E. A. (2005) Binding of the respiratory chain inhibitor
antimycin to the mitochondrial bc1 complex: a new
crystal structure reveals an altered intramolecular hydrogen-bonding
pattern, J. Mol. Biol., 351, 573-597.
116.Dibrova, D. V., Cherepanov, D. A., Galperin, M.
Y., Skulachev, V. P., and Mulkidjanian, A. Y. (2013) Evolution of
cytochrome bc complexes: from membrane-anchored dehydrogenases
of ancient bacteria to triggers of apoptosis in vertebrates,
Biochim. Biophys. Acta, 1827, 1407-1427.
117.Hauss, T., Dante, S., Haines, T. H., and
Dencher, N. A. (2005) Localization of coenzyme Q10 in the center of a
deuterated lipid membrane by neutron diffraction, Biochim. Biophys.
Acta, 1710, 57-62.
118.Lokhmatikov, A. V., Voskoboynikova, N. E.,
Cherepanov, D. A., Steinhoff, H. J., Skulachev, V. P., and
Mulkidjanian, A. Y. (2012) Oxidation of cardiolipin in liposomes: a new
insight into the primary steps of mitochondria-triggered apoptosis,
Biochim. Biophys. Acta, 1817, S100.
119.Mileykovskaya, E., and Dowhan, W. (2000)
Visualization of phospholipid domains in Escherichia coli by
using the cardiolipin-specific fluorescent dye 10-N-nonyl acridine
orange, J. Bacteriol., 182, 1172-1175.
120.Mileykovskaya, E., Dowhan, W., Birke, R. L.,
Zheng, D., Lutterodt, L., and Haines, T. H. (2001) Cardiolipin binds
nonyl acridine orange by aggregating the dye at exposed hydrophobic
domains on bilayer surfaces, FEBS Lett., 507,
187-190.
121.Pustovidko, A. V., Rokitskaya, T. I., Severina,
I. I., Simonyan, R. A., Trendeleva, T. A., Lyamzaev, K. G., Antonenko,
Y. N., Rogov, A. G., Zvyagilskaya, R. A., Skulachev, V. P., and
Chernyak, B. V. (2013) Derivatives of the cationic plant alkaloids
berberine and palmatine amplify protonophorous activity of fatty acids
in model membranes and mitochondria, Mitochondrion, 13,
520-525.
Supplementary Figures S1 and S2 (PDF)