Mitochondria-Targeted Antioxidant SkQ1 Accelerates Maturation in Campbell Dwarf Hamsters (Phodopus campbelli)
K. A. Rogovin1,2*, A. M. Khrushcheva1, O. N. Shekarova1, M. V. Ushakova1, V. N. Manskikh3, and N. Yu. Vasilieva1
1Severtsov Institute of Ecology and Evolution, Russian Academy of Sciences, Leninsky pr. 33, 119071 Moscow, Russia2Biological Faculty, Lomonosov Moscow State University, 119991 Moscow, Russia; E-mail: krogovin@yandex.ru
3Belozersky Institute of Physico-Chemical Biology, Lomonosov Moscow State University, 119991 Moscow, Russia
* To whom correspondence should be addressed.
Received April 9, 2014
We tested two hypotheses. 1) SkQ1 positively affects postnatal development of hamsters in litters born to parents receiving long-term SkQ1 treatment. 2) SkQ1 accelerates maturation of juvenile females receiving the antioxidant treatment from 10 days of age. Parental pairs were kept in an outdoor vivarium under conditions close to natural. At the age of 25 days, juvenile males in litters born to parents treated daily with SkQ1 (50 nmol/kg per os) had higher epididymis mass. Both the size of a litter and SkQ1 affected epididymis mass in young males. Both the litter size and SkQ1 affected uterus mass in 25-day-old females. Juvenile females who received SkQ1 treatment from 10 days of age demonstrated earlier opening of the vagina. This experiment was replicated with the same result. At the age of 2.5 months, virgin females treated with SkQ1 from the early age demonstrated higher ovary mass.
KEY WORDS: SkQ1, antioxidants, mitochondria, maturation, lifespan, Campbell dwarf hamstersDOI: 10.1134/S0006297914100125
Abbreviations: SkQ1, 10-(6′-plastoquinonyl)decyltriphenylphosphonium.
The general question traditionally raised in studies of biological
activity of mitochondria-targeted antioxidants of the SkQ group –
whether SkQs can act as geroprotectors, i.e. whether SkQs have a
positive effect on the fertile and the total lifespan [1-5].
It was found meanwhile that SkQ1, being the most effective compound of the group [6, 7], can affect reproductive properties of animals. In experiments with the fruit fly Drosophila melanogaster, SkQ1 prolonged the lifespan of virgin females and males with a maximum effect demonstrated on the survival of females at an early age [2]. However, when males and females were allowed to breed, SkQ1 solution of the same concentration did not increase lifespan of either males or females; there was no effect on female survival at an early age, but early age fertility and the total number of offspring in SkQ1-treated flies increased [8]. In experiments on mice of outbred SHR line kept in a vivarium with a low pathogen contamination (LP conditions), we found that SkQ1 not only inhibits termination of estrous cycles with age, but it also causes early onset of regular cycles [2-4]. However, a similar study conducted by us on Campbell hamsters (Phodopus campbelli, Thomas, 1905) did not confirm the result obtained on mice. We started treating 10-day-old females with 50 nmol/kg per day of SkQ1, and examined daily the smears of their vaginal discharge from the 30th to the 45th day. Usually a female can breed at the age of 30 days. Most females, both in the experiment and in the control, demonstrated diestrus or irregular change of cycle stages. This lack of effect may be related to reproductive specificity of the species. Contact with the male plays a significant role in the cycle formation in young Campbell hamster females.
However, the effect of SkQ1 on the rate of sexual maturation in hamsters can be traced based on morphological characters showing the pace of postnatal development, indicators of sexual maturity. The results of this analysis are presented in this paper. We tested two working hypotheses. 1) SkQ1 positively affects growth and maturation of hamsters in litters after daily long-term treatment of their parents with SkQ1. 2) SkQ1 accelerates the onset of sexual maturity in female hamsters when they are treated with SkQ1 from 10 days of age.
MATERIALS AND METHODS
The Campbell hamster is a small rodent weighing up to 50 g (average 30-40 g). They are active year-round in their natural habitat, but they can fall in short-term states of torpor at low temperatures. Species range is restricted by the dry steppes and semi-deserts of Central Asia. Their daily activity is polyphasic, but under natural conditions, in warm months they are active in early morning hours, after sunset, and at night. They eat seeds, invertebrates, and in the warmer months also the green parts of plants [9, 10]. During the cold season, they feed almost exclusively on seeds, and outside the breeding season they can survive for a long time on metabolic water. Reduction of water loss is achieved by the very high concentrating capacity of their kidneys [11]. The species is polyestrous. Pregnancy lasts for 18-20 days. Animals live solitary and in pairs in simple, shallow holes. The maximal span of life in captivity is up to 3 years; in the wild about 1-1.5 years [10]. Unlike Siberian hamster (Phodopus sungorus), Campbell hamsters do not turn white in winter. They often inhabit snowless semi-deserts [12]. In comparison to Siberian hamsters, Campbell hamsters are less aggressive and quickly habituate to hands. These features greatly facilitate work with animals. However, it is impossible to keep a group of adult males together.
The present study was carried out as part of a long-term experiment on the geroprotective properties of mitochondria-targeted antioxidant SkQ1 on freely breeding Campbell hamsters kept in an outside vivarium under conditions close to natural. When planning the experiment, we took into account the living conditions of the animals in nature. Pairs (male + female) were kept in plastic cages of 70 × 40 × 40 cm with replaceable underlay (dry sawdust, nesting material from technical cotton wool). We did not use artificial lighting, only natural daylight hours. Temperature and humidity corresponded to the natural conditions throughout the year. Water and food (mixed fodder for rats and mice, oat with the addition of sunflower seeds, vegetables, dry dark bread, protein supplements in the form of low-fat cottage cheese) were provided ad libitum. In winter, the animals received water from vegetables (beets, cabbage). The animals could breed freely. The litter remained with the parents for 25 days after birth, and then it was taken away. In case of natural death of one of the adult hamsters in a pair, a new partner was introduced to the surviving animal from the animal reserve. In the case of death of both partners (male and female), the cage was taken away. Seventy-four pairs of animals aged 10-13 weeks were originally included in the experiment: the experimental group of 37 pairs received daily water solution of SkQ1 at a dose of 50 nmol/kg of body weight, and the control group of 37 pairs received water. The animals received the preparation per os by pipetting. Treatment with SkQ1 was started on October 15, 2010.
We studied males and females in reproductive pairs daily – the state of their external genitalia, late stages of female pregnancy, birth of a litter, animal death (with later autopsy of adults), size of the litter, and weight of young at birth. On the 25th day after birth, we counted the number of surviving young, males and females, their weights, and number of young females with open vagina. We evaluated young males in litters for the state of their external genitalia. Then the litter was taken from the parental cage. The weight of adult animals was determined on a monthly basis. Adult females were also weighed after the birth of the litter. In May-June 2011, four groups of 25-day-old young chosen at random were euthanized and autopsied (25 litters from the experimental series and 22 litters from the control series), for a total of 79 males and 44 females from the experimental series and 67 males and 35 females from the control group.
In addition, in April 2011, 23 young females from the experimental group and 21 young females from the control group (all of them were from litters born around the same time, ±2 days) were treated with the same dose of the preparation (50 nmol/kg) starting from 10 days of age; the age of vaginal opening in these animals was registered by daily examination starting from the age of 15 days. Young females remained in groups with their parents for 25 days, and then the animals were taken to individual cages. At the age of 2.5 months females were euthanized, and the weights of their bodies, uteri, and ovaries were determined; the number of mature follicles was also determined. The experiment with young females treated with SkQ1 was repeated in April 2012 following the same scheme (31 young females in the experimental group and 22 young females in the control).
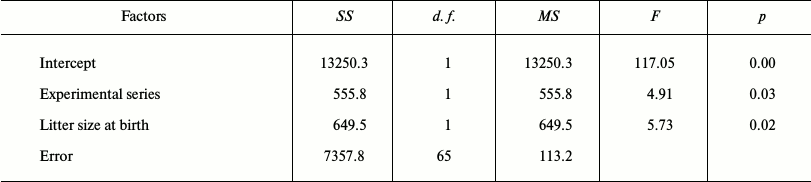
We used the Statistica 6 software (Stat Soft Inc.) for statistical estimation. The correspondence to normality was verified by the Shapiro–Wilk W-test. For normally distributed data we used Student’s t-test for independent samples. The difference between two proportions was evaluated by t-criterion. The General Lineal Model (GLM) was used to evaluate the effect of SkQ1 and other putative factors on reproductive characteristics of 25-day-old male and female hamster. GLM is a generalized version of multiple linear regression analysis that allows simultaneous study of the effects of both categorical and continuous variables. The following parameters were used as dependent variables in different versions of the model: body weight, testicle weight (average of two testicles), and epididymis weight (average of two) in 25-day-old males, and body weight, weight of uterus, and ovary weight (average of two) in 25-day-old females. The following parameters were used as independent variables (factors): experimental series (categorical variable – SkQ1 or control) and continuous variables – body weight, weight and size of the litter at birth, female share in the litter, weight and size of the litter on day 25 after birth, number of previous litters of the parental pair, and number of all young born to the parental pair.
RESULTS
Effect of SkQ1 on growth and development of young hamsters in litters from parents receiving SkQ1. The result of autopsies of 25-day-old hamsters taken from the parental cages in May-July 2011 in the period of their high reproductive activity showed a statistically significant increase of epididymis weight in young males whose parents received SkQ1. Young females showed a statistically insignificant tendency for increased uterus weight (p = 0.11) (Table 1). The use of the GLM showed the presence of significant effects of both SkQ1 (experimental series) and litter size (Table 2). Epididymis weight (average of two) of 25-day-old male hamsters was used as a dependent variable, and the code of experimental series (SkQ1 or Control), body weight, and testicle weight (average of two) at 25 days, litter weight and size at birth, share of females in litter, litter weight and size 25 days after birth, number of prior litters born to the parental pair, and total number of young born to the parental pair were used as putative factors in GLM. Similar analysis of samplings of 25-day-old females, where uterus weight was used as a dependent variable, also showed a statistically significant effect of both SkQ1 and litter size (Table 3).
Table 1. Results of autopsies of 25-day-old
hamsters randomly taken from parental cages in May-July 2011
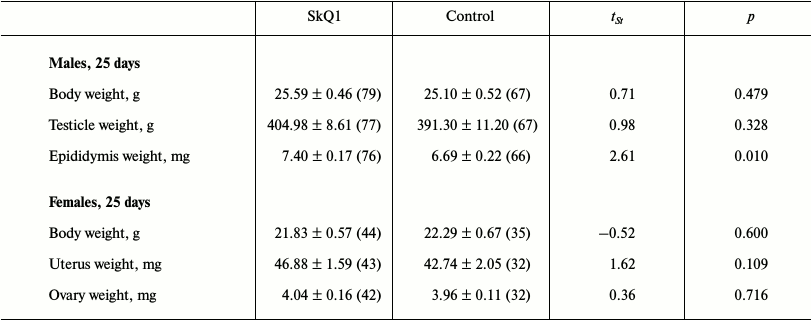
Note: Means with errors are shown. Sample sizes are given in
parentheses.
Table 2. Results of the general linear model
(GLM): epididymis weight (average of two) in 25-day-old male hamsters
of experimental (n = 69) and control (n = 53)
series was used as the dependent variable; the following parameters
were used as probable factors: number of the experimental series, body
weight, testicle weight (average of two) on the 25th day, litter weight
and size at birth, proportion of females in litter, litter weight and
size on the 25th day after birth, number of prior litters born to the
parental pair, and the total number of young born to the parental
pair
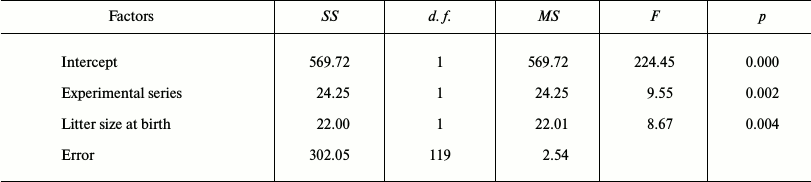
Table 3. Results of the general linear model
(GLM): uterus weight of 25-day-old female hamsters of experimental
(n = 36) and control (n = 39) series was used
as the dependent variable; the following parameters were used as
probable factors: number of the experimental series, body weight, ovary
weight (average of two) on the 25th day, litter weight and size at
birth, proportion of males in litter, litter weight and size on 25th
day after birth, number of prior litters born to the parental pair, and
total number of young born to the parental pair
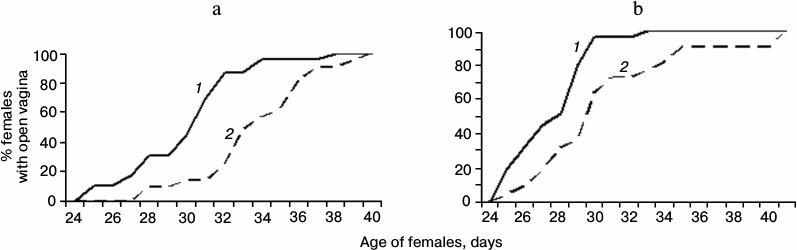
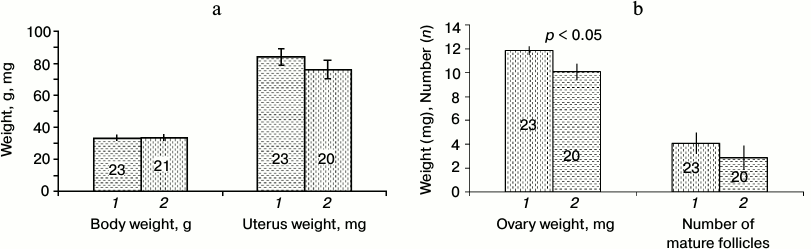
Effect of SkQ1 on the age of vaginal opening in young females. Vagina opening in young Campbell hamster females coincides with the onset of receptivity. In 2011, the proportion of 30-35-day-old young females with opened vagina was higher in the group receiving SkQ1 from 10 days of age than in the control group (the changes were statistically significant, p < 0.05, p < 0.01; Fig. 1a). This experiment was reproduced in spring 2012. The result was confirmed (Fig. 1b). Among young females receiving SkQ1, the proportion of females with open vagina was significantly higher at the age of 29-34 days (p < 0.05, p < 0.01). In 2011, the females receiving SkQ1 from 10 days of age were sacrificed at the age of 2.5 months; body weight, and weight of uterus and ovaries were measured, and the number of mature follicles was determined (Fig. 2). The animals did not differ in their body weight, but the weight of ovaries of females receiving SkQ1 was higher (t = 2.81, p = 0.007, n1 = 23, n2 = 20). Two other parameters showed a statistically insignificant tendency to increased body weight in females receiving SkQ1.
Fig. 1. Cumulative curves characterizing the age of vagina opening in young hamster females treated with SkQ1 from 10 days of age (1) and control group (untreated with SkQ1) (2): a) spring 2011; b) spring 2012.
Fig. 2. Body and uterus weight (a), ovary weight, and the number of mature follicles (b) in 2.5-month-old female hamsters treated with SkQ1 from 10 days of age (1) and control group (untreated with SkQ1) (2).
DISCUSSION
We previously observed that when Campbell hamsters were kept in reproductive pairs outdoors during seasonal changes of day length and temperature (Moscow), 50 nmol/kg per day of SkQ1 increased the fertility of the animals after the state of the winter reproductive break. Although the control and experimental groups showed no difference in total number of litters and young born through the entire lifetime, the pairs receiving SkQ1 started to breed earlier after the winter reproductive pause. As a result, we observed an increased rate of reproduction in pairs receiving SkQ1 in the first half of the year, and in the control pairs receiving water in the second half of the year [13].
This result is consistent with the above-described observations. The daily dose of 50 nmol/kg SkQ1 given to parents had a positive effect on the maturation rate of the young in litters born from these parents, in contrast to young from the water-receiving control parental group. In the period of high reproductive activity of parental pairs in May-July, epididymides of young 25-day-old males from litters of the experimental series had a higher weight compared to the control, and 25-day-old females whose parents received SkQ1 showed a statistically insignificant tendency to higher uterus weight. It is noteworthy that the effect of SkQ1 on epididymis and uterus weight remained with control of the effect of other variables and even despite the statistically significant effect of litter size in the GLM.
This result is consistent with the result of our other specially conducted experiment in which we started treating hamsters with SkQ1 at 10 days of age. We observed earlier opening of vagina in females receiving SkQ1 from 10 days of age. The reliability of this conclusion was confirmed in a repeated experiment. The same females had statistically higher ovary weight measured in 2.5-month-old animals. Thus, our results indicate a positive SkQ1 effect on the rate of sexual maturation of wild-type Campbell hamsters kept in an outdoor vivarium and are consistent with the results obtained on mice [2-4].
We are deeply grateful to Academician V. P. Skulachev who initiated this study and supported us with his constant interest and valuable advice.
This work was commissioned and funded by the Institute of Mitoengineering, Lomonosov Moscow State University.
REFERENCES
1.Skulachev, V. P. (2005) Aging as atavistic program
which can be canceled, Vestnik Ros. Akad. Nauk, 75,
831-843.
2.Anisimov, V. N., Bakeeva, L. E., Egormin, P. A.,
Filenko, O. F., Isakova, E. F., Manskikh, V. N., Mikhelson, V. M.,
Panteleeva, A. A., Pasyukova, E. G., Pilipenko, D. I., Piskunova, T.
S., Popovich, I. G., Roshchina, N. V., Rybina, O. Yu., Samoylova, T.
A., Saprunova, V. B., Semenchenko, A. V., Skulachev, M. V., Spivak, I.
M., Tsybul’ko, E. A., Tyndyk, M. L., Vyssokikh, M. Yu., Yurova,
M. N., Zabezhinsky, M. A., and Skulachev, V. P. (2008)
Mitochondria-targeted plastoquinone derivatives as tools to interrupt
execution of an aging program. 5. SkQ1 prolongs the lifespan and
prevents development of traits of senescence, Biochemistry
(Moscow), 73, 1329-1342.
3.Anisimov, V. N., Egorov, M. V., Krasilschikova, M.
S., Lyamzaev, K. G., Manskikh, V. N., Moshkin, M. P., Novikov, E. A.,
Popovich, I. G., Rogovin, K. A., Shabalina, I. G., Shekarova, O. N.,
Skulachev, M. V., Titova, T. V., Vigodin, V. A., Vyssokikh, M. Yu.,
Yurova, M. N., Zabezhinsky, M. A., and Skulachev, V. P. (2011) Effects
of mitochondria-targeted antioxidant SkQ1 on lifespan of rodents,
Aging, 3, 1-10.
4.Skulachev, V. P., Anisimov, V. N., Antonenko, Yu.
N., Bakeeva, L. E., Chernyak, B. V., Erichev, V. P., Filenko, O. F.,
Kalinina, N. I., Kapelko, V. I., Kolosova, N. G., Kopnin, B. P.,
Korshunova, G. A., Lichinitser, M. R., Obukhova, L. A., Pasyukova, E.
G., Pisarenko, O. I., Roginsky, V. A., Ruuge, E. K., Senin, I. I.,
Severina, I. I., Skulachev, M. V., Spivak, I. M., Tashlitsky, V. N.,
Tkachuk, V. A., Vyssokikh, M. Yu., Yaguzhinsky, L. S., and Zorov, D. B.
(2009) An attempt to prevent senescence: a mitochondrial approach,
Biochim. Biophys. Acta, 1787, 437-461.
5.Shipounova, I. N., Svinareva, D. A., Petrova, T.
V., Lyamzaev, K. G., Chernyak, B. V., Druze, N. I., and Skulachev, V.
P. (2010) Reactive oxygen species produced in mitochondria are involved
in age-dependent changes of hematopoietic and mesenchymal progenitor
cells in mice. A study with novel mitochondria-targeted antioxidant,
Mech. Ageing Dev., 131, 415-421.
6.Skulachev, V. P. (2007) A biochemical approach to
the problem of aging: “megaproject” on membrane-penetrating
ions. The first results and prospects, Biochemistry (Moscow),
72, 1385-1396.
7.Antonenko, Y. N., Avetisyan, A. V., Bakeeva, L. E.,
Chernyak, B. V., Chertkov, V. A., Domnina, L. V., Ivanova, O. Y.,
Izyumov, D. S., Khailova, L. S., Klishin, S. S., Korshunova, G. A.,
Lyamzaev, K. G., Muntyan, O. K., Nepryakhina, A. A., Pashkovskaya, O.
Yu., Pletjushkina, M. S., Pustovidko, A. V., Roginsky, V. A.,
Rokitskaya, T. I., Ruuge, E. K., Saprunova, V. B., Severina, I. I.,
Simonyan, R. A., Skulachev, I. V., Skulachev, M. V., Sumbatyan, N. V.,
Sviryaeva, I. V., Tashlitsky, V. N., Vassiliev, J. M., Vyssokikh, M.
Yu., Yaguzhinsky, L. S., Zamyatnin, A. A., Jr., and Skulachev, V. P.
(2008) Mitochondria-targeted plastoquinone derivatives as tools to
interrupt execution of the aging program. 1. Cationic plastoquinone
derivatives: synthesis and in vitro studies, Biochemistry
(Moscow), 73, 1273-1287.
8.Tsybul’ko, E. A., Roshina, N. V., Rybina, O.
Yu., and Pasyukova, E. G. (2010) Mitochondria-targeted plastoquinone
derivative SkQ1 increases early reproduction of Drosophila
melanogaster at the cost of early survival, Biochemistry
(Moscow), 75, 265-268.
9.Flint, V. E., and Golovkin, A. N. (1961) Essay on
the comparative ecology of Tuva hamsters, Byul. MOIP Otdel.
Biol., 66, 57-75.
10.Feoktistova, N. Yu. (ed.) (2008) Hamsters of
Phodopus Genus. Systematics, Phylogeography, Ecology, Physiology,
Behavior, Chemical Communication [in Russian], Tovarishchestvo
Nauchnykh Izdanii KMK, Moscow.
11.Feoktistova, N. Yu., and Meshersky, I. G. (2008)
Chap. 15, in Hamsters of Phodopus Genus. Systematics,
Phylogeography, Ecology, Physiology, Behavior,
Chemical Communication (Feoktistova, N. Yu., ed.) [in Russian],
Tovarishchestvo Nauchnykh Izdanii KMK, Moscow, pp. 259-272.
12.Sokolov, V. Ye., and Orlov, V. N. (1980) The
Mammals of the Mongolian People’s Republic [in Russian],
Nauka, Moscow.
13.Rogovin, K. A., Khrushcheva, A. M., Shekarova, O.
N., Ushakova, M. V., Manskikh, V. N., Sokolova, O. V., and
Vasilieva, N. Yu. (2014) Effects of mitochondria-targeted plastoquinone
derivative antioxidant (SkQ1) on demography of free breeding Campbell
dwarf hamsters (Phodopus campbelli), kept in outdoor conditions.
Reproduction, and life span: explanation in the framework of ultimate
loads, Biochemistry (Moscow), 79, 1117-1129.