Effects of Mitochondria-Targeted Plastoquinone Derivative Antioxidant (SkQ1) on Demography of Free-Breeding Campbell Dwarf Hamsters (Phodopus campbelli) Kept in Outdoor Conditions. Reproduction and Lifespan: Explanation in the Framework of Ultimate Loads
K. A. Rogovin1,2*, A. M. Khrushcheva1, O. N. Shekarova1, M. V. Ushakova1, V. N. Manskikh3, O. V. Sokolova4, and N. Yu. Vasilieva1
1Severtsov Institute of Ecology and Evolution, Russian Academy of Sciences, Leninsky pr. 33, 119071 Moscow, Russia2Biological Faculty, Lomonosov Moscow State University, 119991 Moscow, Russia; E-mail: krogovin@yandex.ru
3Belozersky Institute of Physico-Chemical Biology, Lomonosov Moscow State University, 119991 Moscow, Russia
4National Hematology Research Centre (NHRC), Novozykovsky Proezd 4, 125167 Moscow, Russia
* To whom correspondence should be addressed.
Received April 9, 2014
We studied demographic effects of the mitochondria-targeted antioxidant SkQ1 on free-breeding Campbell dwarf hamsters (Phodopus campbelli, Thomas, 1905, Rodentia, Cricetidae) in an outdoor vivarium with seasonally varying day length and temperatures. The animals were kept in pairs from their young age. We removed litters from parental cages at their age of 25 days. Experimental hamsters received daily 50 nmol/kg SkQ1 with water by oral dosing, whereas control animals received water. SkQ1 had no effect on the lifespan of either males or females in reproductive pairs. Mortality among females was higher than among males irrespective of SkQ1 treatment, this being related to higher costs of reproduction in females. However, SkQ1 accelerated breeding in pairs in the first half of the reproductive period of a year. Although there were no statistical differences in body mass of males and females between experimental and control animals during most of their life, SkQ1-receiving males had higher body mass at the end of their life. The opposite tendency was characteristic for old females. One-year-old males and females of the experimental and control groups showed no difference in intensity of immune response to sheep red blood cells. The dermal hypersensitivity response to phytohemagglutinin (test for T-cell immunity) was significantly higher in SkQ1-treated 1- and 1.5-year-old males. This was not true for females. There was a tendency toward increased density of the neutrophil population in blood in 1-year-old SkQ1-treated males. However, experimental males showed no difference from control males in the activity of the “peroxidase–endogenous hydrogen peroxide system” of neutrophils. The background level of stress estimated by the concentration of cortisol in blood serum was significantly lower in the SkQ1-treated males during autumn adaptive adjustment of the organism. A similar trend was also observed during the January frosts, when the background level of stress was rather high. We observed no differences between cortisol concentration in experimental and control animals during the reproductive period in early spring and mid-summer. We tend to interpret the absence of geroprotective effect of SkQ1 on free-breeding dwarf hamsters by its ability to intensify breeding. We previously demonstrated the ability of SkQ1 to increase the lifespan of non-breeding females.
KEY WORDS: SkQ1, antioxidants, mitochondria, aging, lifespan, fecundity, Campbell dwarf hamsterDOI: 10.1134/S0006297914100137
Abbreviations: ACTH, adrenocorticotropic hormone; CCI PEHP, cytochemical index characterizing activity of “peroxidase–endogenous hydrogen peroxide system” in neutrophils; DTH, delayed-type hypersensitivity reaction; PHA-P, phytohemagglutinin P; SkQ1, 10-(6′-plastoquinonyl)decyltriphenylphosphonium; SRBC, sheep red blood cells.
Free radical-based signaling pathways may contribute to the mechanisms
regulating lifespan, aging, and reproduction of organisms. In this
regard, integrated effects of antioxidants, which are targeted at
neutralization of the damaging effects of free radicals on cells and
tissues, can be considered as a way to cancel the program of organism
aging and death [1-3]. This
program was given the name phenoptosis by analogy with apoptosis,
programmed cell death [4, 5].
The mitochondria-targeted antioxidant SkQ1 (10-(6′-plastoquinonyl)decyltriphenylphosphonium) is a plastoquinone isolated from plant cell chloroplasts conjugated with the decyltriphenylphosphonium ion (which can penetrate the mitochondrial membrane), wherein the positive charge of the phosphorous atom is delocalized between three substituent aromatic groups [6, 7]. The ability of SkQ1 to increase lifespan by shifting the median of lifespan distribution toward older ages has been studied and confirmed on representatives of such evolutionarily distant taxa as fungi culture (Podospora anserina), cladocerans (Ceriodaphnia affinis), insects (Drosophila melanogaster), and rodents [3, 6, 8-13]. In mice, survival increased primarily in the early stages of the life cycle [9, 10]. In a non-LP (low pathogen) vivarium, median lifespan of SkQ1-treated SHR female outbred mice was shown to be higher than that of the control group; it corresponded to the lifespan of similar mice in an LP vivarium. Male mice of the BALB/c strain kept in the sterile SPF (specific pathogen free) vivarium had shorter lifespan than females, but SkQ1 prolonged the male lifespan to reach that of females. Lifespan of male and female C57Bl/6 mice kept in an SPF vivarium in isolation from each other was the same; SkQ1 increased the lifespan of males, but not of females. SkQ1 increased the lifespan of virgin Campbell dwarf hamster females kept in an outdoor vivarium with seasonally varying day length and temperature.
Males and females were kept separately in the above-described cases, so the rodents could not breed. The effects of SkQ1 on freely breeding rodents in a laboratory with temperature conditions regulated similarly to nature were studied only for the northern mole vole (Ellobius talpinus). In this case, increased lifespan could be observed in both males and females; it was accompanied by a significant increase in birth rate in animals receiving SkQ1. Unfortunately, the age of the voles at the beginning of the experiment varied because the animals were taken directly from nature. The absolute age was evaluated posthumously. Some of the animals died at the initial stage of the experiment, probably due to age heterogeneity of the sample [10].
The above-described studies were carried out in controlled conditions of a laboratory experiment, mainly on laboratory animals, including different strains. However, it is obvious that the final stages of a full research program studying the properties of bioactive geroprotectors should include the evaluation of the effects on animal populations in their natural habitat. This means that the efficiency of the preparation should be evaluated in samples of genetically variable individuals under conditions when the entire range of species-inherent adaptations is realized in the course of individual life histories. It is difficult to implement this requirement in mammals because due to heterogeneity of habitat conditions, it is practically impossible to find experimental and control areas providing equal fitness. In addition, there is another requirement for the experiment – control over drug consumption and state of the animals. It is virtually unattainable in nature when dealing with small short-lived mammals, which provide the possibility for obtaining material fulfilling the statistical requirements in a relatively short time. The solution can be found if testing of potential geroprotectors is conducted on wild-type animals taken from the wild and kept under conditions close to natural.
We chose Campbell dwarf hamsters (Phodopus campbelli Thomas, 1905, Cricetidae, Rodentia) for testing the effects of SkQ1. The choice was determined by the following requirements: a) short lifespan combined with high reproduction rate; b) year-round activity in nature; c) ability to tolerate seasonal changes in temperature and humidity characteristic for Moscow latitude; d) low aggressiveness combined with relatively high resistance to stress (primarily associated with anxiety caused by manipulation of animals).
The Campbell hamster is a small rodent weighing up to 50 g. In the wild, these hamsters are active throughout the year. At low temperatures they can fall into short-term states of torpor. The area of their inhabitance is dry steppes and semi-deserts of Central Asia. Daily activity is polyphasic, but in the wild in the warm season Campbell hamsters are active in the early morning hours, after sunset, and at night. They eat seeds, food of animal origin, and in the warm season also green parts of plants [14, 15]. During the cold season they eat almost exclusively seeds, and outside of the breeding season they can apparently survive for a long time relying on only metabolic water. Reduction of water loss is achieved by very high concentrating ability of the kidneys [16]. The species is polyestrous. Pregnancy lasts for 18-20 days. Campbell hamsters are solitary or live in pairs (male + female) in simple, shallow holes. The maximal lifespan in captivity is up to 3 years, in the wild 1-1.5 years [14]. Unlike the similar Siberian hamster (Phodopus sungorus), Campbell hamsters do not turn white in winter. They are frequent inhabitants of snowless semi-deserts [17]. Compared to Siberian hamsters, they are less aggressive and quickly acclimate to humans, this greatly facilitating work with them. However, it is not possible to keep a group of adult males together.
The purpose of our study was to evaluate the effects of SkQ1 as a geroprotector for the wild-type rodents kept under conditions close to natural: 1) corresponding to socio-demographic characteristics of the species; 2) reproducing seasonal climate variability. In accordance with the above limitations, we sought answers to questions on the effects of SkQ1 on reproductive properties of the animals, on their lifespan, on health indicators (both external signs of health and immunity characteristics), and on the resistance to seasonal stress factors.
MATERIALS AND METHODS
When planning the experiment, we took into account the living conditions of the animals in the wild. The hamsters lived all year-round outside in a specially constructed wire net enclosure under a roof. Pairs (male and female) were kept in spacious plastic cages 70 × 40 × 40 cm with replaceable underlay (dry sawdust, nesting material of technical wool). We used no artificial lighting, only the natural light. Temperature and humidity were determined by outdoor conditions all year round. Water and food (mixed fodder for rats and mice, oat supplemented with sunflower seeds, vegetables, dry dark bread, protein supplements in the form of low-fat cottage cheese) were provided ad libitum. In winter, the animals received water from vegetables (beet, cabbage). The hamsters could breed freely. The litter remained with parents for 25 days, and then was removed. In case of natural death of one hamster in a parental pair, a new partner from the animal reserve was put into the cage. In the case of death of both partners (male and female), the cage was removed. The study was begun with 37 pairs of animals aged 10-13 weeks daily receiving water solution of SkQ1 (50 nmol/kg body weight, experiment) and 37 pairs of animals receiving water (control). The hamsters received SkQ1 preparation per os by pipetting. We started treating the hamsters with SkQ1 on October 15, 2010. We chose this dosage according to the results of a preliminary study of effects of SkQ1 at doses of 50 and 5 nmol/kg on virgin females [10] as well as a preliminary experiment with reproductive pairs. In the latter case, hamsters receiving SkQ1 at the dose of 50 nmol/kg had significantly more litters per 2 months (autumn 2008) than the animals receiving SkQ1 at a dose of 5 nmol/kg (t-test for independent samples: t = 2.54; n1 = 29, n2 = 34; p = 0.014).
We recorded the following parameters on daily examination of the cages. 1) We daily evaluated the state of the external genitalia, the presence of pregnancy (by external characteristics), the fact of giving birth to a litter, its size, weight of the young, the fact of death of an adult individual (in case of death the animal was weighed and frozen to –18oC for subsequent evaluation of the cause of death). 2) Males and females were weighed monthly. Females were also weighed after giving birth to a litter. 3) The number of surviving young was counted on the 25th day after birth; we also determined the number of males and females in the litter, their weights, and the number of young females with open vagina. Then the litter was removed from the parental cage. 4) External characteristics (the quality of fur, vibrissa, teeth, ventral specific skin glands, etc.) were recorded using digital photography of the animals once per season in standard positions. Estimates were made based on digital images. 5) Blood concentration of stress hormones produced by the adrenal cortex (cortisol in case of Campbell hamsters) was measured in males and females during the period of autumn physiological adaptation (first frosts), and in case of males also in the period of winter frosts and in spring and summer (in the reproductive period of life). 6) Immunity characteristics were determined in 1-year-old hamsters.
We used SRBC-test to characterize the state of the adaptive humoral immunity system. This test evaluates immunocompetence in response to immunization of the hamster with 2% suspension of sheep erythrocytes in saline. Blood samples were taken from the sublingual vein 7 days after immunization. The level of antibodies in the serum of hamsters immunized with erythrocytes was determined by the hemagglutination reaction in wells of a 96-well immunological plate by titration of serum samples in wells and the addition of 0.5% suspension of sheep erythrocytes in saline to serial saline dilutions of serum samples. The immune response was evaluated visually by the number of the last well (in the line of successive dilutions) where the amount of antibodies was still sufficient for hemagglutination [18].
T-cell immune response was evaluated 24 h after intradermal injection (heel callus of the hindpaw) of phytohemagglutinin (PHA-P) by the size of swelling. PHA-test is a delayed-type hypersensitivity reaction (DTH) in response to intradermal injection of a mitogen causing attraction and fast proliferation of T-lymphocytes [19].
The cytochemical technique revealing the activity of the “peroxidase–endogenous hydrogen peroxide system” (PEHP) in neutrophils on peripheral blood smears was used to characterize the state of nonspecific innate immunity in the Campbell hamsters [20]. The degree of activation of the system is determined by the density of granules stained with 3,3′-diaminobenzidine tetrahydrochloride. This reaction characterizes the biochemical potential of neutrophil phagocytic activity. To estimate the intensity of the reaction, neutrophils (100 cells) in the smear were ranked according to granule density into three categories: low (Nl – individual, separately located granules), medium (Nm – many granules, which do not merge), and high (Nh – dense clusters of granules). The cytochemical index was calculated per 100 neutrophils with the formula: CCI = 1Nl + 2Nm + 3Nh. We concurrently studied the white blood cell counts in the smears stained by the May-Grunwald method. The leukograms were arrayed by the standard techniques (n leukocytes of a given type per 100 leukocytes).
To assess cortisol level in the hamster blood serum, we used enzyme linked immunosorbent assay (ELISA). Reagent kits for cortisol in human serum were produced by Immunotech (Russia). Testing the applicability of the test system (antibodies) for the particular animal species is a prerequisite for its use to evaluate hormone concentration in blood and excreta [21]. We performed the necessary testing and confirmed the suitability of the reagent kits for evaluating cortisol concentration in blood serum of Campbell hamsters (test on parallelism, response to acute stress factor (social conflict, ACTH administration)). In all cases the blood was taken from the sublingual vein within 2 min after taking the animal, which is twice shorter than the time of cortisol release into blood in response to acute stressor (effect of manipulations with the animal).
Statistical evaluations were performed using the Statistica 6 software (StatSoft). The correspondence of empirical distributions to the model of normal distribution was verified by the Shapiro–Wilk W-test. To normalize distributions of cortisol concentrations in blood serum, data were log10 transformed. We used Student’s t-test for dependent and independent samples for normally distributed data; when distributions differed from normal, we used the Wilcoxon Matched-Pairs test for dependent samples and Mann–Whitney U-test for independent samples. The difference in proportions was evaluated by the t-criterion and by χ2 method.
The general linear model (GLM) was used to assess the effect of SkQ1 and other possible factors on the lifespan or reproductive characteristics of the hamsters. Lifespan was presented as a dependent variable. Independent variables included series of the experiment (categorical variable: 1 = experiment, 2 = control) and continuous variables (number of litters, average litter size, weight at age of four months, immunity characteristics, etc. and interactions between some of them).
The Kaplan–Meier product limit method was used to draw and analyze survival curves. Survival in the control and experimental series was compared using Gehan’s generalized Wilcoxon test adapted for survival analysis.
RESULTS
Breeding. We observed a tendency to earlier breeding in SkQ1-treated hamsters after the autumn–winter delay in both 2011 and 2012. In 2012 we took into account young females put into cages of old males to replace deceased older females (the new females also received SkQ1 in the experiment and water in the control following the standard procedure from the day of joining their partners). This is acceptable because there was no difference in mortality between the control and the experiment, and hence the number of replaced females in these two groups was the same. Only two old females from the experimental and two old females from the control series took part in the spring-summer 2012 breeding. In 2011, the maximal difference in the number of females who had given birth was observed in the second decade of February: 25 females out of 35 reproductive pairs had given birth in the experimental series (71%) and 19 out of 35 (54%) in the control; difference between two proportions: p = 0.14. In 2012 the maximal difference in the number of females who had given birth (seven females) was observed in the first decade of April. Twelve females out of 24 (50%) had given birth in the experimental series and five out of 24 (20.8%) in the control; difference between two proportions: p = 0.04; χ2 with the Yates correction for small samples: χ2 = 3.28; p = 0.07.
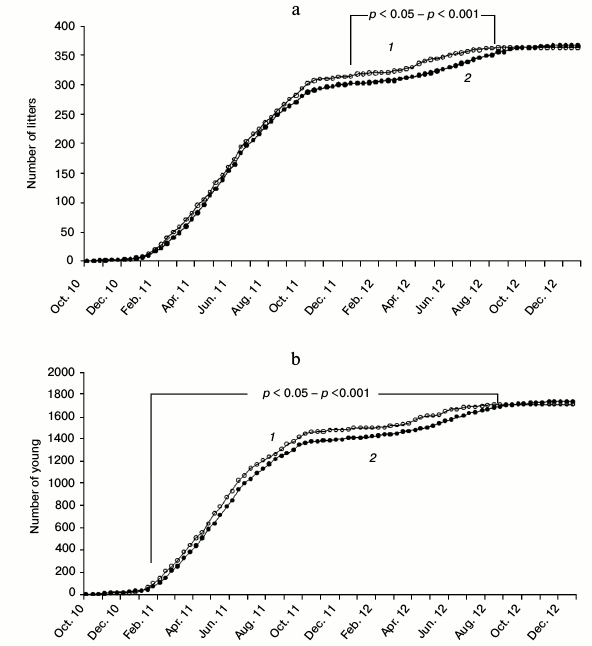
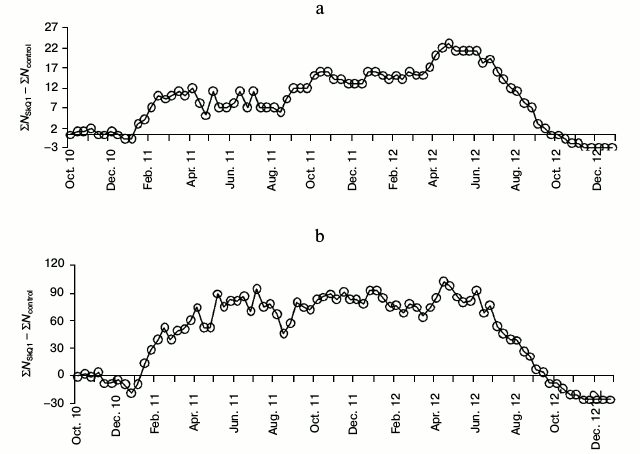
The maximal difference in the accumulated number of litters between the experimental and control series was observed in the third decade of April 2012 (Δ = 23). Significant differences between the proportions of all the litters born before a particular date and the total number of litters were observed between the second decade of December 2011 and the first decade of August 2012 (Figs. 1a and 2a). The maximal difference in the accumulated number of young between the experimental and control series in 2012 was observed in the second decade of April (Δ = 102). Significant differences between the proportions of all the young born before the concrete date and the total number of young were observed in the period between the third decade of January 2011 and the third decade of August 2012 (Figs. 1b and 2b). By the end of 2012 the difference between the sum of litters and the difference between the sum of young in the experimental and control series gradually approached zero. Breeding in the control series continued even in October-November 2012 (Fig. 2, a and b).
Fig. 1. Cumulative curves of the number of litters (a) and the number of young (b) per decade from October 2010 to December 2012, including the core females and those added to replace deceased animals (nexp = 56, ncontr = 58). Statistical evaluation – the difference in proportions of accumulated young or litters on a specific date per total number of young or litters. 1) SkQ1; 2) control.
Fig. 2. Difference in number of accumulated litters (a) and young (b) per decade (Δ = ΣNSkQ1 – ΣNcontr) for the period from October 2010 to December 2012 including the core females and those added to replace deceased animals (nexp = 56, ncontr = 58).
Average litter sizes were decreasing both in the experiment and in the control from spring to autumn 2011. Statistically significant difference in the direction of larger litter size (t-test: t = 2.26, n1 = 12, n2 = 14, p < 0.05) was observed in SkQ1-treated females in the first decade of May 2011, the period of maximal difference in the number of young born by experimental and control females. The size of the first litters of control and experimental females was the same: SkQ1 – 5.26 ± 0.33 young; control – 5.26 ± 0.38, t = –0.01, n1 = 31, n2 = 34, p = 0.99. However, there was a tendency to a larger size of the last litter in the life in the control group: SkQ1 – 2.39 ± 0.30; control – 3.24 ± 0.35, t = –1.84, n1 = 28, n2 = 29, p = 0.07.
For GLM analysis, we took the number of litters or young born during the mother’s entire lifetime as the dependent variable, and the series of the experiment, characteristics of the immunity state, weight in the initial life period (4 months), and lifespan served as probable factors (independent variables). We also took into account the possibility of interaction of these factors. Resulting GLM analysis showed that only the lifespan remained in the final model as a significant factor both for males (sum of litters: F1,53 = 19.9, p < 0.001; sum of young: F1,53 = 14.18, p < 0.001), and females (sum of litters: F1,38 = 6.57, p < 0.014; sum of young: F1,38 = 6.57, p < 0.014).
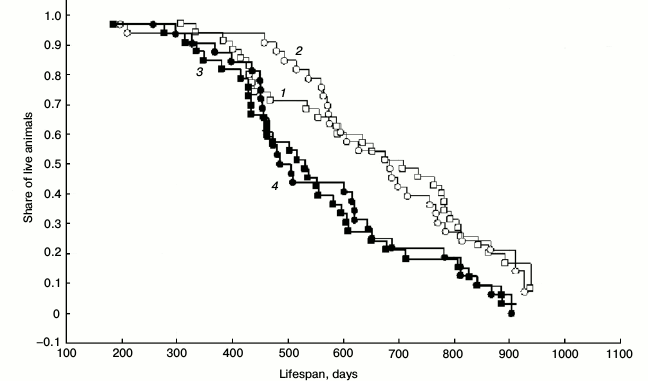
Lifespan. Initially, 37 pairs of Campbell hamsters receiving SkQ1 at 50 nmol/kg perorally from October 15, 2010 (experimental series) and 37 pairs receiving water (control series) were involved in the experiment. By December 31, 2012, only several animals remained alive: 0 females and 6 males in the experimental group; 1 female and 6 males in the control group. There were no differences between the experimental and control groups both among males and females (Survival Analysis: Gehan’s Wilcoxon test: p > 0.05). The absence of experiment–control difference remained also after the strict exclusion of all the cases of “accidental” animal loss (including the cases of female death at parturition): males: Gehan’s Wilcoxon test statistics = –0.18, p = 0.85, nexp = 35, ncontr = 33; females: Gehan’s Wilcoxon test statistics = –0.18, p = 0.85, nexp = 33, ncontr = 32 (Fig. 3). Increased mortality of SkQ1-receiving males was observed at the age of 1-1.5 years (χ2 Yates corrected = 3.88, p < 0.05). However, this might have resulted from more intensive breeding of SkQ1-receiving hamsters in spring 2011.
Fig. 3. Lifespan of hamsters in the outdoor vivarium from October 2010 to December 2012: 1) males receiving SkQ1; 2) control males; 3) females receiving SkQ1; 4) control females. All the cases of accidental animal loss are excluded (female death at delivery; escaped animals). SkQ1: 35 males and 33 females; control: 33 males and 32 females. The curves were derived based on the Kaplan–Meier product limit method using Statistica 6 software.
Higher mortality of females (when compared with males) was observed in both the experiment and the control. It was apparently associated with the higher costs of reproduction in females: experiment: Gehan’s Wilcoxon test statistics = 2.25, p = 0.02, nmales = 35, nfemales = 33; control: Gehan’s Wilcoxon test statistics = 2.59, p = 0.01, nmales = 33, nfemales = 32.
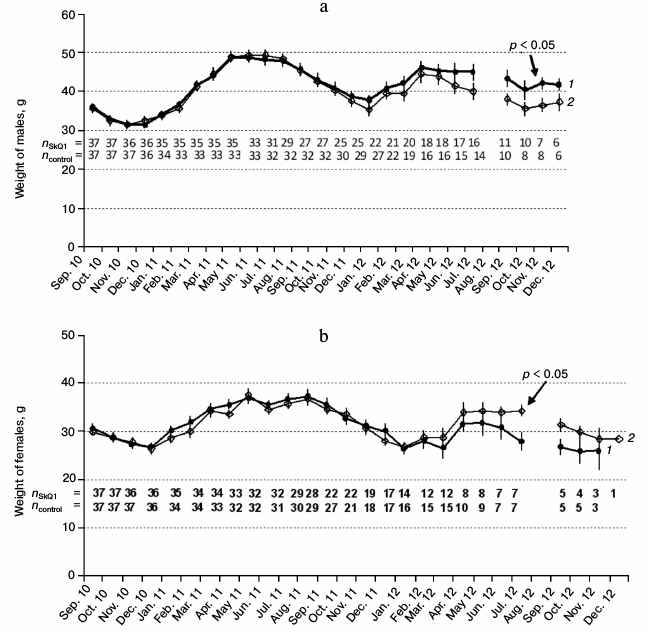
Age dynamics of body weight. Seasonal factor plays a key role in the dynamics of body weight in both male and female hamsters irrespective of SkQ1 treatment (Fig. 4). The weight of the animals decreased from September to December and increased from January to April. The maximal body weight was observed in males and females from May to August 2011. Body weight of both males and females in the experimental and control series decreased by the end of life. We observed no statistically significant differences in body weight dynamics between the experiment and the control throughout most of the experiment. However, at the end of the experiment the SkQ1-receiving males expressed a tendency for higher weight, this observation not being connected to their better survival at the end of life. In contrast, females expressed an opposite tendency at the end of the experiment: SkQ1-receiving females had lower body weight by the end of their lifespan. This may be due to their higher reproduction costs. Higher body weight of females from the control group also was not related to their higher survival. We observed no differences between the experimental and control series when studying the variability of specific ventral gland and external genitals with time (anogenital distance in males, testes size based on their external outlines), as well as color and quality of hair based on the analysis of digital photographs.
Fig. 4. Male (a) and female (b) weight dynamics in experiment (1) and control (2). Numbers indicate the sample size at the date of weighing at the end of month.
Immunity state. SRBC-induced humoral immunity (antibody formation). Young animals with measured immune response to the multifactorial antigen load (SRBC) were selected for the formation of pairs for breeding in 2010 in the outdoor vivarium. The strength of the primary immune response to sheep erythrocytes was the same in the experimental and control animals. One-year-old males underwent repeated immunization, which resulted in the increase in strength of the secondary immune response in both experimental and control animals (t-test for dependent samples: SkQ1: t = –2.198, p = 0.035, n1 = n2 = 31; control: t = –1.94, p = 0.064, n1 = n2 = 34). The experimental series showed no difference from the control in the strength of the secondary immune response (table). In contrast to males, the distributions of antibody titers in females after the first as well as after the second immunizations were strongly asymmetrical with the predominance of low titers. The increase in the strength of the secondary immune response in females had no statistical significance both in the experimental and control series (Wilcoxon Matched Pairs test: experiment: Z = 1.23, p = 0.22, n1 = n2 = 28; control: Z = 0.94, p = 0.34, n1 = n2 = 25). Also, there was no difference between the control and experimental series in the strength of the secondary immune response to erythrocytes (table). Low immunocompetence of many females had no effect on their breeding.
Indicators of immunity of 1-year-old hamsters receiving SkQ1 and water
in outdoor vivarium
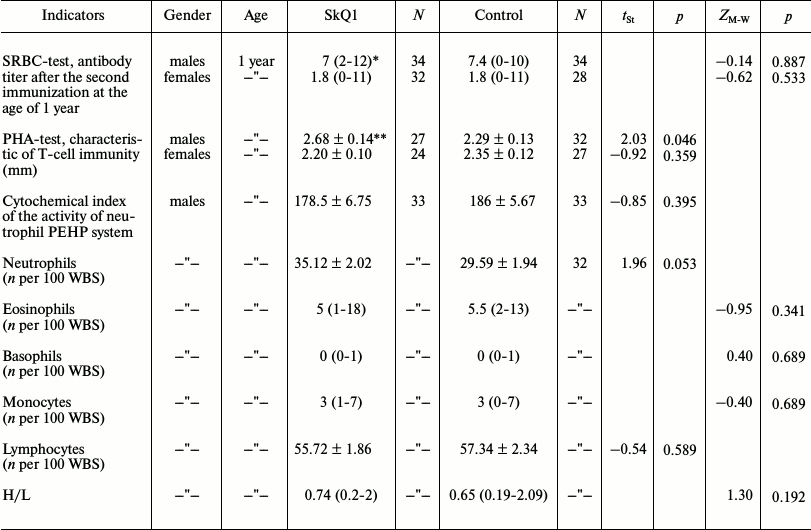
Note: n per 100 WBS, the number of cells of a given type in the
sample of 100 leukocytes; H/L, granulocyte-lymphocyte index;
tSt, t-criterion in the Student’s test;
ZM–W, Z-statistics in the
Mann–Whitney test; p, probability.
* Median and range of variability (in parentheses) for non-normal
distributions.
** Mean value and error for normally distributed data.
Induced T-cell immunity. The dermal delayed-type hypersensitivity reaction to intradermal administration of phytohemagglutinin (PHA-P) was used to test the state of T-cell immunity. The hypersensitivity reaction was shown to be higher in SkQ1-receiving 1-year-old male hamsters (see table). The differences remained also on repeated PHA administration in 1.5-year-old males (t = 2.23, p = 0.033, n1 = 20, n2 = 17). In the case of SkQ1-treated males, their secondary response to PHA was significantly positively associated with the primary (1-year-old males) response (r = 0.65, p = 0.002). In contrast, such a connection was absent in the control group (r = –0.04, p = 0.87). Statistically significant difference between the experimental and control series was absent in the group of 1-year-old females (table).
Nonspecific immunity. In the case of 1-year-old males, there was no difference between the experimental and control series both in cytochemical index of the activity of the PEHP system and the white blood cell counts except some increase (close to statistically significant values) in the number of neutrophils in SkQ1-receiving males (table). This study was not conducted with the females.
Immunity and lifespan. When analyzing the results for the males, we used the general linear model (GLM) where lifespan was the dependent variable and the following parameters were the probable factors: series of experiment (experimental and control), primary and secondary response (antibody titers) to SRBC, DTH skin reaction to phytohemagglutinin, CCI PEHP in neutrophils, granulocyte-lymphocyte index, number of lymphocytes, neutrophils, and monocytes per 100 white blood cells, and body weight. We showed that only the humoral immune response to SRBC at the age of one year has weak, close to statistically significant positive effect on the lifespan: F1,54 = 3.57, p < 0.064.
GLM analysis of the females showed no effects of SkQ1 or immunity characteristics on lifespan.
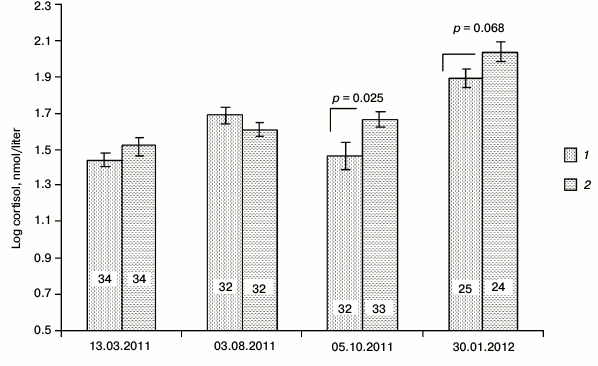
Resistance to physical stressors. Cortisol level in males, measured within the period of first autumn frosts in October 2011, showed a significantly lower level of this main glucocorticoid of adrenal cortex in SkQ1-receiving males (t-test: t = –2.29, p = 0.025, n1 = 32, n2 = 33; Fig. 5). Similar measurements in females showed no significant differences in cortisol levels between the animals from the control and experimental (SkQ1-receiving) groups (t-test: t = –0.34, p = 0.73, n1 = 32, n2 = 27). Repeated cortisol measurement in the peripheral blood of males during the January 2012 frosts (daily temperature range from –20 to –15oC) also demonstrated the tendency to lower cortisol levels in the blood of SkQ1-receiving animals (t-test: t = –1.87, n1 = 25, n2 = 24, p = 0.068). Weakly pronounced differences between the control and experimental (SkQ1-receiving) groups of males were observed against the background of an increased stress level of all the males in the cold compared to other periods (p < 0.05 – p < 0.01). During the reproductive period there were no differences in cortisol levels in blood between the control and experimental groups of males (Fig. 5).
Fig. 5. Seasonal differences in the background level of cortisol in the blood serum of male hamsters receiving SkQ1 (1) and water (2).
DISCUSSION
The SkQ1 dose of 50 nmol/kg per day had no effect on the survival of Campbell hamster males and females kept all year round in reproductive pairs in the outdoor vivarium. The experimental and control groups of animals showed no difference in their lifespan when they were given the opportunity to breed freely and were not subjected to the negative effect of social density as their litters were removed at the age of 25 days. Higher female mortality (when compared to males) could be observed both in the experiment and in the control; this can be naturally explained by higher reproduction costs in the case of females. However, SkQ1 affected birthrate in Campbell hamster pairs kept in outdoor conditions. Although the total number of litters and the number of offspring born during the entire life were the same in the control and experimental groups, SkQ1-receiving pairs started breeding earlier. As a result, we observed a higher reproduction rate in SkQ1-receiving pairs in the first half of the year and in control pairs (receiving water instead of SkQ1) in the second half of the year. Outpacing reproduction after the winter pause was observed in the experimental group both in 2011 and in 2012. Seasonal conditions and age had a similar effect on male and female body weight dynamics in the experimental and control groups. However, the tendency toward higher body weight of SkQ1-receiving males was observed at the end of the experiment. In contrast, among the females it was the animals from the control group that had higher body weight at the end of the experiment, apparently because of being less exhausted by reproduction. It is likely that reproductive costs in the experimental series were in fact higher than we could observe basing on the number of births. We could not take into account the cases of fetal death as well as accurately record the death of offspring at birth or shortly after birth as they are usually eaten by females. This could be evaluated only based on the fact of pregnancy.
Lack of difference in the lifespan of the hamsters from the experimental and control groups (those receiving SkQ1 and receiving free water) is supported by the lack of differences in a number of characteristics of acquired and innate immunity except for the activity of the T-cell immunity. The latter was characterized by DTH response to phytohemagglutinin P. The dermal reaction was statistically higher in 1-year-old male hamsters receiving SkQ1. This result is consistent with the observations of deceleration of age-dependent involution of thymus and spleen follicular structures in SkQ1-treated OXYS rats [3, 22]. The humoral immune response of 1-year-old hamsters to SRBC had a weak, close to statistically significant positive effect on the lifespan of male hamsters independently of SkQ1 treatment. No similar effect was observed in females.
In our first experiment with virgin female Campbell dwarf hamsters kept in groups in outdoors conditions in the environment rich in pathogens, we demonstrated that SkQ1 at dose of 5 and 50 nmol/kg increased their median lifespan [10].
We think that the absence of SkQ1 effect on the lifespan of breeding hamsters does not contradict the earlier data on the geroprotective effect of SkQ1 in the absence of reproductive loads. In our experiment, the possibility to breed freely led to enhanced loads on the organism aggravating the main life history tradeoff between reproduction and survival. The competition between the basic life functions (functional systems) for energy and substrates determines their joint evolution [23, 24]. Natural selection provides (on the evolutionary time scale) the level of function (trait) expression optimal for every period of the individual life history, which is manifested in the form of tradeoff within the tradeoff-permitted limits [23, 25]. It results in optimal for each life stage relocation of limited substrates and energy reserves of the organism maximizing the fitness. Redistribution of energy and substrates between competing systems of procreation-providing reproduction and survival–providing immunity is an example of this phenomenon [26-28]. When considering our results within the framework of the above theory, it seems unlikely to assume simultaneous positive SkQ1 effects on the lifespan and reproduction of a small rodent with very high reproduction potential. Reproductive age of Campbell hamster practically coincides with its absolute age. These animals breed almost until death, the breeding being more exhausting for females. It seems symptomatic that in mice SkQ1-induced increase in lifespan was more pronounced in males than in females even in the absence of reproductive loads [10]. For example, in a vivarium with a low pathogen level (LP conditions), SkQ1 had no effect on outbred SHR female mice, but their lifespan was the same as that of SkQ1-receiving SHR mice in the “dirty” (non-LP conditions) vivarium. Studies of inbred BALB/c and C57Bl/6 male and female mice in the conditions of sterile SPF vivarium showed no SkQ1 effect on the median lifespan of females, but it demonstrated a statistically significant positive effect of SkQ1 on the males.
However, our result does not match the result obtained by our colleagues on the northern mole vole (Ellobius talpinus) [10]. The SkQ1 dose of 50 nmol/kg per day had a positive effect both on the reproductive function and on the lifespan of the voles captured in nature and kept in reproductive groups. However, this is also quite understandable. In the case of the northern mole voles, reproduction is not as exhausting as for Campbell hamsters, the typical r-strategist among rodents. These voles are specialized, long-lived rodents with reproductive loads distributed for years along the life cycle. A set of adaptations providing saving (compared to terrestrial rodents of similar size) consumption of available energy is a characteristic for northern mole voles [29]. The fact that our result does not coincide with the result obtained on northern mole voles is compelling evidence of the need to consider the biology of the species and, accordingly, the conditions, in which we can expect the manifestation of geroprotective properties of mitochondria-targeted antioxidants such as SkQ1.
The positive effect of SkQ1 on the hamster’s reproductive function is the main result of our research. It supports the results previously obtained on northern mole voles, outbred SHR mice (SkQ1 inhibited the cease of female estrous cycles with age and provoked an early onset of regular cycles) [3, 10]. This result is supported by the result of our special study of the development of the young in the litters of the experimental (receiving SkQ1) and control groups (receiving pure water) of Campbell hamsters. This study revealed a positive effect of SkQ1 on the maturation rate of young males and females [30]. In the midst of the reproductive period in May-July, 25-day-old males from the litters of parents receiving SkQ1 had heavier epididymides compared to the control, and 25-day-old experimental females had a tendency toward greater weight of the uterus. It seems significant that the effect of SkQ1 on the weight of epididymis and uterus remained when the litter size and sex ratio in the litter were controlled variables. In addition, a parallel experiment showed that SkQ1 at 50 nmol/kg, administered to young females starting from 10 days of age, caused earlier vaginal opening.
Early breeding of SkQ1-receiving hamsters (after autumn–winter pause) can be explained by the effect of SkQ1 on the hypothalamic–pituitary–adrenal system under the influence of seasonal stressors. SkQ1-induced increase in reproductive activity may be associated with the reduced level of glucocorticoid hormones in SkQ1-treated animals or with reduced sensitivity of the hypothalamic–pituitary–gonadal system to these hormones. Since the classical works of J. Christian [31-33], much data have been accumulated indicating the essential role of stress as a mechanism of conflict resolution between demands for immediate individual reproductive effort and delayed breeding as a factor regulating fertility and mortality in mammalian populations [34, 35]. On the other hand, SkQ1 may have an important protective effect on the organism under acute stress conditions, which require rapid seasonal adjustment of physiology, for example, in adaptation to seasonal temperature decrease. We evaluated the level of stress reaction in hamsters as an indicator of their ability to adapt to the first frosts (intense molting, reduced reproductive activity, accumulation of fat reserves) and low temperatures in January based on cortisol concentration in blood serum.
In both cases, our expectations were confirmed in relation to males. The background level of stress reaction estimated by the cortisol concentration in blood serum was significantly lower in SkQ1-receiving males in the period of autumn seasonal adjustment of the organism. A similar tendency was observed also during January frosts, when the background level of stress reaction was rather high. In the case of females, we observed no difference between the experimental and control series during autumn adaptation (we decided not to take blood samples in January frosts); however, the females had higher (when compared to males) background level of stress reaction, possibly due to unfinished breeding and high reproductive costs. No pronounced differences between the control and experimental groups were observed during the period of active breeding in the beginning of spring or in the middle of summer.
Thus, the absence of an effect of SkQ1 on the lifespan of Campbell hamsters in reproductive pairs that could breed freely while being kept in outdoor conditions can be explained by the positive effect of SkQ1 on reproductive function. SkQ1, while not increasing the overall reproductive success, could activate reproduction in the beginning of the reproductive season, which is also consistent with the positive effect of SkQ1 on the rate of the hamsters’ postnatal development. Our results are in good agreement with the results obtained on insects. In experiments with the fruit fly Drosophila melanogaster, SkQ1 was shown to increase the lifespan of virgin females and males with the maximal effect achieved on the survival of young females [9]. However, when males and females were given an opportunity to breed freely, the SkQ1 solution of the same concentration had no effect either on their lifespan or on female survival at young age [36]. However, the early age fertility and the total number of offspring were shown to increase in SkQ1-treated flies. Interestingly, activation of reproductive function of D. melanogaster males by the female pheromone without the possibility of breeding reduced fat deposits, resistance to starvation, and lifespan, and mating in females removed this effect [37].
We are deeply grateful to Academician V. P. Skulachev, who initiated this study and supported us with his constant interest and valuable advice.
This study was commissioned and funded by the Institute of Mitoengineering, Lomonosov Moscow State University.
REFERENCES
1.Skulachev, V. P. (2005) Aging as atavistic program
that can be canceled, Vestnik Ros. Akad. Nauk, 75,
831-843.
2.Skulachev, V. P. (2010) How to cancel the program
of body aging? Rus. J. Gen. Chem., 80, 1523-1541.
3.Skulachev, V. P., Anisimov, V. N., Antonenko, Yu.
N., Bakeeva, L. E., Chernyak, B. V., Erichev, V. P., Filenko, O. F.,
Kalinina, N. I., Kapelko, V. I., Kolosova, N. G., Kopnin, B. P.,
Korshunova, G. A., Lichinitser, M. R., Obukhova, L. A., Pasyukova, E.
G., Pisarenko, O. I., Roginsky, V. A., Ruuge, E. K., Senin, I. I.,
Severina, I. I., Skulachev, M. V., Spivak, I. M., Tashlitsky, V. N.,
Tkachuk, V. A., Vyssokikh, M. Yu., Yaguzhinsky, L. S., and Zorov, D. B.
(2009) An attempt to prevent senescence: a mitochondrial approach,
Biochim. Biophys. Acta, 1787, 437-461.
4.Skulachev, V. P. (1999) Phenoptosis: programmed death of an
organism, Biochemistry (Moscow), 64, 1418-1426.
5.Skulachev, V. P. (2012) What is
“phenoptosis” and how to fight it? Biochemistry
(Moscow), 77, 689-706.
6.Skulachev, V. P. (2007) A biochemical approach to the problem
of aging: “megaproject” on membrane-penetrating ions. The
first results and prospects, Biochemistry (Moscow), 72,
1385-1396.
7.Antonenko, Y. N., Avetisyan, A. V., Bakeeva, L. E.,
Chernyak, B. V., Chertkov, V. A., Domnina, L. V., Ivanova, O. Y.,
Izyumov, D. S., Khailova, L. S., Klishin, S. S., Korshunova, G. A.,
Lyamzaev, K. G., Muntyan, O. K., Nepryakhina, A. A., Pashkovskaya, O.
Yu., Pletjushkina, M. S., Pustovidko, A. V., Roginsky, V. A.,
Rokitskaya, T. I., Ruuge, E. K., Saprunova, V. B., Severina, I. I.,
Simonyan, R. A., Skulachev, I. V., Skulachev, M. V., Sumbatyan, N. V.,
Sviryaeva, I. V., Tashlitsky, V. N., Vassiliev, J. M., Vyssokikh, M.
Yu., Yaguzhinsky, L. S., Zamyatnin, A. A., Jr., and Skulachev, V. P.
(2008) Mitochondria-targeted plastoquinone derivatives as tools to
interrupt execution of the aging program. 1. Cationic plastoquinone
derivatives: synthesis and in vitro studies, Biochemistry
(Moscow), 73, 1273-1287.
8.Agapova, L. S., Chernyak, B. V., Domnina, L. V.,
Dugina, V. B., Efimenko, A. Y., Fetisova, E. K., Ivanova, O. Y.,
Kalinina, N. I., Khromova, N. V., Kopnin, B. P., Korotetskaya, M. V.,
Lichinitser, M. R., Lukashov, A. L., Pletjushkina, O. Yu., Popova, E.
N., Skulachev, M. V., Shagieva, G. S., Stepanova, E. V., Titova, E. V.,
Tkachuk, V. A., Vasiliev, J. M., and Skulachev, V. P. (2008)
Mitochondria-targeted plastoquinone derivatives as tools to interrupt
execution of the aging program. 3. Inhibitory effect of SkQ1 on tumor
development from p53-deficient cells, Biochemistry (Moscow),
73, 1300-1316.
9.Anisimov, V. N., Bakeeva, L. E., Egormin, P. A.,
Filenko, O. F., Isakova, E. F., Manskikh, V. N., Mikhelson, V. M.,
Panteleeva, A. A., Pasyukova, E. G., Pilipenko, D. I., Piskunova, T.
S., Popovich, I. G., Roshchina, N. V., Rybina, O. Yu., Samoylova, T.
A., Saprunova, V. B., Semenchenko, A. V., Skulachev, M. V., Spivak, I.
M., Tsybul’ko, E. A., Tyndyk, M. L., Vyssokikh, M. Yu., Yurova,
M. N., Zabezhinsky, M. A., and Skulachev, V. P. (2008)
Mitochondria-targeted plastoquinone derivatives as tools to interrupt
execution of an aging program. 5. SkQ1 prolongs the lifespan and
prevents development of traits of senescence, Biochemistry
(Moscow), 73, 1329-1342.
10.Anisimov, V. N., Egorov, M. V., Krasilschikova,
M. S., Lyamzaev, K. G., Manskikh, V. N., Moshkin, M. P., Novikov, E.
A., Popovich, I. G., Rogovin, K. A., Shabalina, I. G., Shekarova, O.
N., Skulachev, M. V., Titova, T. V., Vigodin, V. A., Vyssokikh, M. Yu.,
Yurova, M. N., Zabezhinsky, M. A., and Skulachev, V. P. (2011) Effects
of mitochondria-targeted antioxidant SkQ1 on lifespan of rodents,
Aging, 3, 1-10.
11.Bakeeva, L. E., Barskov, I. V., Egorov, M. V.,
Isaev, N. K., Kapelko, V. I., Kazachenko, A. V., Kirpatovsky, V. I.,
Kozlovsky, S. V., Lakomkin, V. L., Levina, S. B., Pisarenko, O. I.,
Plotnikov, E. Y., Saprunova, V. B., Serebryakova, L. I., Skulachev, M.
V., Stelmashook, E. V., Studneva, I. M., Tskitishvili, O. V.,
Vasilyeva, A. K., Victorov, I. V., Zorov, D. B., and Skulachev, V. P.
(2008) Mitochondria-targeted plastoquinone derivatives as tools to
interrupt execution of the aging program. 2. Treatment of some ROS- and
age-related diseases (heart arrhythmia, heart infarctions, kidney
ischemia, and stroke), Biochemistry (Moscow), 73,
1288-1299.
12.Neroev, V. V., Archipova, M. M., Bakeeva, L. E.,
Fursova, A. Zh., Grigorian, E. N., Grishanova, A. Y., Iomdina, E. N.,
Ivashchenko, Zh. N., Katargina, L. A., Khoroshilova-Maslova, I. P.,
Kilina, O. V., Kolosova, N. G., Kopenkin, E. P., Korshunov, S. S.,
Kovaleva, N. A., Novikova, Yu. P., Philippov, P. P., Pilipenko, D. I.,
Robustova, O. V., Saprunova, V. B., Senin, I. I., Skulachev, M. V.,
Sotnikova, L. F., Stefanova, N. K., Tikhomirova, N. K.,
Khoroshilova-Maslova, I. P., Tsapenko, I. V., Shchipanova, A. I.,
Zinovkin, R. A., and Skulachev, V. P. (2008) Mitochondria-targeted
plastoquinone derivatives as tools to interrupt execution of the aging
program. 4. Age-related eye disease. SkQ1 returns vision to blind
animals, Biochemistry (Moscow), 73, 1317-1328.
13.Skulachev, M. V., Antonenko, Y. N., Anisimov, V.
N., Chernyak, B. V., Cherepanov, D. A., Chistyakov, V. A., Egorov, M.
V., Kolosova, N. G., Korshunova, G. A., Lyamzaev, K. G., Plotnikov, E.
Y., Roginsky, V. A., Savchenko, A. Y., Severina, I. I., Severin, F. F.,
Shkurat, T. P., Tashlitsky, V. N., Shidlovsky, K. M., Vyssokikh, M. Y.,
Zamyatnin, A. A., Zorov, D. B., and Skulachev, V. P. (2011)
Mitochondrial-targeted plastoquinone derivatives. Effect on senescence
and acute age-related pathologies, Curr. Drug. Targets,
12, 800-826.
14.Feoktistova, N. Yu. (2008) Hamsters of
Phodopus Genus. Systematics, Phylogeography,
Ecology, Physiology, Behavior, Chemical
Communication [in Russian], Tovarishchestvo Nauchnykh Izdanii KMK,
Moscow.
15.Flint, V. E., and Golovkin, A. N. (1961) Essay on
the comparative ecology of Tuva hamsters, Byul. MOIP Otd. Biol.,
66, 57-75.
16.Feoktistova, N. Yu., and Meshersky, I. G. (2008)
Chap. 15, in Hamsters of Phodopus Genus. Systematics,
Phylogeography, Ecology, Physiology,
Behavior, Chemical Communication (Feoktistova, N. Yu., ed.)
[in Russian], Tovarishchestvo Nauchnykh Izdanii KMK, Moscow, pp.
259-272.
17.Sokolov, V. Ye., and Orlov, V. N. (1980) The
Mammals of the Mongolian People’s Republic [in Russian],
Nauka, Moscow.
18.Roitt, I., Brostoff, J., and Male, D. (2000)
Immunology [Russian translation], Mir, Moscow.
19.Tella, J. L., Lemu, J. A., Carrete, M., and
Blanco, G. (2008) The PHA test reflects acquired T-cell mediated
immunocompetence in birds, PLoS ONE, 3, 1-9,
e3295; doi: 10.1371/journal.pone.0003295.
20.Rogovin, V. V., and But, P. G. (1989) The method
for determining the activity of peroxidase-endogenous hydrogen peroxide
system in blood leukocytes in smears, Patents: No. 20-21-208 from
14.11.1989, Moscow; No. 20-22-241 from 14.11.1989, Moscow.
21.Palme, R. (2005) Measurement of corticosterone
metabolites in birds’ droppings: an analytical approach, Ann.
N.Y. Acad. Sci., 1046, 17-34.
22.Obukhova, L. A., Skulachev, V. P., and Kolosova,
N. G. (2009) Mitochondria-targeted antioxidant SkQ1 inhibits
age-dependent involution of the thymus in normal and senescence-prone
rats, Aging, 1, 389-401.
23.Roff, D. A. (1992) The Evolution of Life
Histories: Theory and Analysis, Chapmann and Hall, New York.
24.Stearns, S. C. (1992) The Evolution of Life
Histories, Oxford University Press, Oxford.
25.Roff, D., and Fairbairn, D. J. (2007) The
evolution of trade-offs: where are we? J. Evol. Biol.,
20, 433-447.
26.Roberts, M. L., Buchanan, K. L., and Evans, M. R.
(2004) Testing the immunocompetence handicap hypothesis: a review of
the evidence, Anim. Behav., 68, 227-239.
27.Martin, L. B., Weil, Z. M., and Nelson, R. J.
(2008) Seasonal changes in vertebrate immune activity: mediation by
physiological trade-offs, Phil. Trans. R. Soc. B, 363,
321-339.
28.Boonekamp, J. J., Ros, A. H. F., and Verhulst, S.
(2008) Immune activation suppresses plasma testosterone level: a
meta-analysis, Biol. Lett., 4, 741-744.
29.Novikov, Ye. A. (2008) Physiological Costs of
Adaptations to Underground Life: Ellobius talpinus Pall. Compared to
Terrestrial Rodents [in Russian]: author’s abstract of
Doctoral dissertation, Novosibirsk.
30.Rogovin, K. A., Khrushcheva, A. M., Shekarova, O.
N., Ushakova, M. V., Manskikh, V. N., and Vasilieva, N. Yu. (2014)
Mitochondria-targeted antioxidant SkQ1 accelerates maturation in
Campbell dwarf hamsters (Phodopus campbelli, Thomas, 1905,
Rodentia, Cricetidae), Biochemistry (Moscow), 79,
1111-1116.
31.Christian, J. J. (1950) The adreno-pituitary
system and population cycles in mammals, J. Mammal., 31,
247-259.
32.Christian, J. J. (1963) Endocrine adaptive
mechanisms and the physiologic regulation of population growth, in
Physiological Mammalogy (Mayer, W. V., and van Gelder, R. G.,
eds.) Vol. 1, Academic Press, New York, pp. 189-359.
33.Christian, J. J. (1968) Endocrine-behavioral
negative feedback responses to increased population density, Colloq.
Int. Centre Natl. Rech. Sci. Paris, 173, 289-316.
34.Rogovin, K. A., and Moshkin, M. P. (2007)
Autoregulation of mammal population quantity and stress, Zh. Obshch.
Biol., 68, 244-267.
35.Novikov, Ye. A., and Moshkin, M. P. (2009) The
role of stress in the modification of ontogenetic programs, Uspekhi
Sovrem. Biol., 129, 1-12.
36.Tsybul’ko, E. A., Roshina, N. V., Rybina,
O. Yu., and Pasyukova, E. G. (2010) Mitochondria-targeted plastoquinone
derivative SkQ1 increases early reproduction of Drosophila
melanogaster at the cost of early survival, Biochemistry
(Moscow), 75, 265-268.
37.Gendron, C. M., Kuo Tsung-Han, Harvanek, Z. M.,
Chung, B. Y., Yew, J. Y., Dierick, H. A., and Pletcher, S. D. (2014)
Drosophila life span and physiology are modulated by sexual
perception and reward, Science, 343, 544-548.