Heat Shock Induces Production of Reactive Oxygen Species and Increases Inner Mitochondrial Membrane Potential in Winter Wheat Cells
A. V. Fedyaeva1*, A. V. Stepanov1, I. V. Lyubushkina1,2, T. P. Pobezhimova1, and E. G. Rikhvanov1
1Siberian Institute of Plant Physiology and Biochemistry, Siberian Division of the Russian Academy of Sciences, ul. Lermontova 132, 664033 Irkutsk, Russia; fax: (3952) 510-754; E-mail: fedyaeva.anna@mail.ru2Irkutsk State University, ul. Suhe-Bator 5, 664003 Irkutsk, Russia; fax: (3952) 241-870; 241-855; E-mail: estel_86@mail.ru
* To whom correspondence should be addressed.
Received May 23, 2014; Revision received July 7, 2014
Heat shock leads to oxidative stress. Excessive ROS (reactive oxygen species) accumulation could be responsible for expression of genes of heat-shock proteins or for cell death. It is known that in isolated mammalian mitochondria high protonic potential on the inner membrane actuates the production of ROS. Changes in viability, ROS content, and mitochondrial membrane potential value have been studied in winter wheat (Triticum aestivum L.) cultured cells under heat treatment. Elevation of temperature to 37-50°C was found to induce elevated ROS generation and increased mitochondrial membrane potential, but it did not affect viability immediately after treatment. More severe heat exposure (55-60°C) was not accompanied by mitochondrial potential elevation and increased ROS production, but it led to instant cell death. A positive correlation between mitochondrial potential and ROS production was observed. Depolarization of the mitochondrial membrane by the protonophore CCCP inhibited ROS generation under the heating conditions. These data suggest that temperature elevation leads to mitochondrial membrane hyperpolarization in winter wheat cultured cells, which in turn causes the increased ROS production.
KEY WORDS: Triticum aestivum L., reactive oxygen species, mitochondrial membrane potential, heat stressDOI: 10.1134/S0006297914110078
Abbreviations: CCCP, carbonyl cyanide m-chlorophenylhydrazone; DCF, 2′,7′-dichlorofluorescein; FDA, fluorescein diacetate; H2DCF·DA, 2′,7′-dichlorofluorescin diacetate; JC-1, 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide; PI, propidium iodide; ROS, reactive oxygen species.
Elevated level of reactive oxygen species (ROS) is a universal reaction
of an organism in response to practically all stress factors. An
increase in ROS to a certain level triggers the synthesis of stress
proteins that protect the cell from death, but exceeding that level, on
the contrary, it causes death [1, 2]. The sources of ROS in plant cells are the
chloroplasts, peroxisomes, mitochondria, NADPH oxidases of plasmalemma,
and peroxidases [1, 3, 4]. In heterotrophic cell cultures, as well as in
non-photosynthetic or etiolated plant organs, the mitochondrion is one
of the main ROS sources [1-3].
An increase in ROS production was observed at elevated temperatures [5-8]. Participation of
mitochondria in ROS production under heat exposure has been established
[9-11]. Complexes I and III of
the respiratory chain in isolated mitochondria are found to be sites of
ROS formation, where superoxide radical
(O2·−) is formed that is
subsequently converted to H2O2 [2, 3]. However, there is no
evidence whether or not Complexes I and III generate ROS in eukaryotic
cells in situ [12]. However, it was known
that dysfunction of the mitochondrial alternative NAD(P)H-dehydrogenase
Ndb4 [13] and Complex II [14]
suppresses mitochondrial ROS production in plants. Thus, the question
arises what the causes of mitochondrial ROS production during stress
exposure are.
It was suggested by Skulachev that ROS are formed in mitochondria in the absence of stress, when the mitochondrial inner membrane potential is high and components of the respiratory electron transport chain, such as ubisemiquinone, become over-reduced [15]. The potential on the inner mitochondrial membrane is generated by electron transport, which is associated with the protons pumped via Complexes I, III, and IV from the matrix of mitochondria to the outside of the inner mitochondrial membrane and the influx of protons back into the matrix, which are used by the FoF1-ATP-synthase for ATP synthesis. It was shown that ROS production rate in isolated mammalian mitochondria was increased with increase in the mitochondrial membrane potential [16]. A similar situation was observed in mammalian cells, and decrease in the mitochondrial potential usually inhibited the ROS production [17]. Since an increase in mitochondrial ROS production was observed in plant cells during heat shock [9-11], it is logical to assume that the cause of heat-induced ROS production is hyperpolarization of the inner mitochondrial membrane.
Because chloroplasts, one source of ROS in photosynthetic cells, are absent in heterotrophic culture, suspension culture is a useful model to study the role of mitochondria in ROS production. In this connection, the relationship between ROS production and the mitochondrial potential in winter wheat cultured cells under the heat shock conditions was studied in the present work. To do this, the change in ROS level and mitochondrial membrane potential as well as cell viability were analyzed under elevated temperature.
MATERIALS AND METHODS
Cell culture. Heterotrophic suspension cell culture of winter wheat (Triticum aestivum L., cultivar Irkutskaya) in exponential growth phase was used in the study. The cell culture was grown in darkness at 26°C on Murashige and Skoog nutrient medium containing 3.6% of sucrose, 0.6 mg/liter nicotinic acid, 0.6 mg/liter pyridoxine, 1.2 mg/liter thiamine, 3 mg/liter 2,4-dichlorophenoxyacetic acid, 120 mg/liter inositol, and 6 mg/liter sodium dithiocarbamate. The cell culture was subcultured every 2 weeks by threefold dilution with fresh medium.
Temperature treatment. Cell culture (100 μl) was subjected to heat treatment at various temperatures (37, 42, 45, 50, 55, and 60°C) for 0-90 min with shaking on a TS-100 thermo-shaker (BioSan, Latvia). Cells incubated at 26°C were used as the control. After the heat treatment, samples were used for measuring the fluorescence intensity of a dye.
Cell viability determination. Cell viability was evaluated by the method of double staining for 2 min using fluorescent dyes: fluorescein diacetate (FDA) (50 μM) and propidium iodide (PI) (7.5 μM). To quantify the living and dead cells in each experiment, 10 random fields were analyzed using a fluorescence microscope.
Mitochondrial membrane potential determination. The value of mitochondrial potential was determined using the ratiometric cationic fluorescent dye JC-1 (5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide) (10 μM). Cells were stained for 10 min. As positive and negative controls, oligomycin (30 μM) and CCCP (carbonyl cyanide m-chlorophenylhydrazone) (4 μM), respectively, were used. The change in fluorescence intensity of the dye was determined in the red channel and expressed as percentage of control.
ROS content determination. Changes of ROS level were determined using the fluorescent dye 2′,7′-dichlorofluorescin diacetate (H2DCF·DA) (1 μM). Cell staining was performed for 10 min. As positive and negative controls, H2O2 (50 μM) and ascorbic acid (100 mM), respectively, were used. The fluorescence intensity of the dye was evaluated in the green channel and expressed as percentage of control.
Fluorescence of dyes (H2DCF·DA and FDA) in cells is provided by the action of endogenous esterases, which produce fluorescein that emits light in the green channel. Therefore, DCF and FDA fluorescence were compared under different thermal treatments.
An AxioObserverZ1 inverted fluorescence microscope (Carl Zeiss, Germany) with AxioCamMRm3 digital monochrome camera and AxioVisionRel.4.6 software package for image analysis were used.
Experiments were performed at least in triplicate. The results were treated statistically; data in figures represent mean values and standard errors.
RESULTS
Heat exposure was known to increase mitochondrial potential in Arabidopsis thaliana cells [18]. At the same time, elevated temperature increased ROS production in plants [9-11]. First, it was necessary to establish whether or not the given processes occur during heat shock in winter wheat cultured cells. To do that, the JC-1 (Fig. 1a) and H2DCF·DA (Fig. 1b) fluorescence were measured in cells incubated at 26 and 45°C during 30 min.
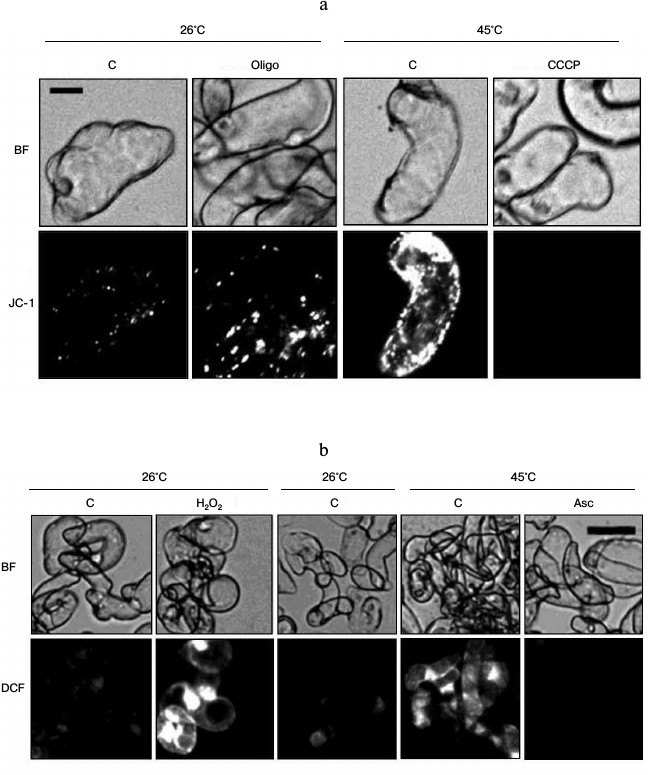
Fig. 1. Heat shock causes an increase in inner mitochondrial membrane potential (a) and ROS content (b). Winter wheat cultured cells were treated for 30 min with 30 μM oligomycin (Oligo) and 50 μM H2O2 (at 26°C), with 4 μM CCCP and 100 mM ascorbic acid (Asc) (at 45°C); control culture (C) was treated at 26 and 45°C, respectively. Fluorescence of dyes (JC-1 – red channel, DCF – green channel) was measured immediately after the treatment. A typical experiment is presented. BF, bright field. Bar, 20 μm (a) and 50 μm (b).
JC-1 is a nontoxic fluorescent probe that is used for determination of mitochondrial membrane potential. JC-1 monomers were selectively accumulated in the mitochondria and form J-aggregates, which emit fluorescence in the red channel if potential is high [17, 19]. As shown in Fig. 1a, the intensity of the JC-1 fluorescence was insignificant under control conditions. Heat treatment led to a sharp fluorescence increase. Mitochondrial potential is formed by a balance between the transfer of protons across the inner mitochondrial membrane during respiratory chain functioning and their backward transport into the mitochondrial matrix due to operation of the FoF1-ATP-synthase [15]. The protonophore CCCP provides free transfer of protons across the mitochondrial membrane and, accordingly, decreases mitochondrial potential. On the contrary, mitochondrial potential is increased by the action of oligomycin, an inhibitor of Fo, the membrane sector of the FoF1-ATP-synthase [17]. Therefore, to prove that the change in intensity of JC-1 fluorescence reflects a change in mitochondrial potential, CCCP and oligomycin were used. Incubation of cells in the presence of CCCP inhibited the increase in JC-1 fluorescence under heat exposure. Instead, the treatment of control cells with oligomycin increased JC-1 fluorescence (Fig. 1a). The ability of CCCP and oligomycin, respectively, to inhibit or increase JC-1 fluorescence gives evidence that a change in JC-1 fluorescence in the red channel corresponds to a change in inner mitochondrial membrane potential.
To study the ROS production in the winter wheat cultured cells, dichlorodihydrofluorescein diacetate acetyl ester H2DCF·DA was used [17]. H2DCF·DA is transformed to H2DCF by deacetylation by esterases. In the presence of ROS, the H2DCF is oxidized to DCF, which emits fluorescence in the green channel. As shown in Fig. 1b, the intensity of DCF fluorescence under control conditions was negligible, but heat treatment led to a significant increase. The antioxidant ascorbic acid and hydrogen peroxide (H2O2) were used as negative and positive controls, accordingly (Fig. 1b). Addition of ascorbic acid under the action of heat shock suppressed an increase in DCF fluorescence (Fig. 1b). On the contrary, the addition of H2O2 to control cells at 26°C led to fluorescence increase. The ability of ascorbic acid and H2O2 to inhibit or increase, respectively, DCF fluorescence indicates that the change in fluorescence of the dye properly reflects the change in the ROS content. Thus, heat treatment leads to the simultaneous increase in mitochondrial potential and ROS content in the winter wheat cells.
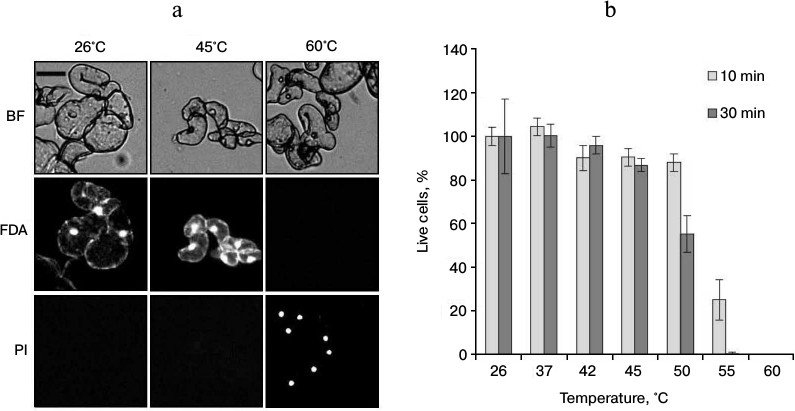
The increase in ROS production under heat shock can lead to cell death [9]. In this regard, it was necessary to determine the viability of cells after different regimes of heat treatment. To do that, the suspension culture was treated at 37, 42, 45, 50, 55, and 60°C (for 10 and 30 min) and the viability was assessed by counting the cells stained by FDA and PI. The FDA dye stains living cells. After penetrating into living cells, this dye due to action of endogenous esterases is transformed into fluorescein, which emits fluorescence in the green channel. PI is permeable only for dead and dying cells with decreased plasmalemma permeability. It interacts with nucleic acids and emits fluorescence in the red channel (Fig. 2a). As shown in Fig. 2b, the treatment at temperatures in the range from 37 to 45°C did not affect cell viability. Heat treatment at 50°C (30 min) reduced viability approximately to 50%. More severe heat exposure at 55 and 60°C for 30 min led to almost complete cell death (Fig. 2b).
Fig. 2. Cell viability of winter wheat culture after heat exposure of various intensities. a) The microphotographs of cells after exposure at the indicated temperatures for 30 min; b) quantitative counting of live cells. The winter wheat cultured cells were treated at the indicated temperatures for 10 and 30 min. Cells were stained with FDA (50 μM) (green channel) and PI (7.5 μM) (red channel). Bar, 50 μm; n = 3; M ± S.E. BF, bright field.
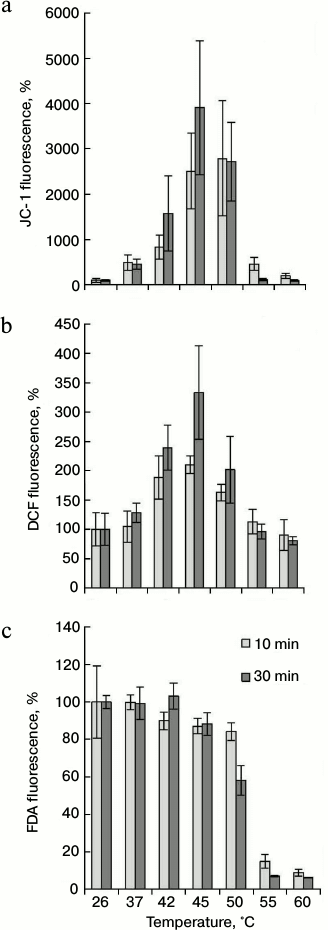
To determine at which temperature the hyperpolarization of the inner mitochondrial membrane and the ROS production are observed, the cells were subjected to the heat treatment, as indicated above, and JC-1 and DCF fluorescence were measured. As shown in Fig. 3a, raising the temperature to 37°C increased the intensity of JC-1 fluorescence. The maximal fluorescence increase was observed at 45°C in comparison with the control (26°C). Increase in JC-1 fluorescence was not observed if the temperature was increased to 55 and 60°C. Significant differences in dye fluorescence were not detected depending on time of thermal exposure (10 and 30 min) (Fig. 3a).
Fig. 3. Change in mitochondrial potential (a), ROS content (b), and intensity of FDA fluorescence (c) during thermal exposure at varying temperatures. The winter wheat cultured cells were treated for 10 and 30 min at the indicated temperatures, and the fluorescence was measured. Quantitative analysis of the fluorescence intensity is presented. Fluorescence is expressed as percentage of control (26°C); n = 3; M ± S.E.
The DCF fluorescence in the winter wheat cultured cells was determined under the same conditions as for JC-1. The treatment of cells at 42°C resulted in a significant increase in the fluorescence intensity in comparison with control (26°C). The maximal peak of fluorescence was observed after heat treatment at 45°C. Further increase in temperature (up to 50°C) reduced the fluorescence somewhat (Fig. 3b). The intensity of fluorescence at higher temperatures, such as 55 and 60°C, did not differ from the control values. Also, it should be noted that long-term exposure at 45°C (30 min) led to more intensive dye fluorescence than short (10 min) exposure (Fig. 3b).
Comparison of JC-1 (Fig. 3a) and DCF (Fig. 3b) fluorescence intensity indicates that the fluorescence of these dyes changed in a similar way. The coefficient of correlation between these two parameters was 0.73 (for 10-min treatment) and 0.94 (for 30-min treatment). This indicates that there is relationship between the mitochondrial membrane potential and ROS level. Heat treatment in the range from 37 to 50°C led to an increase in fluorescence intensity of JC-1 and DCF (Fig. 3, a and b). This phenomenon was not observed at higher temperatures.
It was necessary to exclude the possibility that heat-induced increase in fluorescence of JC-1 and DCF is the result of disturbance of plasmalemma permeability. Therefore, the fluorescence intensity of FDA was studied in the same range of heat treatment. The FDA dye was previously used to determine cell viability (Fig. 2b). As it turned out, the change in FDA fluorescence (Fig. 3c) and cell viability (Fig. 2b) under the action of high temperatures had a similar tendency. Elevation of temperature to 55 and 60°C decreased fluorescence intensity of FDA (Fig. 3c) and living cell number (Fig. 2b). Therefore, a malfunction of esterase activity at 55 and 60°C resulted to FDA fluorescence decrease (Fig. 3c). Probably for the same reason, decrease in DCF fluorescence at 55 and 60°C was observed (Fig. 3b). However, in contrast to DCF and JC-1, an increase in FDA fluorescence did not occur in the range of heat exposure from 37 to 50°C (Fig. 3c). The intensity of FDA fluorescence did not depend on treatment time (10 and 30 min). Therefore, an increase in JC-1 (Fig. 3a) and DCF (Fig. 3b) fluorescence is not a result of plasmalemma permeability disturbance on treatment of temperatures ranging from 37 to 50°C and correctly reflects the mitochondrial potential changes and the ROS production.
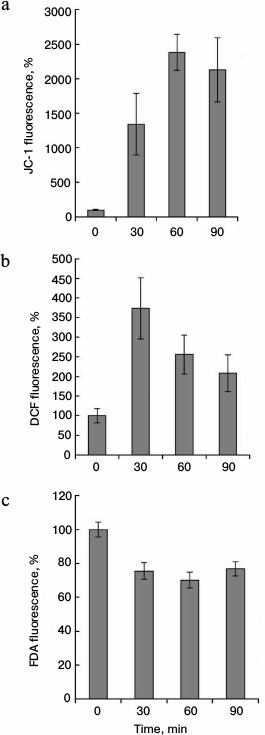
These results indicate that the maximum of the mitochondrial membranes hyperpolarization (Fig. 3a) and the maximal increase in the ROS content (Fig. 3b) occur at 45°C. It was necessary to establish the dependence of these processes on the duration of the heat exposure. Cells were treated at 45°C for 0-90 min, and fluorescence of JC-1, DCF, and FDA was measured. The maximal increase in JC-1 fluorescence was observed after 60-90 min of heat treatment (Fig. 4a). Maximal fluorescence of DCF was registered after 30 min of heat treatment (Fig. 4b). More prolonged incubation (60-90 min) decreased the DCF fluorescence, which, however, did not reach the control level. FDA fluorescence was not changed significantly during heat treatment (Fig. 4c). Thus, changes in the intensity of JC-1 and DCF fluorescence depend not only on the temperature, but also on the duration of the treatment.
Fig. 4. Dynamics of changes of inner mitochondrial membrane potential and of ROS content during thermal exposure at 45°C. The winter wheat cultured cells were treated at 45°C for 0-90 min, and JC-1 fluorescence (red channel) (a), DCF fluorescence (green channel) (b), and FDA fluorescence (green channel) (c) were measured. Quantitative analysis of the fluorescence intensity is presented. Fluorescence is expressed as percentage of control (26°C); n = 3; M ± S.E.
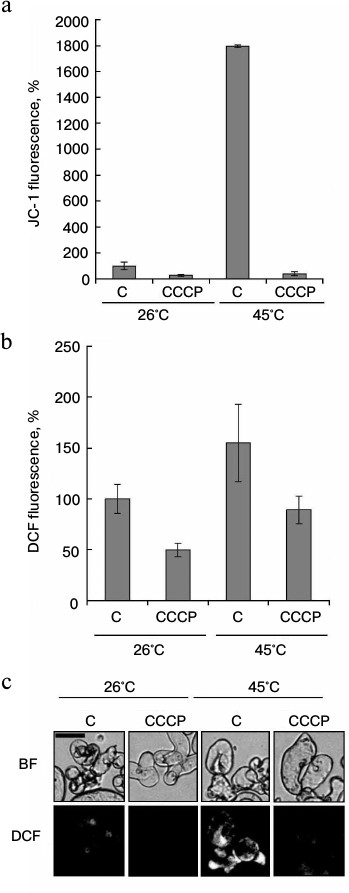
As shown in Fig. 3, there was a strong correlation between increase in mitochondrial potential and ROS production at elevated temperatures. However, the analysis of temporal dynamics at 45°C, presented in Fig. 4, gives evidence that ROS production decreased with increasing time of heat exposure, despite the mitochondrial potential increase. The protonophore CCCP is known to dissipate mitochondrial membrane potential. Therefore, at the next stage the effect of CCCP on heat-induced ROS production was studied. As expected, CCCP decreased intensity of JC-1 fluorescence in control and in heat-shocked samples (Fig. 5a). Similar results were obtained in the study of the CCCP effect on the intensity of DCF fluorescence (Fig. 5, b and c). Heat shock at 45°C increased ROS production, and CCCP addition inhibited this process. In a similarly way, CCCP inhibited ROS production under the control conditions (Fig. 5, b and c). Thus, the protonophore CCCP, by reducing mitochondrial membrane potential, suppresses ROS production under heat shock.
Fig. 5. Effect of the protonophore CCCP on ROS content under heat shock. Winter wheat cultured cells were treated at 26 and 45°C for 30 min in the absence (C) or presence of 4 μM CCCP. JC-1 (red channel) and DCF (green channel) fluorescence was measured immediately after the treatment. Quantitative analysis of JC-1 (a) and DCF (b) fluorescence intensity and fluorescence microphotographs of cells with DCF (c) are presented. Fluorescence is expressed as percentage of control (26°C). BF, bright field. Bar, 50 μm; n = 3; M ± S.E.
DISCUSSION
Wheat is one of the most important crops in the world [20]. Irkutskaya is a frost-resistant winter wheat cultivar from Eastern Siberia [21]. In early spring, winter wheat has a more developed root system in comparison with spring wheat, which allows using the winter and spring soil moisture. For this reason, winter wheat is more resistant to high temperatures and drought. However, drought and unfavorable temperature conditions induce significant yield losses of winter wheat. Therefore, the study of the physiological and biochemical mechanisms occurring in winter wheat at high temperatures is important [21].
One of the factors negatively affecting plants at high temperatures is increased ROS production [22]. In accordance with literature data [5-9], heat shock increased ROS generation in winter wheat cultured cells (Fig. 1b). According to Volkov et al. [6], ROS production was increased after temperature exposure to 37°C. It was required for elevated expression of HSP17.6, HSP18.2, and APX2 in A. thaliana cells. However, ROS content increased after the heat exposure at 44°C, but the elevated expression of genes encoding heat shock proteins (heat shock proteins, HSP) was not observed [6]. Similar data were obtained by us earlier using winter wheat cultured cells. Temperature exposure at 37 and 39°C increased the content of HSP101 and HSP17.6 (I, II class) proteins [23]. At the same time, the maximal ROS production in the cells of winter wheat was observed in the range from 42 to 50°C (Fig. 3b), but HSP protein synthesis did not occur under these conditions [23].
The question arises, why is there no strict correlation between HSP content and ROS level? It is known that ROS have a dual role in cells [1, 2]. HSP synthesis is activated when ROS level does not exceed a threshold values. Signal transduction required for HSP synthesis is performed by heat shock factors (HSF). The expression of HSFA2 was shown to increase under oxidative stress [24]. Thus, ROS production could activate HSF, which induce HSP synthesis and the development of thermotolerance [5]. When the ROS contents exceed the threshold level, HSP synthesis is not observed [23], for example, in the range from 42 to 50°C (Fig. 3b), and cell death begins. However, a significant decrease in viability was observed only at 50°C. Cells remained alive after treatment at 42 and 45°C (Fig. 2) if measurements were performed immediately after the heat exposure. It should be noted that immediately after the shock, the plant cells could remain alive, but they die during some time of incubation under control conditions [25]. Therefore, it cannot be excluded that cells of winter wheat treated in the temperature range from 42 to 50°C will eventually die after some time.
According to Locato et al. [26], treatment at 55°C increased ROS production in cultured tobacco cells. However, as shown in Fig. 3b, elevated ROS level was not observed after the same treatment in the winter wheat cultured cells. Probably the reason for this is the use of another plant and methods of ROS measurement. Tobacco cells did not lose viability after heat shock (55°C) [26], but the treatment at 55°C led to instant death of most winter wheat cells (Fig. 2b).
Chloroplasts can be a source of ROS under heat shock in light-grown A. thaliana cell culture [27]. In our work heterotrophic, dark-grown winter wheat cell culture was used; therefore, the role of chloroplasts in ROS generation under heat shock can be excluded (Fig. 3b). Some authors [6, 7] have shown that diphenylethane chloride suppresses ROS generation under heat shock conditions in plant cell cultures. Because diphenylethane chloride is an inhibitor of the plasmalemma-bound NADPH oxidase, it was suggested that NADPH oxidase is the ROS source under heat shock in heterotrophic culture [7]. However, diphenylethane chloride is also an inhibitor of a mitochondrial flavin-containing enzyme [3], so it cannot be excluded that this agent also suppresses mitochondrial ROS production. For example, the deletion of the NDB4 gene encoding a flavin-containing enzyme of an alternative mitochondrial NAD(P)H dehydrogenase suppresses ROS production in Arabidopsis cells [13].
ROS production is one of the consequences of the functioning of the plant mitochondrial respiratory chain [3]. It is likely that mitochondria are involved in ROS production under heat shock. Increased ROS production was shown to occur under heat shock conditions in mitochondria of Arabidopsis and tobacco cells [9-11]. The results obtained in our work show that in the winter wheat cultured cells, as well as in Arabidopsis and tobacco cells, mitochondria are one of the main sources of ROS generation during heat shock. First, there was a positive correlation between the increase in ROS content and inner mitochondrial membrane potential (Fig. 3, a and b). Second, decrease in the potential by the protonophore CCCP led to inhibition of ROS production (Fig. 5).
The relationship between mitochondrial membrane potential and ROS production suggests a causal relationship between these phenomena. It is likely that the increase in mitochondrial potential induces ROS production under conditions of heat shock. This conjecture is consistent with the theory of V. P. Skulachev. Accordingly, the over-reduced respiratory chain is a result of mitochondrial membrane potential increase, which, in turn, stimulates ROS production [15]. It was shown that in isolated mammalian mitochondria [16] (in absence of stress) an increase in mitochondrial potential was accompanied by increased ROS content. Obviously, the same rule is valid for intact cells under stress conditions. In yeast cells [28] and in poplar cell cultures [29] treatment by amiodarone and extracellular ATP, respectively, increased mitochondrial potential and enhanced ROS production. Depolarization of mitochondrial potential by the protonophore CCCP suppressed ROS production. It should be noted that correlation between increase in mitochondrial potential and in ROS production was observed after heat treatment at 45°C for 10 and 30 min (Fig. 3, a and b). However, ROS production was decreased, but mitochondrial potential, on the contrary, increased after 60 min treatment at 45°C (Fig. 4, a and b). This apparent contradiction can be explained by the fact that the increase in heat exposure duration activates the antioxidant enzymes that scavenge the ROS, despite the high value of mitochondrial membrane potential. It was shown that in A. thaliana cells heat shock at 45°C induced expression of APX1b encoding ascorbate peroxidase [30]. Heat shock at 55°C increased the catalase activity in tobacco cells [8].
It is generally accepted that mitochondrial ROS production is increased with over-reduction of the respiratory chain [2, 15]. To prevent this process, the alternative oxidase and uncoupling proteins are activated in mitochondria [3]. Over-reduction of the respiratory chain can be observed under two conditions. First, when the activity of the terminal oxidase is inhibited. For example, the inhibition of complex III by antimycin A leads to over-reduction of ubiquinone [17]. Second, when the rate of electron input exceeds the ability of respiratory chain to transport them [2]. Probably, the second mechanism was observed in isolated mitochondria, when the rate of ROS production was increasing in parallel with the increase in mitochondrial potential [16].
The question remains about reasons for mitochondrial ROS production under heat shock conditions. Previously it was shown that heat shock leads to increased mitochondrial potential in cell culture of Arabidopsis [18], yeast [31], and mammalian cells [32]. But the link between the ROS production and mitochondrial potential increase has not been previously studied. Our data indicate that high potential on the inner mitochondria membrane leads to over-reduction of the respiratory chain under heat shock, and this causes ROS production in the winter wheat cell culture.
This work for supported by the Russian Foundation for Basic Research (project Nos. 14-04-32126 and 14-04-31677).
REFERENCES
1.Kreslavsky, V. D., Los, D. A., Allakhverdiev, S.
I., and Kuznetsov, V. V. (2012) Signaling role of reactive oxygen
species in plants under stress, Russ. J. Plant Physiol.,
59, 141-154.
2.Rhoads, D. M., Umbach, A. L., Subbaiah, C. C., and
Siedow, J. N. (2006) Mitochondrial reactive oxygen species.
Contribution to oxidative stress and interorganellar signaling,
Plant Physiol., 141, 357-366.
3.Møller, I. M. (2001) Plant mitochondria and
oxidative stress: electron transport, NADPH turnover, and metabolism of
reactive oxygen species, Annu. Rev. Plant Physiol. Plant Mol.
Biol., 52, 561-591.
4.Kolupaev, Y. E., Oboznyi, A. I., and Shvidenko, N.
V. (2013) Role of hydrogen peroxide in generation of a signal inducing
heat tolerance of wheat seedlings, Russ. J. Plant Physiol.,
60, 227-234.
5.Miller, G., and Mittler, R. (2006) Could heat shock
transcription factors function as hydrogen peroxide sensors in plants,
Ann. Bot., 98, 279-288.
6.Volkov, R. A., Panchuk, I. I., Mullineaux, P. M.,
and Schoffl, F. (2006) Heat stress-induced H2O2
is required for effective expression of heat shock genes in
Arabidopsis, Plant Mol. Biol., 61, 733-746.
7.Konigshofer, H., Tromballa, H. W., and Loppert, H.
G. (2008) Early events in signaling high-temperature stress in tobacco
BY2 cells involve alterations in membrane fluidity and enhanced
hydrogen peroxide production, Plant Cell Environ., 31,
1771-1780.
8.Locato, V., Gadaleta, C., De Gara, L., and De
Pinto, M. C. (2008) Production of reactive species and modulation of
antioxidant network in response to heat shock: a critical balance for
cell fate, Plant Cell Environ., 31, 1606-1619.
9.Vacca, R. A., de Pinto, M. C., Valenti, D.,
Passarella, S., Marra, E., and De Gara, L. (2004) Production of
reactive oxygen species, alteration of cytosolic ascorbate peroxidase,
and impairment of mitochondrial metabolism are early events in heat
shock-induced programmed cell death in tobacco Bright-Yellow 2 cells,
Plant Physiol., 134, 1100-1112.
10.Zhang, L., Li, Y., Xing, D., and Gao, C. (2009)
Characterization of mitochondrial dynamics and subcellular localization
of ROS reveal that HsfA2 alleviates oxidative damage caused by heat
stress in Arabidopsis, Exp. Bot., 60,
2073-2091.
11.Schwarzlander, M., Logan, D. C., Johnston,
I. G., Jones, N. S., Meyer, A. J., Fricker, M. D., and Sweetlove, L. J.
(2012) Pulsing of membrane potential in individual mitochondria: a
stress-induced mechanism to regulate respiratory bioenergetics in
Arabidopsis, Plant Cell, 24, 1188-1201.
12.Zorov, D. B., Isaev, N. K., Plotnikov, E. Y.,
Zorova, L. D., Stelmashook, E. V., Vasileva, A. K., Arkhangelskaya, A.
A., and Khrjapenkova, T. G. (2007) The mitochondrion as janus bifrons,
Biochemistry (Moscow), 72, 1115-1126.
13.Smith, C., Barthet, M., Melino, V., Smith, P.,
Day, D., and Soole, K. (2011) Alterations in the mitochondrial
alternative NAD(P)H dehydrogenase NDB4 lead to changes in mitochondrial
electron transport chain composition, plant growth and response to
oxidative stress, Plant Cell Physiol., 52, 1222-1237.
14.Gleason, C., Huang, S., Thatcher, L. F., Foley,
R. C., Anderson, C. R., Carroll, A. J., Millar, A. H., and Singh, K. B.
(2011) Mitochondrial complex II has a key role in mitochondrial-derived
reactive oxygen species influence on plant stress gene regulation and
defense, Proc. Natl. Acad. Sci. USA, 108,
10768-10773.
15.Skulachev, V. P. (1998) Uncoupling: new
approaches to an old problem of bioenergetics, Biochim. Biophys.
Acta, 1363, 100-124.
16.Korshunov, S. S., Skulachev, V. P., and Starkov,
A. A. (1997) High protonic potential actuates a mechanism of production
of reactive oxygen species in mitochondria, FEBS Lett.,
416, 15-18.
17.Suski, J. M., Lebiedzinska, M., Bonora, M.,
Pinton, P., Duszynski, J., and Wieckowski, M. R. (2012) Relation
between mitochondrial membrane potential and ROS formation, Methods
Mol. Biol., 810, 183-205.
18.Pyatrikas, D. V., Rikhvanov, E. G., Fedoseeva, I.
V., Varakina, N. N., Rusaleva, T. M., Tauson, E. L., Stepanov, A. V.,
Borovskii, G. B., and Voinikov, V. K. (2014) Mitochondrial retrograde
regulation of HSP101 expression in Arabidopsis thaliana under
heat stress and amiodarone action, Russ. J. Plant Physiol.,
61, 80-89.
19.Szilagyi, G., Simon, L., Koska, P., Telek, G.,
and Nagy, Z. (2006) Visualization of mitochondrial membrane potential
and reactive oxygen species via double staining, Neurosci.
Lett., 399, 206-209.
20.FAO Statistical Yearbook – World,
Food and Agriculture (2012) Food and Agricultural Organization of
the United Nations, Rome, Italy (www.fao.org).
21.Dorofeev, N. V., Peshkova, A. A., and Voinikov,
V. K. (2003) Winter Wheat in the Irkutsk Region [in Russian],
Art Press, Irkutsk.
22.Kolupaev, Y. Y., and Karpets, Y. V. (2009)
Salicylic acid and plants resistance to abiotic stressors, Byul.
Kharkiv Nat. Agr. Univ., 17, 19-39.
23.Fedyaeva, A. V., Stepanov, A. V., Pobezhimova, T.
P., and Rikhvanov, E. G. (2014) Synthesis of HSP and the death of cell
cultures of plants under thermal influence, Byul. Irkutsk Gos. Tekh.
Univ., 2, 167-171.
24.Nishizawa, A., Yabuta, Y., Yoshida, E., Maruta,
T., Yoshimura, K., and Shigeoka, S. (2006) Arabidopsis heat
shock transcription factor A2 as a key regulator in response to several
types of environmental stress, Plant J., 48, 535-547.
25.Swidzinski, J. A., Sweetlove, L. J., and Leaver,
C. J. (2002) A custom microarray analysis of gene expression during
programmed cell death in Arabidopsis thaliana, Plant J.,
30, 431-446.
26.Locato, V., Gadaleta, C., De Gara, L., and De
Pinto, M. C. (2008) Production of reactive species and modulation of
antioxidant network in response to heat shock: a critical balance for
cell fate, Plant Cell Environ., 31, 1606-1619.
27.Doyle, S. M., Diamond, M., and McCabe, P. F.
(2010) Chloroplast and reactive oxygen species involvement in
apoptotic-like programmed cell death in Arabidopsis suspension
cultures, Exp. Bot., 61, 473-482.
28.Pozniakovsky, A. I., Knorre, D. A., Markova, O.
V., Hyman, A. A., Skulachev, V. P., and Severin, F. F. (2005) Role of
mitochondria in the pheromone- and amiodarone-induced programmed death
of yeast, J. Cell. Biol., 168, 257-269.
29.Sun, J., Zhang, C. L., Deng, S. R., Lu, C. F.,
Shen, X., Zhou, X. Y., Zheng, X. J., Hu, Z. M., and Chen, S. L. (2012)
An ATP signaling pathway in plant cells: extracellular ATP triggers
programmed cell death in Populus euphratica, Plant Cell
Environ., 35, 893-916.
30.Larkindale, J., and Vierling, E. (2008) Core
genome responses involved in acclimation to high temperature, Plant
Physiol., 146, 748-761.
31.Fedoseeva, I. V., Pjatricas, D. V., Varakina, N.
N., Rusaleva, T. M., Stepanov, A. V., Rikhvanov, E. G., Borovskii, G.
B., and Voinikov, V. K. (2012) Effect of amiodarone on thermotolerance
and Hsp104p synthesis in the yeast Saccharomyces cerevisiae,
Biochemistry (Moscow), 77, 78-86.
32.Balogh, G., Horvath, I., Nagy, E., Hoyk, Z.,
Benko, S., Bensaude, O., and Vigh, L. (2005) The hyperfluidization of
mammalian cell membrane acts as a signal to initiate the heat shock
protein response, FEBS Lett., 272, 6077-6086.