Bacterial Lipopolysaccharide Activates CD57-Negative Human NK Cells
L. M. Kanevskiy1, S. A. Erokhina1,2, M. A. Streltsova1, W. G. Telford3, A. M. Sapozhnikov1,2, and E. I. Kovalenko1*
1Shemyakin and Ovchinnikov Institute of Bioorganic Chemistry, Russian Academy of Sciences, ul. Miklukho-Maklaya 16/10, 117997 Moscow, Russia; E-mail: lenkovalen@mail.ru; leonid_kanewski@mail.ru2Lomonosov Moscow State University, Faculty of Biology, 119991 Moscow, Russia
3Experimental Transplantation and Immunology Branch, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA
* To whom correspondence should be addressed.
Received July 27, 2014
NK cells play an important regulatory role in sepsis by induction and augmentation of proinflammatory reactions in early stages of the septic process and by suppression of immune response in later stages of inflammation. The present work was aimed at the effect of bacterial lipopolysaccharide (LPS), the main pathogenic factor of sepsis development, on human NK cells ex vivo. We show that LPS activates immature CD57-negative NK cells, which typically constitute less than half of the normal NK cell population in human peripheral blood. Under conditions of NK cell stimulation with IL-2, addition of LPS provokes an increase in IFN-γ production. However, LPS both increased and inhibited NK cell cytotoxic activity. It is important to note that the activation of NK cells on LPS addition was observed in the absence of TLR4 on the NK cell surface. These results confirm our previous data arguing for a direct interaction of LPS with NK cells and evidence an atypical mechanism of LPS-induced NK cell activation without the involvement of surface TLR4.
KEY WORDS: NK cells, lipopolysaccharide, IFN-γ production, CD57, cytotoxicity, TLR4DOI: 10.1134/S0006297914120074
Natural killer cells (NK cells) are a heterogeneous population of cytotoxic lymphocytes of the innate immune system. They are able to mount a rapid effector response towards virus-infected, tumor, or other damaged cells [1]. NK cells recognize their targets by a restricted set of receptors whose expression does not require gene rearrangement. In addition to their cytotoxic function, NK cells regulate immune reactions by production of various proinflammatory and immunosuppressive cytokines and chemokines. In particular, these cells are the major producers of interferon-gamma (IFN-γ) at an early stage of the immune response [2, 3]. Immunoregulatory activity of NK cells increases significantly under the influence of cytokines secreted by other immune cells during inflammation. Under inflammatory conditions, NK cells receive more access to lymph nodes, where they interact with activated dendritic cells. NK cells influence many elements of innate immunity by cytokine production and contact interactions, and they thereby adjust the subsequent antigen-specific response [4].
In recent years, considerable data have accumulated on the involvement of NK cells in the pathogenesis of sepsis – the systemic inflammatory process induced by bacterial lipopolysaccharide (LPS) [5]. In early stages of sepsis, NK cells exacerbate the development of systemic inflammation by production of cytokines that regulate the activity of macrophages, dendritic cells, and neutrophils. This may be a decisive factor in determining the survival of patients with sepsis. In mouse models, removal of NK cells was shown to lead to decreased production of proinflammatory cytokines (TNF, GM-CSF, and IFN-γ) and to reduced mortality [6]. Moreover, activated NK cells also exert an inhibitory effect on the immune system by production of immunosuppressive cytokines such as IL-10, and by elimination of excessively activated cells, particularly macrophages [7, 8]. It is believed that for the successful treatment of patients with sepsis, a combination of two events is necessary: the induction of systemic inflammation sufficient to remove the infectious agents, and the subsequent deactivation of the immune system to restore homeostasis. It is important that these two processes occur, because excessive induction of inflammation can lead to severe damage to internal organs, and excessive inhibition of the immune system may cause prolonged immunosuppression, in which the body becomes unprotected against penetration by new infectious agents. It is now known that NK cells take part in both processes. In early stages of disease development, they serve as “inflammation multipliers” – they activate mutually macrophages and dendritic cells, the main sensors of pathogen-associated molecules (so-called, PAMPs). In subsequent stages of the inflammatory immune response, the function of NK cells is elimination of activated macrophages and production of IL-10 [9]. The requirement for precise regulation of both activating and inhibitory processes demonstrates the importance of further study of the involvement of NK cells in immunoregulation, especially in development of systemic inflammation.
Until recently, it was assumed that the activating effect of LPS on NK cells occurs only by an indirect mechanism. This assumption was due to the almost complete absence of LPS receptors (TLR4) on the surface of natural killer cells. Therefore, LPS-induced activation of NK cells was considered to be the result of the interaction of LPS with TLR4 on macrophages and dendritic cells, leading to cytokine production and formation of contact interactions that stimulate natural killer cells [10, 11]. However, in recent years there is increasing evidence that LPS, as well as other PAMPs, is able to directly activate NK cells. It has been found that NK cells express a variety of Toll-like receptors (TLRs) including TLR2, TLR3, TLR4, TLR7, TLR8, and TLR9 (at least at the mRNA level), and in some cases they can be activated by various TLR agonists [12-15]. Nevertheless, the question of the participation of TLR4 in the activation of NK cells by LPS remains unclear. Some authors have reported TLR4 expression on the surface of human NK cells [16]. However, other data suggest that the level of surface expression of TLR4 on human NK cells is very low [17-19]. These data question whether the membrane form of TLR4 mediates effects of LPS on NK cells. However, a pool of intracellular TLR4 protein has been found in NK cells, which, in accordance with published data, is able to conduct signal [20, 21]. The significance of this phenomenon is still unclear.
This study was therefore aimed to investigate uncharacterized aspects of NK cells in response to LPS. The main focus was on the effect of LPS on NK cell subpopulations that differ in degree of differentiation. The study then focused on the relationship between LPS-induced IFN-γ production and cytotoxicity of NK cells and the possible mechanism of LPS action mediated by surface and intracellular forms of TLR4 in NK cells.
MATERIALS AND METHODS
Isolation and stimulation of NK cells. Blood samples were taken from healthy adult volunteers who have given informed consent for their blood to be used in this study. Peripheral blood mononuclear cells (PBMC) were isolated by centrifugation on Ficoll density gradient 1.077 g/cm3 (Paneco, Russia). The NK cells were then magnetically separated using a human NK cell negative selection kit (Miltenyi Biotec, Germany). The percentage of CD3–CD56+ cells in the preparations after separation was no less than 97% as verified by flow cytometry. In some cases, NK subpopulations were isolated from magnetically separated NK cells by fluorescent-activated cell sorting after staining with monoclonal antibodies CD3-PC7, CD56-APC (Beckman Coulter, USA), and CD57-FITC (eBioscience, USA). Isolated NK cells were cultivated in RPMI-1640 (Paneco) supplemented with 10% FCS (HyClone, USA) (200 μl) at cell concentration of 1.5·106 cells/ml or 0.5·106 cells/ml (for sorted cells) for 18 h in 96 U-well plates (Costar, USA). Recombinant human IL-2 (500 U/ml) purchased from Hoffmann-La-Roche (Germany) and LPS (5 μg/ml) from E. coli strain 055:B5 (Sigma-Aldrich, USA) were added to the cell culture. After incubation, TLR4 and CD69 expression and cytotoxicity of NK cells were analyzed; cell-free supernatants were collected for estimation of cytokine production.
IFN-γ production. IFN-γ level in supernatants collected after incubation of the NK cells under different conditions was analyzed using IFN-γ ELISA kits (Vector-Best, Russia). Plates were read using a Multiscan FC plate reader (Thermo Fisher Scientific, USA) set to 450 nm absorption wavelength with reference wavelength of 620 nm.
Flow cytometric analysis of CD69 surface expression. After 18 h incubation with IL-2 alone, IL-2 with LPS, or IL-2 with IL-12 (10 ng/ml; Sigma-Aldrich), immunofluorescent staining was performed using CD56-APC, CD57-FITC, and CD69-PE antibodies (eBioscience) followed by multicolor flow cytometric analysis.
Evaluation of TLR4 expression in NK cells. To measure TLR4 surface expression, NK cells were labeled with anti-TLR4-FITC (clone HTA125; HyCult Biotech, USA). For intracellular staining, cells were fixed and permeabilized using the Cytofix-Cytoperm Kit (BD Biosciences, USA), then labeled with the above antibody. In both variants of staining, an isotype mouse antibody (Miltenyi Biotec, Germany) was used as a control. The TLR4 expression level was estimated by flow cytometry in the region of CD56+ lymphocytes. For identification of CD57+ NK cells in experiments with intracellular TLR4, staining of the hybridoma HNK-1 [22] supernatant was used.
Cytotoxicity. Cytolytic activity was assessed by degranulation of NK cells after incubation with target cells as described earlier [19]. Degranulation level was measured by flow cytometry using antibody CD107a-PC5 (Beckman Coulter, USA). K562 cell line was used as target cells.
Flow cytometry and cell sorting. Samples were analyzed on a FACSCalibur flow cytometer (Becton Dickinson, USA). Cell sorting was performed on a FACSVantage DiVa fluorescence-activated cell sorter (Becton Dickinson). Flow cytometric data were analyzed using the CellQuest analysis software (BD Biosciences) and WinMDI (Dr. Joe Trotter, USA).
Statistics. Data were analyzed in SigmaPlot, version 11.0 (Systat Software Inc., USA). For estimation of differences between two groups of data, Student’s t-test was used unless otherwise indicated. Values of P < 0.05 were considered statistically significant.
RESULTS
Activation of CD57-negative NK cells by LPS. We have previously shown that NK cells isolated from human peripheral blood consistently respond to LPS treatment in vitro by a marked increase in IFN-γ production. This effect was observed only under conditions of additional stimulation by cytokines, particularly IL-2 [19]. In this work we have compared the action of LPS on NK cells of various differentiation stages. It is known that NK cells differ in their properties, such as cytotoxicity and ability to proliferate and produce cytokines, depending on their differentiation stages. Traditionally, the main subpopulations of natural killers are characterized by the level of CD56 surface expression. Here we have used the more informative approach allowing discrimination of the different stages of NK cell development and maturity based on the surface expression of not only CD56, but also CD57, the molecule that appears on the cell surface at the terminal stages of differentiation [23].
Analysis of the CD56 and CD57 surface distribution on NK cells isolated by magnetic separation (Fig. 1a) discriminated four subpopulations, the first, CD56brightCD57–, representing less mature NK cells, and the last, CD56dimCD57bright, indicates cells in the latest phase of differentiation. In our experiments, CD56brightCD57– cells usually accounted for 5.7 ± 3.4% of the whole NK cell fraction, CD56dimCD57– – 24.9 ± 9.5%, CD56dimCD57dim – 22.9 ± 4.6%, and CD56dimCD57bright – 46.8 ± 12.2% (here are shown average values of 11 experiments ± standard deviation).
Fig. 1. NK cell subpopulations and IFN-γ production following LPS stimulation. a) Cytogram of NK cell fraction stained with antibodies to CD56 and CD57. Subpopulations isolated by cell sorting are designated by Roman numerals according to the stage of differentiation: I) CD56brightCD57–; II) CD56dimCD57–; III) CD56dimCD57dim; IV) CD56dimCD57bright. b) IFN-γ production by cells of different natural killer subpopulations under the action of LPS. Here are shown representative data of one of three experiments. Here and further asterisks denote significant differences (P < 0.05).
We investigated the involvement of these four NK cell subpopulations isolated by cell sorting in LPS-induced IFN-γ production. NK cells from the different subpopulations markedly differed in their ability to produce cytokines in response to IL-2 stimulation (Fig. 1b). The highest level of IL-2-triggered IFN-γ production was observed in CD56brightCD57– cells, and the lowest was found in supernatants of CD56dimCD57bright cells; moreover, in some experiments IFN-γ was not detected at all in this subpopulation (Fig. 1b). Under the influence of LPS, a significant increase in IFN-γ production was observed in CD56brightCD57– and CD56dimCD57– cells. However, in CD57-positive NK cells IFN-γ production remained at the same level. These results indicate that the phenomenon of LPS-induced increase in cytokine production by NK cells is linked exactly with the subpopulation of CD57– cells.
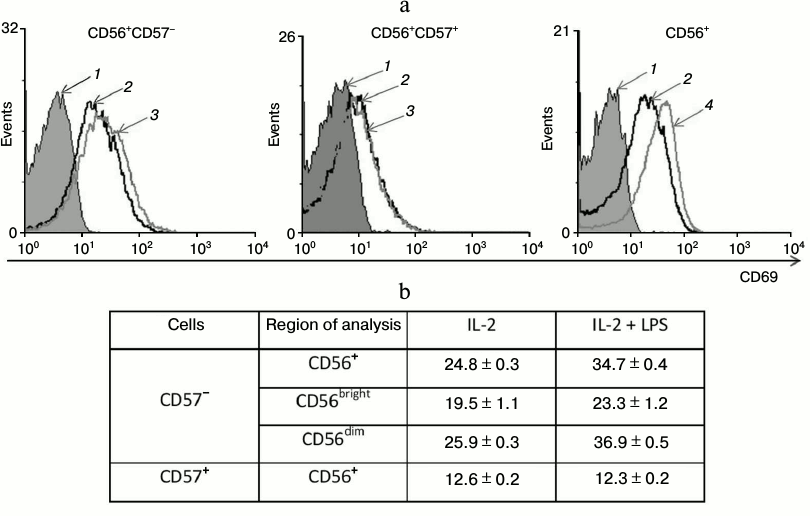
We also investigated the influence of LPS on expression of activation marker CD69 by CD57– and CD57+ NK cells isolated by cell sorting. The increase in CD69 surface expression after cultivation with LPS in the presence of IL-2 was observed only on CD57– cells (Fig. 2). Taken together, our data demonstrate the qualitative difference between CD57– and CD57+ NK cell subpopulations in response to LPS and evidence that LPS specifically activated the CD57 negative cells, which are considered to be less differentiated.
Fig. 2. Effect of LPS on CD69 expression in CD57– and CD57+ NK cells isolated by cell sorting. Representative results of one of two experiments are shown (the data of this experiments are shown also in Fig. 3c, where they are indicated at 3). a) Histograms of the fluorescence intensity distribution of cells stained by CD69-PE: 1) autofluorescence; 2) cells stimulated by IL-2 (500 U/ml); 3) cells stimulated by IL-2 (500 U/ml) and LPS (5 μg/ml); 4) stimulation of NK cells by IL-2 (500 U/ml) and IL-12 (10 ng/ml) (positive control). b) Flow cytometric analysis of CD69 expression on the surfaces of different NK cell subpopulations. Data are mean ± SD.
Influence of LPS on NK cell cytotoxicity. In an earlier study, we obtained preliminary data suggesting that LPS-induced production of IFN-γ can be accompanied by inhibition of the cytotoxic activity of these cells [19]. To analyze more thoroughly the correlation between changes in LPS-induced IFN-γ production and cytolytic NK cell function, we performed additional experiments with NK cells isolated from human blood using magnetic separation. A definite link between changes in IFN-γ production and in NK cytotoxicity in response to LPS was revealed. In experiments where LPS did not significantly change the IFN-γ production, the cytotoxicity remained at the same level (data not shown). However, in experiments where a significant stimulating effect of LPS on cytokine production was observed, the cytotoxicity level had increased, reduced, or remained unchanged depending on the donor (Fig. 3a). Notably, decrease in cytotoxicity was observed in experiments in which a higher basic level of NK cell degranulation in the presence of K562 target cells was recorded (Fig. 3b). We conclude that NK cell activation by LPS can induce multidirectional changes in their cytotoxicity. At the high basic level of cytolytic activity of NK cells, such activation can lead to inhibition of cytotoxicity.
Fig. 3. Effect of LPS on cytotoxic activity of NK cells estimated by their degranulation level in the presence of K562 target cells. a) Action of LPS on magnetically isolated NK cells. The Y-axis shows the change in percentage of cytotoxicity baseline registered in cells stimulated with IL-2 alone. Numbers 1-8 designate independent experiments performed in triplicate in which LPS demonstrated a positive effect on IFN-γ production. b) Basic level of cytotoxic activity of NK cells in the combined group of experiments in which we found a positive effect or lack of LPS effect (1) and in a group of experiments in which the negative influence of LPS on cytotoxic activity was found (2). These groups were compared using the Mann–Whitney U-test, and for each group the median and percentile values (10, 25, 75, and 90) are presented. c) Influence of LPS on cytotoxicity of CD57– and CD57+ NK cells isolated by cell sorting. Changes in cytotoxicity are shown in percentage of cytotoxicity baseline registered in cells stimulated with IL-2 alone. Numbers 1-4 designate individual experiments.
In a separate series of experiments, we analyzed the influence of LPS on cytotoxicity of CD57+ and CD57– NK cells isolated by cell sorting. LPS was shown to act only on CD57– NK cells, with no effect on CD57+ cells observed (Fig. 3c). As in the experiments with magnetically separated NK cells described above, the effects of LPS were activating or inhibiting in NK cell fractions taken from different donors.
Dynamics of surface and intracellular expression of TLR4 in NK cells. In several previous studies, the effect of expression of TLR4 in NK cells on mRNA level was found. However, the question about the participation of TLR4 receptor in the interaction of LPS with NK cells remains open. The expression of TLR4 on the plasma membrane is very low, but there is evidence of an intracellular pool of this protein [17, 19]. We hypothesized that IL-2 stimulation of NK cells can lead to additional biosynthesis of TLR4 or to its translocation to the cell surface, with subsequent binding of TLR4 with LPS and triggering of the signal cascade resulting in induction of IFN-γ biosynthesis. To test this hypothesis, we investigated the dynamics of the level of surface and intracellular forms of TLR4 in cultivated NK cells isolated by magnetic separation. In these experiments, IL-2 (500 U/ml) was added to the NK cell cultures, and after different time intervals the surface and intracellular levels of TLR4 were analyzed. As it was shown earlier, freshly isolated NK cells do not express detectable levels of TLR4 on the cell surface (Fig. 4a). During 18 h of incubation with IL-2, the TLR4 level did not change significantly (Fig. 4b). In the interval between 4 and 6 h, a small apparent increase in binding of TLR4-specific antibody to the cell surface was observed. However, because binding of the control isotype antibody also became greater, this increase can be considered as nonspecific; it might be a consequence of short-term changes in the properties of the cell membrane during cell activation. The level of intracellular TLR4 was not significantly changed during the first 18 h of incubation with IL-2, but it was increased after 48 h of cultivation of NK cells under such conditions (Fig. 4c). Thus, activation of NK cells by IL-2 did not significantly alter the surface expression level of TLR4, but it led to an increase in the intracellular pool of that receptor in NK cells after 2 days of stimulation. However, this fact alone cannot explain the LPS-induced increase in IFN-γ production and changes in NK cell cytotoxicity that are usually observed after 18 h incubation of cells in the presence of IL-2. To evaluate whether the changes in intracellular TLR4 level contributed to LPS-triggered effects on NK cell subpopulations, we analyzed by multicolor flow cytometry the level of intracellular TLR4 in CD57– and CD57+ NK cells stimulated by IL-2 or IL-2 with LPS for a specified time (Fig. 5a). We found no significant changes in intracellular TLR4 level in CD57– and CD57+ subpopulations both under the action of IL-2 or with the combination of IL-2 and LPS for over 18 h (Fig. 5a). In CD56bright cells, such analysis was not performed because their activation by IL-2 caused a significant decrease in the proportion of these cells in the total population of NK cells, presumably due to their transformation into CD56dim cells (Fig. 5b). It should be noted that in all experiments the intracellular level of TLR4 in CD56bright cells, which, as shown above, are the most extensive producers of IFN-γ in response to LPS, was lower compared to CD56dim cells. Thus, our data do not confirm the participation of TLR4 in the reception of LPS signal that leads to the activation of NK cells.
Fig. 4. TLR4 expression in human NK cells. a) Cytogram of NK cells labeled by antibodies to CD56 and TLR4 (surface staining). b) Dynamics of TLR4 surface expression under conditions of NK cell activation by IL-2 and LPS. Representative results of one of three experiments are shown. c) Dynamics of intracellular TLR4 level under conditions of NK cell activation by IL-2 and LPS. Generalized results of three experiments are presented.
Fig. 5. Intracellular expression of TLR4 in different subpopulations of NK cells. a) Influence of LPS on intracellular expression of TLR4 in CD57– and CD57+ NK cells. Generalized results of two independent experiments are presented (mean ± SD). b) Cytograms of NK cells labeled with antibodies to CD56 (surface staining) and to TLR4 (intracellular staining) obtained at different times of activation with IL-2.
DISCUSSION
This study was devoted to investigation of the influence of bacterial LPS on NK cells, the cytotoxic and immunoregulatory lymphocytic element of the innate immune system. The immune system has the ability to respond actively to bacterial infection by recognizing of LPS, which can lead in some cases to the development of systemic inflammatory disease or sepsis. It is known that NK cells actively participate in the development of inflammation by production of cytokines stimulating macrophages, dendritic cells, and neutrophils in the early stages of sepsis, supporting NK cells as enhancers of the inflammatory reaction. On the other hand, in the final stages of inflammation, activated NK cells are able to participate in the formation of an immunosuppressive state by producing IL-10 and elimination of activated macrophages [24]. The inability of NK cells to carry out the immunosuppressive action can lead to so-called “syndrome of macrophage activation” and development of severe sepsis. In contrast, when the immunosuppression is very powerful but the cytolytic activity of NK cells is weak, another pathological situation can occur – in this state the immune system is unable to fight persistent latent viruses in the body and leads to secondary opportunistic infections [5]. According to data that we obtained previously, LPS can directly act on NK cells, causing an increase in IFN-γ secretion. This suggests another potential regulatory role for NK cells in immune reactions caused by bacterial infection [19]. One of a few characterized aspects of action of LPS on NK cells was its effect on cytotoxic activity of NK cells, although we have previously demonstrated that an LPS-induced increase in IFN-γ production by NK cells could be accompanied by a decrease in their cytotoxicity. Based on the results obtained in this study, we conclude that this scenario does take place in many cases and generally correlates with a higher baseline cytotoxicity of NK cells. However, it has been shown that natural cytotoxicity can also increase under the influence of LPS. It can be assumed that the LPS-induced inhibition of cytotoxicity, accompanied by increased production of IFN-γ, may be due to a compensatory mechanism that is able to block the excessive activation of NK cells. Absence of any significant effect on LPS on the cytotoxicity of NK cells observed in several experiments may represent a stage of an intermediate state of NK cells.
Subpopulations of NK cells have different abilities to produce cytokines and engage in cytotoxicity, whereby their roles in systemic inflammatory processes may differ noticeably. The least differentiated cells are CD56brightCD57–. Cells with this phenotype constitute a minority of NK cells in human blood (about 5%), but they are widely present in various organs of the immune system (in lymph nodes and spleen) as well as in several other organs (primarily liver and lungs). Once activated by cytokines such as IL-2, IL-15, and IL-21, these cells intensively proliferate and produce cytokines [25]. Our data show high levels of IFN-γ production in CD56brightCD57– cells (Fig. 1b). On the surface of these cells, the CD16 receptor is virtually absent, indicating that they are not capable of antibody-dependent cellular cytotoxicity (ADCC). In the later stages of maturation, NK cells acquire surface expression of marker CD57, the terminally sulfated carbohydrate epitope [26]. It is still not entirely clear on what NK cell membrane molecule this epitope is exhibited. Unlike the immature cells CD56brightCD57–, NK cells with the phenotype CD56dimCD57bright are considered to be terminally differentiated [23]. They have shorter telomeres and lower ability to proliferate. They differ from the less mature NK cells by lower expression of receptors NKp30, NKp46, and NKG2A. However, these NK cells highly express CD16, whereby they are the most important effectors of ADCC [27]. NK cells CD56dimCD57bright also to produce cytokines, although to a much lesser extent than CD56brightCD57–, wherein the primary stimulus for this is not the other cytokines but the recognition of target cells by these NK cells via CD16 [28]. We also observed very low levels of IFN-γ production in this subpopulation, often below the threshold of detection (Fig. 1b). The NK cells of transitional differentiation stages CD56dimCD57– and CD56dimCD57low have intermediate properties (in a number of articles only the first of them was distinguished, while the second is united with terminally differentiated NK cells) [27]. The sizes of the above subpopulations vary greatly among individuals depending on various factors, the most important being age and the presence of inflammatory processes in the body [28, 29]. According to our data, NK cells with the CD57+ phenotype often predominate in human blood.
In this study, we have shown for the first time that LPS increases IFN-γ production only in CD57-negative NK cells, which represent the least mature NK cells in human blood. This result correlates well with modern concepts about these cells, in particular the CD56brightCD57– subpopulation, which carry out mainly regulatory functions performed by intensive cytokine production. The action of LPS was not limited to this NK cell subpopulation. Increase in IFN-γ production with LPS was also observed in CD56dimCD57– cells, which produce lower levels of IFN-γ compared to the CD56brightCD57– subpopulation. In the CD57-negative subpopulation, unlike the CD57 positives, we observed a small increase in the level of expression of the activation marker CD69. Apparently, CD57–, but not CD57+ NK cells take part in development of a “cytokine storm” in the early stages of sepsis. Our findings also suggest that cells of this subpopulation activated by cytokines acquire the ability to respond directly to LPS by increased production of cytokines (Fig. 1b). One of the objectives of this study was to demonstrate more clearly the existence of a mechanism of direct action of LPS on NK cells. After isolation of NK cells by magnetic separation (purity above 97%), the purified population could still be contaminated by, for example, dendritic cells, and the cytokine production produced by them can lead to indirect activation of the NK cells. Additional separation by cell sorting does minimize this possibility. The data that only a fraction of the NK cells but not the whole population is influenced by LPS confirms the existence of the mechanism of the direct action of LPS on NK cells.
The mechanism of LPS action on human NK cells remains unknown. To date, it is generally recognized that the receptor of lipopolysaccharide is TLR4; however, its surface expression level on NK cells is very low. In this work, we have shown that even after prolonged incubation with IL-2 and LPS, the surface level of this protein remains close to zero, which indirectly indicates that LPS apparently acts on NK cells not through the membrane form of TLR4.
There is evidence that LPS can interact with the intracellular form of TLR4 and trigger its activation [20, 21]. In our study, increase in intracellular TLR4 level in NK cells was registered only 48 h after the start of incubation in the presence of IL-2 and LPS (Fig. 4). However, a marked effect of LPS on functional activity of NK cells was detected much earlier, specifically 18 h after the beginning of stimulation. In this time period we observed no influence of IL-2 or IL-2 in combination with LPS to TLR4 intracellular level in subpopulations CD57– and CD57+ (Fig. 5). Nevertheless, we cannot completely exclude the possibility that LPS interacts with intracellular TLR4. However, it remains unclear how LPS penetrates into the cell. An alternative hypothesis of the possible mechanism of LPS direct action on NK cells is that oligosaccharide fragments of LPS participate in the activation of NK cells. Earlier in a model system, we demonstrated that oligosaccharides presented on the surface of target cells promote the activation of NK cells [30]. However, the possible existence of such mechanism of interaction of lipopolysaccharide with natural killer cells requires further study.
Thus, in this work we have shown that: 1) LPS causes an increase in IFN-γ production only in less differentiated NK cells with phenotype CD57–; 2) the LPS-induced increase in IFN-γ production by NK cells can be accompanied by inhibition or increase in their cytotoxicity; 3) the mechanism of action of LPS on NK cells does not seem to be connected to the membrane form of TLR4; 4) the data obtained in this study do not confirm the participation of the intracellular form of TLR4 in the effects of LPS on the activity of NK cells.
The authors thank Prof. Miguel Lopez-Botet for providing the hybridoma supernatant HNK-1.
This work was supported by the Russian Foundation for Basic Research grants (Nos. 13-04-02041 and 14-04-01842).
REFERENCES
1.Yarilin, A. A. (2010) Immunology [in
Russian], GEOTAR-Media, Moscow.
2.Thale, C., and Kiderlen, A. F. (2005) Sources of
interferon-gamma (IFN-gamma) in early immune response to Listeria
monocytogenes, Immunobiology, 210, 673-683.
3.Artavanis-Tsakonas, K., and Riley, E. M. (2002)
Innate immune response to malaria: rapid induction of IFN-gamma from
human NK cells by live Plasmodium falciparum-infected
erythrocytes, J. Immunol., 169, 2956-2963.
4.Abakushina, E. V., Kuzmina, E. G., and Kovalenko,
E. I. (2012) The main properties and functions of human NK cells,
Immunologiya, 4, 220-225.
5.Chiche, L., Forel, J. M., Thomas, G., Farnarier,
C., Vely, F., Blery, M., Papazian, L., and Vivier, E. (2011) The role
of natural killer cells in sepsis, J. Biomed. Biotechnol.,
2011, 986491.
6.Emoto, M., Miyamoto, M., Yoshizawa, I., Emoto, Y.,
Schaible, U. E., Kita, E., and Kaufmann, S. H. (2002) Critical role of
NK cells rather than V alpha 14(+)NKT cells in
lipopolysaccharide-induced lethal shock in mice, J. Immunol.,
169, 1426-1432.
7.Cooper, M. A., Fehniger, T. A., Fuchs, A., Colonna,
M., and Caligiuri, M. A. (2004) NK cell and DC interactions, Trends
Immunol., 25, 47-52.
8.Gerosa, F., Baldani-Guerra, B., Nisii, C.,
Marchesini, V., Carra, G., and Trinchieri, G. (2002) Reciprocal
activating interaction between natural killer cells and dendritic
cells, J. Exp. Med., 195, 327-333.
9.Goodier, M. R., and Londei, M. (2000)
Lipopolysaccharide stimulates the proliferation of human
CD56+CD3– NK cells: a regulatory role of
monocytes and IL-10, J. Immunol., 165, 139-147.
10.Godshall, C. J., Scott, M. J., Burch, P. T.,
Peyton, J. C., and Cheadle, W. G. (2003) Natural killer cells
participate in bacterial clearance during septic peritonitis through
interactions with macrophages, Shock, 19, 144-149.
11.Tu, Z., Bozorgzadeh, A., Pierce, R. H., Kurtis,
J., Crispe, I. N., and Orloff, M. S. (2008) TLR-dependent cross talk
between human Kupffer cells and NK cells, J. Exp. Med.,
205, 233-244.
12.Chalifour, A., Jeannin, P., Gauchat, J. F.,
Blaecke, A., Malissard, M., N’Guyen, T., Thieblemont, N., and
Delneste, Y. (2004) Direct bacterial protein PAMP recognition by human
NK cells involves TLRs and triggers α-defensin production,
Blood, 104, 1778-1783.
13.Lauzon, N. M., Mian, F., MacKenzie, R., and
Ashkar, A. A. (2006) The direct effects of Toll-like receptor ligands
on human NK cell cytokine production and cytotoxicity, Cell
Immunol., 241, 102-112.
14.Saikh, K. U., Lee, J. S., Kissner, T. L., Dyas,
B., and Ulrich, R. G. (2003) Toll-like receptor and cytokine expression
patterns of CD56+ T cells are similar to natural killer
cells in response to infection with Venezuelan equine encephalitis
virus replicons, J. Infect. Dis., 188, 1562-1570.
15.Mian, M. F., Lauzon, N. M., Andrews, D. W.,
Lichty, B. D., and Ashkar, A. A. (2010) FimH can directly activate
human and murine natural killer cells via TLR4, Mol. Ther.,
18, 1379-1388.
16.O’Connor, G. M., Hart, O. M., and Gardiner,
C. M. (2005) Putting the natural killer cells in its place,
Immunology, 117, 1-10.
17.Souza-Fonseca-Guimaraes, F., Parlato, M.,
Philippart, F., Misset, B., Cavaillon, J. M., and Adib-Conquy, M.
(2012) Toll-like receptors expression and interferon-γ production
by NK cells in human sepsis, Crit. Care, 16, R206.
18.Tadema, H., Abdulahad, W. H., Stegeman, C. A.,
Kallenberg, C. G., and Heeringa, P. (2011) Increased expression of
Toll-like receptors by monocytes and natural killer cells in
ANCA-associated vasculitis, PLoS One, 6, e24315.
19.Kanevskiy, L. M., Telford, W. G., Sapozhnikov, A.
M., and Kovalenko, E. I. (2013) Lipopolysaccharide induces IFN-γ
production in human NK cells, Front. Immunol., 4, 11.
20.Shibata, T., Motoi, Y., Tanimura, N., Yamakawa,
N., Akashi-Takamura, S., and Miyake, K. (2011) Intracellular TLR4/MD-2
in macrophages senses Gram-negative bacteria and induces a unique set
of LPS-dependent genes, Int. Immunol., 23, 503-510.
21.Hornef, M. W., Normark, B. H., Vandewalle, A.,
and Normark, S. (2003) Intracellular recognition of lipopolysaccharide
by Toll-like receptor 4 in intestinal epithelial cells, J. Exp.
Med., 198, 1225-1235.
22.Abo, T., and Balch, C. M. (1981) A
differentiation antigen of human NK and K cells identified by a
monoclonal antibody (HNK-1), J. Immunol., 127,
1024-1029.
23.Luetke-Eversloh, M., Killig, M., and Romagnani,
C. (2013) Signatures of human NK cell development and terminal
differentiation, Front. Immunol., 4, 499.
24.Cavaillon, J. M., and Adib-Conquy, M. (2006)
Bench-to-bedside review: endotoxin tolerance as a model of leukocyte
reprogramming in sepsis, Crit. Care, 10, 233.
25.Cooper, M. A., Fehniger, T. A., Turner, S. C.,
Chen, K. S., Ghaheri, B. A., Ghayur, T., Carson, W. E., and Caligiuri,
M. A. (2001) Human natural killer cells: a unique innate
immunoregulatory role for the CD56(bright) subset, Blood,
97, 3146-3151.
26.Voshol, H., van Zuylen, C. W., Orberger, G.,
Vliegenthart, J. F., and Schachner, M. (1996) Structure of the HNK-1
carbohydrate epitope on bovine peripheral myelin glycoprotein P0, J.
Biol. Chem., 271, 22957-22960.
27.Nielsen, C. M., White, M. J., Goodier, M. R., and
Riley, E. M. (2013) Functional significance of CD57 expression on human
NK cells and relevance to disease, Front. Immunol., 4,
422.
28.Lopez-Verges, S., Milush, J. M., Pandey, S.,
York, V. A., Arakawa-Hoyt, J., Pircher, H., Norris, P. J., Nixon, D.
F., and Lanier, L. L. (2010) CD57 defines a functionally distinct
population of mature NK cells in the human CD56dimCD16+
NK cell subset, Blood, 116, 3865-3874.
29.Gayoso, I., Sanchez-Correa, B., Campos, C.,
Alonso, C., Pera, A., Casado, J. G., Morgado, S., Tarazona, R., and
Solana, R. (2011) Immunosenescence of human natural killer cells, J.
Innate Immun., 3, 337-343.
30.Kovalenko, E. I., Abakushina, E. V., Telford, W.,
Kapoor, V., Korchagina, E. Yu., Khaidukov, S. V., Molotkovskaya, I. M.,
Sapozhnikov, A. M., Vlaskin, P. A., and Bovin, N. V. (2007) Clustered
carbohydrates as a target for natural killer cells: a model system,
Histochem. Cell. Biol., 127, 313-326.