Formation of Compact Aggregates of B-lymphocytes in Lung Tissue during Mycobacterial Infection in Mice Depends on TNF Production by These Cells and Is Not an Element of the Host’s Immunological Protection
T. K. Kondratieva1, I. A. Linge1, E. V. Kondratieva1, A. V. Dyatlov1,2, M. S. Drutskaya2,3, R. V. Zvartsev3, S. A. Nedospasov2,3, and A. S. Apt1,2*
1Central Research Institute of Tuberculosis, Russian Academy of Medical Sciences, Yauzskaya Alley 2, 107564 Moscow, Russia; E-mail: asapt@aha.ru2Lomonosov Moscow State University, Biology Faculty, 119991 Moscow, Russia
3Engelhardt Institute of Molecular Biology, Russian Academy of Sciences, ul. Vavilova 32, 119991 Moscow, Russia; fax: +7 (499) 135-1405; E-mail: isinfo@eimb.ru
* To whom correspondence should be addressed.
Received May 14, 2014; Revision received June 9, 2014
Tumor necrosis factor (TNF) plays a pivotal role in the early control of Mycobacterium tuberculosis and M. avium infections by a host. It was previously shown that both phagocyte-derived and T-cell-derived TNF productions are critical for protective immunity against M. tuberculosis, but the role of TNF produced by B-cells remained unclear. By comparing mice with B-cell-specific TNF deletion to littermate control mice, here we show that TNF production by B-lymphocytes is essential for the formation of infection-specific aggregates of B-cells in the lung. It is likely that these compact foci represent a pathogenic feature of inflammatory response rather than an element of protective immunity, since the capacity to form aggregates has no influence on the severity of M. tuberculosis- and M. avium-triggered diseases.
KEY WORDS: mycobacterial infections, B-lymphocytes, TNF, lung pathology, mouse modelsDOI: 10.1134/S0006297914120098
Tumor necrosis factor (TNF) production is an important part of the host’s control of infection caused by Mycobacterium tuberculosis. Engulfment of mycobacteria by lung-resident macrophages initiates a complex chain of immune reactions among which TNF production is one of the earliest important events leading to macrophage activation and granuloma formation [1]. In vivo studies in mice with genetically disrupted tnfα gene encoding TNF demonstrated a critical role of this cytokine in containment of tuberculosis (TB) infection [2, 3]. Moreover, TNF is indispensable for TB granuloma integrity, the phenotype important for prevention of dissemination of infection [4]. Another evidence indicating the important role of TNF in TB control was obtained in clinical studies: treatment of patients suffering from rheumatoid arthritis and Crohn’s disease with anti-TNF monoclonal antibodies in many cases resulted in reactivation of latent TB [5].
TNF is produced by various cells of the immune system – macrophages, dendritic cells, neutrophils, and lymphocytes. Since CD4+ T-cells and macrophages are generally considered as key elements of anti-TB defense [6], quite naturally, after introduction of methods allowing selective silencing of genes in certain cell populations, it was examined how switching off the tnfα gene in T-cells or macrophages plus neutrophils shifts anti-TB response in mice. It was shown that in T-TNF–/– mice parameters of the disease remained unaltered at the early acute phase of infection, but became more severe at the chronic phase. In contrast, tnfα disruption in macrophages and neutrophils caused more rapid disease progression starting from the early phase of infection, followed by early mortality of animals. Overall, TNF production by myeloid cells appeared to be more important for immune defense than by T-cells. Combined disruption of the tnfα gene in T-cells and myeloid cells led to a severe disease indistinguishable from that caused by the systemic knockout mutation [7].
Participation of B-lymphocytes in TB immunity and pathogenesis has acquired much less attention compared to T-cells and macrophages. However, B-cells may affect anti-TB immune response at different levels not only by antibody production, but also as antigen-presenting cells and the source of cytokines and chemokines. Thus, B-cell-deficient CBA/N-xid mice and B–/– mice with a total lack of B-lymphocytes are characterized by an increased level of IL-10, a cytokine that plays a key role in anti-inflammatory response [8-10]. In some TB mouse models, efficient treatment of the disease with monoclonal antibodies against several mycobacterial antigens has been reported [11, 12].
In numerous publications, it has been reported that TB infection in humans, monkeys, and mice is accompanied by formation of B-cell aggregates (BCA) in lung tissue, resembling B-cell follicles of secondary lymphoid organs [13-17]. In mice and humans, these structures are predominantly located near necrotic granuloma, and in the mouse model it was demonstrated that genetically susceptible mice form more BCA than more resistant mice do. Moreover, necrotization of granuloma and formation of hypoxic zones in the lungs was characteristic exclusively for susceptible mice [16]. Together with the observation that aerosol TB infection leads to increased inflammation and impaired granuloma formation in B-cell-deficient mice [9], this suggests that BCA form either as the attempt to localize immune response or because of insufficient immunity.
Since the importance of TNF for the maintenance of the structure and integrity of lung granuloma is firmly established (see ref. [4] for review), we decided to study whether TNF produced by B-cells themselves participates in BCA formation and influences pathogenesis of and/or protection against mycobacterial infections. In this work, we studied two mycobacterial pathogens, Mycobacterium tuberculosis (MTB) and Mycobacterium avium (MA). Using aerosol challenge route, we compared parameters of the diseases and BCA formation in the lungs between the wild-type B6 mice and B-TNF–/– mice on the B6 genetic background.
MATERIALS AND METHODS
Animals. Mice on the C57BL/6 genetic background with flox sites in the tnfα gene and Cre-recombinase under CD19 promoter (CD19-Cre+/–TNFflox/flox) [18] and their wild-type (TNFflox/flox) littermates (control) were obtained by mating CD19-Cre+/–TNFflox/flox mice with TNFflox/flox mice under SPF (specific pathogen free) conditions in the Pushchino Animal Facility, Moscow Region, a branch of the Institute of Bioorganic Chemistry, Russian Academy of Sciences. Genotyping by PCR was performed as previously described [18, 19]. Adult mice were transferred to the Central Institute for Tuberculosis (Moscow) and maintained under conventional conditions with water and food provided ad libitum.
CBA/LacStoCit (hereafter, CBA) and CBA/NCit (CBA/N) mice were bred and maintained under conventional conditions at the Animal Facilities of the Central Institute for Tuberculosis. Since no sex-related phenotypic differences were observed, mice of both sexes were used at 2-3 months of age.
Mycobacteria and infection. Mice were infected with the highly virulent M. tuberculosis strain H37Rv or M. avium strain 724, which cause lethal infection in mice accompanied by BCA formation in the lung [16]. Both species were from the collection of the Laboratory for Immunogenetics, Central Institute for Tuberculosis. Mycobacteria were grown for 3 weeks in Dubos broth at 37°C, suspended in sterile saline containing 0.05% Tween 20, and kept at –80°C until use. Mice were infected via the respiratory route with 102 CFU of MTB or 104 CFU of MA per mouse using an inhalation exposure system (Glas-Col, USA) as described [20].
CFU counts. To assess mycobacterial multiplication in spleens and lungs, at week 8 after MTB infection or week 16 after MA infection, 50-μl probes from sterile whole-organ 2-ml homogenates were plated onto Dubos agar in serial 10-fold dilutions, and colonies were counted after 18-20 days of incubation at 37°C. The numbers of CFU are displayed as recalculated CFU per the whole organ (CFU/organ).
Histopathology and immune histochemistry. To evaluate BCA formation in lungs, samples of lung tissue were frozen in the regimen of –60 to –20°C temperature gradient in an electronic CryotomeH (ThermoShandon, UK), and serial 6-8-μm-thick sections were made across the widest area of the middle right lobe. Sections were fixed with acetone, dried at room temperature, and rinsed with PBS (phosphate buffered saline). To avoid nonspecific staining, sections were blocked with 10% goat serum for 15 min according to the manufacturer’s instructions. Sections were incubated for 1 h at room temperature with or without (control) monoclonal antibody anti-CD19 (rat anti-mouse; BD-PharMingen, USA) diluted in PBS 1 : 50. Sections were rinsed in PBS (2 min, 3 times) and stained with secondary biotin-conjugated mouse anti-rat IgG mAbs (BD-PharMingen) for 30 min, then rinsed and incubated with labeled horseradish peroxidase for 30 min, rinsed, and incubated with diaminobenzidine solution for 5-6 min. The reaction was stopped by distilled water (2-3 min), and slides were rinsed three times in PBS containing Tween 20, counterstained for 1 min with hematoxylin, and serially incubated in 50, 70, and 90% C2H5OH and xylene with subsequent mounting in HyperMount under cover slips.
RESULTS AND DISCUSSION
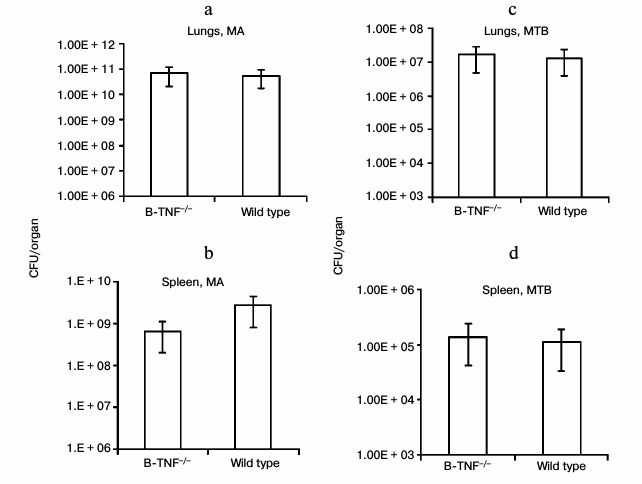
We first assessed whether TNF production by B-cells affects the growth of mycobacteria in lungs and spleens of infected animals. At week 8 after MTB infection and at week 16 after MA infection, we estimated the number of CFU in lungs and spleens of the wild-type and B-TNF–/– mice. As shown in Fig. 1, TNF production by B-cells did not influence the mycobacterial growth.
Fig. 1. TNF production by B-cells does not affect mycobacterial multiplication in organs of infected animals. The wild-type (WT) and B-TNF–/– mice were infected with M. avium (a, b) or M. tuberculosis (c, d) as described in “Materials and Methods”, and the numbers of mycobacteria in lungs (a, c) and spleens (b, d) were assessed by CFU counting. No statistically significant differences between groups were observed.
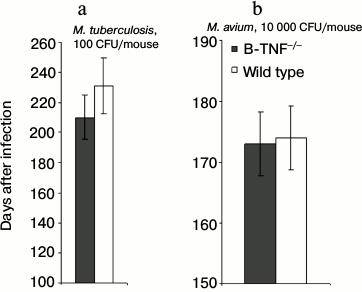
Another important characteristic of a lethal infection is the speed of mortality. Interestingly, during TB infection rapid mycobacterial multiplication in the lungs not always correlates with the early death of infected animals, suggesting that these basic parameters may have different genetic control [21]. We compared mortality curves of animals infected with two mycobacterial species and found no difference in lifespan between the wild-type and B-TNF–/– mice (Fig. 2). Thus, production of TNF by B-cells, unlike that by T-cells and macrophages [7], plays a marginal to no role in host defense against mycobacterial infections.
Fig. 2. TNF production by B-cells does not affect the lifespan of infected animals. The results are displayed as the median survival time ± SEM. No statistically significant differences between groups were observed.
These negative results raised the question whether the absence of TNF in B-cells results in any phenotypic changes during infectious inflammation. BCA formation looked as the most appropriate phenotype to study due to two observations. First, studies in noninfectious models demonstrated that the TNF production by B-cells is required for maintenance of the follicular structure in Payer’s patches and intestine follicular dendritic cells [19]. Second, as mentioned above, TNF is essential for maintenance of the structure of tuberculosis granuloma [4, 22]. Thus, we anticipated that for organization of BCA, which are forming during mycobacterial infections and consist mostly of B-cells, TNF production by B-cells themselves might be essential.
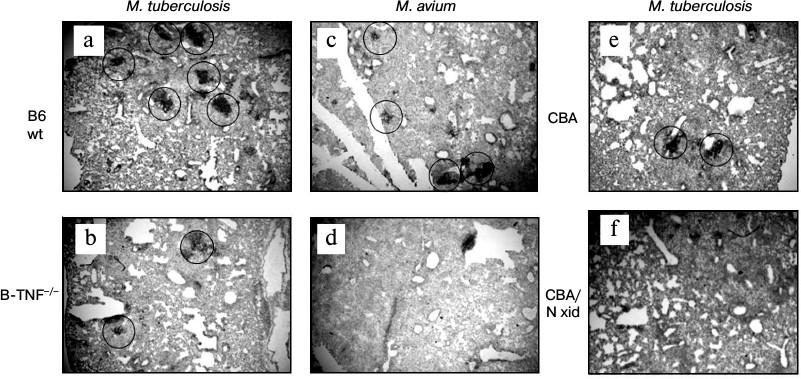
Indeed, BCA formation appeared to depend upon the capacity of B-cells to produce TNF. Figure 3 displays histological evaluation of BCA in lung tissue at weeks 8 and 16 after MTB and MA challenge, respectively (staining with anti-CD19 mAb). MTB challenge leads to quantitative differences: more and larger BCA in the lungs of the wild-type mice compared to B-TNF–/– mice (Fig. 3, a and b). MA infection results in the phenotypic differences that are extreme: BCA appeared only in the lungs of wild-type but not in B-TNF–/– mice (Fig. 3, c and d). We performed control experiments in pleural B1-cell-deficient CBA/N-xid mice and their wild-type CBA counterparts and demonstrated that during MA infection TNF production by B-cells is as crucial for BCA formation as the presence of B-cells themselves. After aerosol infection with MA, no BCA where found in the lungs of CBA/N-xid mice (Fig. 3f), whereas in CBA mice their numbers were normal (Fig. 3e).
Fig. 3. TNF production by B-cells is important for BCA formation in lung tissue following infection. After M. tuberculosis infection, the numbers and sizes of BCA (circled) are larger in the WT (a) compared to the B-TNF–/– (b) mice. After M. avium infection, BCA are formed in the WT (c) but not in the B-TNF–/– (d) mice. Lack of TNF in B-cells has the same effect in BCA formation as the absence of B-cells themselves: BCA are readily formed in the wild-type CBA (e), but not in B-cell-deficient CBA/N-xid mice (f). Anti-CD19 staining with hematoxylin counterstaining. Magnification ×22 (halves of whole middle right lobes are displayed).
Previous data and the results reported herein indicate that TNF production by cells forming compact structures in the lung tissue, e.g. complex tuberculosis granuloma [4, 22] or BCA ([16] and this work), is required for maintaining the integrity of these inflammatory foci. However, the functional importance of these structures is presumably completely different. Stability of granuloma consisting of many types of lymphoid cells is very important for host defense [7], whereas maintenance of the BCA structure does not influence the course of infection (see above).
Thus, TNF-dependent BCA formation should be considered as an additional factor of pathogenesis of mycobacterial infections rather than a part of protective immunity.
This study was financially supported by the Russian Foundation for Basic Research (grant No. 13-04-00120 to T.K.K.).
REFERENCES
1.Russell, D. G. (2007) Who puts the tubercle in
tuberculosis? Nature Rev. Microbiol., 5,
39-47.
2.Flynn, J. L., Goldstein, M. M., Chan, J., Triebold,
K. J., Pfeffer, K., Lowenstein, C. J., Schreiber, R., Mak, T. W., and
Bloom, B. R. (1995) Tumor necrosis factor-alpha is required in the
protective immune response against Mycobacterium tuberculosis in
mice, Immunity, 2, 561-572.
3.Jacobs, M., Marino, M. W., Brown, N., Abel, B.,
Bekker, L. G., Quesniaux, V. J., Fick, L., and Ryffel, B. (2000)
Correction of defective host response to Mycobacterium bovis BCG
infection in TNF-deficient mice by bone marrow transplantation, Lab.
Invest., 80, 901-914.
4.Rook, G. A. W., and Hernandez-Pando, R. (1996) The
pathogenesis of tuberculosis, Ann. Rev. Microbiol., 50,
259-284.
5.Kean, J. (2005) The pathogenesis of tuberculosis
TNF-blocking agents and tuberculosis: new drugs illuminate an old
topic, Rheumatology (Oxford), 44, 714-720.
6.Ottenhoff, T. H. M., and Kaufmann, S. H. E. (2012)
Vaccines against tuberculosis: where are we and where do we need to go?
PLoS Pathogens, 8, e1002607.
7.Allie, N., Grivennikov, S. I., Keeton, R., Hsu, N.
J., Bourigault, M. L., Court, N., Fremond, C., Yeremeev, V.,
Shebzukhov, Y., Ryffel, B., Nedospasov, S. A., Quesniaux, V. F., and
Jacobs, M. (2013) Prominent role for T cell-derived tumor necrosis
factor for sustained control of Mycobacterium tuberculosis
infection, Sci. Rep., 3, 1-14.
8.Junqueira-Kipnis, A. P., Kipnis, A., Henao, T. M.,
Harton, M., Gonzalez Juarrero, M., Basaraba, R. J., and Orme, I. M.
(2005) Interleukin-10 production by lung macrophages in CBA xid mutant
mice infected with Mycobacterium tuberculosis,
Immunology, 115, 246-252.
9.Maglione, P. J., Xu, J., and Chan, J. (2007) B
cells moderate inflammatory progression and enhance bacterial
containment upon pulmonary challenge with Mycobacterium
tuberculosis, J. Immunol., 178, 7222-7234.
10.Maglione, P. J., and Chan, J. (2009) How B cells
shape the immune response against Mycobacterium tuberculosis,
Eur. J. Immunol., 39, 676-686.
11.Glatman-Freedman, A. (2006) The role of
antibody-mediated immunity in defense against Mycobacterium
tuberculosis: advances toward a novel vaccine strategy,
Tuberculosis (Edinb.), 86, 191-197.
12.Balu, S., Reljic, R., Lewis, M. J., Pleass, R.
J., McIntosh, R., van Kooten, C., van Egmond, M., Challacombe, S.,
Woof, J. M., and Ivanyi, J. (2011) A novel human IgA monoclonal
antibody protects against tuberculosis, J. Immunol., 186,
3113-3119.
13.Ulrichs, T., Kosmiadi, G. A., Trusov, V., Jorg,
S., Pradl, L., Titukhina, M., Mishenko, V., Gushina, N., and Kaufmann,
S. H. (2004) Human tuberculous granulomas induce peripheral lymphoid
follicle-like structures to orchestrate local host defense in the lung,
J. Pathol., 204, 217-228.
14.Tsai, M. C., Chakravarty, S., Zhu, G., Xu, J.,
Tanaka, K., Koch, C., Tufariello, J., Flynn, J., and Chan, J. (2006)
Characterization of the tuberculous granuloma in murine and human
lungs: cellular composition and relative tissue oxygen tension, J.
Cell Microbiol., 8, 218-232.
15.Kahnert, A., Hopken, U. E., Stein, M.,
Bandermann, S., Lipp, M., and Kaufmann, S. H. (2007) Mycobacterium
tuberculosis triggers formation of lymphoid structure in murine
lungs, J. Infect. Dis., 195, 46-54.
16.Kondratieva, E., Logunova, N., Majorov, K.,
Averbakh, M., and Apt, A. S. (2010) Host genetics in granuloma
formation: human-like lung pathology in mice with reciprocal genetic
susceptibility to M. tuberculosis and M. avium, PLoS
One, 5, e10515.
17.Phuah, J. Y., Mattila, J., Lin, P. L., and Flynn,
J. L. (2012) Activated B cells in the granulomas of nonhuman primates
infected with Mycobacterium tuberculosis, Am. J. Pathol.,
181, 508-514.
18.Kuprash, D. V., Tumanov, A. V., Liepinsh, D. J.,
Koroleva, E. P., Drutskaya, M. S., Kruglov, A. A., Shakhov, A. N.,
Southon, E., Murphy, W. J., Tessarollo, L., Grivennikov, S. I., and
Nedospasov, S. A. (2005) Novel tumor necrosis factor-knockout mice that
lack Peyer’s patches, Eur. J. Immunol., 35,
1592-1600.
19.Tumanov, A. V., Grivennikov, S. I., Kruglov, A.
A., Shebzukhov, Y. V., Koroleva, E. P., Piao, Y., Cui, C. Y., Kuprash,
D. V., and Nedospasov, S. A. (2010) Cellular source and molecular form
of TNF specify its distinct functions in organization of secondary
lymphoid organs, Blood, 116, 3456-3464.
20.Radaeva, T. V., Kondratieva, E. V., Sosunov, V.
V., Majorov, K. B., and Apt, A. S. (2008) A human-like TB in
genetically susceptible mice followed by the true dormancy in a
Cornell-like model, Tuberculosis (Edinb.), 88,
576-585.
21.Nikonenko, B. V., Averbakh, M. M., Lavebratt, C.,
Schurr, E., and Apt, A. S. (2000) Comparative analysis of mycobacterial
infections in susceptible I/St and resistant A/Sn inbred mice,
Tuber. Lung Dis., 80, 15-25.
22.Zganiacz, A., Santosuosso, M., Wang, J., Yang,
T., Chen, L., Anzulovic, C., Alexander, S., Gicquel, B., Wan, Y.,
Bramson, J., Inman, M., and Xing, Z. (2004) TNF-alpha is a critical
negative regulator of type 1 immune activation during intracellular
bacterial infection, J. Clin. Invest., 113, 401-412.