REVIEW: Acid-Sensing Ion Channels and Their Modulators
D. I. Osmakov, Ya. A. Andreev, and S. A. Kozlov*
Shemyakin and Ovchinnikov Institute of Bioorganic Chemistry, Russian Academy of Sciences, ul. Miklukho-Maklaya 16/10, 117997 Moscow, Russia; E-mail: serg@ibch.ru; osmadim@gmail.com* To whom correspondence should be addressed.
Received May 27, 2014
According to a modern look acid-sensing ion channels (ASICs) are one of the most important receptors that perceive pH change in the body. ASICs represent proton-gated Na+-selective channels, which are expressed in neurons of the central and peripheral nervous system. These channels are attracting attention of researchers around the world, as they are involved in various physiological processes in the body. Drop of pH may occur in tissues in norm (e.g. the accumulation of lactic acid, the release of protons upon ATP hydrolysis) and pathology (inflammation, ischemic stroke, tissue damage and seizure). These processes are accompanied by unpleasant pain sensations, which may be short-lived or can lead to chronic inflammatory diseases. Modulators of ASIC channels activity are potential candidates for new effective analgesic and neuroprotection drugs. This review summarizes available information about structure, function, and physiological role of ASIC channels. In addition a description of all known ligands of these channels and their practical relevance is provided.
KEY WORDS: acid-sensing ion channel, pain perception, ligand, low molecular weight modulator, peptideDOI: 10.1134/S0006297914130069
Abbreviations: AA, arachidonic acid; ASIC, acid-sensing ion channel; CNS, central nervous system; DEG/ENaC, amiloride-sensitive degenerin/epithelial Na+ channel; EPSP, excitable postsynaptic potential; GMQ, 2-guanidine-4-methylquinazolin; NSAIDs, nonsteroidal antiinflammatory drugs; PcTx1, psalmotoxin 1; SLP3, stomatin-like protein 3; TM, transmembrane domain.
One of the most urgent tasks in biochemistry is to search for and/or to
create molecular tools to study the mechanisms of functioning of the
sensory systems of living organisms both in normal and pathological
states. Interesting objects for study are the acid-sensing ion channels
(ASIC) related to the superfamily of amiloride-sensitive
degenerin/epithelial Na+-channels (DEG/ENaC) [1].
ASIC channels are found in large numbers in neurons of the central nervous system (CNS) [2, 3], where at least three (ASIC1a, ASIC2a, and ASIC2b) subunits from the six known are found. Among all the subunits present in brain, ASIC1a is basic. Homomeric ASIC1a and heteromeric ASIC1a/2b channels are able to conduct both Na+ and Ca2+ [4, 5]. It was shown that ASIC1a and ASIC2 are directly involved in synaptic plasticity, learning, transmission of nerve excitation [3, 6], ischemia, and neuronal cell death [7-10], as well as epilepsy [11].
In neurons of the peripheral nervous system, homomeric ASIC3- and heteromeric ASIC3-containing channels are mainly present [12]. These channels are shown to: a) participate in perception of acid-mediated, inflammatory, or postoperative pain [13-15]; b) contribute to development of primary and/or secondary mechanical hypersensitivity in muscles [16]; c) participate in cutaneous and visceral mechanical sensitivity and in perception of pain from mechanical stimuli [17-19]; d) be involved in perception of pain signals from the lungs and gastrointestinal tract [20].
STRUCTURE OF ASICs
Neuronal receptors able to perceive extracellular pH decrease were discovered in 1980 [21], but they were cloned and characterized only in 1997 [22]. In mammals, the presence of four genes (ACCN 1-4) encoding at least six subunits of these channels (ASIC1a, ASIC1b, ASIC2a, ASIC2b, ASIC3, and ASIC4) was established [23]. The primary structure of ASIC channels is quite conservative for a variety of mammalian species (rats, mice, and humans). Thus, ASIC isoforms in rats have amino acid sequence similarity of 45-80%. In addition to mammals, ASICs have been found in animals of other classes such as toadfish (Batrachoididae), lampreys, sharks, and the freshwater aquarium fish Danio rerio [24, 25].
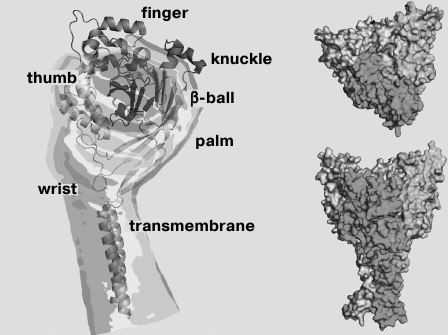
Functional ASICs are trimeric complexes [26, 27] that can be homomeric or heteromeric [3, 5, 9, 28, 29]. In 2007, the spatial structure of chicken ASIC isoform 1a was resolved by X-ray analysis to resolution of 1.9 Å (Fig. 1) [26].
Fig. 1. Crystal model of desensitized chicken ASIC1a channel (cASIC1a) presented without the N- and C-terminal regions.
As in all DEG/ENaC channels, membrane topology of the individual subunit of the ASIC channel consists of two transmembrane domains (TM1 and TM2) linked via a large cysteine-rich extracellular loop with short intracellular N- and C-terminal regions [30]. The domain of the N-terminal region, adjacent to TM1, is responsible for channel selectivity to Na+ [31]. The conducting pore in the membrane is formed by direct contacts of the TM1 and TM2 domains of all three subunits [32]. The TM2 domain is highly conserved not only among the ASIC channels, but also among channels of the DEG/ENaC family in general [33]. It is involved in the formation of the desensitization gate. Amino acid residues (a.a.) Asp433-Gly436 are important for it, and the Asp433 side group is directed into the lumen of the pore [27]. TM2 also contains an amiloride-binding site and a selective filter formed by a.a. 443-445 [32, 33], as well as two amino acid residues responsible for blocking by Ca2+ (channel ASIC1a) [34].
The extracellular region of each subunit has the shape of a “hand clutching a ball” consisting of “wrist”, “palm”, “finger”, “knuckle”, and “β-ball” domains and a cystine-rich “thumb” domain (Fig. 1) [26, 27]. It is formed by seven α-helices (α1-α7) and 12 β-layers (β1-β12) [26] and stabilized by 14 disulfide bonds, which are quite conserved among DEG/ENaC channels [35]. The palm domain contains seven β-strands, it is a central element of the extracellular region and forms numerous contacts with other domains. Part of the extracellular loop near TM1 contains the degenerin site [36]. The extracellular part is connected with the TM domains through the small wrist domain consisting of only two ordered loops.
Numerous studies have established an important functional role for the various amino acid residues. The β10-layer in palm domain and a region located between the α6- and α7-helices in the knuckle domain contain two conserved glycosylation sites Asn367 and Asn394 that play an important structural role in proton-sensing [26, 30, 37]. Residues Asp107 in the α1- and Arg153 in the α3-helices of the finger domain in the ASIC3 channel form an acid–base interaction [38]. The Trp287 residue of the thumb domain and Tyr71 of the wrist domain in ASIC1 interact with each other and participate in channel opening [39]. The Asp79-Glu80 pair is conserved in all ASIC channel subunits, it is associated with inactivation of the ASIC3 channel [40] and in addition influences proton-sensitivity in the ASIC2a channel [41]. His72 is important for the sensing pH changes [42] and is in close proximity to the domain responsible for the desensitization kinetics [43].
At the interface between the thumb, finger, and palm domains, chloride ions are accessed to each subunit while being coordinated by Arg310, Glu314, and Lys212 residues of the neighboring subunit [26, 27]. As shown by experiments with the replacement of chlorine ions and mutagenesis of coordinating amino acid residues, access of Cl– to the channel plays an important role in the channel desensitization [44].
A proton-sensitive sensor is located at the interface between the finger and thumb domains of one subunit and the palm domain of the neighboring subunit. This area, which looks like a cavity on the surface of the channel, is rich in acidic residues, so it was named the “acidic pocket” [26, 41, 45]. Cavities have strongly expressed negative electrostatic potentials due to the presence of 12 acidic residues – Asp79, Glu420, Glu426, Asp433 in the upper part and Glu80, Glu374, Glu412, Glu417 in the central part of the pore. It is supposed that the cavities serve as “cationic reservoirs” concentrating cations near the pore of the ion channel, which provides reliable channel conductivity [46]. Engaging bivalent cations to the cavity reduces the concentration of Na+ near the pore and thus prevents Na+ current. This is observed for ASIC3 at the presence of Ca2+ [47].
It has also been found that the loop region connecting the β9-layer of the palm domain and the α4-helix of the thumb domain plays an important role in signal transduction from the extracellular region to the transmembrane domains, resulting in a conformational rearrangement and opening of the channel [48]. Proline residues located in this loop form a sharp turn followed by the approach of Trp287 and Tyr71 toward each other in vicinity of TM1 [49]. Conformational changes occur also in the wrist domain, which presumably lead to the subsequent twisting (or bending) of TM1 and TM2. During the transition of the channel from the closed to the open or desensitized state, important conformational changes occur also in other domains. Thus, mutagenesis of various residues of human ASIC1a (hASIC1a) identified a number of residues that alter the kinetics of the opening and closing of the pore: in the region between the β1-β2-layers and the β1-layer of the palm domain; in the loop between the palm and finger domains; in the loop between the β-ball and finger domains; and in the finger domain [40, 43, 50-52].
Based on generalization of the results of numerous experiments, the following general mechanism of opening of the channel in response to a change in pH was suggested: protonation/deprotonation of acidic amino acid residues of the thumb domain affects its interaction with amino acid residues located in the finger domain, which moves the thumb domain. Then the signal through the wrist domain is transmitted to the TM domains, which leads to a change in the inclination angle of the helices, expansion of the extracellular part of the pore, narrowing in the selective filter, and channel opening [32]. Pore opening leads to the fact that the backbone carbonyl oxygen atoms as well as the side chain oxygen atoms of residues such as Asp433 align along the third order symmetry axis on the path of sodium ions. Long duration of low pH causes the mutual convergence of the wrist domain and the neighboring β1 and β12 strands, which leads to occlusion of the central region of the pore and closure of the ion channel (the desensitization).
ELECTROPHYSIOLOGICAL PROPERTIES OF ASICs
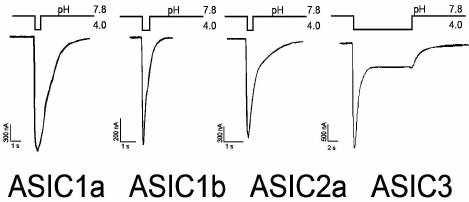
The electrophysiological properties and pharmacological profiles of the ASIC channels have been carefully studied in heterologous expression systems [53] and in neurons of different areas of the brain such as cerebral cortex [9], hippocampus [29], striatum [54], cerebellum [55], net ganglia [56], and spinal cord [57]. Figure 2 shows typical examples of ASIC currents corresponding to homomeric ASIC1a, 1b, 2a, and 3 channels expressed in oocytes of the clawed frog Xenopus laevis. The remaining subunits do not form homomeric actively conducting sodium channels. Homomeric ASIC3 channels are fundamentally different in the form of the response to stimulating pulse. They have a two-component response to pH drop in the extracellular medium. These responses have the form of a fast-inactivating current with greater amplitude (transient component or peak) and slow-inactivating current with a smaller amplitude (sustained component or plateau).
Fig. 2. Characteristic incoming currents of ASIC channels. Measurements were carried out on oocytes of the frog Xenopus laevis expressing homomeric channels of rat in the configuration of the whole cell. Activation of the ion current was caused by a rapid replacement of solutions with different values of pH (data obtained in the Laboratory of Neuroreceptors and Neuroregulators, Institute of Bioorganic Chemistry, Russian Academy of Sciences).
The amino acid sequence similarity between the subunits ASIC1a and ASIC1b is approximately 70%, but ASIC1b with a similar form of the recorded current differs in many other parameters. First, ASIC1b channels are found in mammals only in peripheral sensory neurons [58]. In the example found in rodents, they are impermeable to Ca2+, while ASIC1a channels conduct these ions [4]. It is functionally important that ASIC1b channels are activated at a low pH (~6.5 vs. ~7.0 for ASIC1a) and, accordingly, have lower pH50 (pH at which the activation of the channel reaches half of the maximum value) values (~5.9 vs. 6.8 for ASIC1a) [22].
Homomeric ASIC2a channels are relatively insensitive to protons (their pH50 value is 4.4) [59]. Functional heteromeric channels ASIC2a/1a are found in mammalian brain [9, 29, 54]. Unlike ASIC2a subunits, homomeric ASIC2b subunits do not form functional channels in vivo; they are always present in a complex with other ASIC subunits [5, 53, 59]. For example, channels ASIC2b/1a are involved in triggering neuronal cell death in acidosis caused by ischemia [5].
ASIC3, like ASIC1b, is found mainly in peripheral sensory neurons [57, 60-62]. Moreover, it was shown that ASIC3 from sensory neurons of rat skin gives predominant response under moderate acidification [13]. Electrophysiological data showed that in sensory neurons ASIC3 subunits function as homomeric and heteromeric channels [28, 62, 63]. They are susceptible to acidification both in physiological and pathological processes, such as skin sensitivity, pain perception under inflammatory processes, and ischemia [60, 64-68].
As noted earlier, these channels have a unique two-component response to acidic stimulation. The transient component of the current is highly sensitive to protons (pH50 6.5), has a large amplitude, and is rapidly desensitized [53, 61]. If there is a drop in pH value from 7.4 to 6.5 and <6.0, then there is a slowly developing component of the current ASIC3, which lasts as long as the acidic stimulation [8, 22, 69]. In the generation of the plateau effect for different intervals of pH (i.e. >6.5 and <6.0), various structural elements of the channel are involved [69]. Thus, depending on the intensity and duration of the acid stimulus, the form of the recorded current through ASIC3 can be different: transient component, sustained component, or the sum of both components.
ASIC4 subunits are present in the hypophysis. Like ASIC2b, they do not form functional homomeric channels [70].
ASICs are characterized by the fact that they can go into a state of steady-state desensitization (also known as steady-state inactivation). This process occurs under a weak but long-lasting increasing proton concentration for a period from a few seconds to a few minutes [47, 71]. Thus, subsequent activation by low pH does not result in a significant current. As in the case of the activation, stationary desensitization is mediated by addition of a proton to specific sites in the extracellular loop. These inactivation sites have a higher degree of affinity for protons than sites that are responsible for activation of the channel [71].
ROLE OF ASICs IN PHYSIOLOGICAL PROCESSES
ASIC channels have been found in the postsynaptic membrane and bodies of CNS neurons [6]. Interactions of these channels with the synapse-associated protein PSD-95 (which plays an important role in synaptic plasticity) and kinase 1-interacting proteins have been demonstrated [72, 73]. These facts and the fact that the pH of the synaptic cleft is changed during operation of the synapse suggest that the ASICs can affect synaptic transmission. This was demonstrated by the example in mice of hippocampal neuron knockout of the ASIC1a channel, which showed the absence of long-term potentiation of excitatory postsynaptic potential (EPSP) produced by high-frequency stimulation, in contrast to wild-type mice [6]. With the same mouse knockout model, the absence of inhibitory effect of NMDA-receptor antagonists, D-2-amino-5-phosphonovalerate, on EPSP summation was shown, unlike wild-type mice, which demonstrated a synergistic effect in functioning of ASIC1a channels and NMDA-receptors [74].
ASIC1a channels were found in the amygdala, which plays an important role in anxiety behavior [3]. It was shown that mice with knockout of the gene encoding ASIC1a were considerably less susceptible to anxiety in the “open field” test in response to sudden noise or predator odor [75]. In this case, increased expression of the channels leads, on the contrary, to increased anxiety [76], but it had no effect on the appearance of undue anxiety [77].
The role of ASIC1a channels in the development of CO2-mediated anxious behavior was also established. It was shown that an increase in CO2 in the air breathed by experimental mice resulted in a decrease in pH in the brain and caused the development of anxious behavior. Adding a buffer solution maintaining a normal pH in the amygdala region significantly attenuated the symptoms of anxious behavior, but pH decrease in this region reproduced the effect of CO2. It has been demonstrated that the elimination/inhibition of ASIC1a channel leads to a substantial reduction in alarm activity, whereas overexpression of ASIC1a channel in the amygdala of mice deficient for these channels, conversely, restores CO2-mediated anxious behavior. Thus, a possible molecular mechanism underlying the development of fear and anxiety was shown [78].
The ability of ASICs to sense the change in environmental pH and to generate an ion current across the cell membrane includes this type of receptor among the major sensory receptors. The dominant role in the perception of external stimuli in mammals belongs to ASIC3. In rodents, ASIC3 channels are present in more than half of muscle afferents and in more than a third of DRG neurons innervating the knee joint [66, 68]. They were found in specialized cutaneous mechanosensory structures such as Meissner’s corpuscle, Merkel nerve endings, free nerve endings, and lanceolate nerve endings surrounding the hair follicle [65]. The role of ASIC3 in mechanosensing of the gastrointestinal tract has been described [17, 18].
Mice with a targeted ACCN3 deletion (the gene encoding ASIC3) – asic3–/– mice – showed increased sensitivity to weak mechanical stimuli [65, 79]. It was also shown that stomatin-like protein 3 (SLP3), which is involved in response to the touch in mice, in vitro is associated with ASIC3 and does not interact with the TRPV1 receptor that also plays an important sensory role. SLP3 inhibited ASIC-mediated currents in sensory neurons that indicates participation of ASIC3 in mechanosensory complexes [80]. There is still an absence of evidence about direct activation of ASIC3 channels by mechanical stimuli, leaving open the question of their direct involvement in mechanosensing [81].
ASIC3 channels have been shown to participate in the processes of auditory perception and vision. ASIC3 channels have been found in cells of the spiral ganglion and the organ of Corti, located in the cochlea. ASIC3-deficient mice showed hearing loss in the early stages of development, with its subsequent restoration at the age of two months [82]. ASIC3 inactivation led to negative effects similar to those in glaucoma and chronic ischemia associated with defects in the inner segment of “rods” [83]. This is because the signal transmission in neurons of the retina is strongly dependent on the pH of the extracellular medium [84].
Besides, ASIC3 channels certainly play an important role in chemoperception, sensing signals in the form of reduction of the extracellular medium pH, of Ca2+ level, and of certain metabolites. For example, asic3–/– mice were better protected against age-dependent glucose intolerance and demonstrated an increased sensitivity to insulin. Similar results were obtained on administration in wild-type mice an inhibitor of ASIC3, peptide APETx2, which proved the participation of ASIC3 in the development of age-dependent glucose intolerance and insulin resistance [85].
Peripheral ASIC3 channels play an important role in the perception of pain and signal integration under the inflammation process. Moderate acidosis in combination with hyperosmolarity and arachidonic acid induces inflammation-associated pain, which declines under the action of the peptide APETx2 and ASIC3-specific antisense RNA [13]. Also, ASIC3-deficient mice showed almost no development of thermal and mechanical hypersensitivity during inflammation caused by injection of complete Freund’s adjuvant or carrageenan into footpad [15, 86].
ASICs AND PATHOLOGICAL STATES
Many experiments with models of neurodegenerative diseases affecting the CNS organs have shown that ASICs make a significant contribution to the perception of damaging stimuli. Thus, in experimental models of autoimmune encephalomyelitis in mice not expressing ASIC1a, a reduction in the level of axonal degeneration in comparison with wild-type mice was shown. Measurements of cerebrospinal fluid pH in mice suffering from autoimmune encephalomyelitis showed acidification sufficient to activate the ASIC1a channel, and deletion of the gene encoding ASIC1a demonstrated a protective effect on neuronal explants in vitro. Blockade of ASIC channels by amiloride induced the same neuroprotective effect [10]. Analogously, it has been demonstrated that the blockade of ASIC1 by amiloride protects both myelin and neurons against damage in models of acute pain [87]. Thus, a role of ASIC1a in axonal degeneration was demonstrated, which allowed to propose the use of ASIC1a inhibitors for neuro- and myelo-protection in multiple sclerosis [87].
Parkinson’s disease is characterized by impaired motor function and loss of dopaminergic neurons in the substantia nigra [88]. Although the mechanism of neuronal damage is not clear, there is evidence that the affected neurons express ASIC1a channels [3, 89]. Blockade of this channel type by amiloride and psalmotoxin 1 (PcTx1) provided neuroprotection of the substantia nigra in a mouse model of Parkinson’s disease induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine [90].
Anxiety disorders are debilitating neuropsychiatric diseases. They are treated mainly with benzodiazepines and selective serotonin reuptake inhibitors, but these drugs have a variety of side effects. The use of ASIC1a inhibitors such as PcTx1, A-317567, and amiloride improved parameters associated with the vegetative nervous system and behavioral parameters in models of anxiety in rodents [91].
Under intensive neuronal excitation in epileptic seizures, acidification in the brain was observed [92, 93]. This suggests the participation of ASIC1 channels in epilepsy. In models of epileptic seizures, it was shown that ASIC1a inhibitors significantly reduce the strength and frequency of action potentials and prolonged membrane depolarization [94, 95]. However, in other studies, i.e. on GABA-ergic interneurons, an inhibitory effect exerted by ASIC1a on the development of seizure was demonstrated [11]. These results suggest that the role of ASIC channels, in particular ASIC1a, in epileptic seizures is complicated and ambiguous, depending on numerous factors such as the nature of the stimulus inducing a seizure, localization in the brain, as well as the age of neurons [96].
Accumulation of lactic acid during anaerobic hydrolysis as well as the release of protons from ATP hydrolysis leads to a pH decrease and acidosis of the brain [97] that plays an important role in brain injury during ischemia [98] and is directly related to the strength of myocardial infarction [99]. In cultures of mouse and human cortical neurons, for example, activation of ASIC channels by a solution of low pH led to neuronal damage that was inhibited by ASIC1a-specific blockade and by deletion of the ASIC1a-encoding gene [100]. In models of cerebral ischemia in rodents, intracerebroventricular administration of an ASIC1a blocker reduced the strength of stroke by more than 60% [9, 101]. A similar neuroprotective effect was observed in mice with knockout of the ASIC1a-encoding gene [9].
Similarly, ASIC3 channels in the peripheral nervous system are strongly associated with pathologies of various organs. Studies have shown that decreasing pH to 6.7 activates ASIC3 channels in neurons innervating the cardiac muscle. Based on indirect evidence (effects of low pH and lactic acid), an important sensory role of ASIC3 channels in cardiac ischemia and angina was proposed [8]. Representatives of ASIC channels, particularly ASIC3, are considered as important participants in the perception of visceral pain from mechanical stimuli in the organs of the gastrointestinal tract [18]. In several models of acute and chronic pain (such as thermal, chemical, mechanical, inflammatory, and neuropathy) in rodents, an important contribution of ASIC1a to the development of pain has been demonstrated. Intrathecal or intracerebroventricular administration of a specific ASIC1a inhibitor, PcTx1, or introduction of antisense oligonucleotides leads to a significant analgesic effect [102, 103]. It was found that inhibition of ASIC1a activates an endogenous enkephalin pathway that increases levels of Met-enkephalin in the cerebrospinal fluid, apparently leading to the analgesic effect [102].
MODULATORS OF ASICs ACTIVITY
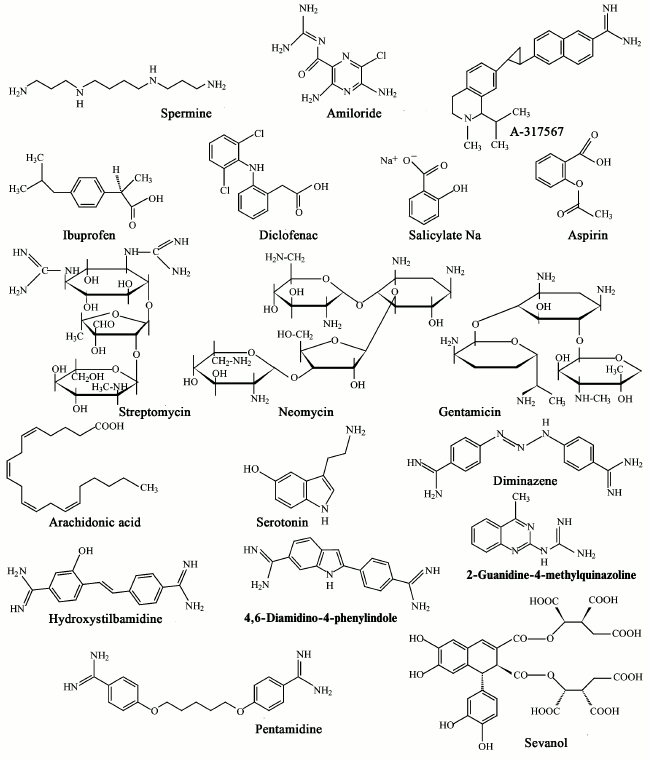
As indicated in the previous two sections, ASICs, especially types 1a and 3, are involved in a wide range of processes in animals. Their malfunction leads to a variety of pathological states that can be corrected and studied in detail through different ligands. A quite a large number of endogenous and exogenous ligands are known that can directly activate acid-sensitive channels or potentiate/inhibit proton-induced currents through the channels. In the table, we summarized all published ASIC modulators. These molecules have different chemical nature and not always selectively interact with ASICs. Low molecular weight compounds (their structures are shown in Fig. 3) are now of the greatest practical interest.
Pharmacology of homomeric ASIC channels*
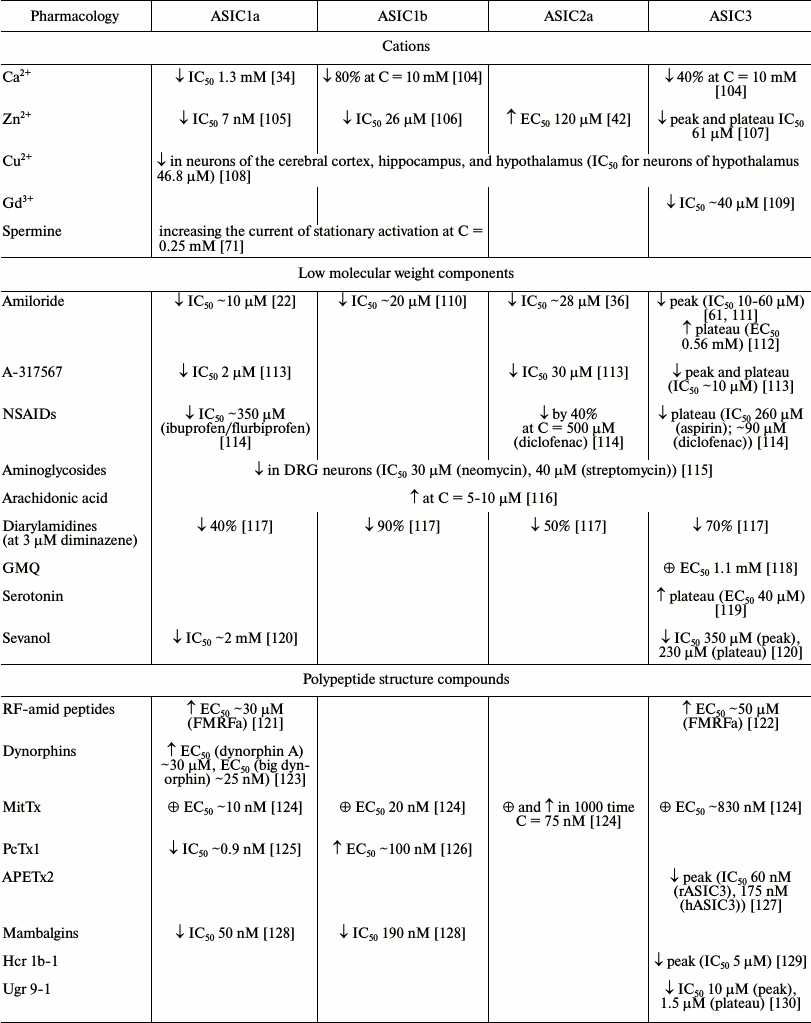
Note: ↓ inhibitory effect; ↑ potentiating
effect; ☼ activation.
* Results presented in the table were obtained in different experimental
systems with various pH-activating stimuli (see original works for
conditions).
Fig. 3. Low molecular weight modulators of ASICs.
Endogenous ligands. Arachidonic acid (AA) is a polyunsaturated fatty acid present in phospholipids of all cell membranes. It functions not only as a secondary messenger, but it also plays an important role in various pathological conditions such as inflammation and ischemic brain injury [131]. It was found that AA enhances activation of ASIC channels. Studies on Purkinje cells showed that preincubation with 5-10 µM AA solution increased the amplitude of both the transient and sustained components of the ASIC current [55].
Serotonin (or 5-hydroxytryptamine), a proinflammatory agent, has a potentiating effect on the sustained component of the ASIC3 current. This effect is observed on activation at pH 6.0 and below. In a model of inflammatory pain in mice, serotonin showed a significant enhancement of the pain effect that was not observed in mice deficient in the gene encoding ASIC3 [119].
Tetrapeptide FMRF-amide and similar peptides are capable of potentiating H+-mediated currents (EC50 ~ 10-50 µM for FMRF-amide) [121]. FMRF-amide does not occur in mammals, but other peptides of this group such as NPFF, NPAF, and NPSF are present in many parts of the CNS. In chronic inflammation, elevated level of NPFF is observed in the spinal cord. NPFF is also found in small and medium-sized DRG neurons. It is assumed that the modulation of ASICs by endogenous RF-amide peptides is a response to a strong acidification of the environment. RF-amides interact with the extracellular domain, which results in an increase in the current amplitude and deceleration of desensitization of ASIC1 and ASIC3 (but not ASIC2a) due to decrease of proton sensitivity for sites responsible for steady-state desensitization [121, 122, 132, 133].
Dynorphins are a class of endogenous opioid neuropeptides abundantly represented in the CNS. They are involved in various physiological processes including analgesia and neuroendocrine signal transmission. At high concentrations, dynorphin A potentiates acidic pH-activated currents in cortical neurons as well as in CHO cells expressing homomeric ASIC1a [123]. In the case of expressed channels, it was shown that the potentiation effect is mediated by a limiting action on the steady-state desensitization process and does not depend on the activity of the opioid receptors, i.e. it occurs under direct action of the peptides on the ASIC1a channel. At the same time, the depressing effect that dynorphin exerted on the steady-state desensitization negatively influenced neurons exposed to prolonged acidosis [123]. It is therefore considered that dynorphin might exacerbate brain damage in pathological conditions associated with ischemia.
ASIC channels are modulated by divalent cations, especially Ca2+ ions, which play an important role in the regulation of various voltage-dependent and ligand-gated ion channels. The effect of Ca2+ concentration changes in the extracellular medium on ASICs depends on whether Ca2+ was present on channel activation or not. Application of Ca2+ together with acid solution inhibits activation of the channels [25, 61]. Conversely, the absence of Ca2+ enhances activation of ASICs by an acid solution [71, 134]. Studies on the mechanisms underlying the modulating action of Ca2+ revealed that Ca2+ reduces the affinity of the channels (for example, ASIC3) to H+ ions [71]. It was suggested that at pH 7.4 ASIC3 channels are closed due to the blockade by Ca2+. By acidification protons attach to the channel, which causes dissociation of Ca2+ from its binding sites and as a consequence an opening of the channel [47]. ASIC1a was found to have two Ca2+-binding sites: one serves for pore blockade by Ca2+ and the other mediates Ca-dependent regulation of activation by protons [34, 135].
At physiological pH and Ca2+ concentration, zinc ions also play an important role in the regulation of ASIC channels. In CHO cells expressing different combinations of ASIC subunits, it was shown that Zn2+ in nanomolar concentration inhibits currents generated by homomeric ASIC1a and heteromeric ASIC1a/2a channels. This cation led to both a decrease in the amplitude of the current and to reduced affinity of ASIC1a to protons. It was shown that Lys133, located at the beginning of the α2-helix in the finger domain, is involved in a high-affinity process of inhibition by Zn2+ [105]. At micromolar concentrations, Zn2+ caused a dose-dependent inhibition of ASIC1b (IC50 26 µM) [106] and ASIC3 channels (IC50 61 µM) [107]. The inhibitory effect was also due to the interaction of Zn2+ with the binding site located in the extracellular portion of ASIC1b and ASIC3 channels. Experiments with mutagenesis of the channels proved that Cys149 residue located in the finger domain of ASIC1b subunit determines the sensitivity to Zn2+ [106]. At concentrations greater than 100 µM, Zn2+ interacts also with the low-affinity binding site on the ASIC2a subunit, which increases affinity for protons and potentiates activity of ASIC2a-containing channels [42].
Copper ions (Cu2+) have a modulating effect on ASIC channels, as shown in cell cultures of hypothalamus, hippocampus, and cortical neurons. Copper dose-dependently reduces the amplitude of ASIC currents as well as slowing desensitization [108]. Micromolar concentrations of copper ions weaken acid-induced membrane depolarization, so Cu2+ can be regarded as an endogenous modulator that reduces increased neuronal excitability [108].
Spermine (a polyvalent cation) exerts a potentiating effect on the activity of ASIC1b and ASIC1a channels [71]. Spermine exacerbates damage of neurons during ischemia through increasing ASIC1a sensitivity to extracellular medium acidification [136]. Pharmacological blockade of ASIC1a or deletion of the gene encoding ASIC1 greatly reduces the effect of spermine on neuron damage during ischemia in a culture of dissociated neurons and in a model of focal ischemia in mice [136]. Spermine reduces the desensitization of ASIC1a in the open state, but also accelerates the recovery after desensitization in response to repeated acid stimuli. Functionally, increase in channel activity is accompanied by increased acid-induced depolarization of neurons and overloading of the cytoplasm by Ca2+, which may explain the increased negative effect spermine on neuron damage. Therefore, spermine significantly contributes to neuron damage in ischemia, in part by enhancing the activity of ASIC1a receptors.
Exogenous ligands. Amiloride – a nonspecific blocker of Na+ channels – is used in medicine as a K+-sparing diuretic agent, but it is also widely used as an inhibitor of ASICs in research. According to data from many studies, it inhibits the ion current conducted by these channels, with IC50 values of 10-60 µM. In the case of ASIC3, amiloride induces a complex response. Only the peak current component is inhibited, whereas for the plateau component no effect at low concentrations and potentiation at higher concentrations (EC50 of 0.5 mM and pH 7.4-6.8) were observed [8, 9, 22, 54, 58, 112]. Based on the data obtained in the study of ENaC, it was proposed that the mechanism of the inhibitory effect of amiloride is a direct blocking the cation-conducting pore of the channel [137]. A site located in front of the TM2 domain plays an important role in the action of amiloride. Mutation of residue Gly430 in this area greatly changes the sensitivity of ASIC2a to amiloride [36]. Amiloride inhibits acid-induced pain in the peripheral sensory system [86, 138] and acidosis-mediated neuronal damage in the CNS [4, 9]. However, because of its adverse effect on ion channels such as ENaC and T-type Ca2+ channels as well as its action on ion-exchange systems (Na+/H+- and Na+/Ca2+-exchangers) it seems unlikely that amiloride can be used as a neuroprotective agent in the future.
A-317567 – a small molecule vaguely reminiscent of the amiloride structure is another nonselective blocker of ASICs. In DRG neurons of rats, this molecule inhibits ASIC1a, ASIC2a, and ASIC3 with IC50 value of 2-30 µM [113]. In the case of ASIC3, it was shown that A-317567 blocks both the peak and sustained current components. Side effects of A-317567 versus amiloride are minimal; it does not show any diuretic or natriuretic activity, suggests its higher specificity to the ASIC. A-317567 can be considered as a perspective analgesic because it has been shown effective for inhibiting pain in rats in a model of thermal hypersensitivity at a dose of 5-10 mg/kg of the animal’s weight, which is a 10-fold lower dose than that of amiloride [113].
Another cation, Gd3+ (gadolinium), exerts an inhibitory effect on both the peak and the sustained component of the current of homomeric ASIC3 channels and is able to inhibit heteromeric ASIC2a/3 [109]. It is known that gadolinium ions block activation of neurons in response to stretching [139]. On this basis, it has been suggested that inhibition of ASIC3 and ASIC2a/3 currents by Gd3+ may be evidence of the participation of ASIC3-containing channels in the perception of mechanical stimuli [109].
Nonsteroidal antiinflammatory drugs (NSAIDs) are widely used as antiinflammatory and analgesic agents. They inhibit the synthesis of prostaglandins, which play a major role in inflammatory processes in tissues. It was shown that NSAIDs inhibit the activity of ASIC channels at concentrations relevant to their analgesic doses. Ibuprofen and flurbiprofen, for example, inhibit ASIC1a-containing channels with IC50 value of 350 µM (measured on COS line cells). Aspirin and salicylate inhibit ASIC3-containing channels with IC50 value of 260 µM (measured on culture of DRG neurons), while diclofenac inhibits ASIC3-containing channels with IC50 value of 92 µM (measured on COS line cells) [114]. In this case, aspirin, salicylate, and diclofenac suppress only the sustained component of the ASIC3 current. It is also known that NSAIDs inhibit the inflammatory-mediated increase in ASIC expression in sensory neurons [114]. The proposed mechanism of action of the NSAIDs is allosteric inhibition of channels by slowing recovery after inactivation [140].
Aminoglycosides (streptomycin, neomycin, and gentamicin) represent a group of antibiotics that have been shown to have a blocking effect on a wide range of receptors: Ca2+ channels, excitatory amino acid receptors, TRPV1 (transient-receptor-potential V1) channels [141]. In DRG neurons, streptomycin and neomycin at 30 µM concentration showed significant reversible reduction in amplitude of ASIC currents and retarding action on the desensitization process. In this case, decrease in concentration of Ca2+ in the extracellular medium enhanced the effect of streptomycin and neomycin on desensitization [115].
Diarylamidines are widely used initially to treat diseases caused by protozoa such as trypanosomiases and leishmaniases. Four representatives of the diarylamidines – 4,6-diamidine-2-phenylindole, diminazene, hydroxystilbamidine, and pentamidine – inhibit currents generated by ASIC receptors in hippocampal neurons with IC50 values of 2.8, 0.3, 1.5, and 38 µM, respectively. Diminazene also showed increased desensitization of ASIC currents in hippocampal neurons. The inhibitory effect of diminazene on ASIC currents in CHO cells decreases in the series ASIC1b > ASIC3 > ASIC2a ≥ ASIC1a [117].
2-Guanidine-4-methylquinazoline (GMQ), a small molecule containing a guanidine group and a heterocycle, activates ASIC3 at neutral pH without any effects on ASIC1a, 1b, and 2a (EC50 value measured in CHO cells is 1 mM). In this case, the activated channels have less pronounced selectivity for Na+. It was proposed that conformational changes of transmembrane domains caused by GMQ–channel interaction differ from conformational changes induced by protons only. Using site-directed mutagenesis of the channel, it has been shown that activation of the ASIC3 channel by the GMQ molecule involve residues Glu79, Glu423, Leu77, and Arg376 located in a small cavity that is located at the base of the extracellular palm domain [118, 142]. GMQ activates sensory neurons and causes pain associated with the activation of ASIC3 [118].
Sevanol (or 9,10-diisocitryl ester of epiphyllic acid), isolated from acetic extract of the Thymus armeniacus plant, is the first natural low molecular weight compound that inhibits both components of the current of the ASIC3 channel. The peak current component is completely inhibited (IC50 353 µM), whereas the inhibition of the sustained component is only 45% (IC50 of 234 ± 53 µM). At the same time, it also inhibits the conductivity of ASIC1a expressed in frog oocytes, although less efficiently. On models of the acid-induced pain and thermal hypersensitivity caused by inflammation, sevanol at doses of 1-10 mg/kg exhibited a pronounced analgesic effect [120].
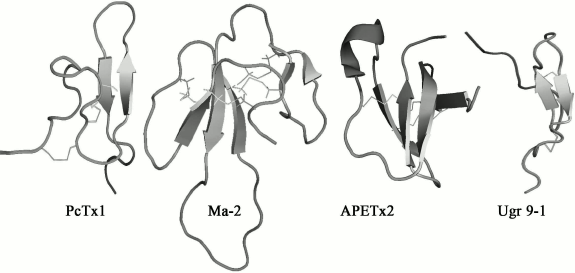
Polypeptide modulators. Natural venoms have long established themselves on equal terms with plant extracts as indispensable sources of biologically active compounds [143]. A series of polypeptides that are capable of modulating the activity of ASICs have been found in animal venoms. All currently known polypeptides are markedly different both in primary and in the spatial structure. Four spatial structures of polypeptide modulators from natural venoms with different folding of polypeptide chains are shown in Fig. 4.
Fig. 4. Spatial structure of polypeptide modulators of ASICs from spider venom – PcTx1 (PDB 2KNI), from snake venom – Ma-2 (PDB 2MFA), and from venom of sea anemone – APETx2 (PDB 1WXN) and Ugr 9-1 (PDB 2LZO).
The greatest progress has been achieved in the study of psalmotoxin 1 isolated from the spider Psalmopoeus cambridgei, which is a highly selective inhibitor of the receptor of ASIC1a [125]. PcTx1 is a small polypeptide consisting of 40 a.a., has a molecular weight of 4689.4 Da, and has pronounced basic properties (pI 10.38, nine positively charged amino acid residues). The spatial structure of PcTx1 is a compact central core stabilized by three disulfide bonds, from which N- and C-terminal loops protrude (Fig. 4). Such folding is named “inhibitory cysteine knot” [144, 145]. Most of the toxins from spider venoms acting on ionotropic receptors have this fold [146].
Natural PcTx1, as well as its synthetic analogs, have equally high-affinity inhibitory effect on homomeric ASIC1a channels in various cellular expression systems (IC50 < 1 nM). Binding occurs at pH 7.4 with the channel existing in the closed state. This process is reversible. Full blocking of the current is observed at 10 nM peptide concentration [125]. An interesting fact is that at pH values above 7.4, application of PcTx1 can lead to the activation of ASIC1a [147]. It should also be noted that PcTx1 has a potentiating effect on ASIC1b [126].
Functional study of the toxin showed that Trp24, Arg26, and Arg27 play significant roles in the activity of PcTx1 [148]. Studies of chimeric ASIC channels constructed from sensitive and insensitive to PcTx1 subunits, computer modeling, and site-directed mutagenesis of ASIC1a showed that the polypeptide is attached to the channel in the acidic pocket that is responsible for pH-dependent channel opening [148-150]. The crystal structure of the PcTx1 complex with the ASIC1a channel demonstrated that both the hydrophobic region of the toxin (which interacts with the thumb domain of the channel) and a positively charged cluster (especially Arg26 and Arg27 which form hydrogen bonds with residues in the acidic pocket) participate in the binding [151].
The mechanism of PcTx1 action involves increasing of ASIC1a affinity to H+, and thus the toxin causes the desensitization of the channel, which ultimately leads to the inability of the channel to be activated [147]. This structural-functional study of the highly specific ligand of ASIC1a defined the important role of the acidic pocket in the pH-dependent opening of the channel.
The polypeptide MitTx was found in the venom of the coral snake Micrurus tener tener. MitTx is an agonist of all functional subtypes of ASICs, activating them at neutral pH. MitTx showed different specificity towards different types of channels expressed in Xenopus oocytes, in the nanomolar concentration acting on ASIC1a and ASIC1b subtypes (EC50 = 9.4 ± 1.3 and 23 ± 3.6 nM, respectively) and at micromolar concentrations acting on ASIC2a and ASIC3 [124]. MitTx consists of two components, MitTx-α and MitTx-β, which are noncovalently associated with each other. MitTx-α is a 6-kDa protein that is structurally similar to Kunitz-type protease inhibitors, and MitTx-β is structurally similar to phospholipase A2 [124]. The entire MitTx complex is structurally similar to a toxin from a snake venom, β-bungarotoxin (PDB 1BUN), which in contrast to MitTx inhibits K+ channels.
The crystal structure of the MitTx complex with ASIC1a channel revealed that the toxin is attached to domains wrist, palm, and thumb, like a “churchkey” bottle opener, wherein the binding region of MitTx-β overlaps with that for PcTx1. Residues Phe14 and Lys16 play an important role in binding of MitTx-α. Phe14 interacts with Ala82 and Thr84 in the β1-β2-linker of one subunit and Val361 and Met364 of the other subunit. Lys16 interacts with a site on the interface of the wrist and TM1 domains [152].
Conformational changes leading to the channel opening after toxin binding occur in the bottom part of the palm domain, followed by expansion of the extracellular pore region and rearrangement in the α-helixes of the TM domains. At the same time, the region of selective filter formed by Gly443, Ala445, and Ser446 extends parallel to the membrane, breaking the helix of the TM2 domain into two parts, and the carbonyl groups of Gly443 form a ring of radius ~3.6 Å providing selectivity for the passage of Na+ ions [152].
Two more polypeptides, mambalgin-1 and mambalgin-2 (Ma-1 and Ma-2), were isolated from the venom of the snake Dendroaspis polylepis polylepis. They both consist of 57 a.a. and include eight cysteines forming four disulfide bonds. The peptides differ by one substitution in position 4, Tyr in Ma-1 or Phe in Ma-2. The mambalgin structure includes tight central area stabilized by four disulfide bonds (Cys3–Cys19, Cys12–Cys37, Cys41–Cys49, and Cys50–Cys55) and three extended loops forming two β-layers (Fig. 4) [153]. Such folding is named “three-finger toxins”, typical for a large number of well-known neurotoxins from snakes.
Mambalgins reversibly inhibit recombinant homomeric ASIC1a, heteromeric ASIC1a/2a and ASIC1a/2b, as well as ASIC1b and ASIC1a/1b channels with IC50 values of 55, 246, 61, 192, and 72 nM, respectively [128]. It was also shown that they inhibit the currents of ASIC channels in neurons of the spinal cord and hippocampus and in sensory neurons. Mambalgins exhibited analgesic effects in vivo in models of acute and inflammatory pain via inhibition of ASIC1b channels in peripheral nervous system or via inhibition of ASIC1a and ASIC1a/2a channels in CNS [128].
Mambalgins have a strong positive electrostatic potential on their surface, which can play an important role in the binding of the peptides to ASIC channels as in case of PcTx1. However, mambalgins have a different mechanism of action, i.e. they bind to the channel in the closed or inactivated state and change the channel affinity for protons [128]. Functional studies of ASIC1a mutant channel revealed the important role of residue Phe350 for the interaction with mambalgins. It should be noted that this region disposed in the vicinity from acidic pocket is considered important also for the interaction with PcTx1. Thus, the binding sites for mambalgins and PcTx1 overlap [154].
Today three polypeptides from different species of sea anemone with inhibitory activity on ASIC3 are known. Two are referred to the most numerous structural class 1, and the other to the rarest structural class 9 [155].
Polypeptide APETx2, the main component of the venom of the sea anemone Anthopleura elegantissima, contains 42 a.a., has mass of 4561.1 Da, and has basic properties (pI 9.59). This polypeptide reversibly inhibits ASIC3 channels expressed in oocytes or mammalian cells (IC50 = 63 nM), suppressing only the peak current component [127]. The spatial structure of the toxin was determined by NMR spectroscopy; it includes a compact central area with three disulfides (Cys4–Cys37, Cys6–Cys30, Cys20–Cys38) and protruding basic loop (residues 15-27) as well as N- and C-termini (Fig. 4) [156]. By spatial structure, APETx2 belongs to the defensins (β-defensin-like peptides) comprising human antimicrobial peptides as well as some of the toxins from venoms of snakes, sea anemones, and the platypus. Calculated on the basis of the peptide structure dipole moment allows to specify a basic-aromatic cluster on the surface of the toxin molecule formed by amino acid residues Phe15, Tyr16, Arg17, Arg31, and Phe33, which probably plays an important role in the interaction of APETx2 with the channel [156]. This suggestion was partially confirmed by studies showing that the replacement of Arg17 and Phe15 reduced the activity APETx2 towards the ASIC3 channel in mice by 25- and 100-fold, respectively [157, 158].
APETx2 showed a significant analgesic effect in vivo in a model of acid-induced muscle pain and in a model of peripheral inflammatory pain in rats [13, 159]. It should be noted that the effect of APETx2 is not selective, and therefore an inhibition of Na+-channels by the toxin also contributes to analgesia. APETx2 exerts an inhibitory effect on voltage-sensitive Na+-channels Nav1.8 (IC50 2.6 µM for rat DRG neurons) [160] and Nav1.2 (IC50 110 nM for human channels expressed in Xenopus oocytes) [161].
A close structural analog of APETx2 – polypeptide Hcr 1b-1 – was isolated from alcoholic extract of the sea anemone Heteractis crispa. It has a molecular weight of 4537 Da and consists of 41 a.a., including six cysteine residues forming three disulfide bonds. The peptide reversibly inhibits the peak component of the current of human ASIC3 channels with an IC50 value of 5.5 µM [129].
Strictly structurally different polypeptide Ugr 9-1 was obtained from the venom of the sea anemone Urticina grebelnyi. Its molecular weight is 3135 Da, and it is the shortest polypeptide capable of modulating the activity of ASIC. The spatial structure of Ugr 9-1 is a β-hairpin and five β-turns stabilized by two disulfide bonds, while a long N-terminus and a short C-terminus protrude from the central area (Fig. 4) [130].
Ugr 9-1 has an inhibiting effect on the peak and sustained current components of ASIC3 without affecting other types of acid-sensitive channels. This distinguishes the biological properties of Ugr 9-1 from other sea anemone toxins – APETx2 and Hcr 1b-1, which inhibit only the peak current component. The peak component is completely inhibited by addition of toxin (IC50 10 µM), and the sustained component only by 48% (IC50 1.44 µM). In vivo Ugr 9-1 at doses of 0.1-0.5 mg/kg showed a significant analgesic effect (against acid-induced pain and thermal hypersensitivity) [130].
CONCLUSION
ASIC channels are a group of proteins with extremely important regulatory and sensory function for neurons of the peripheral and central nervous system. Examples demonstrating involvement of these channels (in particular ASIC1a and ASIC3) in physiological and pathological processes are increasingly found. By a combination of biochemistry and molecular biology, as well as through structural and functional studies, it has been possible to make some progress in establishing mechanisms of these channels, especially through the study of the interaction of these receptors with their ligands. These studies not only reveal the fundamentals of the functioning of these channels but also are a strong foundation for the creation of effective drugs for the treatment of a variety of pathological conditions.
This work was supported by the Russian Foundation for Basic Research (grant Nos. 14-04-31578 and 12-04-01068), programs of the Presidium of the Russian Academy of Sciences “Molecular and Cell Biology” and “Fundamental Science for Medicine”, and the Russian President Grant for State Support of Leading Scientific Schools of the Russian Federation (NSh-1924.2014.4).
REFERENCES
1.Kellenberger, S., and Schild, L. (2002) Epithelial
sodium channel/degenerin family of ion channels: a variety of functions
for a shared structure, Physiol. Rev., 82, 735-767.
2.Alvarez de la Rosa, D., Krueger, S. R., Kolar, A.,
Shao, D., Fitzsimonds, R. M., and Canessa, C. M. (2003) Distribution,
subcellular localization and ontogeny of ASIC1 in the mammalian central
nervous system, J. Physiol., 546, 77-87.
3.Wemmie, J. A., Askwith, C. C., Lamani, E., Cassell,
M. D., Freeman, J. H., and Welsh, M. J. (2003) Acid-sensing ion channel
1 is localized in brain regions with high synaptic density and
contributes to fear conditioning, J. Neurosci., 23,
5496-5502.
4.Yermolaieva, O., Leonard, A. S., Schnizler, M. K.,
Abboud, F. M., and Welsh, M. J. (2004) Extracellular acidosis increases
neuronal cell calcium by activating acid-sensing ion channel 1a,
Proc. Natl. Acad. Sci. USA, 101, 6752-6757.
5.Sherwood, T. W., Lee, K. G., Gormley, M. G., and
Askwith, C. C. (2011) Heteromeric acid-sensing ion channels (ASICs)
composed of ASIC2b and ASIC1a display novel channel properties and
contribute to acidosis-induced neuronal death, J. Neurosci.,
31, 9723-9734.
6.Wemmie, J. A., Chen, J., Askwith, C. C.,
Hruska-Hageman, A. M., Price, M. P., Nolan, B. C., Yoder, P. G.,
Lamani, E., Hoshi, T., Freeman, J. H., et al. (2002) The acid-activated
ion channel ASIC contributes to synaptic plasticity, learning, and
memory, Neuron, 34, 463-477.
7.Gao, J., Duan, B., Wang, D.-G., Deng, X.-H., Zhang,
G.-Y., Xu, L., and Xu, T.-L. (2005) Coupling between NMDA receptor and
acid-sensing ion channel contributes to ischemic neuronal death,
Neuron, 48, 635-646.
8.Yagi, J., Wenk, H. N., Naves, L. A., and McCleskey,
E. W. (2006) Sustained currents through ASIC3 ion channels at the
modest pH changes that occur during myocardial ischemia, Circ.
Res., 99, 501-509.
9.Xiong, Z.-G., Zhu, X.-M., Chu, X.-P., Minami, M.,
Hey, J., Wei, W.-L., MacDonald, J. F., Wemmie, J. A., Price, M. P.,
Welsh, M. J., et al. (2004) Neuroprotection in ischemia: blocking
calcium-permeable acid-sensing ion channels, Cell, 118,
687-698.
10.Friese, M. A., Craner, M. J., Etzensperger, R.,
Vergo, S., Wemmie, J. A., Welsh, M. J., Vincent, A., and Fugger, L.
(2007) Acid-sensing ion channel-1 contributes to axonal degeneration in
autoimmune inflammation of the central nervous system, Nat.
Med., 13, 1483-1489.
11.Ziemann, A. E., Schnizler, M. K., Albert, G. W.,
Severson, M. A., Howard, M. A., Welsh, M. J., and Wemmie, J. A. (2008)
Seizure termination by acidosis depends on ASIC1a, Nat.
Neurosci., 11, 816-822.
12.Babinski, K., Le, K. T., and Seguela, P. (1999)
Molecular cloning and regional distribution of a human proton receptor
subunit with biphasic functional properties, J. Neurochem.,
72, 51-57.
13.Deval, E., Noel, J., Layliquid Alloui, A.,
Diochot, S., Friend, V., Jodar, M., Lazdunski, M., and Lingueglia, E.
(2008) ASIC3, a sensor of acidic and primary inflammatory pain, EMBO
J., 27, 3047-3055.
14.Deval, E., Noel, J., Gasull, X., Delaunay, A.,
Alloui, A., Friend, V., Eschalier, A., Lazdunski, M., and Lingueglia,
E. (2011) Acid-sensing ion channels in postoperative pain, J.
Neurosci., 31, 6059-6066.
15.Yen, Y.-T., Tu, P.-H., Chen, C.-J., Lin, Y.-W.,
Hsieh, S.-T., and Chen, C.-C. (2009) Role of acid-sensing ion channel 3
in sub-acute-phase inflammation, Mol. Pain, 5, 1.
16.Sluka, K. A., Winter, O. C., and Wemmie, J. A.
(2009) Acid-sensing ion channels: a new target for pain and CNS
diseases, Curr. Opin. Drug Discov. Devel., 12,
693-704.
17.Jones, R. C. W., Xu, L., and Gebhart, G. F.
(2005) The mechanosensitivity of mouse colon afferent fibers and their
sensitization by inflammatory mediators require transient receptor
potential vanilloid 1 and acid-sensing ion channel 3, J.
Neurosci., 25, 10981-10989.
18.Page, A. J., Brierley, S. M., Martin, C. M.,
Price, M. P., Symonds, E., Butler, R., Wemmie, J. A., and Blackshaw, L.
A. (2005) Different contributions of ASIC channels 1a, 2, and 3 in
gastrointestinal mechanosensory function, Gut, 54,
1408-1415.
19.Fromy, B., Lingueglia, E., Sigaudo-Roussel, D.,
Saumet, J. L., and Lazdunski, M. (2012) Asic3 is a neuronal
mechanosensor for pressure-induced vasodilation that protects against
pressure ulcers, Nat. Med., 18, 1205-1207.
20.Wultsch, T., Painsipp, E., Shahbazian, A.,
Mitrovic, M., Edelsbrunner, M., Lazdunski, M., Waldmann, R., and
Holzer, P. (2008) Deletion of the acid-sensing ion channel ASIC3
prevents gastritis-induced acid hyperresponsiveness of the
stomach-brainstem axis, Pain, 134, 245-253.
21.Krishtal, O. A., and Pidoplichko, V. I. (1980) A
receptor for protons in the nerve cell membrane, Neuroscience,
5, 2325-2327.
22.Waldmann, R., Champigny, G., Bassilana, F.,
Heurteaux, C., and Lazdunski, M. (1997) A proton-gated cation channel
involved in acid-sensing, Nature, 386, 173-177.
23.Wemmie, J. A., Price, M. P., and Welsh, M. J.
(2006) Acid-sensing ion channels: advances, questions and therapeutic
opportunities, Trends Neurosci., 29, 578-586.
24.Coric, T., Zheng, D., Gerstein, M., and Canessa,
C. M. (2005) Proton sensitivity of ASIC1 appeared with the rise of
fishes by changes of residues in the region that follows TM1 in the
ectodomain of the channel, J. Physiol., 568, 725-735.
25.Paukert, M., Sidi, S., Russell, C., Siba, M.,
Wilson, S. W., Nicolson, T., and Grunder, S. (2004) A family of
acid-sensing ion channels from the zebrafish: widespread expression in
the central nervous system suggests a conserved role in neuronal
communication, J. Biol. Chem., 279, 18783-18791.
26.Jasti, J., Furukawa, H., Gonzales, E. B., and
Gouaux, E. (2007) Structure of acid-sensing ion channel 1 at 1.9 Å
resolution and low pH, Nature, 449, 316-323.
27.Gonzales, E. B., Kawate, T., and Gouaux, E.
(2009) Pore architecture and ion sites in acid sensing ion channels and
P2X receptors, Nature, 460, 599-604.
28.Benson, C. J., Xie, J., Wemmie, J. A., Price, M.
P., Henss, J. M., Welsh, M. J., and Snyder, P. M. (2002)
Heteromultimers of DEG/ENaC subunits form H+-gated channels
in mouse sensory neurons, Proc. Natl. Acad. Sci. USA, 99,
2338-2343.
29.Askwith, C. C., Wemmie, J. A., Price, M. P.,
Rokhlina, T., and Welsh, M. J. (2004) Acid-sensing ion channel 2
(ASIC2) modulates ASIC1 H+-activated currents in hippocampal
neurons, J. Biol. Chem., 279, 18296-18305.
30.Saugstad, J. A., Roberts, J. A., Dong, J.,
Zeitouni, S., and Evans, R. J. (2004) Analysis of the membrane topology
of the acid-sensing ion channel 2a, J. Biol. Chem., 279,
55514-55519.
31.Coscoy, S., de Weille, J. R., Lingueglia, E., and
Lazdunski, M. (1999) The pre-transmembrane 1 domain of acid-sensing ion
channels participates in the ion pore, J. Biol. Chem.,
274, 10129-10132.
32.Li, T., Yang, Y., and Canessa, C. M. (2011)
Outlines of the pore in open and closed conformations describe the
gating mechanism of ASIC1, Nat. Commun., 2, 399.
33.Kellenberger, S., Gautschi, I., and Schild, L.
(2002) An external site controls closing of the epithelial Na+
channel ENaC, J. Physiol., 543, 413-424.
34.Paukert, M., Babini, E., Pusch, M., and Grunder,
S. (2004) Identification of the Ca2+ blocking site of
acid-sensing ion channel (ASIC) 1: implications for channel gating,
J. Gen. Physiol., 124, 383-394.
35.Benos, D. J., and Stanton, B. A. (1999)
Functional domains within the degenerin/epithelial sodium channel
(Deg/ENaC) superfamily of ion channels, J. Physiol., 520,
631-644.
36.Champigny, G., Voilley, N., Waldmann, R., and
Lazdunski, M. (1998) Mutations causing neurodegeneration in
Caenorhabditis elegans drastically alter the pH sensitivity and
inactivation of the mammalian H+-gated Na+
channel MDEG1, J. Biol. Chem., 273,
15418-15422.
37.Jing, L., Chu, X.-P., Jiang, Y.-Q., Collier, D.
M., Wang, B., Jiang, Q., Snyder, P. M., and Zha, X.-M. (2012)
N-glycosylation of acid-sensing ion channel 1a regulates its
trafficking and acidosis-induced spine remodeling, J. Neurosci.,
32, 4080-4091.
38.Yang, Y., Yu, Y., Cheng, J., Liu, Y., Liu, D.-S.,
Wang, J., Zhu, M. X., Wang, R., and Xu, T.-L. (2012) Highly conserved
salt bridge stabilizes rigid signal patch at extracellular loop
critical for surface expression of acid-sensing ion channels, J.
Biol. Chem., 287, 14443-14455.
39.Jing, L., Jiang, Y.-Q., Jiang, Q., Wang, B., Chu,
X.-P., and Zha, X.-M. (2011) The interaction between the first
transmembrane domain and the thumb of ASIC1a is critical for its
N-glycosylation and trafficking, PLoS One, 6, e26909.
40.Cushman, K. A., Marsh-Haffner, J., Adelman, J.
P., and McCleskey, E. W. (2007) A conformation change in the
extracellular domain that accompanies desensitization of acid-sensing
ion channel (ASIC) 3, J. Gen. Physiol., 129, 345-350.
41.Smith, E. S. J., Zhang, X., Cadiou, H., and
McNaughton, P. A. (2007) Proton binding sites involved in the
activation of acid-sensing ion channel ASIC2a, Neurosci. Lett.,
426, 12-17.
42.Baron, A., Schaefer, L., Lingueglia, E.,
Champigny, G., and Lazdunski, M. (2001) Zn2+ and H+
are coactivators of acid-sensing ion channels, J. Biol.
Chem., 276, 35361-35367.
43.Coric, T., Zhang, P., Todorovic, N., and Canessa,
C. M. (2003) The extracellular domain determines the kinetics of
desensitization in acid-sensitive ion channel 1, J. Biol. Chem.,
278, 45240-45247.
44.Kusama, N., Harding, A. M. S., and Benson, C. J.
(2010) Extracellular chloride modulates the desensitization kinetics of
acid-sensing ion channel 1a (ASIC1a), J. Biol. Chem.,
285, 17425-17431.
45.Paukert, M., Chen, X., Polleichtner, G.,
Schindelin, H., and Grunder, S. (2008) Candidate amino acids involved
in H+ gating of acid-sensing ion channel 1a, J. Biol.
Chem., 283, 572-581.
46.Dani, J. A. (1986) Ion-channel entrances
influence permeation. Net charge, size, shape, and binding
considerations, Biophys. J., 49, 607-618.
47.Immke, D. C., and McCleskey, E. W. (2003) Protons
open acid-sensing ion channels by catalyzing relief of Ca2+
blockade, Neuron, 37, 75-84.
48.Yang, H., Yu, Y., Li, W.-G., Yu, F., Cao, H., Xu,
T.-L., and Jiang, H. (2009) Inherent dynamics of the acid-sensing ion
channel 1 correlates with the gating mechanism, PLoS Biol.,
7, e1000151.
49.Li, T., Yang, Y., and Canessa, C. M. (2009)
Interaction of the aromatics Tyr72/Trp288 in the interface of the
extracellular and transmembrane domains is essential for proton gating
of acid-sensing ion channels, J. Biol. Chem., 284,
4689-4694.
50.Li, T., Yang, Y., and Canessa, C. M. (2010)
Asn415 in the beta11-beta12 linker decreases proton-dependent
desensitization of ASIC1, J. Biol. Chem., 285,
31285-31291.
51.Bargeton, B., and Kellenberger, S. (2010) The
contact region between three domains of the extracellular loop of
ASIC1a is critical for channel function, J. Biol. Chem.,
285, 13816-13826.
52.Springauf, A., Bresenitz, P., and Grunder, S.
(2011) The interaction between two extracellular linker regions
controls sustained opening of acid-sensing ion channel 1, J. Biol.
Chem., 286, 24374-24384.
53.Hesselager, M., Timmermann, D. B., and Ahring, P.
K. (2004) pH dependency and desensitization kinetics of heterologously
expressed combinations of acid-sensing ion channel subunits, J.
Biol. Chem., 279, 11006-11015.
54.Jiang, Q., Li, M.-H., Papasian, C. J., Branigan,
D., Xiong, Z.-G., Wang, J. Q., and Chu, X.-P. (2009) Characterization
of acid-sensing ion channels in medium spiny neurons of mouse striatum,
Neuroscience, 162, 55-66.
55.Allen, N. J., and Attwell, D. (2002) Modulation
of ASIC channels in rat cerebellar Purkinje neurons by ischemia-related
signals, J. Physiol., 543, 521-529.
56.Lilley, S., LeTissier, P., and Robbins, J. (2004)
The discovery and characterization of a proton-gated sodium current in
rat retinal ganglion cells, J. Neurosci., 24,
1013-1022.
57.Baron, A., Voilley, N., Lazdunski, M., and
Lingueglia, E. (2008) Acid sensing ion channels in dorsal spinal cord
neurons, J. Neurosci., 28, 1498-1508.
58.Chen, C. C., England, S., Akopian, A. N., and
Wood, J. N. (1998) A sensory neuron-specific, proton-gated ion channel,
Proc. Natl. Acad. Sci. USA, 95, 10240-10245.
59.Lingueglia, E., de Weille, J. R., Bassilana, F.,
Heurteaux, C., Sakai, H., Waldmann, R., and Lazdunski, M. (1997) A
modulatory subunit of acid sensing ion channels in brain and dorsal
root ganglion cells, J. Biol. Chem., 272,
29778-29783.
60.Lingueglia, E. (2007) Acid-sensing ion channels
in sensory perception, J. Biol. Chem., 282,
17325-17329.
61.Waldmann, R. (1997) Molecular cloning of a
non-inactivating proton-gated Na+ channel specific for
sensory neurons, J. Biol. Chem., 272, 20975-20978.
62.Lin, Y.-W., Min, M.-Y., Lin, C.-C., Chen, W.-N.,
Wu, W.-L., Yu, H.-M., and Chen, C.-C. (2008) Identification and
characterization of a subset of mouse sensory neurons that express
acid-sensing ion channel 3, Neuroscience, 151,
544-557.
63.Hattori, T., Chen, J., Harding, A. M. S., Price,
M. P., Lu, Y., Abboud, F. M., and Benson, C. J. (2009) ASIC2a and ASIC3
heteromultimerize to form pH-sensitive channels in mouse cardiac dorsal
root ganglia neurons, Circ. Res., 105, 279-286.
64.Immke, D. C., and McCleskey, E. W. (2001) Lactate
enhances the acid-sensing Na+ channel on ischemia-sensing
neurons, Nat. Neurosci., 4, 869-870.
65.Price, M. P., McIlwrath, S. L., Xie, J., Cheng,
C., Qiao, J., Tarr, D. E., Sluka, K. A., Brennan, T. J., Lewin, G. R.,
and Welsh, M. J. (2001) The DRASIC cation channel contributes to the
detection of cutaneous touch and acid stimuli in mice, Neuron,
32, 1071-1083.
66.Molliver, D. C., Immke, D. C., Fierro, L., Pare,
M., Rice, F. L., and McCleskey, E. W. (2005) ASIC3, an acid-sensing ion
channel, is expressed in metaboreceptive sensory neurons, Mol.
Pain, 1, 35.
67.Sluka, K. A., Radhakrishnan, R., Benson, C. J.,
Eshcol, J. O., Price, M. P., Babinski, K., Audette, K. M., Yeomans, D.
C., and Wilson, S. P. (2007) ASIC3 in muscle mediates mechanical, but
not heat, hyperalgesia associated with muscle inflammation,
Pain, 129, 102-112.
68.Ikeuchi, M., Kolker, S. J., and Sluka, K. A.
(2009) Acid-sensing ion channel 3 expression in mouse knee joint
afferents and effects of carrageenan-induced arthritis, J. Pain,
10, 336-342.
69.Salinas, M., Lazdunski, M., and Lingueglia, E.
(2009) Structural elements for the generation of sustained currents by
the acid pain sensor ASIC3, J. Biol. Chem., 284,
31851-31859.
70.Akopian, A. N., Chen, C. C., Ding, Y., Cesare,
P., and Wood, J. N. (2000) A new member of the acid-sensing ion channel
family, Neuroreport, 11, 2217-2222.
71.Babini, E., Paukert, M., Geisler, H.-S., and
Grunder, S. (2002) Alternative splicing and interaction with di- and
polyvalent cations control the dynamic range of acid-sensing ion
channel 1 (ASIC1), J. Biol. Chem., 277, 41597-41603.
72.Hruska-Hageman, A. M., Wemmie, J. A., Price, M.
P., and Welsh, M. J. (2002) Interaction of the synaptic protein PICK1
(protein interacting with C kinase 1) with the non-voltage gated sodium
channels BNC1 (brain Na+ channel 1) and ASIC (acid-sensing
ion channel), Biochem. J., 361, 443-450.
73.Zha, X., Costa, V., Harding, A. M. S., Reznikov,
L., Benson, C. J., and Welsh, M. J. (2009) ASIC2 subunits target
acid-sensing ion channels to the synapse via an association with
PSD-95, J. Neurosci., 29, 8438-8446.
74.Cho, J.-H., and Askwith, C. C. (2008) Presynaptic
release probability is increased in hippocampal neurons from ASIC1
knockout mice, J. Neurophysiol., 99, 426-441.
75.Coryell, M. W., Ziemann, A. E., Westmoreland, P.
J., Haenfler, J. M., Kurjakovic, Z., Zha, X., Price, M., Schnizler, M.
K., and Wemmie, J. A. (2007) Targeting ASIC1a reduces innate fear and
alters neuronal activity in the fear circuit, Biol. Psychiatry,
62, 1140-1148.
76.Wemmie, J. A., Coryell, M. W., Askwith, C. C.,
Lamani, E., Leonard, A. S., Sigmund, C. D., and Welsh, M. J. (2004)
Overexpression of acid-sensing ion channel 1a in transgenic mice
increases acquired fear-related behavior, Proc. Natl. Acad. Sci.
USA, 101, 3621-3626.
77.Coryell, M. W., Wunsch, A. M., Haenfler, J. M.,
Allen, J. E., McBride, J. L., Davidson, B. L., and Wemmie, J. A. (2008)
Restoring acid-sensing ion channel-1a in the amygdala of knock-out mice
rescues fear memory but not unconditioned fear responses, J.
Neurosci., 28, 13738-13741.
78.Ziemann, A. E., Allen, J. E., Dahdaleh, N. S.,
Drebot, I. I., Coryell, M. W., Wunsch, A. M., Lynch, C. M., Faraci, F.
M., Howard, M. A., Welsh, M. J., et al. (2009) The amygdala is a
chemosensor that detects carbon dioxide and acidosis to elicit fear
behavior, Cell, 139, 1012-1021.
79.Borzan, J., Zhao, C., Meyer, R. A., and Raja, S.
N. (2010) A role for acid-sensing ion channel 3, but not acid-sensing
ion channel 2, in sensing dynamic mechanical stimuli,
Anesthesiology, 113, 647-654.
80.Wetzel, C., Hu, J., Riethmacher, D.,
Benckendorff, A., Harder, L., Eilers, A., Moshourab, R., Kozlenkov, A.,
Labuz, D., Caspani, O., et al. (2007) A stomatin-domain protein
essential for touch sensation in the mouse, Nature, 445,
206-209.
81.Drew, L. J., Rohrer, D. K., Price, M. P., Blaver,
K. E., Cockayne, D. A., Cesare, P., and Wood, J. N. (2004) Acid-sensing
ion channels ASIC2 and ASIC3 do not contribute to mechanically
activated currents in mammalian sensory neurons, J. Physiol.,
556, 691-710.
82.Hildebrand, M. S., de Silva, M. G., Klockars, T.,
Rose, E., Price, M., Smith, R. J. H., McGuirt, W. T., Christopoulos,
H., Petit, C., and Dahl, H.-H. M. (2004) Characterization of DRASIC in
the mouse inner ear, Hear. Res., 190, 149-160.
83.Ettaiche, M., Deval, E., Pagnotta, S., Lazdunski,
M., and Lingueglia, E. (2009) Acid-sensing ion channel 3 in retinal
function and survival, Invest. Ophthalmol. Vis. Sci., 50,
2417-2426.
84.Barnes, C., Tibbitts, T., Sager, J., Deitzer, G.,
Bubenheim, D., Koerner, G., and Bugbee, B. (1993) Accuracy of quantum
sensors measuring yield photon flux and photosynthetic photon flux,
HortScience, 28, 1197-1200.
85.Huang, S.-J., Yang, W.-S., Lin, Y.-W., Wang,
H.-C., and Chen, C.-C. (2008) Increase of insulin sensitivity and
reversal of age-dependent glucose intolerance with inhibition of ASIC3,
Biochem. Biophys. Res. Commun., 371, 729-734.
86.Sluka, K. A., Price, M. P., Breese, N. M.,
Stucky, C. L., Wemmie, J. A., and Welsh, M. J. (2003) Chronic
hyperalgesia induced by repeated acid injections in muscle is abolished
by the loss of ASIC3, but not ASIC1, Pain, 106,
229-239.
87.Vergo, S., Craner, M. J., Etzensperger, R.,
Attfield, K., Friese, M. A., Newcombe, J., Esiri, M., and Fugger, L.
(2011) Acid-sensing ion channel 1 is involved in both axonal injury and
demyelination in multiple sclerosis and its animal model, Brain,
134, 571-584.
88.Dauer, W., and Przedborski, S. (2003)
Parkinson’s disease: mechanisms and models, Neuron,
39, 889-909.
89.Pidoplichko, V. I., and Dani, J. A. (2006)
Acid-sensitive ionic channels in midbrain dopamine neurons are
sensitive to ammonium, which may contribute to hyperammonemia damage,
Proc. Natl. Acad. Sci. USA, 103, 11376-11380.
90.Arias, R. L., Sung, M.-L. A., Vasylyev, D.,
Zhang, M.-Y., Albinson, K., Kubek, K., Kagan, N., Beyer, C., Lin, Q.,
Dwyer, J. M., et al. (2008) Amiloride is neuroprotective in an MPTP
model of Parkinson’s disease, Neurobiol. Dis., 31,
334-341.
91.Dwyer, J. M., Rizzo, S. J., Neal, S. J., Lin, Q.,
Jow, F., Arias, R. L., Rosenzweig-Lipson, S., Dunlop, J., and Beyer, C.
E. (2009) Acid sensing ion channel (ASIC) inhibitors exhibit
anxiolytic-like activity in preclinical pharmacological models,
Psychopharmacology, 203, 41-52.
92.Somjen, G. G. (1984) Acidification of
interstitial fluid in hippocampal formation caused by seizures and by
spreading depression, Brain Res., 311, 186-188.
93.Chesler, M., and Kaila, K. (1992) Modulation of
pH by neuronal activity, Trends Neurosci., 15,
396-402.
94.Ali, A., Pillai, K. P., Ahmad, F. J., Dua, Y.,
and Vohora, D. (2006) Anticonvulsant effect of amiloride in
pentetrazole-induced status epilepticus in mice, Pharmacol.
Rep., 58, 242-245.
95.N’Gouemo, P. (2008) Amiloride delays the
onset of pilocarpine-induced seizures in rats, Brain Res.,
1222, 230-232.
96.Weng, J.-Y., Lin, Y.-C., and Lien, C.-C. (2010)
Cell type-specific expression of acid-sensing ion channels in
hippocampal interneurons, J. Neurosci., 30,
6548-6558.
97.Rehncrona, S. (1985) Brain acidosis, Ann.
Emerg. Med., 14, 770-776.
98.Siesjo, B. K., Katsura, K. I., Kristian, T., Li,
P. A., and Siesjo, P. (1996) Molecular mechanisms of acidosis-mediated
damage, Acta Neurochir. Suppl., 66, 8-14.
99.Siesjo, B. K. (1988) Acidosis and ischemic brain
damage, Neurochem. Pathol., 9, 31-88.
100.Li, M., Inoue, K., Branigan, D., Kratzer, E.,
Hansen, J. C., Chen, J. W., Simon, R. P., and Xiong, Z.-G. (2010)
Acid-sensing ion channels in acidosis-induced injury of human brain
neurons, J. Cereb. Blood Flow Metab., 30, 1247-1260.
101.Pignataro, G., Simon, R. P., and Xiong, Z.-G.
(2007) Prolonged activation of ASIC1a and the time window for
neuroprotection in cerebral ischemia, Brain, 130,
151-158.
102.Mazzuca, M., Heurteaux, C., Alloui, A.,
Diochot, S., Baron, A., Voilley, N., Blondeau, N., Escoubas, P., Gelot,
A., Cupo, A., et al. (2007) A tarantula peptide against pain via ASIC1a
channels and opioid mechanisms, Nat. Neurosci., 10,
943-945.
103.Duan, B., Wu, L.-J., Yu, Y.-Q., Ding, Y., Jing,
L., Xu, L., Chen, J., and Xu, T.-L. (2007) Upregulation of acid-sensing
ion channel ASIC1a in spinal dorsal horn neurons contributes to
inflammatory pain hypersensitivity, J. Neurosci., 27,
11139-11148.
104.Sutherland, S. P., Benson, C. J., Adelman, J.
P., and McCleskey, E. W. (2001) Acid-sensing ion channel 3 matches the
acid-gated current in cardiac ischemia-sensing neurons, Proc. Natl.
Acad. Sci. USA, 98, 711-716.
105.Chu, X.-P., Wemmie, J. A., Wang, W.-Z., Zhu,
X.-M., Saugstad, J. A., Price, M. P., Simon, R. P., and Xiong, Z.-G.
(2004) Subunit-dependent high-affinity zinc inhibition of acid-sensing
ion channels, J. Neurosci., 24, 8678-8689.
106.Jiang, Q., Inoue, K., Wu, X., Papasian, C. J.,
Wang, J. Q., Xiong, Z. G., and Chu, X. P. (2011) Cysteine 149 in the
extracellular finger domain of acid-sensing ion channel 1b subunit is
critical for zinc-mediated inhibition, Neuroscience, 193,
89-99.
107.Jiang, Q., Papasian, C. J., Wang, J. Q., Xiong,
Z. G., and Chu, X. P. (2010) Inhibitory regulation of acid-sensing ion
channel 3 by zinc, Neuroscience, 169, 574-583.
108.Wang, W., Yu, Y., and Xu, T.-L. (2007)
Modulation of acid-sensing ion channels by Cu2+ in cultured
hypothalamic neurons of the rat, Neuroscience, 145,
631-641.
109.Babinski, K., Catarsi, S., Biagini, G., and
Seguela, P. (2000) Mammalian ASIC2a and ASIC3 subunits co-assemble into
heteromeric proton-gated channels sensitive to Gd3+, J.
Biol. Chem., 275, 28519-28525.
110.Ugawa, S., Ueda, T., Takahashi, E.,
Hirabayashi, Y., Yoneda, T., Komai, S., and Shimada, S. (2001) Cloning
and functional expression of ASIC-beta2, a splice variant of ASIC-beta,
Neuroreport, 12, 2865-2869.
111.De Weille, J. R., Bassilana, F., Lazdunski, M.,
and Waldmann, R. (1998) Identification, functional expression and
chromosomal localization of a sustained human proton-gated cation
channel, FEBS Lett., 433, 257-260.
112.Li, W.-G., Yu, Y., Huang, C., Cao, H., and Xu,
T.-L. (2011) Nonproton ligand sensing domain is required for
paradoxical stimulation of acid-sensing ion channel 3 (ASIC3) channels
by amiloride, J. Biol. Chem., 286, 42635-42646.
113.Dube, G. R., Lehto, S. G., Breese, N. M.,
Baker, S. J., Wang, X., Matulenko, M. A., Honore, P., Stewart, A. O.,
Moreland, R. B., and Brioni, J. D. (2005) Electrophysiological and
in vivo characterization of A-317567, a novel blocker of acid
sensing ion channels, Pain, 117, 88-96.
114.Voilley, N., de Weille, J., Mamet, J., and
Lazdunski, M. (2001) Nonsteroid anti-inflammatory drugs inhibit both
the activity and the inflammation-induced expression of acid-sensing
ion channels in nociceptors, J. Neurosci., 21,
8026-8033.
115.Garza, A., Lopez-Ramirez, O., Vega, R., and
Soto, E. (2010) The aminoglycosides modulate the acid-sensing ionic
channel currents in dorsal root ganglion neurons from the rat, J.
Pharmacol. Exp. Ther., 332, 489-499.
116.Smith, E. S., Cadiou, H., and McNaughton, P. A.
(2007) Arachidonic acid potentiates acid-sensing ion channels in rat
sensory neurons by a direct action, Neuroscience, 145,
686-698.
117.Chen, X., Qiu, L., Li, M., Durrnagel, S.,
Orser, B. A., Xiong, Z.-G., and MacDonald, J. F. (2010) Diarylamidines:
high potency inhibitors of acid-sensing ion channels,
Neuropharmacology, 58, 1045-1053.
118.Yu, Y., Chen, Z., Li, W.-G., Cao, H., Feng,
E.-G., Yu, F., Liu, H., Jiang, H., and Xu, T.-L. (2010) A nonproton
ligand sensor in the acid-sensing ion channel, Neuron,
68, 61-72.
119.Wang, X., Li, W.-G., Yu, Y., Xiao, X., Cheng,
J., Zeng, W.-Z., Peng, Z., Xi Zhu, M., and Xu, T.-L. (2013) Serotonin
facilitates peripheral pain sensitivity in a manner that depends on the
nonproton ligand sensing domain of ASIC3 channel, J. Neurosci.,
33, 4265-4279.
120.Dubinnyi, M. A., Osmakov, D. I., Koshelev, S.
G., Kozlov, S. A., Andreev, Y. A., Zakaryan, N. A., Dyachenko, I. A.,
Bondarenko, D. A., Arseniev, A. S., and Grishin, E. V. (2012) Lignan
from thyme possesses inhibitory effect on ASIC3 channel current, J.
Biol. Chem., 287, 32993-33000.
121.Askwith, C. C., Cheng, C., Ikuma, M., Benson,
C., Price, M. P., and Welsh, M. J. (2000) Neuropeptide FF and FMRFamide
potentiate acid-evoked currents from sensory neurons and proton-gated
DEG/ENaC channels, Neuron, 26, 133-141.
122.Deval, E., Baron, A., Lingueglia, E.,
Mazarguil, H., Zajac, J.-M., and Lazdunski, M. (2003) Effects of
neuropeptide SF and related peptides on acid sensing ion channel 3 and
sensory neuron excitability, Neuropharmacology, 44,
662-671.
123.Sherwood, T. W., and Askwith, C. C. (2009)
Dynorphin opioid peptides enhance acid-sensing ion channel 1a activity
and acidosis-induced neuronal death, J. Neurosci., 29,
14371-14380.
124.Bohlen, C. J., Chesler, A. T., Sharif-Naeini,
R., Medzihradszky, K. F., Zhou, S., King, D., Sanchez, E. E.,
Burlingame, A. L., Basbaum, A. I., and Julius, D. (2011) A heteromeric
Texas coral snake toxin targets acid-sensing ion channels to produce
pain, Nature, 479, 410-414.
125.Escoubas, P., De Weille, J. R., Lecoq, A.,
Diochot, S., Waldmann, R., Champigny, G., Moinier, D., Menez, F., and
Lazdunski, M. (2000) Isolation of a tarantula toxin specific for a
class of proton-gated Na+ channels, J. Biol. Chem.,
275, 25116-25121.
126.Chen, X., Kalbacher, H., and Grunder, S. (2006)
Interaction of acid-sensing ion channel (ASIC) 1 with the tarantula
toxin psalmotoxin 1 is state dependent, J. Gen. Physiol.,
127, 267-276.
127.Diochot, S., Baron, A., Rash, L. D., Deval, E.,
Escoubas, P., Scarzello, S., Salinas, M., and Lazdunski, M. (2004) A
new sea anemone peptide, APETx2, inhibits ASIC3, a major acid-sensitive
channel in sensory neurons, EMBO J., 23, 1516-1525.
128.Diochot, S., Baron, A., Salinas, M., Douguet,
D., Scarzello, S., Dabert-Gay, A.-S., Debayle, D., Friend, V., Alloui,
A., Lazdunski, M., et al. (2012) Black mamba venom peptides target
acid-sensing ion channels to abolish pain, Nature, 490,
552-555.
129.Kozlov, S. A., Osmakov, D. I., Andreev, Y. A.,
Koshelev, S. G., Gladkikh, I. N., Monastyrnaya, M. M., Kozlovskaya, E.
P., and Grishin, E. V. (2012) A sea anemone polypeptide toxin
inhibiting the ASIC3 acid-sensitive channel, Russ. J. Bioorg.
Chem., 38, 578-583.
130.Osmakov, D. I., Kozlov, S. A., Andreev, Y. A.,
Koshelev, S. G., Sanamyan, N. P., Sanamyan, K. E., Dyachenko, I. A.,
Bondarenko, D. A., Murashev, A. N., Mineev, K. S., et al. (2013) Sea
anemone peptide with uncommon β-hairpin structure inhibits
acid-sensing ion channel 3 (ASIC3) and reveals analgesic activity,
J. Biol. Chem., 288, 23116-23127.
131.Farooqui, A. A., and Horrocks, L. A. (2006)
Phospholipase A2-generated lipid mediators in the brain: the good, the
bad, and the ugly, Neuroscientist, 12, 245-260.
132.Sherwood, T. W., and Askwith, C. C. (2008)
Endogenous arginine-phenylalanine-amide-related peptides alter
steady-state desensitization of ASIC1a, J. Biol. Chem.,
283, 1818-1830.
133.Ostrovskaya, O., Moroz, L., and Krishtal, O.
(2004) Modulatory action of RFamide-related peptides on acid-sensing
ionic channels is pH dependent: the role of arginine, J.
Neurochem., 91, 252-255.
134.Wang, W.-Z., Chu, X.-P., Li, M.-H., Seeds, J.,
Simon, R. P., and Xiong, Z.-G. (2006) Modulation of acid-sensing ion
channel currents, acid-induced increase of intracellular
Ca2+, and acidosis-mediated neuronal injury by intracellular
pH, J. Biol. Chem., 281, 29369-29378.
135.Zhang, P., Sigworth, F. J., and Canessa, C. M.
(2006) Gating of acid-sensitive ion channel-1: release of Ca2+
block vs. allosteric mechanism, J. Gen. Physiol.,
127, 109-117.
136.Duan, B., Wang, Y.-Z., Yang, T., Chu, X.-P.,
Yu, Y., Huang, Y., Cao, H., Hansen, J., Simon, R. P., Zhu, M. X., et
al. (2011) Extracellular spermine exacerbates ischemic neuronal injury
through sensitization of ASIC1a channels to extracellular acidosis,
J. Neurosci., 31, 2101-2112.
137.Adams, C. M., Snyder, P. M., and Welsh, M. J.
(1999) Paradoxical stimulation of a DEG/ENaC channel by amiloride,
J. Biol. Chem., 274, 15500-15504.
138.Ugawa, S., Ueda, T., Ishida, Y., Nishigaki, M.,
Shibata, Y., and Shimada, S. (2002) Amiloride-blockable acid-sensing
ion channels are leading acid sensors expressed in human nociceptors,
J. Clin. Invest., 110, 1185-1190.
139.Hamill, O. P., and McBride, D. W. (1996) The
pharmacology of mechanogated membrane ion channels, Pharmacol.
Rev., 48, 231-252.
140.Dorofeeva, N. A., Barygin, O. I., Staruschenko,
A., Bolshakov, K. V., and Magazanik, L. G. (2008) Mechanisms of
non-steroid anti-inflammatory drugs action on ASICs expressed in
hippocampal interneurons, J. Neurochem., 106,
429-441.
141.Raisinghani, M., and Premkumar, L. S. (2005)
Block of native and cloned vanilloid receptor 1 (TRPV1) by
aminoglycoside antibiotics, Pain, 113, 123-133.
142.Yu, Y., Li, W.-G., Chen, Z., Cao, H., Yang, H.,
Jiang, H., and Xu, T.-L. (2011) Atomic level characterization of the
nonproton ligand-sensing domain of ASIC3 channels, J. Biol.
Chem., 286, 24996-25006.
143.Andreev, Y. A., Kozlov, S. A., Korolkova,
Y. V., Dyachenko, I. A., Bondarenko, D. A., Skobtsov, D. I.,
Murashev, A. N., Kotova, P. D., Rogachevskaja, O. A., Kabanova,
N. V., et al. (2013) Polypeptide modulators of TRPV1 produce
analgesia without hyperthermia, Mar. Drugs, 11,
5100-5115.
144.Norton, R. S., and Pallaghy, P. K. (1998) The
cystine knot structure of ion channel toxins and related polypeptides,
Toxicon, 36, 1573-1583.
145.Swartz, K. J., and MacKinnon, R. (1995) An
inhibitor of the Kv2.1 potassium channel isolated from the venom of a
Chilean tarantula, Neuron, 15, 941-949.
146.Vassilevski, A. A., Kozlov, S. A., and Grishin,
E. V. (2009) Molecular diversity of spider venom, Biochemistry
(Moscow), 74, 1505-1534.
147.Chen, X., Kalbacher, H., and Grunder, S. (2005)
The tarantula toxin psalmotoxin 1 inhibits acid-sensing ion channel
(ASIC) 1a by increasing its apparent H+ affinity, J. Gen.
Physiol., 126, 71-79.
148.Saez, N. J., Mobli, M., Bieri, M., Chassagnon,
I. R., Malde, A. K., Gamsjaeger, R., Mark, A. E., Gooley, P. R., Rash,
L. D., and King, G. F. (2011) A dynamic pharmacophore drives the
interaction between psalmotoxin-1 and the putative drug target
acid-sensing ion channel 1a, Mol. Pharmacol., 80,
796-808.
149.Qadri, Y. J., Berdiev, B. K., Song, Y.,
Lippton, H. L., Fuller, C. M., and Benos, D. J. (2009) Psalmotoxin-1
docking to human acid-sensing ion channel-1, J. Biol. Chem.,
284, 17625-17633.
150.Sherwood, T., Franke, R., Conneely, S., Joyner,
J., Arumugan, P., and Askwith, C. (2009) Identification of protein
domains that control proton and calcium sensitivity of ASIC1a, J.
Biol. Chem., 284, 27899-27907.
151.Baconguis, I., and Gouaux, E. (2012) Structural
plasticity and dynamic selectivity of acid-sensing ion channel-spider
toxin complexes, Nature, 489, 400-405.
152.Baconguis, I., Bohlen, C. J., Goehring, A.,
Julius, D., and Gouaux, E. (2014) X-Ray structure of acid-sensing ion
channel 1-snake toxin complex reveals open state of a
Na+-selective channel, Cell, 156, 717-729.
153.Kini, R. M., and Doley, R. (2010) Structure,
function and evolution of three-finger toxins: mini proteins with
multiple targets, Toxicon, 56, 855-867.
154.Schroeder, C. I., Rash, L. D., Vila-Farres, X.,
Rosengren, K. J., Mobli, M., King, G. F., Alewood, P. F., Craik, D. J.,
and Durek, T. (2014) Chemical synthesis, 3D structure, and ASIC binding
site of the toxin mambalgin-2, Angew. Chem. Int. Ed. Engl.,
53, 1017-1020.
155.Kozlov, S., and Grishin, E. (2012) Convenient
nomenclature of cysteine-rich polypeptide toxins from sea anemones,
Peptides, 33, 240-244.
156.Chagot, B., Escoubas, P., Diochot, S., Bernard,
C., Lazdunski, M., and Darbon, H. (2005) Solution structure of APETx2,
a specific peptide inhibitor of ASIC3 proton-gated channels, Protein
Sci., 14, 2003-2010.
157.Anangi, R., Chen, C.-C., Lin, Y.-W., Cheng,
Y.-R., Cheng, C.-H., Chen, Y.-C., Chu, Y.-P., and Chuang, W.-J. (2010)
Expression in Pichia pastoris and characterization of APETx2, a
specific inhibitor of acid sensing ion channel 3, Toxicon,
56, 1388-1397.
158.Anangi, R., Rash, L. D., Mobli, M., and King,
G. F. (2012) Functional expression in Escherichia coli of the
disulfide-rich sea anemone peptide APETx2, a potent blocker of
acid-sensing ion channel 3, Mar. Drugs, 10,
1605-1618.
159.Karczewski, J., Spencer, R. H., Garsky, V. M.,
Liang, A., Leitl, M. D., Cato, M. J., Cook, S. P., Kane, S., and Urban,
M. O. (2010) Reversal of acid-induced and inflammatory pain by the
selective ASIC3 inhibitor, APETx2, Br. J. Pharmacol.,
161, 950-960.
160.Blanchard, M. G., Rash, L. D., and
Kellenberger, S. (2012) Inhibition of voltage-gated Na+
currents in sensory neurons by the sea anemone toxin APETx2,
Br. J. Pharmacol., 165, 2167-2177.
161.Peigneur, S., Beress, L., Moller, C., Mari, F.,
Forssmann, W.-G., and Tytgat, J. (2012) A natural point mutation
changes both target selectivity and mechanism of action of sea anemone
toxins, FASEB J., 26, 5141-5151.