Effect of Cationic Plastoquinone SkQ1 on Electron Transfer Reactions in Chloroplasts and Mitochondria from Pea Seedlings
V. D. Samuilov* and D. B. Kiselevsky
Lomonosov Moscow State University, Faculty of Biology, 119991 Moscow, Russia; fax: +7 (495) 939-3807; E-mail: vdsamuilov@mail.ru* To whom correspondence should be addressed.
Received August 14, 2014; Revision received December 4, 2014
Plastoquinone bound with decyltriphenylphosphonium cation (SkQ1) penetrating through the membrane in nanomolar concentrations inhibited H2O2 generation in cells of epidermis of pea seedling leaves that was detected by the fluorescence of 2′,7′-dichlorofluorescein. Photosynthetic electron transfer in chloroplasts isolated from pea leaves is suppressed by SkQ1 at micromolar concentrations: the electron transfer in chloroplasts under the action of photosystem II or I (with silicomolybdate or methyl viologen as electron acceptors, respectively) is more sensitive to SkQ1 than under the action of photosystem II + I (with ferricyanide or p-benzoquinone as electron acceptors). SkQ1 reduced by borohydride is oxidized by ferricyanide, p-benzoquinone, and, to a lesser extent, by silicomolybdate, but not by methyl viologen. SkQ1 is not effective as an electron acceptor supporting O2 evolution from water in illuminated chloroplasts. The data on suppression of photosynthetic O2 evolution or consumption show that SkQ1, similarly to phenazine methosulfate, causes conversion of the chloroplast redox-chain from non-cyclic electron transfer mode to the cyclic mode without O2 evolution. Oxidation of NADH or succinate in mitochondria isolated from pea roots is stimulated by SkQ1.
KEY WORDS: programmed cell death, mitochondria-targeted quinones, SkQ1, electron transfer, retardation in chloroplasts, stimulation in mitochondriaDOI: 10.1134/S0006297915040045
Abbreviations: Asc, ascorbate; BQ, p-benzoquinone; DCF, 2′,7′-dichlorofluorescein; DCMU, 3-(3,4-dichlorophenyl)-1,1-dimethylurea; DTPP+, decyltriphenylphosphonium cation; EC, epidermal cells; FeCy, ferricyanide; GC, guard cells; MitoQ, 10-(6′-ubiquinonyl)decyltriphenylphosphonium; MV, methyl viologen; PCD, programmed cell death; PMS, phenazine methosulfate; PS I(II), photosystem I(II); ROS, reactive oxygen species; SH, salicylhydroxamate; SiMo, silicomolybdate; SkQ1, 10-(6′-plastoquinonyl)decyltriphenylphosphonium; SkQ3, 10-(6′-methylplastoquinonyl)decyltriphenylphosphonium; SkQR1, 10-(plastoquinonyl)decylrhodamine; TMPD, N,N,N′,N′-tetramethyl-p-phenylenediamine; Δψ, electric potential transmembrane difference.
Programmed cell death (PCD) is a physiological process of cell
autodestruction. Mitochondria act in PCD as providers of reactive
oxygen species (ROS) and of some apoptogenic factors including
cytochrome c and the flavoprotein AIF [1].
In plants, chloroplasts providing ROS and (presumably) activating a
specific protein kinase regulated by the redox state of quinones are
involved in PCD [2, 3].
Mitochondria generate the membrane potential (Δψ) with “minus” sign in the matrix and accumulate cations penetrating through the membranes, in particular, methyl triphenylphosphonium cations [4]. Mitochondria selectively absorb MitoQ – ubiquinone covalently bound with the penetrating decyltriphenylphosphonium cation (DTPP+) [5]. During the interaction with the mitochondrial respiratory chain, MitoQ manifests itself as an effective antioxidant preventing peroxidation of membrane lipids and having antiapoptotic action [5-7].
Some new antioxidants have been synthesized consisting of plastoquinone, a penetrating cation, and a decane or pentane linker [8]: 10-(6′-plastoquinonyl)-DTPP+ (SkQ1), 10-(plastoquinonyl)decylrhodamine 19 (SkQR1), and 10-(6′-methylplastoquinonyl)-DTPP+ (SkQ3). These compounds display antioxidant features at pico- and nanomolar concentrations, but at higher (micromolar) concentrations act as prooxidants. The antioxidant activity changes in the series as follows: SkQ1 = SkQR1 > SkQ3 > MitoQ. Cationic quinones are reduced by complexes I and II of the mitochondrial respiratory chain, i.e. they are regenerable antioxidants of reiterative action [8].
Cyanide induces death of cells in epidermis of plant leaves that is recorded by destruction of the cellular nuclei. The epidermis is a monolayer of guard cells (GC) of stomata containing mitochondria and chloroplasts and epidermis basal cells (epidermal cells (EC)) containing only mitochondria. Destruction of the nuclei of GC and EC caused by CN– as a PCD inducer is prevented by cationic quinones [9]. Electron transfer in illuminated thylakoids of chloroplasts generates in them Δψ with positive sign inside; therefore, quinones bound with the penetrating cations will not be accumulated in the energized chloroplasts but will be ejected from them. In fact, the protective effect of DTPP+-derivatives of quinones is absent under conditions of illumination [9].
Will cationic quinones act on chloroplasts? The aim of the present work was to test the effect of plastoquinone covalently bound with DTPP+ (SkQ1) on electron transfer in chloroplasts from leaves and in mitochondria from roots of pea seedlings.
MATERIALS AND METHODS
Pea (Pisum sativum L. cv. Alpha) seedlings were grown for 7-15 days under conditions of periodic illumination with light of a DRiZ (250 W) metal-halogen lamp with the intensity of ~100 mE×m–2×s–1 (light for 16 h, darkness for 8 h) at 20-24°C.
Chloroplasts were isolated as described earlier [10] by triturating leaves of pea seedlings in a ceramic mortar in medium containing 50 mM Tricine-KOH, 35 mM NaCl, and 0.4 M sucrose (pH 7.8), washed, and suspended in the same medium. Mitochondria were isolated from roots of pea seedlings as described in [11] by triturating in a mortar, then washed and kept similarly to chloroplasts in the same medium. The chloroplasts and mitochondria were kept at 4°C and used within 3-4 h after the isolation. The chlorophyll content in the chloroplasts was determined by Arnon’s method [12]; the protein content in the mitochondria was determined using bicinchoninic acid and copper sulfate [13].
The generation of ROS in cells of pea seedlings leaves was determined by fluorescence of 2′,7′-dichlorofluorescein (DCF). The DCF fluorescence was excited by a laser beam at 488 nm and recorded at 500-530 nm with an Axiovert 200M microscope equipped with an LSM 510 Meta confocal device (Carl Zeiss, Germany).
The light-dependent evolution of O2 by chloroplasts and consumption of O2 by pea mitochondria were measured by polarography with a closed platinum electrode. The chloroplasts were illuminated with focused light of a halogen lamp (250 W) with intensity of ~11.4 mE×m–2×s–1.
RESULTS AND DISCUSSION
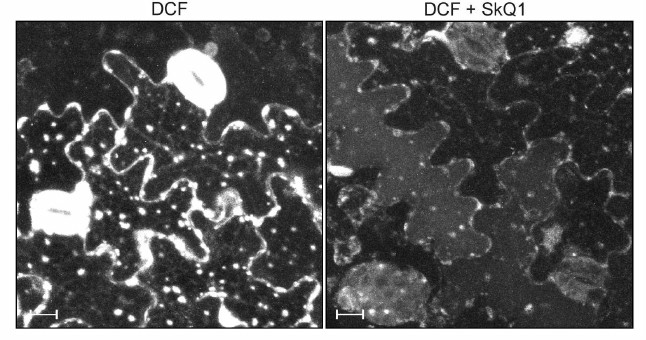
Figure 1 shows the generation of H2O2 in GC and EC of pea leaves recorded by fluorescence of DCF. Nonfluorescent 2′,7′-dichlorofluorescin (DCFH) is oxidized in cells to fluorescent DCF under the influence of H2O2 enzymatically (with involvement of peroxidase) or non-enzymatically (in the presence of H2O2 + Fe2+) [14]. DCFH is also oxidized by OH·, CO2–·, slower by NO2·, but not by O2–· [15]. In GC, the fluorescence of DCF is mainly observed in chloroplasts, whereas in EC the DCF fluorescence is mainly recorded in spherical structures, which are mitochondria [16], and along plasma membranes, which contain NADPH oxidase generating ROS [17]. SkQ1 quenches the DCF fluorescence in GC and EC (Fig. 1) preventing the generation of H2O2.
Fig. 1. Effect of SkQ1 on DCF fluorescence in GC and EC of pea leaves. The leaf pieces were supplemented with 100 nM SkQ1, incubated for 1 h, and stained with 20 µM 2′,7′-dichlorofluorescin diacetate for 20 min; the DCF fluorescence was detected at the edge of the leaf pieces. The images present a summarized picture (the maximal projection) of the DCF fluorescence from 20 optical slices with the distance of 1 µm between them. The scale rule represents 10 µm. The DCF fluorescence was excited by light with wavelength 488 nm and recorded at 500-530 nm.
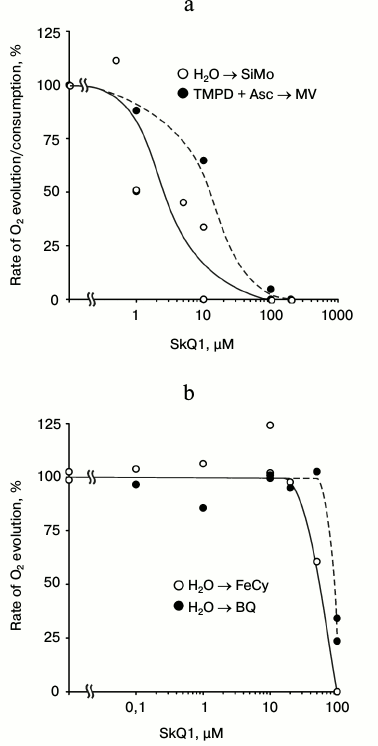
The SkQ1 effect on the non-cyclic electron transfer was studied in different regions of the photosynthetic redox chain. The evolution of O2 by chloroplasts with silicomolybdate (SiMo: H4SiO4×12 MoO3×H2O) as an electron acceptor is realized by photosystem II (PS II) and is resistant to 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU), which inhibits electron transfer at the level of the secondary plastoquinone QB of PS II: SiMo displaces DCMU from its binding site and is reduced by the primary plastoquinone QA of PS II [18]. Photosystem I (PS I) was taken into action using an electron-donor pair of ascorbate (Asc) and N,N,N′,N′-tetramethyl-p-phenylenediamine (TMPD) interacting with the b6f-cytochrome complex, plastocyanin, and PS I reaction center complex. As an electron acceptor, we used methyl viologen (MV), which is mainly reduced by the FeS center of FB [19] of the PS I redox chain and is spontaneously oxidized by O2. As a result, the electron transfer from the TMPD + ascorbate pair to methyl viologen and further to O2 leads to consumption of O2 by illuminated chloroplasts. SkQ1 suppresses the non-cyclic electron transfer in both PS II and PS I (Fig. 2a); the half-maximal concentration (I50) for SkQ1 is about 2-3 and 10-20 µM, respectively.
Fig. 2. Effect of SkQ1: a) on evolution of O2 during electron transfer from H2O to silicomolybdate (SiMo) and on the consumption of O2 during electron transfer from ascorbate (Asc) + N,N,N′,N′-tetramethyl-p-phenylenediamine (TMPD) to methyl viologen (MV); b) on evolution of O2 during electron transfer from H2O to ferricyanide (FeCy) or p-benzoquinone (BQ) in illuminated chloroplasts from pea leaves. Before measurements, chloroplasts were preincubated with SkQ1 at concentrations indicated in the figure for 1-2 h. The chloroplasts containing chlorophyll (10-20 µg/ml) were introduced into an oximetric cell (1.5 ml). The chloroplasts were incubated in medium containing 50 mM Tricine-KOH, 35 mM NaCl, and 0.4 M sucrose (pH 7.8). Additions: 0.1 mM SiMo, 0.1 mM TMPD, 1 mM Asc, 1 mM MV, 2 mM FeCy, 0.1 mM BQ. 100% rates of O2 evolution with SiMo, FeCy, and BQ were 35-50 and those of O2 consumption with MV were 200-350 µmol O2/h per mg chlorophyll.
The photosynthetic evolution of O2 by chloroplasts with ferricyanide (FeCy) or with p-benzoquinone (BQ) is resistant to SkQ1 up to concentrations of 20 and 50 µM, respectively. Further increase in SkQ1 concentration suppresses the evolution of O2 with I50 of about 50-60 and 90-100 µM, respectively (Fig. 2b). The differences in the electron transfer sensitivity to SkQ1 in the presence of electron acceptor pairs seem to be associated not only with their redox properties.
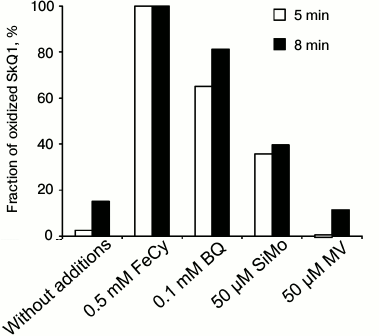
Thus, SkQ1 reduced with borohydride NaBH4 is oxidized by ferricyanide, p-benzoquinone, to the lesser degree by SiMo, but not by methyl viologen (Fig. 3). The lower rate of oxidation by silicomolybdate can be caused by its structural features. The variant with methyl viologen is similar to the spontaneous aerobic oxidation of SkQ1.
Fig. 3. Oxidation of SkQ1 (reduced with NaBH4) by various electron acceptors. The SkQ1 oxidation was recorded by the difference between the optical densities of its oxidized and reduced forms at 270 nm (D270). A solution of 50 µM SkQ1 in water was supplemented with 0.2 mM NaBH4, and the D270 difference was detected before and 1 min after the addition of NaBH4. The electron acceptors were added 1.5-2 min after the addition of NaBH4. The fraction of oxidized SkQ1 was measured after 5 or 8 min. The contribution of NaBH4 and the electron acceptors to D270 was also taken into account.
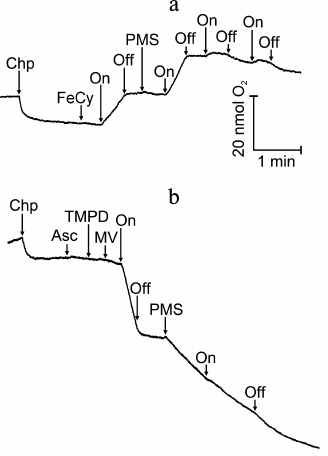
SkQ1, as an electron acceptor, competing with SiMo and methyl viologen, inhibits non-cyclic electron transfer and seems to shift it to the cyclic pathway, being reduced by plastoquinones QB, QP, QZ, and QC of the P680 complex and of b6f cytochrome complex, by phylloquinone and FeS-centers of the P700 complex, and being oxidized by (Mn)4 and Tyr components of PS II reaction center complexes, by b6f-complex, by plastocyanin, and even by the reaction center P700 (Fig. 4). Certainly, such an explanation of the redox interaction of SkQ1 with components of the electron transfer chain of chloroplasts is not without alternatives, and so further studies are required. The E0′ value of SkQ1 measured under standard conditions cannot be used here because it will depend on the interaction with components of the membranes: as Fig. 4 shows, the same plastoquinone functions as QA, QB, QP, QZ, and QC. On being oxidized by ferricyanide or p-benzoquinone, SkQ1 inhibits the photosynthetic evolution of O2 only at high concentrations switching the non-cyclic electron transfer to the cyclic mode. Phenazine methosulfate (PMS), which is an effective redox mediator of cyclic electron transfer [20] on reduction by ascorbate, itself causes the absorption of O2 and similarly to SkQ1 suppresses both the evolution and consumption of O2 by illuminated chloroplasts (Fig. 5).
Fig. 4. Non-cyclic and cyclic electron transfer chains in chloroplasts. (Mn)4, H2O dehydrogenase containing four Mn atoms; Tyr, tyrosine 161 of subunit D1 of PS II reaction center complex P680; QA and QB, primary and secondary plastoquinones of complex P680; QP, plastoquinone of the membrane pool in chloroplasts; QZ and QC, plastoquinones of the b6f-cytochrome complex (b6f); PC, plastocyanin; P700, PS I reaction center complex that includes phylloquinone PhQ and FeS-centers FX, FA, and FB; Fd, ferredoxin; FNR, ferredoxin:NADP+ reductase. The arrows indicate stages of electron transfer.
Fig. 5. Effect of phenazine methosulfate (PMS): a) on O2 evolution during electron transfer from H2O to ferricyanide (FeCy); b) on O2 consumption during electron transfer from ascorbate (Asc) + N,N,N′,N′-tetramethyl-p-phenylenediamine (TMPD) to methyl viologen (MV). For incubation conditions of the chloroplasts, see Fig. 2. Chp, chloroplasts; On and Off, switching on and off the light. Additions: 0.1 mM FeCy, 0.1 mM PMS, 0.1 mM Asc, 0.1 mM TMPD, 50 µM MV.
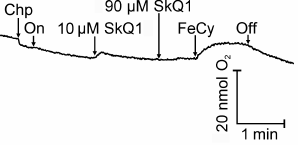
SkQ1 as an electron acceptor at concentration 10 µM supported only a slight O2 evolution that terminated on increasing the concentration to 100 µM, when the subsequent addition of ferricyanide was ineffective (Fig. 6).
Fig. 6. SkQ1 as electron acceptor in illuminated chloroplasts. For incubation conditions of chloroplasts, see Fig. 2. Chp, chloroplasts; On and Off, switching on and off the light. Additions: SkQ1, 2 mM FeCy.
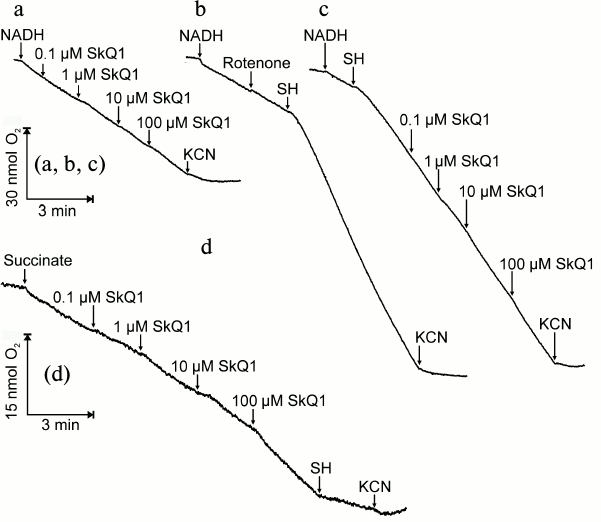
Figure 7 shows the effect of SkQ1 on the respiration of mitochondria isolated from roots of pea seedlings. SkQ1 does not influence oxidation of added NADH and only slightly stimulates it on increase in concentration. As observed earlier [21], added NADH is oxidized only through the major (cytochrome) pathway of the respiratory chain omitting the alternative oxidase; therefore, the process is fully inhibited by KCN (Fig. 7a). Rotenone, an inhibitor of respiratory chain complex I, does not influence, whereas the alternative oxidase inhibitor salicylhydroxamate (SH) does not inhibit but stimulates KCN-sensitive oxidation of added NADH (Fig. 7b), and this suggests that the cytochrome branch of the respiratory chain can be regulated by the alternative oxidase. The respiration of mitochondria with NADH as a substrate is insignificantly stimulated by SkQ1 in both presence and absence of SH (Figs. 7c and 7a, respectively). The oxidation of succinate in mitochondria is stimulated by SkQ1 up to its concentration of 100 µM, significantly inhibited by SH, and blocked by further addition of KCN (Fig. 7d). The stimulation of mitochondrial respiration by SkQ1 seems to be associated with its uncoupling effect; at micromolar concentrations, SkQ1 suppresses the generation of Δψ in rat heart mitochondria [22].
Fig. 7. Effect of SkQ1 on respiration of mitochondria from roots of pea seedlings in the presence of NADH or succinate as substrates of oxidation. The incubation medium for mitochondria (50 mM Tricine-KOH, 35 mM NaCl, and 0.4 M sucrose, pH 7.8) in oximetric cell (1.5 ml) was supplemented with mitochondria to protein concentration 0.21-0.42 mg/ml, 1 mM NADH, SkQ1, 2.5 mM KCN, 1 µM rotenone, 2 mM salicylhydroxamate (SH), and 5 mM succinate.
Findings obtained show that SkQ1 in nanomolar concentrations acts as an antioxidant suppressing production of ROS in mitochondria and chloroplasts (Fig. 1), whereas at increased concentration to 100 µM it suppresses non-cyclic electron transfer in PS II and PS I (Fig. 2). As differentiated from p-benzoquinone, SkQ1 does not support non-cyclic electron transfer with the evolution of O2 (Fig. 6), and it inhibits reactions with ferricyanide, p-benzoquinone, silicomolybdate, and methyl viologen as electron acceptors (Fig. 2). The differences in the sensitivity of these reactions to SkQ1 are caused by features of these acceptors (Fig. 3): being reduced by NaBH4, SkQ1 is oxidized by ferricyanide, p-benzoquinone, and to the lesser degree by silicomolybdate, but not by methyl viologen. The suppression of non-cyclic electron transfer seems to be associated with switching of the photosynthetic redox chain from the non-cyclic pathway into the mode of cyclic electron transfer. In fact, the effect of SkQ1 is similar to that of phenazine methosulfate, switching the redox chain of chloroplasts into the cyclic mode (Fig. 5).
Authors are grateful to Academician V. P. Skulachev and researchers of the Institute of Mitoengineering of M. V. Lomonosov Moscow State University (MSU) for the presented mitochondria-targeted quinones.
This study was supported by the Russian Foundation for Basic Research (projects 12-04-31622 mol_a, 14-04-00507 a).
Equipment of the MSU Collective Use Center was used, which was purchased with funds of the MSU Development Program with the support of the Russian Federation Ministry of Education and Science.
REFERENCES
1.Skulachev, V. P. (2006) Bioenergetic aspects of
apoptosis, necrosis and mitoptosis, Apoptosis, 11,
473-485.
2.Samuilov, V. D., Lagunova, E. M., Dzyubinskaya, E.
V., Izyumov, D. S., Kiselevsky, D. V., and Makarova, Y. V. (2002)
Involvement of chloroplasts in the death of plant cells,
Biochemistry (Moscow), 67, 627-634.
3.Samuilov, V. D., Lagunova, E. M., Kiselevsky, D.
B., Dzyubinskaya, E. V., Makarova, Y. V., and Gusev, M. V. (2003)
Participation of chloroplasts in plant apoptosis, Biosci. Rep.,
23, 103-117.
4.Liberman, E. A., and Skulachev, V. P. (1970)
Conversion of biomembrane-produced energy into electric form. IV.
General discussion, Biochim. Biophys. Acta, 216,
30-42.
5.Kelso, G. F., Porteous, C. M., Coulter, C. V.,
Hughes, G., Porteous, W. K., Ladgerwood, E. C., Smith, R. A., and
Murphy, M. P. (2001) Selective targeting of a redox-active ubiquinone
to mitochondria within cells. Antioxidant and antiapoptotic properties,
J. Biol. Chem., 276, 4588-4596.
6.Dhanasekaran, A., Kotamraju, S., Kalivendi, S. V.,
Matsunaga, T., Shang, T., Keszler, A., Joseph, J., and Kalyanaraman, B.
(2004) Supplementation of endothelial cells with mitochondria-targeted
antioxidants inhibit peroxide-induced mitochondrial iron uptake,
oxidative damage, and apoptosis, J. Biol. Chem., 279,
37575-37587.
7.James, A. M., Cocheme, H. M., Smith, R. A., and
Murphy, M. P. (2005) Interactions of mitochondria-targeted and
untargeted ubiquinones with the mitochondrial respiratory chain and
reactive oxygen species. Implications for the use of exogenous
ubiquinones as therapies and experimental tools, J. Biol. Chem.,
280, 21295-21312.
8.Skulachev, V. P. (2007) A biochemical approach to
the problem of aging: “megaproject” on membrane-penetrating
ions. The first results and prospects, Biochemistry (Moscow),
72, 1385-1399.
9.Vasil’ev, E. V., Dzyubinskaya, E. V.,
Kiselevsky, D. B., Shestak, A. A., and Samuilov, V. D. (2011)
Programmed cell death in plants: protective effect of
mitochondrial-targeted quinines, Biochemistry (Moscow),
76, 1120-1132.
10.Barsky, E. L., Gubanova, O. N., and Samuilov, V.
D. (1991) Inhibition of photosynthetic electron transfer in
chloroplasts by carbonyl cyanide m-chlorophenylhydrazone,
Biokhimiya, 56, 434-438.
11.Millenaar, F. F., Benschop, J. J., Wagner, A. M.,
and Lambers, H. (1998) The role of the alternative oxidase in
stabilizing the in vivo reduction state of the ubiquinone pool
and the activation state of the alternative oxidase, Plant
Physiol., 118, 599-607.
12.Arnon, D. I. (1949) Copper enzymes in isolated
chloroplasts. Polyphenol oxidase in Beta vulgaris, Plant
Physiol., 24, 1-15.
13.Smith, P. K., Krohn, R. I., Hermanson, G. T.,
Mallia, A. K., Gartner, F. H., Provenzano, M. D., Fujimoto, E. K.,
Goeke, N. M., Olson, B. J., and Klenk, D. C. (1985) Measurement of
protein using bicinchoninic acid, Anal. Biochem., 150,
76-85.
14.LeBel, C. P., Ischiropoulos, H., and Bondy, S. C.
(1992) Evaluation of the probe 2′,7′-dichlorofluorescin as
an indicator of reactive oxygen species formation and oxidative stress,
Chem. Res. Toxicol., 5, 227-231.
15.Wrona, M., Patel, K., and Wardman, P. (2005)
Reactivity of 2′,7′-dichlorodihydrofluorescein and
dihydrorhodamine 123 and their oxidized forms toward carbonate,
nitrogen dioxide, and hydroxyl radicals, Free Radic. Biol. Med.,
38, 262-270.
16.Vasil’ev, L. A., Dzyubinskaya, E. V.,
Zinovkin, P. A., Kiselevsky, D. B., Lobesheva, N. V., and Samuilov, V.
D. (2009) Chitosan-induced programmed cell death in plants,
Biochemistry (Moscow), 74, 1035-1044.
17.Sagi, M., and Fluhr, R. (2006) Production of
reactive oxygen species by plant NADPH oxidases, Plant Physiol.,
141, 336-340.
18.Graan, T. (1986) The interaction of
silicomolybdate with the photosystem II herbicide-binding site, FEBS
Lett., 206, 9-14.
19.Fujii, T., Yokoyama, E., Inoue, K., and Sakurai,
H. (1990) The sites of electron donation of photosystem I to methyl
viologen, Biochim. Biophys. Acta, 1015, 41-48.
20.Braun, G., Driesenaar, A. R. J., Shalgi, E., and
Malkin, S. (1992) Manipulation of the imbalance for linear electron
flow activities between photosystems I and II of photosynthesis by
cyclic electron flow cofactors, Biochim. Biophys. Acta,
1099, 57-66.
21.Moore, A. L., and Siedow, J. N. (1991) The
regulation and nature of the cyanide-resistant alternative oxidase of
plant mitochondria, Biochim. Biophys. Acta, 1059,
121-140.
22.Severina, I. I., Severin, F. F., Korshunova, G.
A., Sumbatyan, N. V., Ilyasova, T. M., Simonyan, R. A., Rogov, A. G.,
Trendeleva, T. A., Zvyagilskaya, R. A., Dugina, V. B., Domnina, L. V.,
Fetisova, E. K., Lyamzaev, K. G., Vyssokikh, M. Yu., Chernyak, B. V.,
Skulachev, M. V., Skulachev, V. P., and Sadovnichii, V. A. (2013) In
search of novel highly active mitochondria-targeted antioxidants:
thymoquinone and its cationic derivatives, FEBS Lett.,
587, 2018-2024.