Identification and Biochemical Characterization of a New Antibacterial and Antifungal Peptide Derived from the Insect Sphodromantis viridis
Hadi Zare-Zardini1,2,3, Asghar Taheri-Kafrani3, Mahtab Ordooei4*, Leila Ebrahimi5, Behnaz Tolueinia6, and Mojgan Soleimanizadeh7
1Young Researchers and Elite Club, Yazd Branch, Islamic Azad University, Yazd, Iran2Hematology and Oncology Research Center, Shahid Sadoughi University of Medical Sciences and Health Services, Yazd, Iran
3Department of Biotechnology, Faculty of Advanced Sciences and Technologies, University of Isfahan, Isfahan, Iran
4Pediatric Endocrinologist, Yazd Diabetes Research Center, Shahid Sadoughi University of Medical Sciences, Yazd, Iran; fax: +98-351-8229104; E-mail: dr.rdooei@yahoo.com; mahtab.ordooei@gmail.com
5Blood Transfusion Research Center, High Institute for Research and Education in Transfusion Medicine, Tehran, Iran
6Department of Biology, Faculty of Sciences, Iranshahr Branch, Payam Noor University of Sistan and Baluchestan, Iranshahr, Iran
7Biotechnology Department, Agriculture Faculty, Ferdowsi University of Mashhad, Mashhad, Iran
* To whom correspondence should be addressed.
Received September 19, 2014; Revision received November 26, 2014
Antimicrobial peptides are members of the immune system that protect the host from infection. In this study, a potent and structurally novel antimicrobial peptide was isolated and characterized from praying mantis Sphodromantis viridis. This 14-amino acid peptide was purified by RP-HPLC. Tandem mass spectrometry was used for sequencing this peptide, and the results showed that the peptide belongs to the Mastoparan family. The peptide was named Mastoparan-S. Mastoparan-S demonstrated that it has antimicrobial activities against a broad spectrum of microorganisms (Gram-positive and Gram-negative bacteria and fungi), and it was found to be more potent than common antibiotics such as kanamycin. Mastoparan-S showed higher antimicrobial activity against Gram-negative bacteria compared to Gram-positive ones and fungi. The minimum inhibitory concentration (MIC) values of Mastoparan-S are 15.1-28.3 µg/ml for bacterial and 19.3-24.6 µg/ml for fungal pathogens. In addition, this newly described peptide showed low hemolytic activity against human red blood cells. The in vitro cytotoxicity of Mastoparan-S was also evaluated on monolayer of normal human cells (HeLa) by MTT assay, and the results illustrated that Mastoparan-S had significant cytotoxicity at concentrations higher than 40 µg/ml and had no any cytotoxicity at the MIC (≤30 µg/ml). The findings of the present study reveal that this newly described peptide can be introduced as an appropriate candidate for treatment of topical infection.
KEY WORDS: antimicrobial peptides, cytotoxicity, Sphodromantis viridis, immune system, hemolytic activityDOI: 10.1134/S0006297915040069
Innate immunity is the most important line of defense in both vertebrates and invertebrates against pathogenic microbes such as bacteria, fungi, and viruses [1]. Due to the lack of an adaptive immune system in insects, antimicrobial peptides (AMP) play a decisive role in the struggle against pathogens, and, consequently, they are regarded as important factors in innate immunity. Insects synthesize these peptides in response to microbial infection in their fat body and in blood cells, which results in synthesis of peptides into hemolymph.
Defense peptides have broad antimicrobial activity against different microbes [2-4]. AMPs are small, typically cationic, and in many cases made of less than 100 a.a. [5-7]. Different AMPs have been purified from various animals such as amphibians, mammals, birds, reptiles, etc. [8-12]. Initially, Boman’s group purified an AMP from hemolymph of Hyalophora cecropia [13]. Scientists have described over 1.7 million of the world’s species of animals, plants and algae as of 2010, and one million species are insects [14]. Similar to other animals, insects have innate immune systems with a wide range of antimicrobial peptides [15-17], which have been identified from different types of insects [18-21]. Mantodea is an order of insects that contains 2200 species in 15 families [22, 23]. Most of the species belongs to the Mantidae family, such as praying mantis Sphodromantis viridis [24-26]. Since few studies have examined the AMP existing in this kind of animal [27], this study attempts to investigate the purification and characterization of new AMP from S. viridis.
MATERIALS AND METHODS
Extraction. The data for this study came from 100 adult S. viridis insects that were collected from different natural environmental locations in Iran. To isolate the antibacterial peptides, adult insects were kept in small cages in the laboratory during the study. The animals were frozen in liquid nitrogen and reduced to a powder in the presence of liquid nitrogen. The powder was acidified with water containing 1% trifluoroacetic acid (TFA). The acidified solution was incubated for 30 min in an ice-cold water bath under gentle shaking and centrifuged at 15,000g for 30 min, and then the supernatant was collected and lyophilized.
Peptide isolation. To purify peptides, lyophilized extract was dissolved in the smallest possible volume of distilled water and was purified using a C18 semi-preparative reverse-phase high-performance liquid chromatography (RP-HPLC) column. The injected amount of sample was 400 µl each time. Elution was performed using solution A (0.1% TFA in water) combined with a 5% to 65% gradient of solution B (0.098% TFA in acetonitrile) for 70 min at a flow rate of 1 ml/min. According to the absorbance at 220 nm, the fractions were collected and lyophilized. To evaluate the sample purity, a small amount of each peak was checked on an analytical C18 column using RP-HPLC. Each fraction was tested for antimicrobial activity. All fractions were concentrated and lyophilized. Each fraction was assayed to find the major fraction containing antimicrobial peptides as indicated in the section “Hemolytic and cell viability assay”. For further purification, the peak containing antimicrobial activity was purified using the method described above except that the elution was conducted using a 0.5% per minute increasing gradient of solution B.
Antimicrobial assay. The initial antimicrobial activity was determined by an ultrasensitive assay described in our previous studies [28, 29]. The microbes were grown in 3% (w/v) Trypticase soy broth (TSB) at 37°C overnight. The cell concentrations were estimated by measuring the optical density at 620 nm, and then the cells were added to 6 ml of under-layer agar broth (10 mM sodium phosphate, 1% (v/v) TSB, 1% agarose, pH 6.5). Then the agar was poured into a Petri dish. Samples were added directly to 3-mm-diameter wells that were made on the solidified under-layer agar. After incubation for 3 h at 37°C, the under-layer agar was covered with a nutrient-rich agar overlay and incubated overnight at 37°C. The antimicrobial activities were assayed by observing the suppression of bacterial growth around the 3-mm-diameter wells.
For antifungal activity, 1 ml of fungal suspension was inoculated into 20 ml of potato dextrose agar (PDA). Then it was poured into germ culture plates. Holes were then created by a punch into the medium, and they were filled with the plant extract. The plates were incubated for 7 days at 30-35°C, and the results were recorded during this period.
For quantifying antimicrobial activity, Minimal Inhibitory Concentrations (MIC) was determined through the following process: first, stock serial dilutions of 0.1 to 1 mg/ml of the extract were prepared. Then, 20 µl of the peptide stocks were added to a solution containing 106 CFU/ml of bacteria. Then it was poured into a plate. The ultimate concentrations were 10, 20, 40, 80, and 100 µg/ml of the peptides. The microplate was incubated at 37°C for 18 h. Then the absorbance of each well was read at 630 nm using an enzyme-linked immunosorbent assay (ELISA) reader. The results were compared to the control samples. The MIC was defined as the lowest concentration that the bacterial growth was completely inhibited.
To determine the fungi-associated MIC, 180 µl of Sabouraud Dextrose Agar culture medium along with 10 µl of fungal suspension (106 CFU/ml) as well as 10 µl of serial concentration of the peptide were poured into microplates. Then they were incubated at 37°C for 24 h. The MIC was similarly defined as minimum concentration at which no growth was observed. Escherichia coli PTCC2433, Klebsiella pneumonia PTCC4231, Pseudomonas aeruginosa PTCC2834, Bacillus subtilis PTCC4533, Leuconostoc mesenteroides PTCC1445, Bacillus cereus PTCC1435, Aspergillus niger, Aspergillus fumigates, and Candida albicans were used for MIC determination.
Hemolytic assay. To determine the hemolytic activity of a peptide, fresh human erythrocytes were incubated with AMP. The hemolytic assay was then carried out according to methods reported in our previous study [28].
Assay for cell viability. The MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide)-based assay was used to determine peptide cytotoxicity against normal human cervical cells (HeLa). In this way, prior to drug exposure, cells were cultured into 96-well plates for 24 h. Then the culture medium was replaced with fresh medium containing the drug at concentrations ranging from 10 to 100 µg/ml, e.g. in the range of antimicrobial activity assay. These plates were incubated for 30 min, 3 h, or 6 h before adding MTT solution (5 µg/ml in PBS) to each well. The wells containing medium without drug were used as controls in MTT assay. The tetrazolium/formazan reaction was allowed to proceed for 4 h at 37°C, the supernatant was removed, and 200 µl of a DMSO-containing solution was added to dissolve the formazan crystals. After an overnight incubation at 37°C, the optical density at 540 nm was measured with a 96-well multiscanning auto-reader by resorting to solubilization as blank. To translate the OD540 values into the number of live cells in each well, the OD540 values were compared with those of standard OD540-versus-cell number curves generated for each cell line. The percentage of cell survival was expressed as live cell number in the test group [30].
Mass spectrometry and sequencing. The molecular mass determination and de novo sequencing of isolated peptides were performed using a MALDI-TOF/TOF instrument. Purified and lyophilized peptide was reconstituted through 10 µl of 0.1% TFA (v/v). A 1-µl aliquot of each peptide solution was directly applied to a ground steel MALDI target plate, immediately followed by an equal volume of a freshly prepared 5 mg/ml solution of 4-hydroxy-α-cyano-cinnamic acid (Sigma, USA) in 50% aqueous (v/v) acetonitrile containing 0.1% TFA (v/v). Bruker flex analysis software (v.3.3) was used to perform the spectral processing and peak list generation for both the MS and the MS/MS spectra. De novo sequencing was performed by hand, allowing for a maximum mass error of 0.5 Da for any given fragmentation ion. Deduced b- and y-ion series were overlaid onto their fragmentation spectra by applying the Bruker flex analysis software (v.3.3).
Sequence and structure analysis. The search for similar sequences was performed onto the antimicrobial peptide database (http://aps.unmc.edu/AP/main.php). The CLC main workbench v.5.5 software was used to discover identity and similarity of peptides. Evolutionary relationship prediction between similar peptides was performed using the same software by the neighbor joining method. The phylogenetic tree was created to estimate the reproducibility of the tree topology.
RESULTS
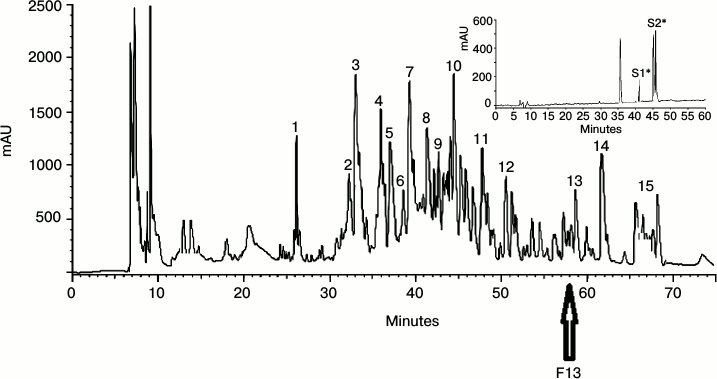
Purification of antimicrobial peptides and assay of antimicrobial activity. The lyophilized extract was fractionated by RP-HPLC, and the major peaks were selected to assay their antimicrobial activities. Figure 1 shows the chromatogram of the RP-HPLC of the lyophilized powder of the insect. The fractions were numbered sequentially. To select the most active one, all fractions were analyzed. As demonstrated in Fig. 1, F13 is the most active fraction that was further purified by applying a second run of C18 RP-HPLC (inset to Fig. 1). S1 and S2 were found to be the most active peaks against the studied microbes (Fig. 2).
Fig. 1. Chromatogram of RP-HPLC for lyophilized powder from Sphodromantis viridis. The most active peak is indicated with an arrow. The most active fraction, F13, was greatly purified using RP-HPLC (inset). The inset of the figure indicates the purity of the labeled fraction. The most active fractions are indicated with an asterisk. S1 and S2 are the most active peaks against the studied microbes.
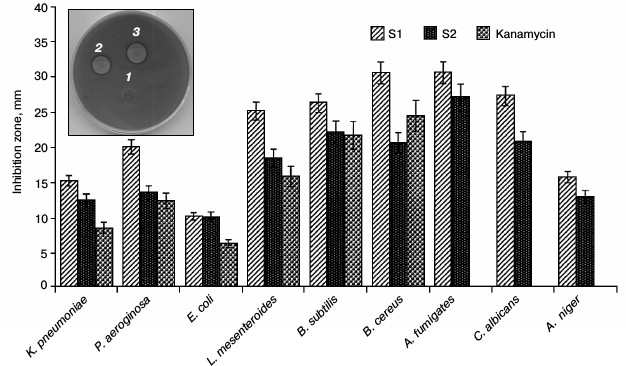
Antimicrobial activity. The radial diffusion assay (RDA) results obtained for S1, S2, and kanamycin are shown in Fig. 2. As shown in this figure, S1 and S2 have effective antimicrobial activity at a concentration of 5 µg/ml. In all studied microbes, the diameters of growth inhibition zone of S1 and S2 fractions were larger than that for kanamycin, and S1 fraction in all microbial species are greater than for S2. The inset of Fig. 2 indicates the non-growth halos of S1, S2, and kanamycin against B. subtilis for example, and antimicrobial activity of S1 fraction was quantified by the MIC method. The results are consistent with the RDA results. Among the tested microorganisms, S1 fraction exhibited highest antimicrobial activities against B. cereus and A. fumigates. The MIC values of this fraction on B. cereus ATCC2592 and A. fumigates are 15.1 and 19.3 µg/ml, respectively (Table 1). The antimicrobial activity of S1 fraction was proved lethal for the sensitive strain.
Fig. 2. Antimicrobial activity of purified peptides against bacteria and fungi using the radial diffusion assay method (inhibition zone). The diameter of the non-growth halo was measured, and its value is shown on the diagram. Kanamycin was taken as a control. The inset indicates the non-growth halos of S1 (2), S2 (3), and kanamycin (1) against B. subtilis as an example.
Table 1. MIC values and hemolytic activities
of Mastoparan-S (S1 fraction)
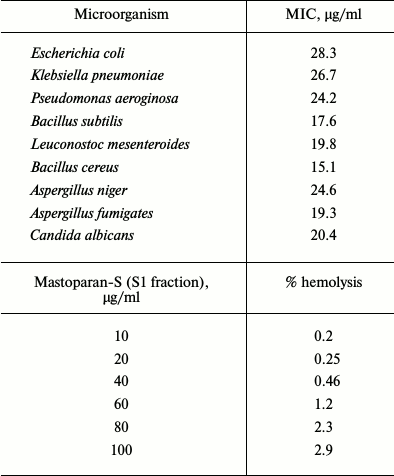
Note: Each experiment was repeated at least three times.
Hemolytic activity. Human red blood cells were used for hemolytic activity experiments in this study and the results were shown in Table 1. As shown in this table, the S1 fractions have low hemolytic activity. This peptide lysed only 3% of human red blood cells at the concentration up to 100 µg/ml.
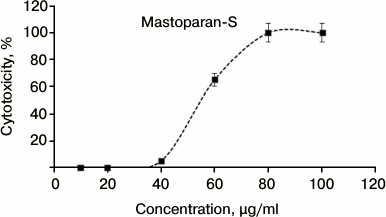
Cytotoxicity against human cells. The cytotoxic effects of S1 fractions on HeLa cells were determined and the results are shown in Fig. 3. As shown in this figure, the S1 fraction has significant cytotoxicity at concentrations higher than 40 µg/ml. According to the data of antimicrobial activity and in vitro toxicity, this peptide has no cytotoxicity effect at the MIC point. Only 5% cytotoxicity effect was observed at the concentration of 40 µg/ml.
Fig. 3. Dose-dependent effect of Mastoparan-S viability on normal human cervical cell (HeLa). Cells were incubated with serial dilutions of Mastoparan-S for 24 h, and the conversion of MTT dye to formazan was measured spectrophotometrically. The data represent mean ± SD of three independent experiments.
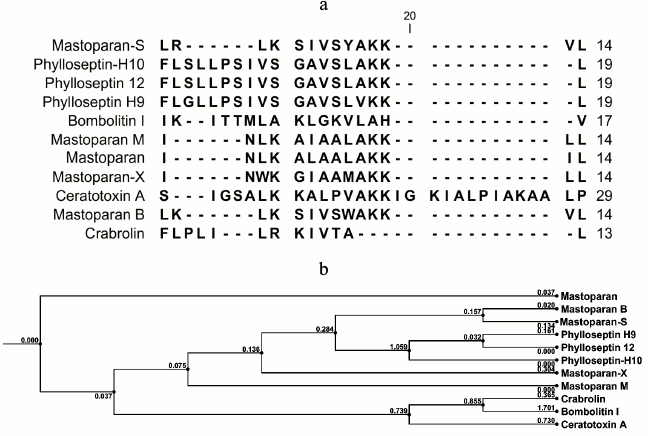
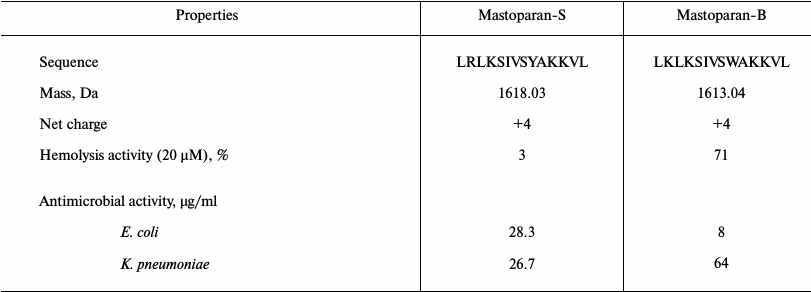
Structural analysis. Because of potent biological activity, the structure of S1 fraction was analyzed with mass spectrometry and the CLC Main Workbench software. The results are shown in Table 2 and Fig. 4. Table 2 indicates the estimated amino acid sequences, molecular masses, total hydrophobic ratio, total net charge, and calculated isoelectric points of the S1 fraction. The comparison of the peptide sequence and other identified AMP sequences revealed that S1 exhibits no sequence homology to any AMP in the database, suggesting that this peptide is a newly discovered AMP (Fig. 4a). This newly identified AMP was named Mastoparan-S based on the systematic nomenclature for AMPs [34]. The molecular mass of Mastoparan-S is 1618.03 Da. The amino acid composition of this peptide was identified as LRLKSIVSYAKKVL. Mastoparan-S achieves the predicted pI (isoelectric point) of 10.46. This analysis was done with the ExPASy MW/pI tool (http://www.expasy.ch/tools/pi-tool.html).
Table 2. Structural properties of S1
fraction

Fig. 4. a) Alignment of Mastoparan-S with other AMPs from various sources. The alignment was carried out with CLC Main Workbench v.5.5 software. b) Phylogenetic tree of Mastoparan-S. Amino acid sequences of the 10 reference peptides obtained from the AMP database were incorporated into the tree using the neighbor-joining method. The name of each sequence is given at the end of the corresponding branch. Reliability of the tree was assessed by bootstrap analysis with 100 replications. The substitutions per amino acid position are indicated above each branch.
Multiple sequence alignment of this new peptide was carried out along with 10 AMPs obtained from the antimicrobial peptide database. The results of the BLAST revealed that Mastoparan-S has high homology to Mastoparan-B. This data was also supported by the phylogenetic tree (Fig. 4b).
DISCUSSION
A large number of AMPs have been isolated from animals and plants. These agents are recognized as important components of the innate defense system [31-33]. A considerable variety of AMPs has been characterized from different insects [15, 34]. In this study, a peptide isolated from S. viridis exhibited strong activity against Gram-negative and Gram-positive bacteria as well as fungal species. The structural studies performed on this peptide showed that it has 14 residues. The evidence from amino acid sequencing also indicated a positive net charge (+4). The molecular mass of this peptide was found to be 1618.03 Da. This peptide has no sequence homology with other insect antimicrobial peptides but shows striking similarities with Mastoparan-B, a peptide toxin from the venom of the hornet Vespa basalis [35]. This new peptide was named Mastoparan-S. Its net charge is similar to Mastoparan-B. Mastoparan is a family of AMPs that is commonly found in Hymenoptera [36]. The AMPs of this family exhibits various biological and pharmacological activities [37-41]. Mastoparan-S has potent antimicrobial activities against different bacterial and fungal pathogens. The results show that Mastoparan-S acts as an effective antimicrobial compound, and it is capable of killing several bacterial and fungal species at low dosages. Unlike other AMPs of the Mastoparan family, Mastoparan-S has no hemolytic activity on human red blood cells [42, 43]. Hydrophobicity and net positive charge are two important factors that effect biological activity of AMPs such as antimicrobial activity [44, 45]. The net positive charge of Mastoparan-S is similar or more than other AMPs in the Mastoparan family, and this property increases antimicrobial activity. Mastoparan-S inhibits microbial growth in the range of 15.1-28.3 µg/ml for bacterial and 19.3-24.6 µg/ml for fungal pathogens. The comparison of these data with other AMPs from the Mastoparan family showed that this peptide is more suitable than other members [46-48]. Considering the great similarity of Mastoparan-S to Mastoparan-B, the biological activities of Mastoparan-S and Mastoparan-B were compared and are summarized in Table 3.
Table 3. Comparison of chemical and
biological properties of Mastoparan-S and Mastoparan-B
In addition, Mastoparan-S has no cytotoxicity on HeLa cells at the MIC points. Moreover, the majority of AMPs have cytotoxicity on natural cells such as intestinal epithelial cells. This effect limits the application of AMPs as suitable drugs [49-52]. Mastoparan-S has no activity against HeLa and red blood cells. Regarding these finding, Mastoparan-S can be considered as a novel candidate for treatment of microbial disease without side effects. However, its activity in vivo needs further investigation.
REFERENCES
1.Bulet, P., Hetru, C., Dimarcq, J.-L., and Hoffmann,
D. (1999) Antimicrobial peptides in insects; structure and function,
Dev. Comp. Immunol., 23, 329-344.
2.Irving, P., Troxler, L., and Hetru, C. (2004) Is
innate enough? The innate immune response in Drosophila, C.
R. Biologies, 327, 557-570.
3.Tassanakajon, A., Somboonwiwat, K., and Amparyup,
P. (2015) Sequence diversity and evolution of antimicrobial peptides in
invertebrates, Dev. Comp. Immunol., 48, 324-341.
4.Tzou, P., De Gregorio, E., and Lemaitre, B. (2002)
How Drosophila combats microbial infection: a model to study
innate immunity and host–pathogen interactions, Curr. Opin.
Microbiol., 5, 102-110.
5.Yang, J., Furukawa, S., Sagisaka, A., Ishibashi,
J., Taniai, K., Shono, T., and Yamakawa, M. (1999) cDNA cloning and
gene expression of cecropin D, an antibacterial protein in the silkworm
Bombyx mori, Comp. Biochem. Physiol. B, 122,
409-414.
6.Padhi, A., Sengupta, M., Sengupta, S., Roehm, K.
H., and Sonawane, A. (2014) Antimicrobial peptides and proteins in
mycobacterial therapy: current status and future prospects,
Tuberculosis, 94, 363-373.
7.Pei, Z., Sun, X., Tang, Y., Zhang, D., Gao, Y., and
Ma, H. (2014) Cloning, expression, and purification of a new
antimicrobial peptide gene from Musca domestica larva,
Gene, 549, 41-45.
8.Che, Q., Zhou, Y., Yang, H., Li, J., Xu, X., and
Lai, R. (2008) A novel antimicrobial peptide from amphibian skin
secretions of Odorrana grahami, Peptides,
29, 529-535.
9.Rollins-Smith, L. A. (2009) The role of amphibian
antimicrobial peptides in protection of amphibians from pathogens
linked to global amphibian declines, Biochim. Biophys. Acta
(Biomembranes), 1788, 1593-1599.
10.Chen, W., Yang, B., Zhou, H., Sun, L., Dou, J.,
Qian, H., Huang, W., Mei, Y., and Han, J. (2011)
Structure–activity relationships of a snake cathelicidin-related
peptide BF-15, Peptides, 32, 2497-2503.
11.McQuade, R., Roxas, B., Viswanathan, V. K., and
Vedantam, G. (2012) Clostridium difficile clinical isolates exhibit
variable susceptibility and proteome alterations upon exposure to
mammalian cationic antimicrobial peptides, Anaerobe, 18,
614-620.
12.Nan, Y. H., Lee, S. H., Kim, H. J., and Shin, S.
Y. (2010) Mammalian cell toxicity and candidacidal mechanism of Arg- or
Lys-containing Trp-rich model antimicrobial peptides and their
d-enantiomeric peptides, Peptides, 31, 1826-1831.
13.Hetru, C. (1994) Antimicrobial Peptides
(Boman, H., Marsh, J., and Goode, J. A., eds.) John Wiley & Sons,
N. Y.
14.Brown, M. J. F. (2010) Parasites and insects:
aspects of social behavior, in Encyclopedia of Animal Behavior
(Breed, M. D., and Moore, J., eds.) Academic Press, Oxford, pp.
632-635.
15.Erler, S., Lhomme, P., Rasmont, P., and Lattorff,
H. M. G. (2014) Rapid evolution of antimicrobial peptide genes in an
insect host–social parasite system, Infect. Genet. Evol.,
23, 129-137.
16.Chesnokova, L. S., Slepenkov, S. V., and Witt, S.
N. (2004) The insect antimicrobial peptide, l-pyrrhocoricin, binds to
and stimulates the ATPase activity of both wild-type and lidless DnaK,
FEBS Lett., 565, 65-69.
17.Li, Y., Xiang, Q., Zhang, Q., Huang, Y., and Su,
Z. (2012) Overview on the recent study of antimicrobial peptides:
origins, functions, relative mechanisms and application,
Peptides, 37, 207-215.
18.Dassanayake, R. S., Silva Gunawardene, Y. I. N.,
and Tobe, S. S. (2007) Evolutionary selective trends of insect/mosquito
antimicrobial defensin peptides containing cysteine-stabilized
α/β motifs, Peptides, 28, 62-75.
19.Ahn, H.-S., Cho, W., Kang, S.-H., Ko, S.-S.,
Park, M.-S., Cho, H., and Lee, K. H. (2006) Design and synthesis of
novel antimicrobial peptides on the basis of α-helical domain of
tenecin 1, an insect defensin protein, and structure–activity
relationship study, Peptides, 27, 640-648.
20.Mak, P., Zdybicka-Barabas, A., and Cytrynska, M.
(2010) A different repertoire of Galleria mellonella
antimicrobial peptides in larvae challenged with bacteria and fungi,
Dev. Comp. Immunol., 34, 1129-1136.
21.Lehrer, R. I., and Ganz, T. (1999) Antimicrobial
peptides in mammalian and insect host defence, Curr. Opin.
Immunol., 11, 23-27.
22.Wipfler, B., Wieland, F., DeCarlo, F., and
Hornschemeyer, T. (2012) Cephalic morphology of Hymenopus
coronatus (Insecta: Mantodea) and its phylogenetic implications,
Arthropod Struct. Dev., 41, 87-100.
23.Hurd, L. E. (2009) Chap. 157. Mantodea: (Praying
Mantids), in Encyclopedia of Insects (Resh, V. H., and
Carde, R. T., eds.) 2nd Edn., Academic Press, San Diego, pp.
597-599.
24.Matsuda, R. (1976) 27. The Mantodea, in
Morphology and Evolution of the Insect Abdomen (Matsuda, R.,
ed.) Pergamon, pp. 187-191.
25.Carle, T., Toh, Y., Yamawaki, Y., Watanabe, H.,
and Yokohari, F. (2014) The antennal sensilla of the praying mantis
Tenodera aridifolia: a new flagellar partition based on the
antennal macro-, micro- and ultrastructures, Arthropod Struct.
Dev., 43, 103-116.
26.Popkiewicz, B., and Prete, F. R. (2013)
Macroscopic characteristics of the praying mantis electroretinogram,
J. Insect Physiol., 59, 812-823.
27.Koehler, R., and Predel, R. (2010) CAPA-peptides
of praying mantids (Mantodea), Peptides, 31, 377-383.
28.Memarpoor-Yazdi, M., Zare-Zardini, H., and
Asoodeh, A. (2013) A novel antimicrobial peptide derived from the
insect Paederus dermatitis, Int. J. Pept. Res. Ther.,
19, 99-108.
29.Zardini, H. Z., Amiri, A., Shanbedi, M.,
Maghrebi, M., and Baniadam, M. (2012) Enhanced antibacterial activity
of amino acids-functionalized multi walled carbon nanotubes by a simple
method, Colloids Surf. B Biointerfaces, 92, 196-202.
30.Lupu, A. R., and Popescu, T. (2013) The
noncellular reduction of MTT tetrazolium salt by TiO2
nanoparticles and its implications for cytotoxicity assays, Toxicol.
in vitro, 27, 1445-1450.
31.Simmaco, M., Mignogna, G., and Barra, D. (1998)
Antimicrobial peptides from amphibian skin: what do they tell us?
Biopolymers, 47, 435-450.
32.McGillivary, G., Ray, W. C., Bevins, C. L.,
Munson, R. S., Jr., and Bakaletz, L. O. (2007) A member of the
cathelicidin family of antimicrobial peptides is produced in the upper
airway of the chinchilla and its mRNA expression is altered by common
viral and bacterial co-pathogens of otitis media, Mol. Immunol.,
44, 2446-2458.
33.Boman, H. G. (1991) Antibacterial peptides: key
components needed in immunity, Cell, 65, 205-207.
34.Saido-Sakanaka, H., Ishibashi, J., Momotani, E.,
Amano, F., and Yamakawa, M. (2004) In vitro and in vivo
activity of antimicrobial peptides synthesized based on the insect
defensin, Peptides, 25, 19-27.
35.Ho, C. L., and Hwang, L. L. (1991) Structure and
biological activities of a new mastoparan isolated from the venom of
the hornet Vespa basalis, Biochem. J., 274,
453-450.
36.Nakajima, T., Yasuhara, T., Uzu, S., Wakamatsu,
K., Miyazawa, T., Fukuda, K., and Tsukamoto, Y. (1985) Wasp venom
peptides; wasp kinins, new cytotrophic peptide families and their
physicochemical properties, Peptides, 6, 425-430.
37.Ohara-Imaizumi, M., Nakamichi, Y., Ozawa, S.,
Katsuta, H., Ishida, H., and Nagamatsu, S. (2001) Mastoparan stimulates
GABA release from MIN6 cells: relationship between SNARE proteins and
mastoparan action, Biochem. Biophys. Res. Commun., 289,
1025-1030.
38.Wu, T.-M., Chou, T.-C., Ding, Y.-A., and Li,
M.-L. (1999) Stimulation of TNF-[agr], IL-1[bgr] and nitrite release
from mouse cultured spleen cells and lavaged peritoneal cells by
mastoparan-M, Immunol. Cell. Biol., 77, 476-482.
39.Amin, R. H., Chen, H. Q., Veluthakal, R., Silver,
R. B., Li, J., Li, G., and Kowluru, A. (2003) Mastoparan-induced
insulin secretion from insulin-secreting βTC3 and INS-1 cells:
evidence for its regulation by Rho subfamily of G proteins,
Endocrinology, 144, 4508-4518.
40.Straub, S. G., James, R. F., Dunne, M. J., and
Sharp, G. W. (1998) Glucose augmentation of mastoparan-stimulated
insulin secretion in rat and human pancreatic islets, Diabetes,
47, 1053-1057.
41.Yang, M. J., Lin, W.-Y., Lu, K.-H., and Tu, W.-C.
(2011) Evaluating antioxidative activities of amino acid substitutions
on mastoparan-B, Peptides, 32, 2037-2043.
42.Mendes, M. A., de Souza, B. M., and Palma, M. S.
(2005) Structural and biological characterization of three novel
mastoparan peptides from the venom of the neotropical social wasp
Protopolybia exigua (Saussure), Toxicon, 45,
101-106.
43.Ho, C. L., and Hwang, L. L. (1991) Structure and
biological activities of a new mastoparan isolated from the venom of
the hornet Vespa basalis, Biochem. J., 274,
453-456.
44.Lee, S. Y., Park, N. G., and Choi, M.-U. (1998)
Effects of mastoparan B and its analogs on the phospholipase D activity
in L1210 cells, FEBS Lett., 432, 50-54.
45.Russell, A. L., Kennedy, A. M., Spuches, A. M.,
Venugopal, D., Bhonsle, J. B., and Hicks, R. P. (2010) Spectroscopic
and thermodynamic evidence for antimicrobial peptide membrane
selectivity, Chem. Phys. Lipids, 163, 488-497.
46.Sample, C. J., Hudak, K. E., Barefoot, B. E.,
Koci, M. D., Wanyonyi, M. S., Abraham, S., Staats, H. F., and Ramsburg,
E. A. (2013) A mastoparan-derived peptide has broad-spectrum antiviral
activity against enveloped viruses, Peptides, 48,
96-105.
47.Leal Denis, M. F., Incicco, J. J., Espelt, M. V.,
Verstraeten, S. V., Pignataro, O. P., Lazarowski, E. R., and
Schwarzbaum, P. J. (2013) Kinetics of extracellular ATP in mastoparan
7-activated human erythrocytes, Biochim. Biophys. Acta (General
Subjects), 1830, 4692-4707.
48.Zhang, P., Ray, R., Singh, B. R., and Ray, P.
(2013) Mastoparan-7 rescues botulinum toxin-A poisoned neurons in a
mouse spinal cord cell culture model, Toxicon, 76,
37-43.
49.Walsh, E. G., Maher, S., Devocelle, M.,
O’Brien, P. J., Baird, A. W., and Brayden, D. J. (2011) High
content analysis to determine cytotoxicity of the antimicrobial
peptide, melittin and selected structural analogs, Peptides,
32, 1764-1773.
50.Koyama, Y., Motobu, M., Hikosaka, K., Yamada, M.,
Nakamura, K., Saido-Sakanaka, H., Asaoka, A., Yamakawa, M., Isobe, T.,
Shimura, K., Kong, C. B., Hayashidani, H., Nakai, Y., and Hirota, Y.
(2006) Cytotoxicity and antigenicity of antimicrobial synthesized
peptides derived from the beetle Allomyrina dichotoma defensin
in mice, Int. Immunopharmacol., 6, 748-753.
51.Maher, S., and McClean, S. (2006) Investigation
of the cytotoxicity of eukaryotic and prokaryotic antimicrobial
peptides in intestinal epithelial cells in vitro, Biochem.
Pharmacol., 71, 1289-1298.
52.Dawson, R. M., and Liu, C.-Q. (2011) Analogues of
peptide SMAP-29 with comparable antimicrobial potency and reduced
cytotoxicity, Int. J. Antimicrob. Agents, 37,
432-437.