REVIEW: On Local Coupling of Electron Transport and ATP-Synthesis System in Mitochondria. Theory and Experiment
S. A. Eremeev* and L. S. Yaguzhinsky
Lomonosov Moscow State University, Belozersky Institute of Physico-Chemical Biology, 119991 Moscow, Russia; fax: +7 (495) 939-0338; E-mail: s.eremeev@gmail.com; yag@genebee.msu.su* To whom correspondence should be addressed.
Received December 25, 2014; Revision received January 25, 2015
A brief description of the principal directions for searching and investigating the model of local coupling between respiration and phosphorylation proposed by R. Williams is given in this paper. We found conditions where it was possible to reveal typical functional special features of the mitochondrial phosphorylating system. According to the theory, such special features should be observed experimentally if the mitochondrial phosphorylating system operated in the state of a supercomplex. It was proved that the phosphorylating system is able to operate in two states: P. Mitchell state and R. Williams state. It was demonstrated that in the ATP synthesis reaction, ATP-synthase (F1F0) was able to use thermodynamic potential of Bronsted acids as a source of energy. It was shown using a double-inhibitor titration technique that when the phosphorylating system operated in the supercomplex state, the electron transfer system and ATP-synthesis system were docked rigidly. A model system of chemical synthesis of membrane-bound proton fraction (Bronsted acids), carrying a free energy excess, was developed on the model of bilayer lipid membrane. Catalysts selectively accelerating proton detachment of this fraction were also found. The formation of a Bronsted acids fraction carrying free energy excess was recorded during the operation of proton pumps on mitochondrial and mitoplastic membranes. In the experimental part of the work, a brief description is given of studies on new uncouplers that transfer the phosphorylation system from the local coupling state to the state of transmembrane proton transfer. Thus, they accelerated the respiration of mitochondria and decreased the ADP/O parameter.
KEY WORDS: local coupling, phosphorylating system of mitochondria, membrane-bound protons carrying free energy excess, uncouplers deactivating local couplingDOI: 10.1134/S0006297915050089
Abbreviations: BLM, bilayer lipid membrane; SkQ3, [10-(2,4,5-trimethyl-3,6-dioxocyclohexan-1,4-dien-1-yl)decyl]triphenylphosphonium chloride; TCP-C15, 2,4,6-trichloro-3-pentadecylphenol.
Two models of proton coupling of respiration and phosphorylation of
mitochondria were proposed in 1961. The mechanism proposed by P.
Mitchell [1] is now adopted universally. This
mechanism suggests that the initial stage of transformation of
oxidative reaction energy can be represented by induction of
electrochemical potential of hydrogen ions on the inner mitochondrial
membrane realized by proton pumps through a transmembrane transfer of
hydrogen ions. According to this model, the autonomously functioning
ATP synthase system uses the membrane potential energy for synthesis of
ATP. The local coupling mechanism proposed by R. Williams [2] suggests that the energy can be directly
transferred from the respiratory proton pumps to ATP synthase with the
involvement of hydrogen ions within a membrane supercomplex including
both H+-pumps and ATP synthase complex.
It should be noted that, according to Eugen, the full energy of proton solvation in an aqueous phase is >260 kcal/mol. Considering that synthesis of 1 mol of ATP is associated with storage of 7.3 kcal/mol [3] and that 2.7-3.3 protons are spent for synthesis of one ATP molecule [4], it is concluded that energy needed for synthesis of ATP can be stored with a great excess due to energy of a partial dehydration of the proton. The model proposed by Williams admits this mechanism of energy storage because, according to this model, during ATP synthesis energy is transferred onto ATP-synthase not by “free” fully hydrated protons, but by protons bound with the membrane complex, or Bronsted acids, which are well screened from the free water phase and therefore can be transferred onto ATP synthase in a partially dehydrated form.
Our purpose was to test the correctness of the hypothesis of Williams. Doing this, we had also to answer the question whether Mitchell’s model was true. After some unsuccessful attempts to execute this second and not easy task, we found a decision promising for study of the local coupling mechanism. It occurred that the oxidative phosphorylation system could function in two forms: in a dissociated form corresponding to Mitchell’s model and in a supercomplex form corresponding to Williams’ model. During our studies, we found that at low tonicity of the incubation medium (120 mOsm) during transition to the low-amplitude swelling of mitochondria, the functional parameters of the system corresponded to its functioning in the supercomplex state [5, 6].
DETECTION OF TWO FUNCTIONING STATES OF THE MITOCHONDRIAL
PHOSPHORYLATING SYSTEM
Changes in functional parameters of the mitochondrial phosphorylating system were accompanied by a deep rearrangement of the mitochondrial membrane ultrastructure that we recorded for mitochondria by electron microscopy [6] and by the method of small-angle diffraction of neutrons on “living” functioning mitochondria [7]. Moreover, strongly pronounced structural changes in the protein and lipid components of the mitochondrial membrane were recorded with a fluorescent probe [6]. Using double inhibitory analysis, it was shown that in a functioning supercomplex the electron transfer system of enzymes and the system of ATP synthesis were rigidly coupled to each other similarly to the clock mechanism details, which stopped working when rotation of any element was blocked [5].
Thus, both variants of proton coupling in the phosphorylating system were found to be true. This discovery allowed us to continue experiments to find out and study conditions of generation and properties of membrane-bound hydrogen ions with excess of free energy (more accurately, membrane-bound Bronsted acids). The experiments were conducted on mitochondria and on model systems.
These studies included a systematic search for specific functional features of the mitochondrial phosphorylating system, which, according to the theory, could be observed during operating of this system in the supercomplex state.
BRONSTED ACIDS AS SUBSTRATES OF ATP SYNTHASE
At the initial stage of the study, we obtained a response to the key question associated with energy transfer onto ATP synthase during the system functioning in the supercomplex state. A positive response to the question whether ATP synthase could use the thermodynamic potential of Bronsted acids as a source of energy was obtained on a binary octane–water system. In this system, ATP synthase (F1F0) isolated from heart mitochondria was accumulated on the interphase border (on the aqueous phase side). Substrates of phosphorylation were added into the aqueous phase of this system. As a source of energy in the reaction of ATP synthesis, on the interphase border the thermodynamic potential was used of a Bronsted acid (pentachlorophenol), which was added into the hydrophobic octane phase [8].
FORMATION OF A FRACTION OF MEMBRANE-BOUND HYDROGEN IONS WITH
EXCESS FREE ENERGY ON A BILAYER LIPID MEMBRANE
The first successful attempt of recording the proton binding with the membrane surface during operating of the proton pump was described for a model of rhodopsin patches in work of Drachev et al. [9]. The authors found that dissociation of membrane-bound Bronsted acids produced during operating of the proton pumps there was a rather high kinetic barrier promoting the proton holding on the surface of rhodopsin patches.
A model system of proton transfer was developed in our laboratory based on a bilayer lipid membrane (BLM) [10, 11]. In this system, the transmembrane transfer of hydrogen ions was limited by the reaction of proton detachment from the lipid bilayer surface. Appearance of the membrane-bound fraction of hydrogen ions with excess of free energy was recorded directly. This finding was in agreement with our theory that energy of oxidative reactions could be stored as partially dehydrated membrane-bound protons (more accurately, Bronsted acids) [12]. Results of this work allowed us to suggest that Bronsted acids cold be specifically dehydrated during their transfer across the hydrophobic barrier of the lipid bilayer.
ABOUT CATALYSTS OF DISSOCIATION OF BRONSTED ACIDS BOUND WITH THE
MEMBRANE SURFACE
Work of Antonenko et al. [10] revealed an unusual feature of weak bases – the citric acid anion (citrate) and HEPES usually considered as pH buffers. However, under conditions of experiments conducted on BLM, they catalyzed the detachment of membrane-bound hydrogen ions carrying excess free energy from the membrane surface. If the medium pH was maintained constant, an increase in the citrate (or HEPES) concentration decreased the positive surface potential induced by the transmembrane transfer of proton in the system (the potential was measured by compensation of the internal field of the membrane). It was also shown that weak bases significantly strengthened the rate of proton transfer across the bilayer and thus decreased pH value (measured directly) in the membrane-adjacent unstirred layer. The sign of the latter effect was opposite to the sign expected in the case of citrate acting as a buffer because an increase in the buffer concentration had to decrease but not increase pH gradients in the system. Thus, a specific catalytic effect was shown on the interaction of weak bases with membrane-bound Bronsted acids during their dissociation on the membrane surface. The catalysis in combination with the observed significant increase in the transmembrane flow of protons confirmed once more an imbalance of the state and of a high free energy of membrane-bound protons (Bronsted acids) generated under the experimental conditions.
FRACTION OF “ENERGIZED” PROTONS ON THE SURFACE OF
MEMBRANES OF MITOPLASTS AND MITOCHONDRIA
The detection of catalysts selectively accelerating the dissociation of membrane-bound Bronsted acids carrying free energy excess allowed us to easily detect the presence of this fraction on the surface of mitochondrial membranes [13-15]. On the membrane of mitoplasts under conditions of operating respiratory H+-pumps, we recorded generation of the above-mentioned fraction of “energized” protons. In these experiments, changes in zeta-potential on the surface of mitoplasts were recorded with a Zetasizer. These changes were caused by switching on the H+-pumps and the subsequent addition of a catalyst (HEPES) of the detachment of the “energized” protons from the membrane surface [13].
The same effect was observed on phosphorylating mitochondria (in the presence of substrates: succinate, ADP, phosphate) under conditions of close docking of the inner and outer mitochondrial membranes in medium with decreased tonicity (120 mOsm) [15].
In our works, we showed experimentally the appearance of membrane-bound Bronsted acids carrying free energy excess on membranes of mitoplasts [13] and mitochondria [14, 15]. Based on these experiments, we wrote a full equation of the thermodynamic potential of hydrogen ions (ΔG) as the sum of the electrochemical Mitchell’s potential (ΔµH) and the solvation potential (Δµsolv) resulting due to partial dehydration of membrane-bound protons:
ΔG = ΔµH + Δµsolv.
It was shown that the solvation potential energy could be used for synthesis of ATP and that the catalyst of the proton detachment decreased by 15-30% the ADP/O parameter [12, 16]. This result suggested that the fraction of membrane-bound protons was involved in the synthesis of ATP.
ABOUT TWO NEW MECHANISMS OF RESPIRATION AND PHOSPHORYLATION
UNCOUPLING ASSOCIATED WITH OPERATION OF THE PHOSPHORYLATING SYSTEM IN
THE LOCAL COUPLING STATE
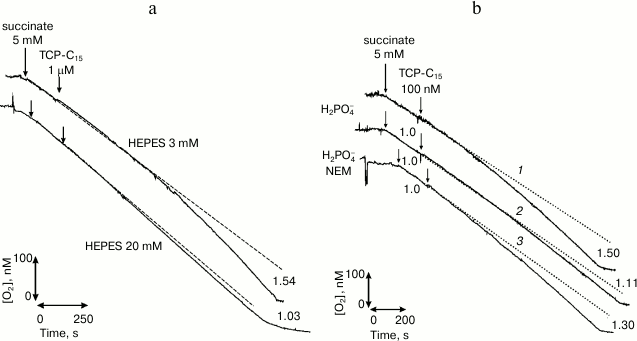
In our laboratory, a new class of protonophores was synthesized purposefully for selective interaction with the fraction of surface-bound hydrogen ions. We synthesized a surface-active derivative of phenol, 2,4,6-trichloro-3-pentadecylphenol (TCP-C15) [17], which was experimentally shown to selectively interact with the fraction of membrane-bound hydrogen ions. TCP-C15 was shown in many series of experiments [17, 18] to stimulate the respiration of mitochondria in the concentration range from 10 nM to tens of µM. If this fraction was removed with a catalyst [10] or by switching on the system of phosphate transfer [19] (Fig. 1), the uncoupling effect of this compound virtually disappeared.
Fig. 1. Abolishment of stimulation of the respiration of rat mitochondria with the protonophore TCP-C15 through removal from the mitochondrial membrane surface of the fraction of energized protons under the influence of catalyst HEPES (20 mM) (a) and through switching on the system of phosphate transfer (b) [18] (printed by permission of Springer).
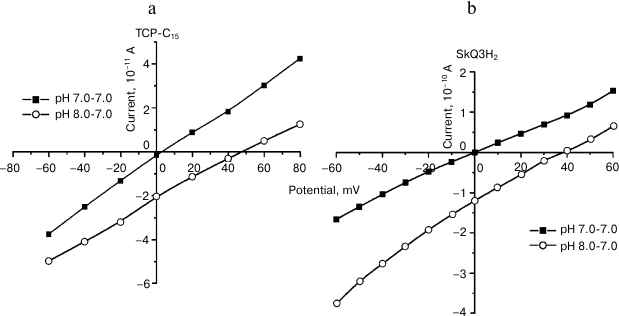
Works on a model system (a bilayer membrane) revealed that TCP-C15 had features of a weak protonophore (Fig. 2). The creation of pH gradient between the cells was accompanied by appearance of transmembrane potential of 45-48 mV.
Fig. 2. Evidence of protonophore features of TCP-C15 (a) and of the SkQ3H2 reduced form (b). Volt-ampere characteristics of surface-active protonophores in the absence and presence of pH gradient on BLM [23] (reprinted by permission of Springer).
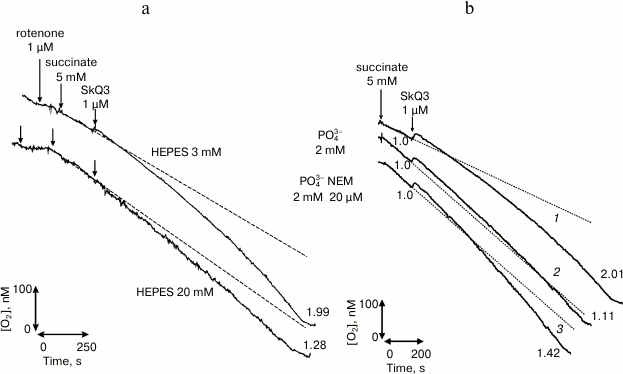
We also worked with the surface-active compound [10-(2,4,5-trimethyl-3,6-dioxocyclohexan-1,4-dien-1-yl)decyl]triphenylphosphonium chloride (SkQ3) [20, 21]. Comparative studies of SkQ3 and TCP-C15 on mitochondria gave quite similar results (Fig. 3). Obviously, this was associated with reduction of SkQ3 to hydroquinone in mitochondrial membranes and was partially associated with the presence of fatty acids in the experimental system. Experiments on BLM were conducted with a beforehand reduced form of SkQ3 (SkQ3H2) on a membrane formed from diphytanyl phosphatidylcholine analog of diphytanoyl phosphatidylcholine that did not have fatty acid radicals, and complex ether bonds were replaced by simple ether bonds. The experiments revealed that this compound had protonophore activity (Fig. 2). The creation of pH gradient, equal to 1, was associated with induction of the transmembrane potential with the value of 40-45 mV that was commensurable with the theoretical value. The experiments were performed by a standard method in media containing 81 mM KCl and 3 mM HEPES at pH 7.0. According to our work [20], pK(SkQ3) = 9.5. Taking into account the decrease by 1.0-1.5 in the dissociation constant pH value on binding surface-active acids on the membrane/water interphase border, this compound had to be an effective protonophore under conditions of the experiment. The result (Fig. 2) did not contradict data of the work by F. Severin et al. [22] that the features of a protonophore displayed by SkQ derivatives were associated with their ability to effectively form heterodimers with fatty acids. They used the oxidized (quinoid) form of SkQ, which virtually does not interact with protons and, as differentiated from the freshly prepared reduced form used by us, does not have features of a protonophore.
Fig. 3. Abolishment of stimulation of rat liver mitochondria respiration with the protonophore SkQ3 at the mitochondrial membrane surface of the fraction of energized protons by addition of the catalyst HEPES (20 mM) (a) and by activating the phosphate transfer system (b) [21] (printed by permission of Springer).
Thus, it has been shown that, independently of the molecule structure, surface-active compounds have the same features of weak uncouplers that selectively interact with the fraction of membrane-bound protons that is produced on the mitochondrial membrane surface during the activity of H+-pumps.
Studies of our laboratory performed for many years established that weak bases incapable of penetrating across membranes could act as weak uncouplers of oxidative phosphorylation [12]. Our work on BLM [10] has directly shown that addition of a catalyst increases the transmembrane flow of protons, because under the experimental conditions just the stage of proton detachment from the membrane surface is the slow stage of the proton transfer. It was shown in the work by Solodovnikova et al. [12] that addition of a catalyst (20 mM HEPES) decreased the efficiency of the phosphorylating system ADP/O functioning. The decrease in the ADP/O parameter in these experiments was coupled with the switching of the phosphorylating system from the local coupling regimen using solvation potential in the ATP synthesis to the state of the transmembrane transfer of a proton.
The study was financially supported by the Russian Foundation for Basic Research (grant 13-04-01-630a).
REFERENCES
1.Mitchell, P. (1961) Coupling of phosphorylation to
electron transfer by a chemiosmotic type of mechanism, Nature,
191, 144-148.
2.Williams, R. J. (1961) Possible functions of chain
of catalysists, J. Theor. Biol., 1, 1-17.
3.Severin, E. S. (2004) Biochemistry [in
Russian], 2nd Edn., GEOTAR-MED, Moscow.
4.Ferguson, S. J. (2010) ATP synthase: from sequence
to ring size to the P/O ratio, Proc. Natl. Acad. Sci. USA,
107, 16755-16756.
5.Krasinskaya, I. P., Marshansky, V. N., Dragunova,
S. F., and Yaguzhinsky, L. S. (1984) Relationships of respiratory chain
and ATP-synthase in energized mitochondria, FEBS Lett.,
167, 176-180.
6.Krasinskaya, I. P., Litvinov, I. S., Zakharov, S.
D., Bakeeva, L. E., and Yaguzhinsky, L. S. (1989) Relationships of
respiratory chain and ATP-synthase in energized mitochondria,
Biokhimiya, 54, 1550-1556.
7.Murugova, T. N., Gordeliy, V. I., Islamov, A. K.,
Kovalev, Y. S., Kuklin, A. I., Vinogradov, A. D., and Yaguzhinsky, L.
S. (2006) Structure of membrane of submitochondrial particles studied
by small angle neutron scattering, Mat. Struct., 2,
68-70.
8.Yaguzhinsky, L. S., Boguslavsky, L. I., Volkov, A.
G., and Rakhmaninova, A. B. (1976) Synthesis of ATP coupled with action
of membrane protonic pumps at the octane–water interface,
Nature, 259, 494-496.
9.Drachev, A. L., Kaulen, A. D., and Skulachev, V. I.
(1984) Reconstitution of biological molecular generators of electric
current H+-ATPase, FEBS Lett., 178,
331-336.
10.Antonenko, Y. N., Kovbasnjuk, O. N., and
Yaguzhinsky, L. S. (1993) Evidence in favor of the existence of a
kinetic barrier for proton transfer from a surface of bilayer
phospholipid membrane to bulk water, Biochim. Biophys. Acta,
1150, 45-50.
11.Kovbasnjuk, O. N., Antonenko, Y. N., and
Yaguzhinsky, L. S. (1991) Proton dissociation from nigerisin at the
membrane-water interface, the rate-limiting step of K/W exchange on the
bilayer lipid membrane, FEBS Lett., 289, 176-178.
12.Solodovnikova, I. M., Yurkov, V. L.,
Ton’shin, A. A., and Yaguzhinsky, L. S. (2004) Local coupling of
respiration processes and phosphorylation in rat liver mitochondria,
Biofizika, 49, 47-56.
13.Moiseeva, V. S., Motovilov, K. A., Lobysheva, N.
V., Orlov, V. N., and Yaguzhinsky, L. S. (2011) The formation of
metastable bond between protons and mitoplast surface, Dokl.
Biochem. Biophys., 438, 127-130.
14.Yurkov, V. I., Fadeeva, M. S., and Yaguzhinsky,
L. S. (2005) Proton transfer through the membrane–water
interfaces in uncoupled mitochondria, Biochemistry (Moscow),
70, 195-199.
15.Eroshenko, L. V., Marakhovskaya, A. S., Vangeli,
I. M., Semenyuk, P. I., Orlov, V. N., and Yaguzhinsky, L. S. (2012)
Bronsted acids bounded to the mitochondrial membranes as a substrate of
ATP synthase, Dokl. Biochem. Biophys., 444, 158-161.
16.Yaguzhinsky, L. S., Yurkov, V. I., and
Krasinskaya, I. P. (2006) On the localized coupling of respiration and
phosphorylation in mitochondria, Biochim. Biophys. Acta,
1757, 408-414.
17.Motovilov, K. A., Yurkov, V. I., Volkov, E. M.,
and Yaguzhinsky, L. S. (2009) Properties and nnew methods of
non-equilibrium membrane bounded proton fraction research under
conditions of proton pump activation, Biol. Membr. (Moscow),
26, 408-418.
18.Yaguzinsky, L. S., Motovilov, K. A., Volkov, E.
M., and Eremeev, S. A. (2013) Interaction of a surface-active base with
the fraction of membrane-bound Williams protons, Biofizika,
58, 95-102.
19.Kramer, R. (1998) Mitochondrial carrier proteins
can reversibly change their transport mode: the cases of the
aspartate/glutamate and the phosphate carrier, Exp. Physiol.,
83, 259-265.
20.Eremeev, S. A., Kargin, V. I., Motovilov, K. A.,
Tashlitsky, V. N., Markov, V. Y., Korshunova, G. A., Sumbatyan, N. V.,
Vyssokikh, M. Y., and Yaguzhinsky, L. S. (2009) Molecular mechanisms of
transformation of SkQ myotropic quinones and the search for new
approaches to creation of selective free radical traps, Biochemistry
(Moscow), 74, 1114-1124.
21.Eremeev, S. A., Motovilov, K. A., Volkov, E. M.,
and Yaguzhinsky, L. S. (2011) A new member of membranotropic
uncouplers, SkQ3, Biol. Membr. (Moscow), 28, 339-345.
22.Severin, F. F., Severina, I. I., Antonenko, Y.
N., Rokitskaya, T. I., Cherepanov, D. A., Mokhova, E. N., Vyssokikh, M.
Y., Pustovidko, A. V., Markova, O. V., Yaguzhinsky, L. S., Korshunova,
G. A., Sumbatyan, N. V., Skulachev, M. V., and Skulachev, V. P. (2010)
Penetrating cation/fatty acid anion pair as a mitochondria-targeted
protonophore, Proc. Natl. Acad. Sci. USA, 107,
663-668.
23.Dzhumashev, D. B., Byvshev, I. M., Eremeev, S.
A., and Yaguzinsky, L. S. (2015) A specific interaction of a
surface-active protonophore 2,4,6-trichlor-3-pentadecylphenol with
artificial and mitochondrial membranes, Biol. Membr. (Moscow),
32, 11-19.