Different Effects of Identical Symmetry-Related Mutations near the Bacteriochlorophyll Dimer in the Photosynthetic Reaction Center of Rhodobacter sphaeroides
L. G. Vasilieva1*, T. Y. Fufina1, A. G. Gabdulkhakov2, and V. A. Shuvalov1
1Institute of Basic Problems of Biology, 142290 Pushchino, Moscow Region, Russia; fax: (496) 733-0532; E-mail: vsyulya@mail.ru2Institute of Protein Research, 142290 Pushchino, Moscow Region, Russia; fax: (495) 514-0218; E-mail: azat@vega.protres.ru
* To whom correspondence should be addressed.
Received December 3, 2014; Revision received February 11, 2015
In the bacterial photosynthetic reaction center (RC), asymmetric protein environment of the bacteriochlorophyll (BChl) dimer largely determines the photophysical and photochemical properties of the primary electron donor. Previously, we noticed significant differences in properties of Rhodobacter sphaeroides RCs with identical mutations in symmetry-related positions – I(M206)H and I(L177)H. The substitution I(L177)H resulted in covalent binding of BChl PA with the L-subunit, as well as in 6-coordination of BChl BB, whereas in RC I(M206)H no such changes of pigment–protein interactions were found. In addition, the yield of RC I(M206)H after its isolation from membranes was significantly lower than the yield of RC I(L177)H. This study shows that replacement of amino acid residues in the M203-M206 positions near BChls PB and BA by symmetry-related residues from the L-subunit near BChls PA and BB leads to further decrease in RC amount in the membranes associated obviously with poor assembly of the complex. Introduction of a new hydrogen bond between BChl PB and its protein environment by means of the F(M197)H mutation stabilized the mutant RC but did not affect its low yield. We suggest that the mutation I(M206)H and substitution of amino acid residues in M203-M205 positions could disturb glycolipid binding on the RC surface near BChl BA that is important for stable assembly of the complex in the membrane.
KEY WORDS: bacterial photosynthesis, Rhodobacter sphaeroides, photosynthetic reaction center, site-directed mutagenesis, bacteriochlorophyll, magnesium atom coordination, pigment–protein interactionsDOI: 10.1134/S0006297915060012
Abbreviations: BA and BB, monomer bacteriochlorophyll; BChl, bacteriochlorophyll; LDAO, lauryldimethylamine N-oxide; P, special pair of bacteriochlorophylls; PA and PB, bacteriochlorophylls of the special pair; RC, reaction center; WT, wild type.
Photosynthesis is one of the most important biochemical processes that
support life on the Earth. The first steps of light energy
transformation into energy of chemical bonds occur in photosynthetic
reaction centers (RCs) of plants, algae, and bacteria. The RC of the
purple nonsulfur bacterium Rhodobacter (Rba.)
sphaeroides consists of three protein subunits, L, M, and H,
and 10 cofactors noncovalently associated with protein. The cofactors
include four bacteriochlorophyll (BChl) molecules, two molecules of
bacteriopheophytin (BPheo), two molecules of ubiquinone, a nonheme iron
atom, and a carotenoid molecule [1]. A substantial
number of water molecules are also included in the RC structure [1-5], and some of them were shown
to play important roles in the functioning of the complex [6, 7]. In addition, in many
crystallographic structures of Rba. sphaeroides RCs, there are
from one to three lipid molecules that appear to be involved in firm
binding of the cofactors [4, 8].
One of the distinctive features of the bacterial RC is the combination of relative structural symmetry and strict functional asymmetry. Although the cofactors are organized into two pseudo-symmetrical branches, A and B, normally only one of them, branch A, is functionally active [9]. It is known that the amino acid sequence homology of the L- and M-subunits that bind all the cofactors is only about 30% [2]. There have been a number of studies devoted to artificial “symmetrization” of Rba. capsulatus RC by increasing homology of particular sections of the L- and M-subunits [10, 11]. For a long time, it was thought that the distinction of the protein environment of the cofactors in the two branches of electron transfer is the basis for the functional activity of the A-branch and inactivity of the B-branch. According to recent data, lipids associated with the surface of the bacterial RC can also contribute to structural and functional asymmetry of the complex [4, 8].
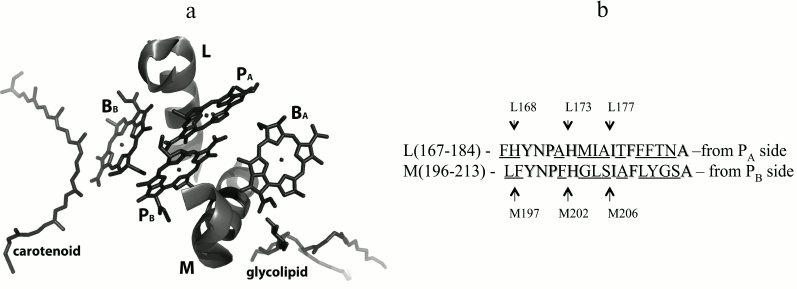
In the 3D-structure of the Rba. sphaeroides RC on the periplasmic side of the photosynthetic membrane, two BChl molecules, PA and PB, form a special pair P overlapping on the level of the first tetrapyrrole rings (Fig. 1a). The nearest protein environment of the BChl dimer P is known to be highly conservative with low percentage of homology of its L- and M-constituent parts (Fig. 1b). Numerous studies have shown that the protein surrounding of the BChl dimer largely determines its photophysical and photochemical properties as the primary electron donor [9, 12, 13]. It was also shown that thermal stability of mutant RCs could be altered depending on the number of hydrogen bonds organized between P and its nearest environment [14].
Fig. 1. a) Fragments L(167-183) and M(196-212) of the α-helices of the L- and M-subunits near bacteriochlorophylls in the Rba. sphaeroides RC structure (PDB 1M3X [4]). Phytol tails of bacteriochlorophylls are not shown for better view. b) Amino acid sequences of the α-helices shown on the left [5]. Homologous sequences are marked, and nonhomologous ones are underlined. Positions of isoleucine residues M206 and L177, histidine ligands M202 and L173, as well as the histidine L168 residue and phenylalanine M197 residue are emphasized by arrows.
Recently, we showed that the single amino acid substitution Ile–His in two symmetrical positions M206 and L177 near the BChl dimer (Fig. 1) leads to similar spectral changes in Rba. sphaeroides RC [15-17]. In particular, in both cases in the absorption spectrum of the mutant RCs blue shift of the QY P band were observed together with decrease in its dipole strength. Besides, a decline of the RC stability was noticed [15-17]. At the same time, significant differences of the mutant RCs properties were observed. Mutation I(L177)H resulted in covalent bond of BChl PA with the L-subunit, as well as in hexa-coordination of BChl BB [5, 17, 18], whereas in the RC I(M206)H no such changes of the pigment–protein interactions were found [15]. It should also be noted that decrease in RC stability was more pronounced after I(M206)H mutation, and the yield of this RC after its purification from membranes was significantly lower than that of the RC I(L177)H.
The aim of this work was to explore possible reasons of different effects of similar substitutions in symmetrical positions near the BChl dimer and to elucidate the nature of the BChl–protein covalent binding in the RC I(L177)H. To increase homology of the protein environment near BChls PB and BA with that near BChls PA and BB, amino acid substitutions were introduced in the shorter, M203-M206, and the longer, M203-M212, section of the transmembrane α-helix of the M-subunit. An additional amino acid substitution F(M197)H was introduced to stabilize the mutant RCs by means of hydrogen bond formation between the 31-acetyl-carbonyl group of BChl PB and the imidazole group of the His M197.
MATERIALS AND METHODS
Rhodobacter sphaeroides recombinant strains were grown on Huttner medium in the presence of tetracycline (1 µg/ml), kanamycin (5 µg/ml), and streptomycin (5 µg/ml) as described earlier [16]. The procedure of preparation of chromatophores and purification of RCs from “no-antenna” strains of Rba. sphaeroides by ion-exchange chromatography was described previously [16, 17]. The yield of reaction centers after their purification from membranes was estimated using absorption near 800 nm (QY B band) measured in 1 cm cuvette (ODV800) calculated per liter of 3-day culture. Pigments were extracted from reaction centers by acetone–methanol mixture (7 : 2) as described earlier [19]. Ground state absorption spectra of the RCs were measured at room temperature with a Shimadzu UV-1601PC spectrophotometer (Japan). The 3D structure was modeled using PyMOL (http://www.pymol.org).
RESULTS
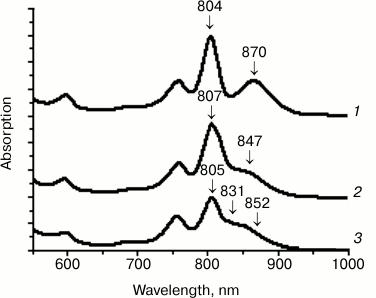
Figure 2 presents the absorption spectra of the wild type (WT) and the mutant RCs I(M206)H and I(L177)H. In the spectra of RCs I(M206)H and I(L177)H, similar blue shifts of the QY P bands were observed: 870 nm for the WT RC, 852 nm for RC I(M206)H, and 847 nm for RC I(L177)H (Fig. 2). In addition, in the spectrum of RC I(M206)H a new absorption band of unknown origin appeared at 831 nm. Amounts of RC purified per liter of culture were estimated as 4-5 ODV800 for the WT, 2-3 ODV800 for RC I(L177)H, and 0.1 ODV800 for RC I(M206)H.
Fig. 2. Absorption spectra of wild type RC (1), RC I(L177)H (2), and RC I(M206)H (3).
Upon amino acid substitutions in M203-M206 positions by symmetry-related residues from L-subunit, L174-L176, the properties and molecular volume of the BChl PB protein environment were gradually changing (Fig. 1b). The values of partial molecular volumes of amino acid residues measured in water at 25oC were taken from [20] (they are given in parentheses and indicated in cm3/mol). In the M203 site, the Gly (37.5) residue was replaced by the bulkier Met (99.6) residue. The Leu (101.9) residue in the M204 site was replaced by the Ile (99.9) residue. Both amino acid residues are aliphatic, and they have similar molecular volumes but different length of their side chains. The weakly polar Ser (55.0) residue in the M205 site was replaced by the nonpolar Ala (54.7) residue with similar molecular volume [20].
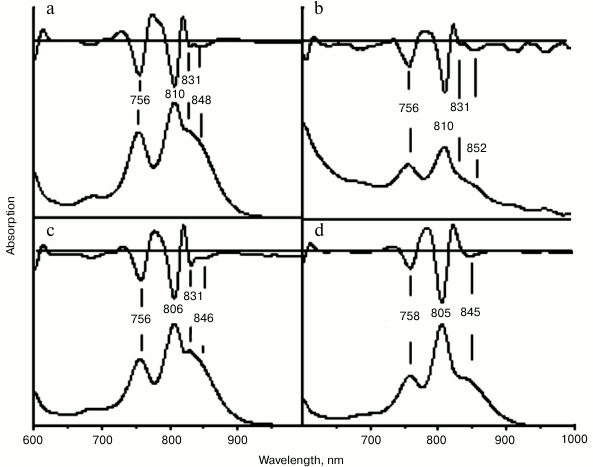
In the absorption spectrum of the RC with double substitution G(M203)M + I(M206)H, blue shift of the QY P band was observed from 870 nm in the wild type RC to 848 nm in the mutant spectrum. Red shift of the QY B absorption band from 804 to 810 nm was also noted. Similar to that in the spectrum of the RC I(M206)H, a new absorption band was observed at 831 nm. The yield of RC G(M203)M + I(M206)H after its purification was about 0.1 ODV800 per liter of culture, as for RC I(M206)H. Similar spectral changes were observed in RCs with double mutation S(M205)A + I(M206)H and with four substitutions G(M203)M + L(M204)I + S(M205)A + I(M206)H (abbreviated to M203-M206) (Fig. 3, b and c). These mutant RCs were extremely unstable after their purification from the membranes, and their yields were less than 0.01 ODV800 per liter of culture.
Fig. 3. Absorption spectra of reaction centers G(M203)M + I(M206)H (a), S(M205)A + I(M206)H (b), M203-M206 (c), and F(M197)H + (M203-M206) (d). Second derivatives of the spectra are shown above the spectra.
In the wild type RC, His-L168 is hydrogen bonded with the 31-acetyl-carbonyl group of BChl PA. By means of the mutation F(M197)H, a symmetrical H-bond was introduced between His-M197 and BChl PB. The purpose of this new H-bond was not only to enhance the symmetry of the BChl dimer protein environment, but also to stabilize its structure. Addition of the mutation F(M197)H did stabilized the RC complex, and the yield of the RC with five substitutions F(M197)H + G(M203)M + L(M204)I + S(M205)A + I(M206)H was an order of magnitude higher compared to that of RC M203-M206, namely 0.1 ODV800 per liter of culture. Absorption spectrum of this RC became similar to the spectrum of RC I(L177)H (Fig. 3d). In addition, no absorption band at 831 nm was observed in the spectrum of the RC with five substitutions. These data suggest that appearance of this band can be explained by instability of the primary electron donor structure in mutant RCs and, therefore, by heterogeneity of the RCs, namely, by presence of an RC fraction that has damaged excitonic interaction between the special pair BChls.
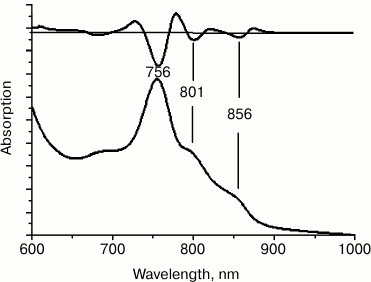
To further increase the symmetry of the BChl dimer protein environment, six more amino acid substitutions were introduced at positions M207-M212, reproducing the amino acid sequence of the symmetrical L-subunit segment L178-L183. In the long wavelength region of the absorption spectrum of membrane-bound RC F(M197)H + (M203-M212), three main bands were observed, i.e. at 756, 801, and 856 nm. The amplitude of the QY BPheo absorption band at 756 nm considerably prevailed over other bands, pointing to instability of the membrane-associated RCs and to accumulation of free and aggregated forms of BPheo (Fig. 4). All attempts to isolate this RC from the membranes failed.
Fig. 4. Absorption spectrum of chromatophores F(M197)H + (M203-M212). The second derivative of the spectra is shown at the top.
According to the results of the pigment analysis, the BChl/BPheo ratio in all newly obtained and purified mutant RCs was similar to that in the wild type RC, 2 : 1. Thus, it was shown that gradual enhancement of the symmetry of the BChl dimer protein environment combined with amino acid substitution I(M206)H not only fails to reproduce the covalent bond of BChl PA with L-subunit detected earlier in RC I(L177)H, but also leads to poor assembly of the mutant complexes in the membranes.
DISCUSSION
Although Rba. sphaeroides RCs displays two-fold rotational structural symmetry, there are some fundamental differences in interactions of different sides of the BChl dimer with its protein environment: 1) highly conservative amino acid residues of transmembrane α-helices of the L- and M-subunits on both sides of P molecule reveal low homology; 2) the His-L168 residue is hydrogen-bonded with the 31-acetyl-carbonyl group of BChl PA, while the Phe residue in symmetric position M197 does not form such contacts with BChl PB; 3) there are few lipid-binding sites in the RC structure, and their location on the surface of the complex is asymmetrical.
In previous studies [10, 11], symmetrization of the cofactor protein environment in Rba. capsulatus RC was carried out to study origins of the photosynthetic electron transfer asymmetry. Near the P molecule, replacements of the long (M187-M204, Sym1 mutant) and short (M194-M204, Sym1-2 mutant; M205-M211, Sym2-1 mutant) fragments of the M-subunit by symmetric fragments of the L-subunit were accomplished. Without focusing on properties of these mutants, for Sym1 mutant decreased RC yield after its purification from the membranes was mentioned [11], while no such information was available for well-characterized Sym1-2 and Sym2-1 mutants [21]. Given very high homology of the amino acid sequences from the P surrounding in Rba. capsulatus and Rba. sphaeroides RCs [22], one can assume that symmetrization of the short M203-M205 fragment should not disturb assembly of the Rba. sphaeroides RC in the membrane, although in case of the longer fragment M203-M212 such effect is possible.
Apparently, reduction of the yields of the RC I(M206)H and other mutant RCs obtained in this work is primarily associated with Ile–His substitution at M206 position. After introduction of the additional H-bond between the BChl PB and protein using F(M197)H mutation, spectral heterogeneity of the P absorption band in the mutant RCs disappeared, but the yield after purification remained low. It is noteworthy that spectral properties of RC with five amino acid substitutions F(M197)H + G(M203)M + L(M204)I + S(M205)A + I(M206)H and RC I(L177)H are very similar, but new pigment–protein interactions observed in RC I(L177)H, namely, covalent bond of the BChl PA with the L-subunit and hexa-coordination of BChl BB were detected in none of the other mutant RCs obtained in this study.
Given the close distance between the M206 position and the glycolipid-binding site (Fig. 1), it can be assumed that the I(M206)H mutation and possibly further replacements aimed to enhance the symmetry of the BChl dimer protein environment may have disturbed the lipid binding on the RC surface that is important for the complex stability [4]. It is known that from one to several lipids can be found in bacterial RC structures currently deposited in PDB [8]. One of them is cardiolipin, which is detected more often. There are also data on phosphatidylcholine and glycolipid binding [4]. It is noted that the lipids have conservative binding sites on the RC surface and appear to play a structural role, reinforcing unstable protein areas by electrostatic and hydrophobic interactions [8]. In a recent study [23], it was shown that residues contacting cardiolipin are also highly conservative, and the mutations that disrupt the lipid binding result in decrease in the thermostability of the RCs [24]. Some of the lipids may be lost during RC isolation and purification; therefore, in many bacterial RC structures detergent molecules are modeled in the putative sites of lipid binding.
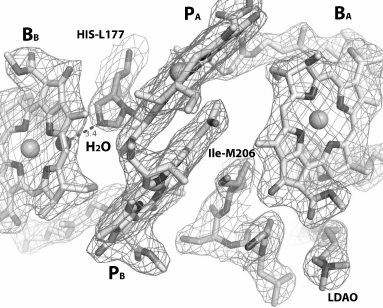
In the RC structure obtained at 2.55 Å resolution (PDB 1M3X), the glycolipid glucosyl-galactosyl diacylglycerol was detected, which interacted mainly with the M-subunit, as well as with single transmembrane helix of the H-subunit (Fig. 1a) [4]. The polar disaccharide moieties of the lipid reside at 3.5 Å distance from BChl BA, and the nonpolar glycolipid tail interacts with the isoprenoid chain of the QA molecule. It is considered that glycolipid provides a hydrophobic environment for the monomer BChl in the active electron transfer branch, and it possibly stabilizes the RC structure in the area of the H- and M-subunits interaction. In Rhodopseudomonas (Blastochloris) viridis RC, electron density was found in the same location, which was interpreted as a detergent molecule [2]. These data support the suggestion that the glycolipid-binding site is conservative in many bacterial RCs. In the structure of RC I(L177)H at 2.98 Å distance from BChl BA, an LDAO molecule was also detected (Fig. 5).
Fig. 5. Structure of RC I(L177)H near bacteriochlorophylls at resolution 2.9 Å (PDB ID 1E6D [5]). BChl dimer PA and PB, monomer BChls BA and BB, LDAO molecule, fragment M203-M206 in the lower part of the figure between BChl PB and LDAO, as well as His L177 and Ile M206, are shown.
It was shown that in the RC structure near the BChl monomer, the polar disaccharide moiety of glycolipid that is able to participate in ionic interactions is close to Leu-M204, Phe-M208, and Trp-M268 residues from the M-subunit and to Leu-H24 and Ile-H28 residues from the H-subunit [4]. It can be assumed that upon replacement of the M203-M206 fragment by symmetrical L174-L176 fragment, the properties of the protein-binding site of the polar glycolipid moiety are changing due to substitution of Gly-M203 by Met residue, Leu-M204 by Ile residue, and weakly polar Ser-M205 by nonpolar Ala residue. However, it is obvious that the main destabilizing influence on the RC assembly in the membranes is caused by introduction of the polar His M206, which significantly violates the protein–protein and protein–lipid interactions near BChls of the A branch of electron transfer. It is known that the dipole moment of the His imidazole group in its neutral state is the largest among side groups of other polar residues [25]. Without establishing stable interactions with nearby molecules, appearance of a His residue in a hydrophobic environment can drastically destabilize a membrane protein structure. In RC I(L177)H, the His-L177 introduced in symmetric site forms a covalent bond with the BChl PA and a H-bond with the new water molecule from the β-side of the BChl BB, as evidenced by the mutant structure obtained at 2.9 Å resolution (Fig. 5). Although RC I(L177)H is less stable than the WT RC, their yields after purification are comparable. Probably the presence of the glycolipid near BChl BA puts obstacles in the way of such interactions in RC I(M206)H, which leads to destabilization of the complex as well as preventing reproduction of the covalent BChl binding with protein observed in the RC I(L177)H.
The mutant RC I(M206)H + H(M202)L can serve as an example of a stable RC in which the I(M206)H substitution does not create problems for the complex assembly in the membranes. In our recent study, it was shown that His ligands of the BChl dimer P can be moved from their native positions L173 (ligand of BChl PA) and M202 (ligand of BChl PB) one turn down to positions L177 and M206, respectively [5, 26, 27]. Carrying over the ligands results in outstanding blue shifts of the QY P absorption bands that are obviously provoked by displacement of the BChl macrocycle planes in the dimer relative to each other and, as a result, by change in exciton interaction inside the special pair P. Molecular modeling based on the RC I(L177)H structure suggests that the imidazole side group of His-L177 in RC I(L177)H + H(L173)L reorients and turns towards the central Mg2+ atom of BChl PB. Similarity of the spectral properties and pigment composition of the two double mutant RCs suggests similar structural changes near the sites of the mutations. It was noted that, despite the presence of “destabilizing” mutation I(M206)H, reaction center I(M206)H + H(M202)L is stable and its yield after purification is comparable to that of the wild type RC. Apparently, presumably stable interaction of His-M206 with the Mg2+ atom of BChl PB removes obstacles to glycolipid binding and thus contributes to the normal assembly of the complex. More detailed information on changes in the protein conformation in RC I(M206)H + H(M202)L can be provided by its crystal structure, and such work is currently under way.
Thus, different effects of similar amino acid substitutions in the symmetric positions M206 and L177 in the photosynthetic reaction center of Rba. sphaeroides are apparently related to the presence of the polar moiety of glycolipid near BChl BA. Introduction of His-M206, as well as other amino acid residues, during enhancement of the symmetry of the P protein environment, probably prevents binding of the glycolipid and, as a result, destabilizes the RC structure.
This work was supported by the Federal Agency for Science and Innovations, by the program “Molecular and Cell Biology”, by a grant of the President of the Russian Federation (Science Schools-4771.2014.4), and by the Russian Foundation for Basic Research (12-04-00332, 13-04-40297 H, 15-04-03041).
REFERENCES
1.Allen, J. P., Feher, G., Yeates, T. O., Komiya, H.,
and Rees, D. S. (1987) Structure of reaction center from
Rhodobacter sphaeroides R-26: the cofactors, PNAS,
84, 5730-5734.
2.Deisenhofer, J., and Michel, H. (1989) Nobel
lecture. The photosynthetic reaction center from the purple bacterium
Rhodopseudomonas viridis, EMBO, 8,
2149-2170.
3.Ermler, U., Fritzsch, G., Buchanan, S. K., and
Michel, H. (1994) Structure of the photosynthetic reaction center from
Rhodobacter sphaeroides at 2.65 Å resolution: cofactors
and protein–cofactor interactions, Structure, 2,
925-936.
4.Camara-Artigas, A., Magee, C., Goetsch, A., and
Allen, J. P. (2002) The structure of the heterodimer reaction center
from Rhodobacter sphaeroides at 2.55 Å resolution,
Photosynth. Res., 74, 87-93.
5.Vasilieva, L. G., Fufina, T. Y., Gabdulkhakov, A.
G., Leonova, M. M., Khatypov, R. A., and Shuvalov, V. A. (2012) The
site-directed mutation I(L177)H in Rhodobacter sphaeroides
reaction center affects coordination of PA and BB
bacteriochlorophylls, Biochim. Biophys. Acta, 1817,
1407-1417.
6.Potter, J. A., Fyfe, P. K., Frolov, D., Wakeham, M.
C., van Grondelle, R., Robert, B., and Jones, M. R. (2005) Strong
effects of an individual water molecule on the rate of primary charge
separation in the Rhodobacter sphaeroides reaction center, J.
Biol. Chem., 280, 27155-27164.
7.Yakovlev, A. G., Jones, M. R., Potter, J. A., Fyfe,
P. K., Vasilieva, L. G., Shkuropatov, A. Y., and Shuvalov, V. A. (2005)
Primary charge separation between P* and BA:
electron-transfer pathways in native and mutant GM203L bacterial
reaction centers, Chem. Phys., 319, 297-307.
8.Jones, M. R. (2007) Lipids in photosynthetic
reaction centers: structural roles and functional holes, Progr.
Lipid Res., 46, 56-87.
9.Leonova, M. M., Fufina, T. Y., Shuvalov, V. A., and
Vasilieva, L. G. (2014) Study of pigment–protein interactions in
the photosynthetic reaction center of the purple bacteria, in Modern
Problems in Photosynthesis (Allakhverdiev, S. I., Rubin, A. B., and
Shuvalov, V. A., eds.) [in Russian], Institute of Computer Studies,
Moscow-Izhevsk, pp. 157-196.
10.Robles, S. J., Breton, J., and Youvan, D. C.
(1990) Partial symmetrization of the photosynthetic reaction center,
Science, 248, 1402-1405.
11.Taguchi, A. K. W., Stocker, J. W., Alden, R. G.,
Causgrove, T. P., Peloquin, J. M., Boxer, S. J., and Woodbury, N. W.
(1992) Biochemical characterization and electron-transfer reactions of
syml, a Rhodobacter capsulatus reaction center symmetry
mutant which affects the initial electron donor, Biochemistry,
31, 10345-10355.
12.Hughes, A. V., Rees, P., Heathcote, P., and
Jones, M. R. (2006) Kinetic analysis of the thermal stability of the
photosynthetic reaction center from Rhodobacter sphaeroides,
Biophys. J., 90, 4155-4166.
13.Jones, M. R. (2009) in Advances in
Photosynthesis and Respiration, Vol. 28 (Hunter, C. N., Daldal, F.,
Thurnauer, M. C., and Beatty, J. T., eds.) Springer, pp. 295-321.
14.Holden-Dye, K., Crouch, L. I., Williams, C. M.,
Bone, R. A., Cheng, J., Bohles, F., Heathcote, P., and Jones, M. R.
(2011) Opposing structural changes in two symmetrical polypeptides
bring about opposing changes to the thermal stability of a complex
integral membrane protein, Arch. Biochem. Biophys., 505,
160-170.
15.Bolgarina, T. I., Khatypov, R. A., Vasilieva, L.
G., Shkuropatov, A. Y., and Shuvalov, V. A. (2004) Substitution of the
Ile M206 by His in reaction center of Rhodobacter sphaeroides
affects structure of the special pair bacteriochlorophyll, Doklady
Biol. Sci., 394, 265-268.
16.Khatypov, R. A., Vasilieva, L. G., Fufina, T. U.,
Bolgarina, T. I., and Shuvalov, V. A. (2005) Biochemistry
(Moscow), 70, 1256-1261.
17.Fufina, T. Y., Vasilieva, L. G., Khatypov, R. A.,
Shkuropatov, A. Y., and Shuvalov, V. A. (2007) Substitution of
isoleucine L177 by histidine in Rhodobacter sphaeroides reaction
center results in the covalent binding of PA
bacteriochlorophyll to the L subunit, FEBS Lett., 581,
5769-5773.
18.Fufina, T. Y., Vasilieva, L. G., Khatypov, R. A.,
and Shuvalov, V. A. (2011) Properties of the Rhodobacter sphaeroides
photosynthetic reaction center with double amino acid substitution
I(L177)H + H(M182)L, Biochemistry (Moscow), 76,
450-454.
19.Van der Rest, M., and Gingras, G. (1974) The
pigment complement of the photosynthetic reaction center isolated from
Rhodospirillum rubrum, J. Biol. Chem., 249,
6446-6453.
20.Nolding, B. (2004) Methods in Modern
Biophysics, Springer-Verlag, Berlin-Heidelberg.
21.Taguchi, A. K., Eastman, J. E., Gallo, D. M.,
Sheagley, E., Xiao, W., and Woodbury, N. W. (1996) Asymmetry
requirements in the photosynthetic reaction center of Rhodobacter
capsulatus, Biochemistry, 35, 3175-3186.
22.El-Kabbani, O., Chang, C. H., Tiede, D., Norris,
J., and Schiffer, M. (1991) Comparison of reaction centers from
Rhodobacter sphaeroides and Rhodopseudomonas viridis:
overall architecture and protein–pigment interactions,
Biochemistry, 30, 5361-5369.
23.Wakeham, M. C., Jones, M. R., Sessions, R. B.,
and Fyfe, P. K. (2001) Is there a conserved interaction between
cardiolipin and the type II bacterial reaction center? Biophys.
J., 80, 1395-1405.
24.Wakeham, M. C., Frolov, D., Fyfe, P. K., van
Grondelle, R., and Jones, M. R. (2003) Acquisition of photosynthetic
capacity by a reaction center that lacks the Q(A) ubiquinone; possible
insights into the evolution of reaction centers, Biochim. Biophys.
Acta, 1607, 53-63.
25.Sandberg, L., and Edholm, O. (2001) Calculated
solvation free energies of amino acids in a dipolar approximation,
J. Phys. Chem., 105, 273-281.
26.Fufina, T. Y., Vasilieva, L. G., Khatypov, R. A.,
and Shuvalov, V. A. (2013) Spectral properties of the Rhodobacter
sphaeroides mutant photoreaction center with double amino acid
substitution I(L177)H + H(L173)L, in Materials of the 15th Int.
Conf. “Photosynthesis: Research for Food, Fuel, and
Future, Adv. Top. Sci. Technol. China, pp. 46-49.
27.Vasilieva, L. G., Fufina, T. Y., Gabdulkhakov, A.
G., Khatypov, R. A., and Shuvalov, V. A. (2014) Relocation BChl axial
ligands in Rhodobacter sphaeroides mutant reaction centers, in
Abstr. Int. Meet. “Photosynthesis Research for
Sustainability”, Pushchino, Russia, p. 52.