REVIEW: Carbonic Anhydrases in Photosynthetic Cells of Higher Plants
N. N. Rudenko, L. K. Ignatova, T. P. Fedorchuk, and B. N. Ivanov*
Institute of Basic Biological Problems, Russian Academy of Sciences, 142290 Pushchino, Moscow Region, Russia; fax: (4967) 330-532; E-mail: ivboni@rambler.ru* To whom correspondence should be addressed.
Received January 13, 2015; Revision received February 27, 2015
This review presents information about carbonic anhydrases, enzymes catalyzing the reversible hydration of carbon dioxide in aqueous solutions. The families of carbonic anhydrases are described, and data concerning the presence of their representatives in organisms of different classes, and especially in the higher plants, are considered. Proven and hypothetical functions of carbonic anhydrases in living organisms are listed. Particular attention is given to those functions of the enzyme that are relevant to photosynthetic reactions. These functions in algae are briefly described. Data about probable functions of carbonic anhydrases in plasma membrane, mitochondria, and chloroplast stroma of higher plants are discussed. Update concerning carbonic anhydrases in chloroplast thylakoids of higher plants, i.e. their quantity and possible participation in photosynthetic reactions, is given in detail.
KEY WORDS: carbonic anhydrase, carbonic anhydrase families, plants, photosynthesis, chloroplasts, thylakoidsDOI: 10.1134/S0006297915060048
Abbreviations: CA, carbonic anhydrase; PSI(II), photosystem I(II); Rubisco, ribulose-bisphosphate carboxylase/oxygenase.
Carbonic anhydrases catalyze the reaction of the reversible hydration of
carbon dioxide:
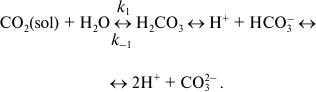 (1)
(1)This reaction in the absence of carbonic anhydrase (CA) has second-order rate constant k1 of 0.0027 M–1·s–1 at 37°C and neutral pH, which corresponds to a pseudo first-order rate constant of 0.15 s–1. The dehydration reaction constant k–1 is 50 s–1. These constants determine [CO2]/[H2CO3] ratio of 340 : 1 in aqueous solutions. Carbonic acid, H2CO3, dissociates in aqueous solution with pK1 of 6.35 and pK2 of 10.25, i.e. at neutral pH the inorganic carbon in the form of bicarbonate ion (HCO3–) predominates. Therefore, reaction (1) is often written without carbonic acid. Carbonic anhydrases (CAs) greatly accelerate both reactions, especially the hydration reaction. The most active enzyme, human carbonic anhydrase II, has kcat of CO2 hydration reaction greater than 106 s–1, while the rate constant of the dehydration reaction is 2.5·105 s–1 [1]. The catalyzed reaction of CO2 hydration is one of the fastest enzymatic reactions. CAs from plants usually have lower values of the respective reaction constants; the value of kcat of the hydration reaction for the soluble CA from pea is 4·105 s–1 [2].
CARBONIC ANHYDRASE FAMILIES AND THEIR REPRESENTATIVES IN LIVING
ORGANISMS
CA was found for the first time in red blood cells in 1933 [3]. Later, many biochemical studies revealed these enzymes in all organs, tissues, and cells of living organisms from prokaryotes to humans [4]. Based on the conserved sequences of nucleic acids in CAs gene sequences, all CAs are subdivided into six evolutionarily independent families [5, 6] that are named α, β, γ, δ, ε, and ζ. Although these enzymes do not have homology in structure and they differ in the organization of the active center, they are all called carbonic anhydrases because they catalyze the same reaction, using similar catalytic mechanisms, which are different in details of the environment of the metal atom (mostly, zinc) in the active site. CAs are believed to have originated repeatedly in the course of evolution, and it is intriguing that many organisms express genes of CAs from more than one family. There is a striking example, the unicellular diatom Thalassiosira pseudonana, that expresses CAs of at least four families: two α-CAs, four γ-CAs, four δ-CAs, and one ζ-CA [6].
α-CA. Representatives of the α-CA family are widespread and are found in eubacteria [7, 8], ascomycetes [9], algae [10, 11], higher plants [12-14], and animals. In tissues of vertebrate animals, there are 16 isoforms of CAs, and all are from the α-family [15]. Despite this fact, they have significant differences in active site structure, and this determines their activity levels.
The first α-CAs that were found in plant cells were periplasmic enzymes from the unicellular green alga Chlamydomonas reinhardtii, and they were named CAH1 and CAH2 [10, 11]. Despite high structural similarity, CAH1 was highly expressed at low CO2 concentration in water, whereas CAH2 had rather low expression level under all growth conditions, but it was somewhat higher at high concentrations of carbon dioxide. Another α-CA is the membrane-bound form of the enzyme called CAH3, which was found in photosystem II (PSII) particles isolated from Ch. reinhardtii [16].
In the Arabidopsis thaliana genome, eight genes encoding α-CAs have been revealed, but convincing data about the location of only one of them, α-CA1, were obtained. α-CA1 is present in all plant organs except roots [12]. This CA was found using western blot analysis in the study of a recently discovered pathway of newly synthesized proteins into the chloroplast with participation of the Golgi apparatus membrane system [13]. Information concerning the other α-CAs from A. thaliana is scarce. The gene encoding α-CA2 is expressed in stems and roots, and α-CA3 in flowers and pods [12]. The latter CA was found using proteomic analysis of proteins isolated from mature pollen [17, 18]. Using the same approach, α-CA4 was found among the proteins of thylakoid membranes [19, 20].
Most α-CAs are monomers with molecular mass of about 30 kDa; one of the few exceptions is CAH1 from Ch. reinhardtii; it is heterotetramer composed of two subunits of 37 kDa and two subunits of 4 kDa [21]. The active site of α-CAs is a conical cavity with slightly distorted tetrahedral geometry with a Zn atom located at the bottom that is coordinated with three histidine residues [22]. In addition to interconversion of CO2 and HCO3–, α-CAs catalyze a number of other reactions such as hydration of aldehydes [23] and hydrolysis of carboxylated esters [24] and various halogen derivatives [25].
β-CA. In 1939, Neish [26] reported the presence of CA among the proteins of chloroplasts, but only 50 years later, after elaboration of DNA sequence technology, the fact that the enzyme is not homologous to CAs from animal tissues was shown. After that CAs were divided into two families – α-CAs from animal tissues and β-CAs from plant tissues [27]. Representatives of the β-CAs were found in bacteria cells [28-31], fungi from the genus Saccharomyces [32], algae [33, 34], and dicotyledonous and monocotyledonous higher plants, both C3 and C4 [35-37]. There is no other family of CAs with such broad structural diversity. The molecular mass of β-CAs varies from 45 to 200 kDa [38]. Dimers (or pseudo-dimers) are fundamental structural units of β-CAs, and these can associate to form tetramers and octamers. The monomers contain one zinc atom coordinated by two cysteine residues and one histidine residue per reaction center [39].
In C3 higher plants, the soluble CA in chloroplasts is the enzyme with highest catalytic rate (see above). This is the most abundant enzyme in cells after the Rubisco (ribulose-bisphosphate carboxylase/oxygenase) protein, and it is one of the main components of the soluble proteins of leaves (0.5-2.0% of total) [40]. Two isoforms of the soluble enzyme had been observed in leaf tissues [41], and later the expression of two different genes of β-CAs in arabidopsis leaves was revealed [37]. For a long time these two CAs were considered as the only ones in C3 plant cells, with the second CA located in the cytoplasm [42]. In the A. thaliana genome, there are six genes encoding β-CAs, and all these genes are expressed [12]. The authors of [12] proposed the numbering of α- and β-CAs that is now used in the literature. The same group of researchers, using incorporation of the green fluorescent protein gene (GFP-fusion), confirmed the location of two of the most active of CAs, called β-CA1 (stromal) and β-CA2 (cytoplasmic). The locations of the other β-CAs in Arabidopsis cell have also been shown: β-CA3 in the cytoplasm, β-CA4 in plasmalemma, β-CA6 in the mitochondrial matrix, and β-CA5 in chloroplasts. The precise position of the latter has not yet been established.
γ-CA. The first representative of the γ-CA family was found in the Archaean Methanosarcina thermophila [43], and it is now known that γ-CAs are present not only in bacteria cells but also in diatoms and green algae [6]. In higher plants, γ-CAs were found in mitochondria (see below). The structure of γ-CAs is significantly different from those of α- and β-CAs. The former function as trimers consisting of identical subunits with mostly β-helix fold structure [44], and they contain one atom of zinc per subunit. Unlike α- and β-CAs, each active site of γ-CAs is located at the interface between two neighboring subunits. The active site of γ-CAs is formed by three residues of histidine and H2O, which coordinate the Zn atom, like the active site of α-CAs, but histidines in γ-CAs are residues of two opposing subunits [44].
δ-CA and ε-CA. Representatives of these families that were found in cells of the diatom Thalassiosira weissfloggi and of the bacterium Thiobacillus neapolitanus, respectively, have no homology in amino acid sequences with the CAs of the other families, although the structure of their active site is similar to those of representatives of the α-family [45-47].
ζ-CA. These CAs were found in marine unicellular organisms, in particular, the diatom Th. pseudonana [6], and its structure is similar to those of β-CAs [48, 49]. All ζ-CAs are pseudo-trimers with three slightly different catalytic centers [50] containing Cd2+ instead of Zn2+. It is believed that in the diatom cells that δ- and ζ-CAs play a role in CO2 fixation [50].
FUNCTIONS OF CARBONIC ANHYDRASES
The presence of carbonic anhydrases in all living organisms probably originates because the components of the catalyzed reaction are involved in almost all metabolic processes. We suppose that the main reason for the abundance of CAs in nature is the ubiquitous involvement of bicarbonate buffers in aqueous media and then in the cytoplasm at every stage of evolution; these enzymes acceleration achieving appropriate pH, which is important for homeostasis of every living cell. The role of carbonic anhydrases in accelerating the conversion of inorganic forms of carbon was probably not their initial main function, but now it is established that these enzymes are critical regulators of the exchange of these forms in many metabolic processes.
Functions of Carbonic Anhydrases in Animals
Although the main purpose of this review is to consider the available experimental data and ideas concerning the role of CAs in higher plants, we should present, at least briefly, information about CA functions in animal tissues, which has been studied in much more detail and is rather well known. It is now established that CAs in animals provide quick access to “stored” bicarbonate, for example, in lungs, the increase in metabolic CO2 efflux from the eye lens [51], and the catalysis of the production of HCO3– as an ion participating in Na+ symport in secretory tissues [52]. CAs play a role in maintaining the balance of electrolytes and pH in cells and tissues [53] and in electrolyte transport in the cells of the Malpighian vessels in Drosophila [54]. The soluble CA in mitochondria of the cells of vertebrates regulates the activity of the oxidative phosphorylation enzymes: bicarbonate converted with the participation of CA from CO2 released in the Krebs cycle activates the soluble adenylyl cyclase, which produces cyclic AMP and triggers protein kinase, which in its turn affects the operating of all mitochondrial complexes [55]. These studies have shown that the mechanisms of CO2, HCO3–, and/or pH sensing in vertebrate cells include CA, and the connection between chemoreception and signaling is executed with participation of widespread secondary messengers.
Functions of Carbonic Anhydrases in Algae
The functions of CAs related to photosynthesis are well studied in aquatic photoautotrophs, cyanobacteria, and algae. Despite the fact that the concentration of carbon dioxide in water is approximately same as in air, the rate of diffusion of CO2 molecules in water is about 1000 times less [56], and without CA cyanobacteria and algae would experience deficiency of CO2 in the vicinity of Rubisco, which would not permit effective photosynthesis. In the course of evolution, these organisms have developed mechanisms of CO2-concentrating in cells. These mechanisms necessarily include CAs and consist of a system of active transport of inorganic carbon through the plasma membrane and/or chloroplast envelope and systems of CO2-concentrating near Rubisco molecules. The role of CAs in the CO2-concentrating mechanism in cyanobacteria has been thoroughly reviewed recently [57].
The mechanisms of CO2 concentration in the unicellular alga Ch. reinhardtii are similar to those in cyanobacteria [58]. In algal cells, CAs of α-, β-, and γ-families function mutually for CO2 concentrating. In Ch. reinhardtii, at least 12 CAs have been found in different cell compartments [6, 58]. CAH1, CAH8, and CAH9 are probably responsible for the delivery of CO2 in the cells. The first is located in the periplasmic space. If the activity of the protein is suppressed by an inhibitor that could not penetrate into the cell, photosynthesis at high environmental pH with large amounts of inorganic carbon in the form of bicarbonate was significantly reduced [59]. This suggests that CAH1 facilitates the entry of CO2 into the cell. CAH8 is located in the plasma membrane, and it facilitates penetration of CO2 through the membrane, enhancing the conversion of HCO3– into CO2 on the cell surface [60]. CAH9 apparently promotes CO2 transport from cytoplasm to chloroplast. CAH3, located on the lumenal side of thylakoid membrane [16], presumably catalyzes the conversion of HCO3– in the lumen to CO2, i.e. it helps CO2 “generation” to a higher level in lumen than in the rest of the volume of the chloroplast. Mutants of Ch. reinhardtii, either without CAH3 or with greatly reduced CAH3 content, could not grow under normal CO2 levels in the air, although they could survive under increased CO2 concentration [16].
In Chara algae, bicarbonate can enter the cell by the H+/HCO3– symport mechanism, and extracellular CA serves as a “trap” of CO2 [61, 62]. In this context, it should also be mentioned that water-living angiosperms, in order to increase the CO2 uptake, pump protons into the periplasmic space [63]. This process in the presence of external CA, which is situated there, increases external CO2 concentration. This facilitates its diffusion into the cell.
Functions of Carbonic Anhydrases in Higher Plants
Plasma membrane carbonic anhydrase. One of the authors of this review, using inhibitor analysis, showed the presence of CA in plasma membrane of photosynthetic cells from pea leaves [64], and soon after this CA activity of plasma membrane preparations was registered for a number of plant species [65]. Later the CA activity in plasma membrane was confirmed to be important for inorganic carbon transport into the cell [66]. It was found that the CA activity of intact protoplasts protected from adhesion of CA freed from disrupted cells during the separation procedure was about 5% of the total activity of lysed protoplasts. Since the plasma membrane volume is only 0.3% of the protoplast volume, the density of the carrier of CA activity in plasma membranes is very high. The value of Km(CO2) was determined for intact protoplasts from pea [67]. The value reflects mainly the property of CA in plasma membrane; its magnitude, 104 mM, is significantly higher than Km(CO2) of soluble CA that was determined in the same study as 20 mM. Based on experiments with pea protoplasts, the function of plasma membrane CA was proposed [68]. The sense is that the reaction catalyzed by the plasma membrane CA consumes intramembranous CO2, whose concentration within the membrane is significantly higher than in water due to its higher solubility in lipids. It is assumed that the protons that are released in this reaction enter the cytoplasm down the concentration gradient, and the bicarbonate ions move there in symport with them. Using the GFP-fusion method, it was recently shown that one of the A. thaliana CAs, namely, β-CA4, is associated with the plasma membrane [12].
Carbonic anhydrase in cytoplasm. The CA content in cytoplasm is rather high; in potato leaves, it is up to 13% of the total content of soluble CAs [42]. In the cytoplasm of A. thaliana, there are two CAs of the β-family: β-CA2 and β-CA3 [12]. The expression level of one of the cytoplasmic CAs, β-CA2, decreased when A. thaliana plants were grown under high light conditions (Rudenko, unpublished data). The total activity of cytoplasmic CA from pea increased by the action of abscisic and jasmonic acids. These facts allow us to classify cytoplasmic CAs as enzymes significant for plant stress response [69].
In addition to the assumptions that cytoplasmic CAs are involved in inorganic carbon transport into chloroplasts and apparently mitigate pH changes under the influence of external factors (see details in review [70]), it is considered that cytoplasmic β-CA in mesophilic cells of C4 plants provides phosphoenolpyruvate carboxylase with bicarbonate [71], thus promoting the primary reaction of carbon fixation in C4 photosynthesis.
Carbonic anhydrase in mitochondria. After three genes that code γ-CAs from A. thaliana were inserted into the E. coli genome, three proteins that were able to incorporate into the mitochondria were obtained [72]. Later, in mitochondrial complex I from A. thaliana, a spherical domain consisting of five γ-CAs was revealed. This domain was attached to the central part of its membrane arm and was very tightly attached to the respiratory complex [73]; according to the authors, this domain was physiologically linked to a pore-like structure within complex I involved in proton translocation. Three CAs of the domain, γ-CA1, γ-CA2, and γ-CA3, were homologous to CAs from M. thermophila, and two were called γ-CAL1 and γ-CAL2 (“gamma carbonic anhydrase-like”) [74]. Besides membrane-bound CAs, β-CA6, the product of the At1g58180 gene, was found in the mitochondrial matrix [12].
At high rate of CO2 fixation, its concentration in chloroplasts can be significantly reduced if for some reason (for example, when stomata are closed) its flow from air is limited. It has been suggested that CO2 that is released in the reactions of Krebs cycle in mitochondria may enter the chloroplasts. According to this hypothesis, a system of active transport of HCO3– from mitochondria into chloroplasts is formed with participation of both the CA domain and β-CA6 [75].
Carbonic anhydrases in chloroplast stroma. In C3 higher plants, as previously noted, the main CA is β-CA1 located in the chloroplast stroma. This compartment is responsible for incorporation of inorganic carbon into organic compounds with the participation of the Rubisco enzyme. Carbon dioxide is the substrate for this reaction, while the bicarbonate is the main form of inorganic carbon in weakly alkaline medium in stroma. According to calculations, relatively slow conversion of bicarbonate into CO2 during high rate of consumption of the latter in the reaction with ribulose bisphosphate (so-called CO2 fixation) would limit the photosynthetic process, therefore not providing the experimentally observed rate of this process. It seems logical to assume the participation of this CA in acceleration of interconversion of inorganic carbon forms and, accordingly in supply of CO2 molecules for their fixation. In vitro studies that were done using partially purified Rubisco from wheat have shown that in the presence of CA, Km(CO2) of carboxylase was reduced [76]. In plants grown at elevated CO2 concentrations, there was a significant decline in the activities of both CA and Rubisco [77]. Experiments with spinach showed that the inhibition of exogenously added CA led to suppression of carboxylase activity and increase in oxygenase activity of Rubisco [77]. Later, study of CA gene expression in cotton seedlings showed that the steady-state levels of Rubisco and CA gene transcripts were increased in response to decrease in CO2 concentration, whereas the enzyme activities changed at the posttranslational level along with the development of functional chloroplasts after illumination of seedlings [78].
Despite the apparent interaction of the activities of CA and Rubisco, in subsequent studies the direct participation of stromal CA in photosynthesis was not established. Price et al. [79] found that in transgenic Nicotiana tabacum plants, where stromal CA level was suppressed using an antisense construct directed against mRNA of this CA, and in primary transformants with (expression) levels of stromal CA as low as 2% of the wild-type levels, there were no significant morphological alterations. Despite the reduction of stromal CA content, there were no significant differences in Rubisco activity, chlorophyll content, stomatal conductance, dry weight per unit leaf area, as well as in the intercellular to ambient CO2 partial pressures ratio. However, the carbon isotope composition of leaf dry matter was changed for the mutant plants; it corresponded to reduction in the CO2 partial pressure of at sites of carboxylation to 15 µBar. The authors concluded that even a reduction in stromal CA activity of two orders of magnitude had no significant effect on photosynthesis at ambient CO2 level; however, they assumed that 100% activity of CA is likely to contribute to the facilitation of CO2 transfer within the chloroplast, thereby making photosynthesis of C3 plants as efficient as possible.
Also, in transgenic tobacco plants with inhibition of up to 99% of the stromal CA activity, there were no significant effect on CO2 assimilation, but an increase in stomatal conductance and susceptibility to water stress was found [80]. Western blot analysis confirmed the absence of stromal CA in the transgenic plants, but the total CA activity in leaves was only 3.5 times less than in wild-type plants. Obviously, there were other CAs in leaves, but at that time, the researchers did not pay attention to this point. According to the same authors, in leaves of transgenic stromal CA-overexpressing tobacco plants, there were no effect on CO2 assimilation, but concomitant increase in Rubisco activity was observed.
The next attempt to investigate the physiological role of β-CA1 was made 14 years later, after the development of methods to produce knockout mutants at a desired gene. During germination of seeds of A. thaliana mutant plants with knockout of the gene encoding β-CA1 on sterile artificial media at ambient CO2 level, a significant decrease in germinating ability was observed. This ability was recovered to that of the wild type during growth under elevated CO2 level (1500 µl/liter) or after addition of sucrose to the medium [81]. Seeds from mutant plants had no significant differences in protein or lipid content or mobilization of reserve components during seedling growth and exhibited reduced capacity for light-dependent 14CO2 assimilation only prior to the development of true leaves. It was previously found that biosynthesis of ethylene, which is required for seed germination, was CO2-dependent [82], and for the normal development of a seedling the fast interconversion of HCO3– to CO2 in alkaline stroma may be necessary. Perhaps the data of [81] could be explained by the fact that the decrease in intracellular CA activity led to reduced production of ethylene and therefore the processes necessary for normal development of seeds were slowed.
There is increasing evidence that the stromal CA plays a role in plant stress defense. The expression in yeast of the alfalfa gene encoding CA revealed that the protein produced possessed antioxidant activity [32]. It was established that the stromal CA exhibited not only CA activity, but also the ability to bind salicylic acid (SA), which plays an important role in initiating a signaling cascade leading to increase in expression of genes as well as to an increase at a posttranslational level of the activity of proteins involved in plant protection from oxidative stress [83]. It was found that in potato plants infected with Phytophthora, the expression of the gene encoding this CA increased during the first 12 h after inoculation, but in the following 24 h, it showed significant repression [84]. The same effect was observed in tomato leaves exposed to fusicoccin fungal toxin [85] and in Arabidopsis plants after treatment with methyl jasmonate [86]. In Nicotiana benthamiana plants with knockout of the gene encoding stromal CA, more rapid development of late blight appeared [84]. The authors hypothesized that the role of stromal CA in protecting from stress may be connected with its participation in lipid biosynthesis, since it was previously shown [78] that reducing CA content led to suppression of fatty acid biosynthesis and, as a consequence, to a decrease in the expression level of jasmonic acid (JA), which induces the expression of defense response genes. For biosynthesis of JA, ω-6 fatty acids that are synthesized in chloroplasts are necessary. According to our opinion, this interpretation ignores the facts that JA and SA are antagonists [87], and for the activation of the JA-dependent defense response genes, SA should be bound, and CA may be important for this. Accordingly, the ambiguous CA gene expression profile after Phytophthora inoculation, namely, first increase and then decrease, may occur because at the beginning JA-dependent defense response genes should be activated by SA-binding, and then, on the contrary, the SA-dependent pathway should be activated. The inhibition of genes of fatty acid biosynthesis after reducing CA content may happen in the same way, because this content may be insufficient for SA binding and thus suppression of JA defense response pathway, but not because CA supplies CO2 for fatty acid biosynthesis.
It was shown that β-CA1 together with β-CA4 is involved in the control of gas exchange between plants and the atmosphere via regulation of stomatal movements [88]. It is known that increase in carbon dioxide concentration in the atmosphere causes stomatal pores in leaves to close; however, the CO2-binding proteins that control this response remained unknown. Knockout of the At3g01500 gene encoding β-CA1 or the At1g70410 gene encoding plasma membrane protein β-CA4 had no effect on the functioning of the stomata apparatus of arabidopsis. However, so-called “double” mutants, plants with two single mutations in genes encoding β-CA4 and β-CA1 showed both impaired regulation of stomata movement and increased stomata conductance. The repressed function apparently relies on the CAs located in guard cells. According to the same authors, plants over-expressing these CA genes demonstrated high efficiency of water use. This led to recognition of the fact that despite the extremely high expression level of the At3g01500 gene, β-CA1 content was still insufficient for plants, at least for agricultural ones, and manipulations with CA genes can give a new approach for developing improved agricultural crops with reduced water loss by transpiration, and improved productivity as a result.
Thus, diverse studies of β-CA1 have shown that its role in higher plants metabolism is complicated, and that the enzyme operates with interaction with other CAs. The results of investigations that were carried out by different research groups using a variety of approaches to study the functions of this CA; however, they are in agreement with the opinion that it is not involved in the delivery of CO2 to the active site of Rubisco.
In one report [89], there is a reference to data for mutant plants with knockout of the gene encoding another chloroplast CA, α-CA1, a decrease in photosynthetic activity and the ability to accumulate starch was observed. This might evidence significance of this CA for photosynthesis, and it is possible that this CA, but not β-CA1, is involved in CO2 transfer to Rubisco.
Carbonic anhydrase in chloroplast thylakoids. Data on the participation of CAs in photosynthesis of algae and the fact that the key reaction of photosynthesis is the fixation into organic compounds of CO2, which is one of the carbonic anhydrase reaction components, continue to stimulate researchers to search for the ways that CAs participate in photosynthesis of higher plants. In the processes taking place in thylakoids, which contain the photosynthetic electron transport chain, the role of other component of the carbonic anhydrase reaction, the proton, is crucial. Moreover, electron transfer on the acceptor side of PSII is dependent on the presence of another component of the reaction, inorganic carbon in the form of bicarbonate. The functioning of CAs in the thylakoids of higher plants has been suggested several times, but their presence there long remained a matter of debate.
The presence of specific carbonic anhydrases in thylakoids. The first information about the presence of CA activity in thylakoids of higher plants was published in the early 1980s [90, 91], but for many years this activity was considered to be the result of contamination of thylakoids during isolation with active and abundant stromal CA. However, experiments showed that the characteristics of the CA activity of thylakoids were different from those of soluble stromal CA: the CA activity of thylakoid membranes was suppressed with 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU) and hydroxylamine, whereas soluble CA was insensitive to them [91]; the CA activity of thylakoids was dependent on the redox potential of the medium [92]; CA inhibitors such as acetazolamide and azide in submicromolar concentrations stimulated CA activity of thylakoids, though the activity of soluble CA was gradually suppressed [93]. The CA activities of thylakoids and soluble enzyme had significantly different values of Km(CO2), 9 and 20 mM, respectively; their dehydration activities had different pH dependences, namely the CA activity of thylakoids had pH maximum of 6.8-7.0, whereas the activity of soluble CA was pH-independent [67]. Finally it was shown with preparations from pea that the soluble CA was cross-reactive with antibodies against spinach β-soluble CA, though the reaction was not observed with thylakoid protein fraction demonstrating the same CA activity [94].
Heterogeneity of carriers of carbonic anhydrase activity in thylakoids. In the early 2000s, information about the presence in thylakoid membranes of more than one carrier of CA activity began to appear. Both preparations of thylakoid membranes enriched with PSI (PSI-membranes) and those enriched with PSII (PSII-membranes) showed CA activity, but only PSII-membranes cross-reacted with antibodies against CAH3, the carbonic anhydrase of the α-family from the green alga Chlamydomonas reinhardtii [95]. The presence of at least two types of CAs in thylakoids free from contamination with stromal CAs was established [94]. One of these activity carriers could be easily removed by treatment with salts at high concentrations, and the second was tightly bound to the membrane. It was also found that after incubation of thylakoids from pea with Triton X-100, there were two peaks of CA activity at Triton/chlorophyll ratios of 0.3 and 1.0 [96, 97]. Bell-shaped response of the activity after treatments with detergents is typical for membrane proteins [98]. The presence of two peaks indicated the presence of at least two CA activity carriers in thylakoid membranes.
Carbonic anhydrase activity of PSII. After treatment of PSII-membranes from pea with Triton, there was only one CA activity maximum at Triton/chlorophyll ratio of 1.0 [96, 97], while there was no effect of the detergent on the CA activity of soluble protein fraction. These facts were additional evidence of the specific nature of the CA activity of thylakoids. Similar stimulation of the CA activities of PSII-membranes from wheat [99] and from arabidopsis [100] was observed after treatment with the same detergent.
Lu and Stemler [101] revealed two sources of carbonic anhydrase activity with distinct properties not only in thylakoids, but also in PSII-membranes from mesophyll of maize. The first (called by those authors “external CA”) can be extracted with salts at high concentrations and with detergents and was available for the measurement of CA activity in both directions, the other (“internal CA”) remained tightly bound to the membrane and showed only hydration activity. However, earlier, using other research materials, the dehydration CA activity of PSII preparations treated with either detergents or salts was observed [102].
The presence of two CA activity sources of high and low molecular mass could be observed after nondenaturing gel electrophoresis of PSII-membranes from pea followed by specific gel staining for CA activity visualization [103]. The presence of CA activity in the stained bands in the gel was confirmed by measurements of the activity of eluates from these bands [103] with a standard method of measurement of this activity by tracking pH changes. A similar distribution of CA activities was observed after electrophoresis of PSII-membranes from arabidopsis [100]. It has been shown that the standard CA inhibitor acetazolamide at concentration of about 10–7 M stimulated CA activity of the low molecular mass fraction eluted from the corresponding gel band after the electrophoresis of PSII-membranes from pea and arabidopsis [100, 103]. At the same time, the specific lipophilic CA inhibitor ethoxyzolamide in nanomolar concentrations suppressed the CA activity of the low molecular mass fraction [103]. The stimulating effect of acetazolamide and sodium azide at low concentrations on the CA activity was observed earlier with undamaged pea thylakoids [93]. Based on the unusual effect of acetazolamide on the CA activity of the low molecular mass protein from PSII, we assume that the low molecular mass CA, which is located close to or directly in this photosystem, belongs to the α-family because the same stimulation was found for α-family CAs from mammals. This stimulation of CA activity by various chemicals, including azoles, one being acetazolamide, resulted from the fact that the rate-limiting step of the CA reaction is the removal of the proton from the reactive center [104], and azoles in low concentrations are able to act as a proton shuttle [105].
The CA activity of the high molecular mass fraction eluted from the corresponding gel band was significantly inhibited by acetazolamide at 10–7 M concentration, but it was weakly sensitive to ethoxyzolamide (Ignatova, unpublished data). Data concerning the nature of the high molecular mass CA activity carrier in PSII-membranes are almost absent. In [94, 99], it was convincingly shown that PSII core-complex possessed CA activity. In work [99], it was assumed that the component of PSII core-complex with CA activity has an active metal-containing center with structure close to those of the known CAs, but the metal in the active center is not necessarily zinc (e.g. the replacement of the zinc with divalent iron in γ-CA from M. thermophila led to increase in the CA activity under anaerobic conditions [39]). According to the authors of [106], the activity of PSII core-complex results from the activity of CaMn4 complex together with its immediate surroundings. It is possible that the high molecular mass activity carrier is not the individual protein but a structure made by amino acids of neighboring proteins, which resembles the structure of CAs of γ-family in which the active site is formed by amino acids of different protein subunits. The different nature of two CA activity carriers in PSII-membranes, namely, a protein and protein complex, may be the reason for the presence of only one peak of CA activity after incubation with Triton X-100 (see above).
In work [107], evidences of CA activities of hydrophilic proteins from the water-oxidation complex, PsbO, PsbP, and PsbQ, were obtained. This activity of PsbO protein appeared only in the presence of Mn2+. Since the CA activity sources had properties that were not characteristics of typical CAs, the authors suggested that they represent a special class of Mn-dependent carbonic anhydrases associated with PSII. According to the same study, after the removal of these proteins the PSII core-complex retained some CA activity that was inhibited by sulfonamides and in like manner, like the hydrophilic PSII proteins, was stimulated by manganese.
Carbonic anhydrase activity of PSI. The CA activity of PSI-membranes from higher plants was first reported in [95]. The CA activity of PSI membranes was equally sensitive to acetazolamide and ethoxyzolamide with I50 of 10–6 M [103]. After Triton X-100 treatment of the PSI-membrane preparations from pea and arabidopsis, the CA activity in both cases had maximum at Triton/chlorophyll ratio of 0.3, which coincided with one of the peaks observed after the treatment of undamaged thylakoids with Triton X-100 [97, 100]. There are still no details concerning the CA that is associated with PSI.
Soluble carbonic anhydrase in thylakoid lumen. After disrupting pea thylakoids with Triton X-100 at high concentrations, soluble protein with CA activity having characteristics different from those of the above-described membrane CA activity carriers was revealed [97]. The mass spectrum of this protein corresponded to those of CAs of the β-family (Rudenko, unpublished). The presence in thylakoid lumen of a specific soluble CA was suggested, and this was later confirmed in experiments with thylakoids from arabidopsis of both the wild-type and mutants with knocked-out gene At3g01500 encoding the stromal β-CA1. These mutants were used to get thylakoids without contaminations by this abundant CA [108]. The protein having the CA activity in the fraction of lumenal proteins was isolated and purified; its apparent molecular mass as determined by native electrophoresis was about 132 kDa. The CA activity of membrane-bound carriers was inhibited by dithiothreitol, while the activity of this soluble CA increased after addition of dithiothreitol, and the latter is typical for CAs of the β-family because they contain many sulfur-containing amino acids [109]. The sensitivity of the soluble lumenal CA to ethoxyzolamide was characterized by I50 value of 5·10–6 M [108], which is also typical for CAs of the β-family [110].
It is tempting to suggest that the soluble lumenal thylakoid CA belonging to the β-family of CAs is β-CA5, which has been found in chloroplasts [12]. The expression level of the At4g33580 gene encoding β-CA5 was two or three orders lower than that of the At3g01500 gene encoding stromal β-CA1, but At4g33580 gene knockout led to significant decrease in plant growth (J. Moroney, private communication). So far, β-CA5 is the only carbonic anhydrase whose lack leads to significant changes in arabidopsis phenotype under normal growth conditions.
Hypothetical functions of thylakoid carbonic anhydrases. Using tobacco leaf discs, it has been shown that ethoxyzolamide, the CA inhibitor that is able to easily pass through membranes, suppresses the photoacoustic signal associated with CO2 absorption [111]. In work [68], it was found that the same inhibitor significantly suppressed CO2-dependent release of O2 in protoplasts of pea leaves. These data can be considered as evidence of the important role of intracellular CA in higher plant photosynthesis. Although we cannot completely exclude the effect of ethoxyzolamide on soluble CAs to be the cause of the inhibition of photosynthesis, nevertheless, taking into account data of [79, 112, 113], as well as the fact that in [68] the effect of ethoxyzolamide was observed at saturating concentrations of CO2, it is likely that this effect is associated with inhibition of CA in thylakoids. Possible functions of thylakoid CAs were earlier considered by Stemler [114]. At that time, there were no data about the presence of several CAs in thylakoids. Ever since, unfortunately, no physiological functions of thylakoid CA have been reliably established, and to describe them we have to compare the CA reaction and the enzyme locations in thylakoid membrane with the stage of photosynthesis that takes place there. We assume a priori that the functioning of CAs in the thylakoids, the main function of which is photosynthesis, is connected directly or indirectly with this process.
Most experimental data and suggestions concerning the role of thylakoid CAs in photosynthetic reactions refers to the processes in PSII, where water oxidation takes place and the electrons derived from water are delivered to terminal acceptors of this photosystem, QA and QB. The conclusion that the functional activity of PSII is associated with CA was made after the first data about the inhibition of thylakoid CA activity by DCMU and hydroxylamine, which are PSII inhibitors [91]. This relationship was confirmed by the fact that specific inhibitors of CA, sulfanilamide compounds and some anions inhibiting CA, suppressed the activity of PSII [115]. It could not be excluded that the activity of PSII was suppressed because of the dysfunction of the water-oxidizing complex. It was found that the features of dependence of the oxygen-evolving function of PSII from maize and pea on Cl– or Ca2+ concentrations was similar to the same dependence of its CA activity [106]. As to the nature of such connection, contradictory results have been obtained in different laboratories: dehydration CA activity was significantly increased after removing external proteins of the water-oxidizing complex [94, 102]; the CA activity of PSII-membranes was decreased by removing the proteins of this complex [116]; relatively high CA activity of PSII-membranes was quite variable, but after removal of proteins of the water-oxidizing complex, CA activity was not observed either in the solubilized proteins or in the core-complex [117]. The ambiguity of experimental data may originate from the different methods of CA activity measuring as well as from the presence of more than one CA activity source even in preparations of PSII-membranes (see above).
It is known that for the physiologically required rate of electron transfer at the acceptor side of PSII, the presence of bicarbonate ions is necessary [118]. In thylakoids, there are two pools of membrane-bound bicarbonate [119]. The first, being the lesser, amounts to one bicarbonate ion per 380-400 chlorophyll molecules; this is approximately matched to the number of PSII reaction centers. The removal of this bicarbonate leads to more than 90% inhibition of oxygen evolution [119], which can be completely restored by addition of bicarbonate. In recent years, evidence in favor of the need for bicarbonate for the normal functioning of the donor side of PSII has accumulated [120, 121]. In both places, researchers suggest the presence of CA, which may participate in bicarbonate exchange [96, 103, 126].
The presence of CA in the aqueous phase accelerates the appearance or disappearance of bicarbonate and proton there, while CO2 can enter the membrane and be stored in the membrane interior, as the solubility of CO2 in lipids is 3-8 times higher than in water [122]. It is unclear which of the products of the CA reaction plays the decisive role in the reactions on the acceptor and donor sides of PSII. On the acceptor side, this product is most likely bicarbonate, which ensures electron transport from QA to QB, which is under control of nonheme iron. In this case, the participation of CA in the protonation of plastoquinone QB at this place, as previously suggested in some studies [123-125], can be a concomitant reaction. The high molecular mass CA [100, 103] could be this CA. On the donor side of PSII, the release of protons produced by water decomposition may be the primary necessary reaction, which may protect the photosystem from photoinhibition. In this case, proton binding is the main function of CA, and bicarbonate plays a role of convenient proton acceptor. A similar role has been suggested for CAH3, the carbonic anhydrase of α-family in green algae Ch. reinhardtii, which is associated with the lumenal side of thylakoid membrane. The absence of this CA was accompanied by a decrease in resistance of the algae to treatment with high illumination [126]. It is still impossible to assign the observed CA activities in PSII to specific proteins on the donor and acceptor sides of the photosystem, as well as to those proteins in chloroplasts that were identified as CAs according to their DNA sequences.
α-CA4 has been detected among the proteins of thylakoid membranes [19]. We found that in arabidopsis plants with knocked-out gene encoding α-CA4, the effective PSII quantum yield at both saturating light intensity and CO2 concentration was higher than in the wild-type plants of ecotype Columbia, while the non-photochemical quenching of fluorescence (NPQ) was 30-50% lower [127]. The NPQ value indicates changes in the photosynthesis apparatus that lead to energy dissipation in the light-harvesting complexes into a heat, and under the conditions of the measurements, the quenching was dependent on proton concentration in the thylakoid lumen. Protons play a dual role in NPQ, leading to conformational changes in light-harvesting antenna proteins due to protonation of PsbS protein [128, 129] and initiation of the process of de-epoxidation of violaxanthin by activation of violaxanthin deepoxidase [130]. In mutant plants, significant accumulation of starch in chloroplasts was also observed that was 2-3 times higher than that in wild-type plants [127]. It seems that the absence of α-CA4 leads to a decrease in proton supply for PsbS and violaxanthin deepoxidase, thus increasing ATP synthesis and, consequently starch synthesis. The absence of α-CA4 in mutant plants had an effect on PSII-membrane isolation. PSII-membrane preparations could be obtained only with significantly increased Mg2+ concentration (up to 10 mM) in the isolation medium (Ignatova, unpublished); this implies the localization of α-CA4 in granal parts of thylakoid membrane. These experimental data indicate the involvement of this CA into the regulation of the functional activity of the PSII external light-harvesting antenna complexes.
The second pool (mentioned above) of bicarbonate in thylakoids is comparable quantitatively with chlorophyll content and can be removed without loss of PSII activity [119]. It is possible that this pool is involved in proton consumption by thylakoids, because their light-dependent uptake is increased after the addition of bicarbonate to thylakoids, while the CA inhibitors acetazolamide and ethoxyzolamide reduced the number of protons absorbed by thylakoids in the light, reducing the concentration of bound bicarbonate as well as the buffering capacity of thylakoids [131]. The same inhibitors, as shown by this group, without affecting the rate of an electron transport in photosynthetic electron transport chain, significantly reduced the rate of photophosphorylation coupled with this transport [132, 133]. The authors suggested the functioning of a particular CA in thylakoid lumen that can facilitate use of protons in the bicarbonate pool in photophosphorylation [132]. The existence of lumenal CA was shown in [108] (see above), and the authors of that study, in turn, suggested that this CA can help stabilize pH in the lumen as the result of both facilitation of proton removal from places of their entry into the lumen and transport within the lumen. The two assumptions can be combined if we imagine that the lumenal CA accelerates proton supply to the membrane channels of ATP synthase.
The functions of the CA that was found near PSI have not been studied as thoroughly as the functions of the CAs located near PSII. The CA(s) located close to PSI should be situated likewise in stroma lamellae and thus to be in contact with the compartment that contains Rubisco. In 1984 in [134], a fruitful hypothesis concerning the role of the CA in supply of Rubisco with CO2 at the expense of the “acidic” thylakoid lumen was offered. According to this hypothesis, the membrane CA facing the lumen (at that moment, the existence of several CAs in thylakoids was not yet known) accelerates the conversion of bicarbonate to CO2 there, and thereafter CO2 diffuses out from the lumen and is used by Rubisco in the stroma. However, realization of such a scheme requires additional assumptions, namely, about the possibility of the charged molecule HCO3– to enter into the lumen with the rate necessary for observed photosynthesis, and also, how to avoid the immediate conversion of CO2 back into HCO3– in the stroma, where not only high pH, but also β-CA1 is plentiful. We proposed a scheme of intrathylakoid proton use for conversion of HCO3– into CO2 [70]; the sense of the hypothesis is that the CA located in the thylakoid membrane is involved in such conversion so that it is attached to the stromal outlet of the transmembrane proton channel; thus, CA would use for the above reaction the protons that leave the lumen through this channel, i.e. this device would operate similarly to the ATP synthase. Such use of lumenal protons would not result in their deficiency for ATP synthesis because linear and cyclic electron transports, both being coupled with the proton consumption, can be easily adjusted to maintain the desired proton concentration in the lumen in response to their changing outflow.
To prevent diffusion into stroma of CO2 molecules that appear in the reaction centers of the proposed thylakoid CA, Rubisco should contact this CA. It has been shown that a many Rubisco molecules are in supramolecular complexes situated in contact with the thylakoid membrane [135]. Previously, experimental results consistent with this hypothesis were obtained, and they were in some measure the cause of its appearance [113], namely, it has been shown that the stimulation of the dehydration CA activity of thylakoids when thylakoid lumen was acidified under light conditions was suppressed by CA inhibitor. In work [136], the suppression by the CA inhibitor acetazolamide of H2O2 release from intact chloroplasts to media through aquaporins was found; also, the presence of CA activity in chloroplast envelope has been shown. These findings allowed the authors to suggest a functional association of CA with aquaporin channel. In work [137], the existence of structural cooperation of at least two human CAs located externally on the cell membrane with the transmembrane anion transporter was convincingly demonstrated. These data may serve as confirmation of the possibility of direct contact of CAs with channels of various kinds, which is the main sense of the proposed hypothesis.
In summary, on reviewing possible CA functions in green plant cells, we perhaps should state it is with regret that despite the large number of publications and the proposed hypotheses, these functions in specific physiological processes are not yet understood. Good prospects for clarifying these functions are opened by the use of knockout mutants that are defective in particular CA genes. However, due to possibility of cooperation of several CAs to a particular process, as occurs in mitochondria, such study may require, in some cases, the use of double and even triple mutants. The presence of six CAs in mitochondria of A. thaliana puts forward a reason to suppose that the carbonic anhydrase system of chloroplasts and even of thylakoids may also include several CAs, and this is confirmed by the results of the studies considered in this review.
The work was supported by the Russian Foundation for Basic Research (grant Nos. 14-04-32323 and 15-04-03883).
REFERENCES
1.Sanyal, G., Pessah, N. I., and Maren, T. H. (1981)
Kinetics and inhibition of membrane-bound carbonic anhydrase from
canine renal cortex, Biochim. Biophys. Acta, 657,
128-137.
2.Johansson, I. M., and Forsman, C. (1993) Kinetic
studies of pea carbonic anhydrase, Eur. Biochem., 218,
439-446.
3.Meldrum, N. N., and Roughton, F. J. (1933) Carbonic
anhydrase: its preparation and properties, Nature, 80,
113-142.
4.Elleuche, S., and Poggeler, S. (2010) Evolution of
carbonic anhydrases in fungi, Microbiology, 156,
23-29.
5.Hewett-Emmett, D., and Tashian, R. E. (1996)
Functional diversity, conservation, and convergence in the evolution of
the α-, β-, and γ-carbonic anhydrase gene families,
Mol. Phylogenet. Evol., 5, 50-77.
6.Moroney, J. V., Ma, Y., Frey, W. D., Fusilier, K.
A., Pham, T. T., Simms, T. A., Di Mario, R. J., Yang, J., and
Mukherjee, B. (2011) The carbonic anhydrase isoforms of
Chlamydomonas reinhardtii: intracellular location, expression,
and physiological roles, Photosynth. Res., 109,
133-149.
7.Soltes-Rak, E., Mulligan, M. E., and Coleman, J. R.
(1997) Identification and characterization of a gene encoding a
vertebrate-type carbonic anhydrase in cyanobacteria,
Bacteriology, 179, 769-774.
8.Elleby, B., Chirica, L. C., Tu, C., Zeppezauer, M.,
and Lindskog, S. (2001) Characterization of carbonic anhydrase from
Neisseria gonorrhoeae, Eur. J. Biochem., 268,
1613-1619.
9.Elleuche, S., and Poggeler, S. (2009) Evolution of
carbonic anhydrases in fungi, Curr. Genet., 55,
211-222.
10.Fukuzawa, H., Fujiwara, S., Yamamoto, Y.,
Dionisio-Sese, M. L., and Miyachi, S. (1990) cDNA cloning, sequence,
and expression of carbonic anhydrase in Chlamydomonas reinhardtii:
regulation by environmental CO2 concentration, Proc.
Natl. Acad. Sci. USA, 87, 4383-4387.
11.Fujiwara, S., Fukuzawa, H., Tachiki, A., and
Miyachi, S. (1990) Structure and differential expression of two genes
encoding carbonic anhydrase in Chlamydomonas reinhardtii,
Proc. Natl. Acad. Sci. USA, 87, 9779-9783.
12.Fabre, N., Reiter, I. M., Becuwe-Linka, N.,
Genty, B., and Rumeau, D. (2007) Characterization and expression
analysis of genes encoding alpha and beta carbonic anhydrases in
Arabidopsis, Plant Cell Environ., 30,
617-629.
13.Villarejo, A., Buren, S., Larsson, S., Dejardin,
A., Monne, M., Rudhe, Ch., Karlsson, J., Jansson, S., Lerouge, P.,
Rolland, N., von Heijne, G., Grebe, M., Bako, L., and Samuelsson, G.
(2005) Evidence for a protein transported through the secretory pathway
en route to the higher plant chloroplast, Nature Cell
Biol., 7, 1224-1231.
14.Tuskan, G. A., Difazio, S., Jansson, S.,
Bohlmann, J., Grigoriev, I., Hellsten, U., Putnam, N., Ralph, S.,
Rombauts, S., Salamov, A., Schein, J., Sterck, L., Aerts, A., Bhalerao,
R. R., Bhalerao, R. P., Blaudez, D., Boerjan, W., Brun, A., Brunner,
A., Busov, V., Campbell, M., Carlson, J., Chalot, M., Chapman, J.,
Chen, G. L., Cooper, D., Coutinho, P. M., Couturier, J., Covert, S.,
Cronk, Q., Cunningham, R., Davis, J., Degroeve, S., Dejardin, A.,
Depamphilis, C., Detter, J., Dirks, B., Dubchak, I., Duplessis, S.,
Ehlting, J., Ellis, B., Gendler, K., Goodstein, D., Gribskov, M.,
Grimwood, J., Groover, A., Gunter, L., Hamberger, B., Heinze, B.,
Helariutta, Y., Henrissat, B., Holligan, D., Holt, R., Huang, W.,
Islam-Faridi, N., Jones, S., Jones-Rhoades, M., Jorgensen, R., Joshi,
C., Kangasjarvi, J., Karlsson, J., Kelleher, C., Kirkpatrick, R.,
Kirst, M., Kohler, A., Kalluri, U., Larimer, F., Leebens-Mack, J.,
Leple, J. C., Locascio, P., Lou, Y., Lucas, S., Martin, F., Montanini,
B., Napoli, C., Nelson, D. R., Nelson, C., Nieminen, K., Nilsson, O.,
Pereda, V., Peter, G., Philippe, R., Pilate, G., Poliakov, A.,
Razumovskaya, J., Richardson, P., Rinaldi, C., Ritland, K., Rouze, P.,
Ryaboy, D., Schmutz, J., Schrader, J., Segerman, B., Shin, H.,
Siddiqui, A., Sterky, F., Terry, A., Tsai, C. J., Uberbacher, E.,
Unneberg, P., Vahala, J., Wall, K., Wessler, S., Yang, G., Yin, T.,
Douglas, C., Marra, M., Sandberg, G., Van de Peer, Y., and Rokhsar, D.
(2006) The genome of black cottonwood Populus trichocarpa,
Science, 313, 1596-1604.
15.Hewett-Emmett, D. (2000) Evolution and
distribution of the carbonic anhydrase gene families, in The
Carbonic Anhydrases: New Horizons (Chegwidden, W. R., Carter, N.
D., and Edwards, Y. H., eds.) Birkhauser Verlag, Basel, pp. 29-76.
16.Karlsson, J., Clarke, A. K., Chen, Z. H. I.,
Hugghins, S. Y., Park, Y. I., Husic, H. D., Moroney, J. V., and
Samuelsson, G. (1998) A novel alpha-type carbonic anhydrase associated
with the thylakoid membrane in Chlamydomonas reinhardtii is
required for growth at ambient CO2, EMBO J.,
17, 1208-1216.
17.Holmes-Davis, R., Tanaka, C. K., Vensel, W. H.,
Hurkman, W. J., and McCormick, S. (2005) Proteome mapping of mature
pollen of Arabidopsis thaliana, Proteomics,
5, 4864-4884.
18.Noir, S., Brautigam, A., Colby, T., Schmidt, J.,
and Panstruga, R. (2005) A reference map of the Arabidopsis
thaliana mature pollen proteome, Biochem. Biophys. Res.
Commun., 337, 1257-1266.
19.Friso, G., Giacomelli, L., Ytterberg, A. J.,
Peltier, J.-B., Rudella, A., Sun, Q., and van Wijk, K. J. (2004)
In-depth analysis of the thylakoid membrane proteome of Arabidopsis
thaliana chloroplasts: new proteins, new functions, and a plastid
proteome database, Plant Cell, 16, 478-499.
20.Sun, Q., Zybailov, B., Majeran, W., Friso, G.,
Olinares, P. D. B., and van Wijk, K. J. (2009) PPDB, The plant
proteomics database at Cornell, Nucleic Acids Res., 37,
969-974.
21.Kamo, T., Shimogawara, K., Fukuzawa, H., Muto,
S., and Miyachi, S. (1990) Subunit constitution of carbonic anhydrase
from Chlamydomonas reinhardtii, Eur. J. Biochem.,
192, 557-562.
22.Eriksson, A. E., Jones, T. A., and Liljas, A.
(1988) Refined structure of human carbonic anhydrase II at 2.0 Å
resolution, Proteins, 4, 274-282.
23.Pocker, Y., and Meany, J. E. (1965) The catalytic
versatility of carbonic anhydrase from erythrocytes. The
enzyme-catalyzed hydration of acetaldehyde, J. Am. Chem. Soc.,
87, 1809-1811.
24.Pocker, Y., and Stone, J. T. (1965) The catalytic
versatility of erythrocyte carbonic anhydrase. III. Kinetic studies of
the enzyme-catalyzed hydrolysis of p-nitrophenyl acetate, J.
Am. Chem. Soc., 87, 5497-5498.
25.Henkart, P., Guidotti, G., and Edsall, J. T.
(1968) Catalysis of the hydrolysis of l-fluoro-2,4-dinitrobenzene by
carbonic anhydrase, J. Biol. Chem., 243, 2447-2449.
26.Neish, A. C. (1939) Studies on chloroplasts. II.
Their chemical composition and the distribution of certain metabolites
between the chloroplasts and the remainder of the leaf, Biochem.
J., 33, 300-308.
27.Fawcett, T. W., Browse, J. A., Volokita, M., and
Bartlett, S. G. (1990) Spinach carbonic anhydrase primary structure
deduced from the sequence of a cDNA clone, J. Biol. Chem.,
265, 5414-5417.
28.Smith, K. S., and Ferry, J. G. (1999) A
plant-type (beta-class) carbonic anhydrase in the thermophilic
metanoarchaeon Methanobacterium thermoautotrophicum, J.
Bacteriol., 181, 6247-6253.
29.So, A. K. C., and Espie, G. S. (1998) Cloning,
characterization and expression of carbonic anhydrase from the
cyanobacterium Synechocystis PCC6803, Plant Mol. Biol.,
37, 205-215.
30.Smith, K. S., Jakubzick, C., Whittam, T. S., and
Ferry, J. G. (1999) Carbonic anhydrase is an ancient enzyme widespread
in prokaryotes, Proc. Natl. Acad. Sci. USA, 96,
15184-15189.
31.Sawaya, M. R., Cannon, G. C., Heinhorst, S.,
Tanaka, S., Williams, E. B., Yeates, T. O., and Kerfeld, C. A. (2006)
The structure of beta-carbonic anhydrase from the carboxysomal shell
reveals a distinct subclass with one active site for the price of
two, J. Biol. Chem., 281, 7546-7555.
32.Gotz, R., Gnann, A., and Zimmermann, F. K. (1999)
Deletion of the carbonic anhydrase-like gene NCE103 of the
yeast Saccharomyces cerevisiae causes an oxygen-sensitive growth
defect, Yeast, 15, 855-864.
33.Eriksson, M., Karlsson, J., Ramazanov, Z.,
Gardestrom, P., and Samuelsson, G. (1996) Discovery of an algal
mitochondrial carbonic anhydrase: molecular cloning and
characterization of a low-CO2-induced polypeptide in
Chlamydomonas reinhardtii, Proc. Natl. Acad. Sci. USA,
93, 12031-12034.
34.Mitsuhashi, S., Ohnishi, J., Hayashi, M., and
Ikeda, M. (2004) A gene homologous to β-type carbonic anhydrase is
essential for the growth of Corynebacterium glutamicum under
atmospheric conditions, Appl. Microbiol. Biotechnol., 63,
592-601.
35.Majeau, N., and Coleman, J. R. (1991) Isolation
and characterization of a cDNA coding for pea chloroplastic carbonic
anhydrase, Plant Physiol., 95, 264-268.
36.Roeske, C. A., and Ogren, W. L. (1990) Nucleotide
sequence of pea cDNA encoding chloroplast carbonic anhydrase,
Nucleic Acids Res., 18, 3413-3413.
37.Fett, J. P., and Coleman, J. R. (1994)
Characterization and expression of two cDNAs encoding carbonic
anhydrase in Arabidopsis thaliana, Plant Physiol.,
105, 707-713.
38.Rowlett, R. S. (2010) Structure and catalytic
mechanism of the β-carbonic anhydrases, Biochim. Biophys.
Acta, 1804, 362-373.
39.Tripp, B. C., Smith, K., and Ferry, J. G. (2001)
Carbonic anhydrase: new insights for an ancient enzyme, J. Biol.
Chem., 276, 48615-48618.
40.Badger, M. R., and Price, G. D. (1994) The role
of carbonic anhydrase in photosynthesis, Ann. Rev. Plant Physiol.
Plant. Mol. Biol., 45, 369-392.
41.Atkins, C. A., Patterson, B. D., and Graham, D.
(1972) Plant carbonic anhydrases. I. Distribution of types among
species, Plant Physiol., 50, 214-217.
42.Rumeau, D., Cuine, S., Fina, L., Gault, N.,
Nicole, M., and Peltier, G. (1996) Subcellular distribution of carbonic
anhydrase in Solanum tuberosum L. leaves:
characterization of two compartment-specific isoforms, Planta,
199, 79-88.
43.Alber, B. E., and Ferry, J. G. (1994) A carbonic
anhydrase from the archaeon Methanosarcina thermophila, Proc.
Natl. Acad. Sci. USA, 91, 6909-6913.
44.Kisker, C., Schindelin, H., Alber, B. E., Ferry,
J. G., and Rees, D. C. (1996) A left-hand beta-helix revealed by the
crystal structure of a carbonic anhydrase from the archaeon
Methanosarcina thermophila, EMBO J., 15,
2323-2330.
45.Roberts, S. B., Lane, T. W., and Morel, F. M.
(1997) Carbonic anhydrase in the marine diatom Thalassiosira
weissflogii (Bacillariophyceae), J. Phycol.,
33, 845-850.
46.Cox, E. H., McLendon, G. L., Morel, F. M., Lane,
T. W., Prince, R. C., Pickering, I. J., and George, G. N. (2000) The
active site structure of Thalassiosira weissflogii carbonic
anhydrase 1, Biochemistry, 39, 12128-12130.
47.So, A. K.-C., Espie, G. S., Williams, E. B.,
Shively, J. M., Heinhorst, S., and Cannon, G. C. (2004) A novel
evolutionary lineage of carbonic anhydrase (epsilon class) is a
component of the carboxysome shell, J. Bacteriol., 186,
623-630.
48.Lane, T. W., and Morel, F. M. (2000) A biological
function for cadmium in marine diatoms, Proc. Natl. Acad. Sci.
USA, 97, 4627-4631.
49.Park, H., Song, B., and Morel, F. M. (2007)
Diversity of the cadmium-containing carbonic anhydrase in marine
diatoms and natural waters, Environ. Microbiol., 9,
403-413.
50.Xu, Y., Feng, L., Jeffrey, P. D., Shi, Y., and
Morel, F. M. (2008) Structure and metal exchange in the cadmium
carbonic anhydrase of marine diatoms, Nature, 452,
56-61.
51.Friedland, B. R., and Maren, T. H. (1981) The
relation between carbonic anhydrase activity and ion transport in
elasmobranch and rabbit lens, Exp. Eye Res., 33,
545-561.
52.Maren, T. H. (1988) The kinetics of
HCO3ˉ synthesis related to fluid secretion, pH
control, and CO2 elimination, Annu. Rev. Physiol.,
50, 695-717.
53.Davies, D. D. (1973) Control of and by pH,
Symp. Soc. Exp. Biol., 27, 513-529.
54.Wessing, A., Zierold, K., and Bertram, G. (1997)
Carbonic anhydrase supports electrolyte transport in Drosophila
malpighian tubules. Evidence by X-ray microanalysis of cryosections,
J. Insect Physiol., 43, 17-28.
55.Acin-Perez, R., Salazar, E., Kamenetsky, M.,
Buck, J., Levin, L. R., and Manfredi, G. (2009) Cyclic AMP produced
inside mitochondria regulates oxidative phosphorylation, Cell
Metab., 9, 265-276.
56.Walker, N. A. (1983) The uptake of inorganic
carbon by freshwater plants, Plant Cell Environ., 6,
323-328.
57.Kupriyanova, E. V., Sinetova, M. A., Cho, S. M.,
Park, Y.-I., Los, D. A., and Pronina, N. A. (2013)
CO2-concentrating mechanism in cyanobacterial
photosynthesis: organization, physiological role, and evolutionary
origin, Photosynth. Res., 117, 133-146.
58.Moroney, J. V., and Ynalvez, R. A. (2007)
Proposed carbon dioxide concentrating mechanism in Chlamydomonas
reinhardtii, Eukaryot. Cell, 6, 1251-1259.
59.Moroney, J. V., Husic, H. D., and Tolbert, N. E.
(1985) Effect of carbonic anhydrase inhibitors on inorganic carbon
accumulation by Chlamydomonas reinhardtii, Plant
Physiol., 79, 177-183.
60.Ynalvez, R. A., Xiao, Y., Ward, A. S., Cunnusamy,
K., and Moroney, J. V. (2008) Identification and characterization of
two closely related beta-carbonic anhydrases from Chlamydomonas
reinhardtii, Physiol. Plant., 133, 15-26.
61.Lucas, W. J., and Berry, J. A. (1985) Inorganic
carbon transport in aquatic photosynthetic organisms, Physiol.
Plant., 65, 539-543.
62.Price, G. D., Badger, M. R., Bassett, M. E., and
Whitecross, M. I. (1985) Involvement of plasmalemmasomes and carbonic
anhydrase in photosynthetic utilization of bicarbonate in Chara
corallina, Aust. J. Plant Physiol., 12, 241-256.
63.Prins, H. B. A., and Helder, R. J. (1984)
HCO3ˉ assimilation by Potamogeton lucens: polar
cation transport and the role of H+ extrusion, in
Inorganic Carbon Uptake by Aquatic Photosynthetic Organisms
(Lucas, W. J., and Berry, J. A., eds.) Waverly Press, Baltimore, pp.
271-286.
64.Ignatova, L. K., and Romanova, A. K. (1992)
Participation of carbonic anhydrase in inhibition of photosynthesis in
pea protoplasts by CO2 excess, Russ. J. Plant
Physiol., 39, 82-88.
65.Utsunomiya, E., and Muto, S. (1993) Carbonic
anhydrase in the plasma membranes from C3 and C4 plants, Physiol.
Plant., 88, 413-419.
66.Ignatova, L. K., Moskvin, O. V., Ivanov, B. N.,
and Romanova, A. K. (1993) The effect of CO2 uptake by pea
protoplasts on O2 evolution rate and parameters of
chlorophyll fluorescence quenching, Plant Physiol. Biochem.,
31, 295-301.
67.Ignatova, L. K., Moskvin, O. V., Romanova, A. K.,
and Ivanov, B. N. (1998) Carbonic anhydrases in the C3-plant leaf
cell, Aust. J. Plant Physiol., 25, 673-677.
68.Ignatova, L. K., Moskvin, O. V., and Ivanov, B.
N. (2001) Effect of carbonic anhydrase inhibitors on proton exchange
and photosynthesis in pea protoplasts, Russ. J. Plant
Physiol., 48, 467-472.
69.Popova, L., Lasova, G. H., and Miteva, T. S.
(1991) Abscisic acid, jasmonic and NaCl effect on carbonic anhydrase
activity in barley leaves, C. R. Acad. Bulg. Sci., 44,
51-54.
70.Ivanov, B. N., Ignatova, L. K., and Romanova, A.
K. (2007) Variety of forms and functions of carbonic anhydrases in
higher land plants, Russ. J. Plant Physiol., 54,
143-162.
71.Ku, M. S., Kano-Murakami, Y., and Matsuoka, M.
(1996) Evolution and expression of C4 photosynthesis genes, Plant
Physiol., 111, 949-957.
72.Parisi, G., Perales, M., Fornasari, M. S.,
Gonzalez-Schain, A. C. N., Gomez-Casati, D., Zimmermann, S., Brennicke,
A., Araya, A., Ferry, J. G., Echave, J., and Zabaleta, E. (2004) Gamma
carbonic anhydrases in plant mitochondria, Plant Mol. Biol.,
55, 193-207.
73.Sunderhaus, S., Dudkina, N. V., Jansch, L.,
Klodmann, J., Heinemeyer, J., Perales, M., Zabaleta, E., Boekema, E.
J., and Braun, H.-P. (2006) Carbonic anhydrase subunits form a
matrix-exposed domain attached to the membrane arm of mitochondrial
complex I in plants, J. Biol. Chem., 281, 6482-6488.
74.Perales, M., Parisi, G., Fornasari, M. S.,
Colaneri, A., Villarreal, F., Gonzalez-Schain, N., Gomez-Casati, D.,
Braun, H. P., Araya, A., Echave, J., and Zabaleta, E. (2004) Gamma
carbonic anhydrase like complex interact with plant mitochondrial
complex I, Plant. Mol. Biol., 56, 947-957.
75.Zabaleta, E., Martin, M. V., and Braun, H. P.
(2012) A basal carbon concentrating mechanism in plants? Plant
Sci., 187, 97-104.
76.Bird, I. F., Cornelius, M. J., and Keys, A. J.
(1980) Effect of carbonic anhydrase on the activity of ribulose
bisphosphate carboxylase, J. Exp. Bot., 31, 365-369.
77.Porter, M. A., and Grodzinski, B. (1984)
Acclimation to high CO2 in bean. Carbonic anhydrase and
ribulose bisphosphate carboxylase, Plant Physiol., 74,
413-416.
78.Hoang, C. V., and Chapman, K. D. (2002)
Biochemical and molecular inhibition of plastidial carbonic anhydrase
reduces the incorporation of acetate into lipids in cotton embryos and
tobacco cell suspensions and leaves, Plant Physiol., 128,
1417-1427.
79.Price, G. D., von Caemmerer, S., Evans, J. R.,
Yu, J.-W., Lloyd, J., Oja, V., Kell, P., Harrison, K., Gallagher, A.,
and Badger, M. R. (1994) Specific reduction of chloroplast carbonic
anhydrase activity by antisense RNA in transgenic tobacco plants has a
minor effect on photosynthetic CO2 assimilation,
Planta, 193, 331-340.
80.Majeau, N., Arnoldo, M. A., and Coleman, J. R.
(1994) Modification of carbonic anhydrase activity by antisense and
over-expression constructs in transgenic tobacco, Plant Mol.
Biol., 25, 377-385.
81.Ferreira, F. J., Guo, C., and Coleman, J. R.
(2008) Reduction of plastid-localized carbonic anhydrase activity
results in reduced Arabidopsis seedling survivorship, Plant
Physiol., 147, 585-594.
82.Kende, H. (1993) Ethylene biosynthesis, Annu.
Rev. Plant Physiol. Plant Mol. Biol., 44, 283-307.
83.Slaymaker, D. H., Navarre, D. A., Clark, D., del
Pozo, O., Martin, G. B., and Klessig, D. (2002) The tobacco salicylic
acid-binding protein 3 (SABP3) is the chloroplast carbonic anhydrase,
which exhibits antioxidant activity and plays a role in the
hypersensitive defense response, Proc. Natl. Acad. Sci. USA,
99, 11640-11645.
84.Restrepo, S., Myers, K. L., del Pozo, O., Martin,
G. B., Hart, A. L., Buell, C. R., Fry, W. E., and Smart, C. D. (2005)
Gene profiling of a compatible interaction between Phytophthora
infestans and Solanum tuberosum suggests a role for carbonic
anhydrase, Mol. Plant–Microbe Interact., 18,
913-922.
85.Frick, U. B., and Schaller, A. (2002) cDNA
microarray analysis of fusicoccin-induced changes in gene expression in
tomato plants, Planta, 216, 83-94.
86.Schenk, P. M., Kazan, K., Wilson, I., Anderson,
J. P., Richmond, T., Somerville, S. C., and Manners, J. M. (2000)
Coordinated plant defense responses in Arabidopsis revealed by
microarray analysis, Proc. Natl. Acad. Sci. USA, 97,
11655-11660.
87.Pieterse, C. M. J., Van der Does, D., Zamioudis,
C., Leon-Reyes, A., and Van Wees, S. C. M. (2012) Hormonal modulation
of plant immunity, Annu. Rev. Cell Dev. Biol., 28,
489-521.
88.Hu, H., Boisson-Dernier, A.,
Israelsson-Nordstrom, M., Bohmer, M., Xue, S., Ries, A., Godoski, J.,
Kuhn, J. M., and Schroeder, J. I. (2010) Carbonic anhydrases are
upstream regulators of CO2-controlled stomatal movements in
guard cells, Nature Cell Biol., 12, 87-93.
89.Buren, S. (2010) Targeting and Function of
CAH1-Characterisation of a Novel Protein Pathway to the Plant Cell
Chloroplast: PhD Thesis, Umea University, Sweden.
90.Komarova, Yu. M., Doman, N. G., and Shaposhnikov,
G. L. (1982) Two forms of carbonic anhydrase in beans chloroplasts,
Biokhimiya, 47, 1027-1034.
91.Vaklinova, S. G., Goushtina, L. M., and Lazova,
G. N. (1982) Carboanhydrase activity in chloroplasts and chloroplast
fragments, C. R. Acad. Bulg. Sci., 35, 1721-1724.
92.Moubarak-Milad, M., and Stemler, A. (1994)
Oxidation-reduction potential dependence of photosystem II carbonic
anhydrase in maize thylakoids, Biochemistry, 33,
4432-4438.
93.Moskvin, O. V., Ignatova, L. K., Ovchinnikova, V.
I., and Ivanov, B. N. (1995) Membrane-bound carbonic anhydrase of pea
thylakoids, Biochemistry (Moscow), 60,
859-864.
94.Moskvin, O. V., Shutova, T. V., Khristin, M. S.,
Ignatova, L. K., Villarejo, A., Samuelsson, G., Klimov, V. V., and
Ivanov, B. N. (2004) Carbonic anhydrase activities in pea thylakoids. A
photosystem II core complex-associated carbonic anhydrase,
Photosynth. Res., 79, 93-100.
95.Pronina, N. A., Allakhverdiev, S. I.,
Kupriyanova, E. V., Klyachko-Gurvich, G. L., and Klimov, V. V. (2002)
Localization of carbonic anhydrase in pea subchloroplast particles,
Russ. J. Plant Physiol., 49, 303-310
96.Rudenko, N. N., Ignatova, L. K., Kamornitskaya,
V. B., and Ivanov, B. N. (2006) Presence of several carbonic anhydrases
in thylakoids of pea leaves, Dokl. Biochem. Biophys.,
408, 155-157.
97.Rudenko, N. N., Ignatova, L. K., and Ivanov, B.
N. (2007) Multiple sources of carbonic anhydrase activity in pea
thylakoids: soluble and membrane-bound forms, Photosynth. Res.,
91, 81-89.
98.Grinshtein, S. V., and Kost, O. A. (2001)
Structure–functional characteristics of membrane proteins,
Uspekhi Biol. Khim., 41, 77-104.
99.Khristin, M. S., Ignatova, L. K., Rudenko, N. N.,
Ivanov, B. N., and Klimov, V. V. (2004) Photosystem II associated
carbonic anhydrase activity in higher plants is situated in core
complex, FEBS Lett., 577, 305-308.
100.Ignatova, L. K., Rudenko, N. N., Mudrik, V. A.,
Fedorchuk, T. P., and Ivanov, B. N. (2011) Carbonic anhydrase activity
in Arabidopsis thaliana thylakoid membrane and fragments
enriched with PSI or PSII, Photosynth. Res., 110,
89-98.
101.Lu, Y. K., and Stemler, A. J. (2002) Extrinsic
photosystem II carbonic anhydrase in maize mesophyll chloroplasts,
Plant Physiol., 128, 643-649.
102.Moskvin, O. V., Razguljayeva, A. Y., Shutova,
T. V., Khristin, M. S., Ivanov, B. N., and Klimov, V. V. (1999)
Carbonic anhydrase activity of different photosystem II preparations,
in Photosynthesis: Mechanism and Effects, Vol. II (Garab, G.,
ed.) Kluver Academic Press, Dordrecht, pp. 1201-1204.
103.Ignatova, L. K., Rudenko, N. N., Khristin, M.
S., and Ivanov, B. N. (2006) Heterogeneous nature of carbonic anhydrase
activity in thylakoid membranes, Biochemistry (Moscow),
71, 525-632.
104.Silverman, D. N. (1991) The catalytic mechanism
of carbonic anhydrase, Can. J. Botany, 69, 1070-1078.
105.Ilies, M., Banciu, M. D., Ilies, M. A.,
Scozzafava, A., Caproiu, M. T., and Supuran, C. T. (2002) Carbonic
anhydrase activators: design of high affinity isozymes I, II, and IV
activators, incorporating tri-/tetra-substituted-pyridinium-azole
moieties, J. Med. Chem., 45, 504-510.
106.Lu, Y. K., and Stemler, A. J. (2007) Differing
responses of the two forms of photosystem II carbonic anhydrase to
chloride, cations, and pH, Biochim. Biophys. Acta, 1767,
633-638.
107.Shitov, A. V., Pobeguts, O. V.,
Smolova, T. N., Allakhverdiev, S. I., and Klimov, V. V. (2009)
Manganese-dependent carbonic anhydrase activity of photosystem 2
proteins, Biochemistry (Moscow), 74, 509-517.
108.Fedorchuk, T. P., Rudenko, N. N., Ignatova, L.
K., and Ivanov, B. N. (2014) The presence of soluble carbonic anhydrase
in the thylakoid lumen of chloroplasts from Arabidopsis
leaves, J. Plant Physiol., 171, 903-906.
109.Bjorkbacka, H., Johansson, I. N., Skarfstad,
E., and Forsman, C. (1997) The sulfhydryl groups of Cys269 and Cys272
are critical for the oligomeric state of chloroplast carbonic anhydrase
from Pisum sativum, Biochemistry, 36,
4287-4294.
110.Atkins, C. A., Patterson, B. D., and Graham, D.
(1972) Plant carbonic anhydrases. II. Preparation and some properties
of monocotyledon and dicotyledon enzyme types, Plant Physiol.,
50, 218-223.
111.Reising, H., and Shreiber, U. (1994) Inhibition
by ethoxyzolamide of a photo-acoustic uptake signal in leaves: evidence
for carbonic anhydrase catalyzed CO2-solubilization,
Photosynth. Res., 42, 65-73.
112.Moskvin, O. V., Ignatova, L. K., and Ivanov, B.
N. (1999) Uncoupler-sensitive light-induced stimulation of carbonic
anhydrase activity of pea thylakoids, in Photosynthesis: Mechanism
and Effects, Vol. II (Garab, G., ed.) Kluwer Academic Press,
Dordrecht, pp. 1205-1208.
113.Moskvin, O. V., Ivanov, B. N., Ignatova, L. K.,
and Kollmeier, M. A. (2000) Light-induced stimulation of carbonic
anhydrase activity in pea thylakoids, FEBS Lett., 470,
375-377.
114.Stemler, A. (1997) The case for chloroplast
thylakoid carbonic anhydrase, Physiol. Plant., 99,
348-353.
115.Stemler, A., and Jursinic, P. (1983) The
effects of carbonic anhydrase inhibitors formate, bicarbonate,
acetazolamide, and imidazole on photosystem II in maize
chloroplasts, Arch. Biochem. Biophys., 221, 227-237.
116.Dai, X. B., Yu, Y., Zhang, R. X., Yu, X. J.,
He, P. M., and Xu, C. H. (2001) Relationship among photosystem II
carbonic anhydrase, extrinsic polypeptides and manganese cluster,
Chin. Sci. Bull., 46, 406-408.
117.McConnell, I. L., Badger, M. R., Wydrzynski,
T., and Hillier, W. (2007) A quantitative assessment of the carbonic
anhydrase activity in photosystem II, Biochim. Biophys. Acta,
1767, 639-647.
118.Wydrzynski, T., and Govindjee (1975) A new site
of bicarbonate effect in photosystem II of photosynthesis: evidence
from chlorophyll fluorescence transients in spinach chloroplasts,
Biochim. Biophys. Acta, 387, 403-408.
119.Stemler, A. (1977) The binding of bicarbonate
ions to washed chloroplast grana, Biochim. Biophys. Acta,
460, 511-522.
120.Klimov, V. V., Hulsebosch, R. J.,
Allakhverdiev, S. I., Wincencjusz, Y., and van Gorkom, B. (1997)
Bicarbonate may be required for ligation of manganese in the
oxygen-evolving complex of photosystem II, Biochemistry,
36, 16277-16281.
121.Klimov, V. V., and Baranov, S. V. (2001)
Bicarbonate requirement for the water-oxidizing complex of photosystem
II, Biochim. Biophys. Acta, 1503, 187-196.
122.Mitz, M. A. (1979) CO2 biodynamics:
a new concept of cellular control, J. Theor. Biol.,
80, 537-551.
123.Stemler, A. (1979) A dynamic interaction
between the bicarbonate ligand and photosystem II reaction center
complexes in chloroplasts, Biochim. Biophys. Acta, 545,
36-45.
124.Van Rensen, J. J., Tonk, W. J. M., and Bruijn,
S. M. (1988) Involvement of bicarbonate in the protonation of the
secondary quinone electron acceptor of photosystem II via the non-heme
iron of the quinone-iron acceptor complex, FEBS Lett.,
226, 347-351.
125.Govindjee, Xu, C., and van Rensen, J. J. S.
(1997) On the requirement of bound bicarbonate for photosystem II
activity, Z. Naturforschung, 52, 24-32.
126.Villarejo, A., Shutova, T., Moskvin, O.,
Forssen, M., Klimov, V. V., and Samuelsson, G. (2002) A photosystem
II-associated carbonic anhydrase regulates the efficiency of
photosynthetic oxygen evolution, EMBO J., 21,
1930-1938.
127.Zhurikova, E. M., Ignatova, L. K., Semenova, G.
A., Rudenko, N. N., Mudrik, V. A., and Ivanov, B. N. (2015) Effect of
α-carbonic anhydrase 4 gene knockout on photosynthetic
characteristics of Arabidopsis thaliana and starch accumulation
in leaves, Russ. J. Plant Physiol., 62, in
press.
128.Crouchman, S., Ruban, A., and Horton, P. (2006)
PsbS enhances nonphotochemical fluorescence quenching in the absence of
zeaxanthin, FEBS Lett., 580, 2053-2058.
129.Johnson, M. P., and Ruban, A. V. (2011)
Restoration of rapidly reversible photoprotective energy dissipation in
the absence of PsbS protein by enhanced ΔpH, J. Biol.
Chem., 286, 19973-19981.
130.Gilmore, A. M., and Yamamoto, H. Y. (1992) Dark
induction of zeaxanthin-dependent nonphotochemical fluorescence
quenching mediated by ATP, Proc. Natl. Acad. Sci. USA,
89, 1899-1903.
131.Podorvanov, V. V., Zolotareva, E. K., and
Chernoshtan, A. A. (2005) Role of hydrocarbonate in light-dependent
proton uptake by isolated chloroplasts, Fiziol. Biokhim. Kult.
Rast., 37, 326-332.
132.Zolotareva, E. K. (2008) Proton Regulation
of Energy Transformation in Thylakoid Membranes: PhD Thesis,
Kharkov National University, Kharkov, Ukraine.
133.Onoiko, E. B., Polishchuk, A. V., and
Zolotareva, E. K. (2010) Stimulation of photophosphorylation in spinach
isolated chloroplasts by exogenous bicarbonate: role of carbonic
anhydrase, Rep. Nat. Acad. Sci. Ukr., 10, 160-165.
134.Pronina, N. A., and Semenenko, V. E. (1984)
Localization of membrane-bound and soluble forms of carbonic anhydrase
in Chlorella cell, Fiziol. Rast., 31, 241-251.
135.Jebanathirajah, J. A., and Coleman, J. R.
(1998) Association of carbonic anhydrase with a Calvin cycle enzyme
complex in Nicotiana tabacum, Planta, 204,
177-182.
136.Borisova-Mubarakshina, M. M., Kozuleva, M. A.,
Rudenko, N. N., Naydov, I. A., Klenina, I. B., and Ivanov, B. N. (2012)
Photosynthetic electron flow to oxygen and diffusion of hydrogen
peroxide through the chloroplast envelope via aquaporins, Biochim.
Biophys. Acta, 1817, 1314-1321.
137.Sterling, D., Alvarez, B. V., and Casey, J. R.
(2002) The extracellular component of a transport metabolon.
Extracellular loop 4 of the human AE1
Cl–/HCO3ˉ exchanger binds carbonic
anhydrase IV, J. Biol. Chem., 277, 25239-25246.