Photodynamic Inactivation of Gramicidin Channels in Bilayer Lipid Membranes: Protective Efficacy of Singlet Oxygen Quenchers Depends on Photosensitizer Location
T. I. Rokitskaya*, A. M. Firsov, E. A. Kotova, and Y. N. Antonenko
Lomonosov Moscow State University, Belozersky Institute of Physico-Chemical Biology, 119991 Moscow, Russia; fax: +7 (495) 939-3181; E-mail: rokitskaya@genebee.msu.ru* To whom correspondence should be addressed.
Received December 23, 2014; Revision received February 27, 2015
The impact of double bonds in fatty acyl tails of unsaturated lipids on the photodynamic inactivation of ion channels formed by the pentadecapeptide gramicidin A in a planar bilayer lipid membrane was studied. The presence of unsaturated acyl tails protected gramicidin A against photodynamic inactivation, with efficacy depending on the depth of a photosensitizer in the membrane. The protective effect of double bonds was maximal with membrane-embedded chlorin e6-monoethylenediamine monoamide dimethyl ester, and minimal – in the case of water-soluble tri-sulfonated aluminum phthalocyanine (AlPcS3) known to reside at the membrane surface. By contrast, the protective effect of the hydrophilic singlet oxygen scavenger ascorbate was maximal for AlPcS3 and minimal for amide of chlorin e6 dimethyl ester. The depth of photosensitizer position in the lipid bilayer was estimated from the quenching of photosensitizer fluorescence by iodide. Thus, the protective effect of a singlet oxygen scavenger against photodynamic inactivation of the membrane-inserted peptide is enhanced upon location of the photosensitizer and scavenger molecules in close vicinity to each other.
KEY WORDS: photodynamic action, gramicidin A, photosensitizer, unsaturated lipids, ascorbate, singlet oxygen, bilayer lipid membraneDOI: 10.1134/S0006297915060097
Abbreviations: AlPcS3, tri-sulfonated aluminum phthalocyanine; BLM, bilayer lipid membrane; DOPC, dioleoylphosphatidylcholine; DPhPC, diphytanoylphosphatidylcholine; PDA, photodynamic action.
Photodynamic therapy is a promising method for the treatment of cancer.
It is based on selective accumulation of photosensitizers in tumors and
oxidative action of reactive oxygen species (in most cases, singlet
oxygen) that are produced under photosensitizer excitation by visible
light. The lifetime of singlet oxygen (1O2) is
known to be longer in artificial lipid membranes than in aqueous
solutions [1-3]. This causes a
correlation between the photosensitizer immersion depth into the lipid
bilayer and the oxidation efficacy of different membrane target
molecules [4-6].
Due to their high content in living organisms and the high oxidation rate constants of reaction of some amino acids with reactive oxygen species [1, 7, 8], proteins are main targets for singlet oxygen and radicals [9] in photodynamic action (PDA). Many studies indicate that damaging of membrane-bound proteins, such as succinate dehydrogenase, NADH dehydrogenase, and others, plays a crucial role in PDA on mitochondria and leads to a breakdown of their functions [10, 11]. During the oxidation of integral and peripheral proteins, unsaturated lipids can serve as antioxidants and prevent the loss of functionality of such proteins [12].
The gramicidin A peptide, which forms an ion channel in lipid membranes and has four tryptophan residues in its structure, was earlier shown [13-16] to be inactivated by singlet oxygen during PDA, losing its ability to form a channel. This model system proved very useful for analyzing different properties of photosensitizers [17-21]. We recently showed that unsaturated lipids protect the gramicidin A peptide from photodynamic damage in the presence of boronated chlorin e6 derivative (BACE [22]) and that the degree of protection increases with increasing number of double bonds in the lipid [23]. In this work, we studied the dependence of unsaturated lipid and ascorbate antioxidant activity on the photosensitizer membrane immersion depth through the example of gramicidin photoinactivation in planar bilayer lipid membrane (BLM). For this purpose, photosensitizers of varying hydrophobicity were selected: tri-sulfonated aluminum phthalocyanine, chlorin e6, and amide of chlorin e6 dimethyl ester (Fig. 1). The relative chlorin location depth in the membrane was estimated by quenching of its fluorescence by potassium iodide. Comparing the results of changes in peptide photoinactivation with the introduction of hydrophobic or hydrophilic singlet oxygen quenchers for different photosensitizers, we found significant differences in quencher effects. The antioxidant effects of the quenchers were shown to be dependent on the hydrophobicity of the chosen photosensitizer, which determines the depth of its location in a lipid membrane.
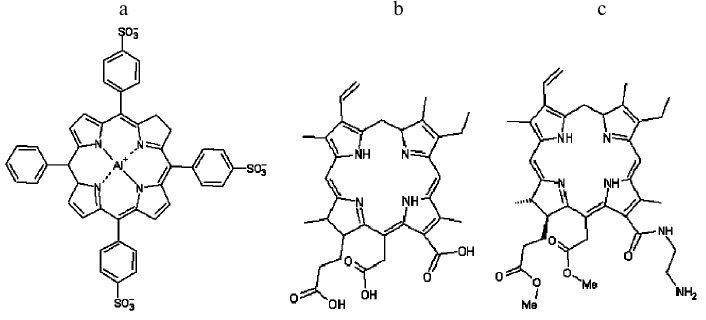
Fig. 1. Structural formulas of tri-sulfonated aluminum phthalocyanine (a), chlorin e6 (b), and amide of chlorin e6 dimethyl ester (c).
MATERIALS AND METHODS
13(1)-N-(2-aminoethyl)-amide-15(2),17(3)-dimethyl ester of chlorin e6, synthesized as described earlier [24], was provided by V. A. Olshevskaya (A. N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences). Chlorin e6 and tri-sulfonated aluminum phthalocyanine (AlPcS3) were obtained from Porphyrin Products (USA).
Bilayer lipid membrane (BLM) was formed from 2% diphytanoylphosphatidylcholine (DPhPC) or dioleoylphosphatidylcholine (DOPC) (Avanti Polar Lipids, USA) solutions in decane on a hole in a Teflon partition separating a cell with buffer solution into two compartments [25]. The hole diameter was 0.5 mm. Gramicidin A (Sigma, USA) was added from stock solution in ethanol into aqueous solution at both sides of the membrane and carefully stirred for 15 min. Photosensitizers were added from aqueous or dimethyl sulfoxide stock solutions into the aqueous solution on the trans-side of the membrane (the cis-side is the front side with respect to the flash lamp) and carefully stirred for 20 min. The aqueous solution contained 100 mM KCl, and 10 mM Tris, pH 7.4. All experiments were conducted at room temperature (23-25°C).
The electrical current was measured under voltage-clamp conditions. Voltage was applied to silver chloride electrodes placed into the Teflon cell. The current was measured using a Keithley 428 amplifier (Keithley Instruments Inc., USA), digitized by the NI-DAQmx digitizer (National Instruments, USA), and analyzed with the WinWCP Strathclyde Electrophysiology Software developed by J. Dempster (University of Strathclyde, GB).
The BLM was illuminated by a xenon flash lamp with flash energy of ~400 mJ/cm2 and flash time <2 ms. A glass filter cutting off light with wavelengths <500 nm was placed between the lamp and the cell. According to data published earlier [26], in the presence of a photosensitizer a single light flash caused a drop in current (I) through the gramicidin channels in the membrane. The dependence of the current on time is well approximated by a monoexponential curve: I(t) = (I0 – I∞)·exp(–t/τ) Ι∞, where I0, I, and τ are the initial current before illumination, the steady-state current established after the flash, and the characteristic time of photoinactivation, respectively. The relative photoinactivation amplitude (α) was defined as α = (I0 – I∞)/I0. The current was induced by applying a voltage of 50 mV to the BLM.
Liposomes were prepared from egg phosphatidylcholine (Avanti Polar Lipids) suspended in a solution containing 10 mM Tris, 100 mM KCl, pH 7.4, with an Avanti Mini-Extruder (Avanti Polar Lipids) using polycarbonate filters with 100 nm pore size (Nucleopore). Photosensitizers were added to liposomes from stock solutions and incubated for 24 h in the dark to reach full equilibrium binding. To measure photosensitizer fluorescence quenching, potassium iodide was added to the liposome suspension. To prevent I2 formation during I– oxidation, Na2S2O3 (10–3 M) was also added to liposomes. Fluorescence intensity was measured using a FluoroMax-3 fluorometer (Horiba Jobin Yvon, Japan) with λex = 407 nm.
Liposomes loaded with 5,6-carboxyfluorescein (Sigma) at self-quenching concentration were prepared from DOPC suspended in 100 mM carboxyfluorescein solution by extrusion through a polycarbonate filter with 100-nm pore size. The resulting liposomes were washed from free carboxyfluorescein by passing through a Sephadex G-50 column with buffer solution containing 100 mM KCl, 10 mM Tris, 10 mM MES, pH 7.0. To measure carboxyfluorescein leakage, the liposomes were diluted to the final concentration of 5 µg/ml in the same buffer solution, and the fluorescence at 520 nm (excitation at 490 nm) was measured with a Panorama fluorometer (Russia). To excite photosensitizers and induce photodynamic permeabilization, liposomes were illuminated by a source of constant light – a halogen lamp (Novaflex, USA; World Precision Instruments, USA) with power density of 0.77 W/cm2 for 60 s. At the end of each experiment, 0.1% Triton X-100 solution was added to register full leakage of carboxyfluorescein. The amount of carboxyfluorescein leakage was calculated as (Ft – F0)/(F100 – F0), where F0 and Ft are the levels of fluorescence at starting and arbitrary times, and F100 is the fluorescence level after full leakage of carboxyfluorescein caused by the addition of Triton X-100.
RESULTS
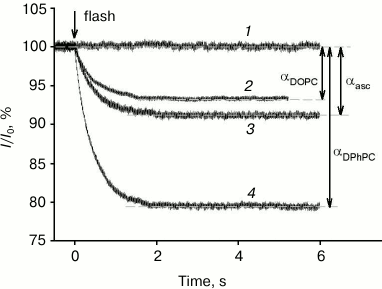
All photosensitizers analyzed in this study caused a drop in gramicidin A-induced current across a BLM after its illumination by a light flash. Figure 2 shows examples of records of gramicidin A-induced current across a BLM, normalized to the initial level (I/I0), in the presence of 0.5 µM of the amide of chlorin e6 dimethyl ester (curves 2-4) and without a photosensitizer (curve 1). At time 0, the membrane was illuminated by a light flash, after which a decrease in gramicidin current occurred in the presence of a photosensitizer, caused by damaging of a fraction of the peptide molecules by singlet oxygen. The control curve 1 shows that in the absence of a photosensitizer the current did not change after the light flash. The BLM was formed from a DPhPC (curves 1, 3, and 4) or DOPC (curve 2) solution in decane. Current kinetics after the flash are well described by a monoexponential dependence with time constants of τ = 0.44 s (curve 4), 0.45 s (curve 3), and 0.49 s (curve 2). These time constants had practically no dependence on the lipid used (DPhPC or DOPC) or the presence of ascorbate. However, the relative gramicidin photoinactivation amplitude (α) depended significantly on the lipid used and was equal to 20.5 and 6.4% for DPhPC and DOPC, respectively, and 9% for DPhPC in the presence of 10 mM ascorbate.
Fig. 2. Recording of relative current (I/I0) kinetics after BLM illumination by a light flash at time t = 0 s in the presence of 0.5 µM of the amide of chlorin e6 dimethyl ester (2-4) or in the absence of a photosensitizer (1). The BLM was formed from DPhPC (1, 3, and 4) or from DOPC (2). Curve 3 was obtained in the presence of 10 mM ascorbate. The membrane current was 0.3 ± 0.11 µA.
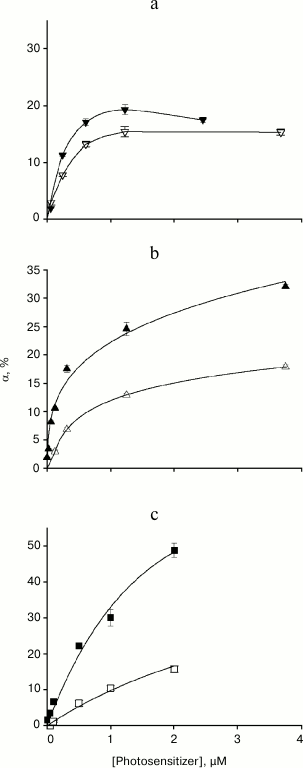
Figure 3 shows the relationship between the relative amplitude of gramicidin current photoinactivation and photosensitizer concentration for membranes formed from DPhPC (filled symbols) and DOPC (empty symbols). In the case of high photosensitizer concentrations (>1 µM) and a membrane formed from DPhPC, α value increased according to the following series: AlPcS3 < chlorin e6 < amide of chlorin e6 dimethyl ester. When the bilayer lipid membrane was formed from the unsaturated lipid DOPC, gramicidin photoinactivation amplitude decreased for all photosensitizers (Fig. 3, empty symbols), but the degree of α decrease for these photosensitizers varied significantly. The smallest change in α was observed in the presence of AlPcS3, in which case the αDOPC/αDPhPC ratio was 0.75. The maximum gramicidin inhibition occurred in the presence of amide of chlorin e6 dimethyl ester, with αDOPC/αDPhPC of ~0.3. For chlorin e6, the ratio of photoinactivation amplitudes was ~0.5. Therefore, the antioxidant effect of unsaturated double bonds in lipids during gramicidin photoinactivation depends on the photosensitizer used.
Fig. 3. Dependence of the relative amplitude of photoinactivation of gramicidin current α and standard error on the concentration of the photosensitizer in the aqueous environment: a) AlPcS3; b) chlorin e6; c) amide of chlorin e6 dimethyl ester. The BLM was formed from DPhPC (filled symbols) or DOPC (empty symbols).
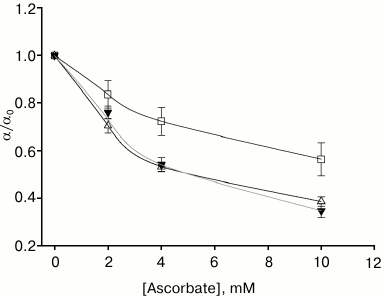
We compared the effect of ascorbate (a water-soluble singlet oxygen quencher) on gramicidin photoinactivation amplitude in the presence of the studied photosensitizers on membranes formed from DPhPC. For all of them, ascorbate at 10 mM concentration significantly decreased α (Fig. 4). The maximum decrease in photoinactivation amplitude occurred in the presence of AlPcS3 (αascorbate/αcontrol = 0.35) and chlorin e6, and the least effect of ascorbate was observed in the presence of amide of chlorin e6 dimethyl ester (αascorbate/αcontrol = 0.56).
Fig. 4. Dependence of the gramicidin photoinactivation relative amplitude on the concentration of ascorbate in the aqueous medium in the presence of 0.5 µM amide of chlorin e6 dimethyl ester (empty squares, α0 = 20.1 ± 2.3%), 0.5 µM chlorin e6 (empty triangles, α0 = 21.7 ± 2.4%), and 1.3 µM AlPcS3 (filled triangles, α0 = 20.7 ± 1.2%).
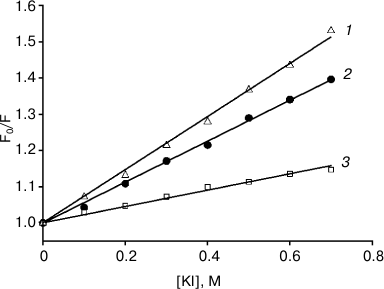
The relative location depth of the compounds studied in this work – AlPcS3, chlorin e6, and amide of chlorin e6 dimethyl ester – inside lipid membranes, was estimated by photosensitizer fluorescence quenching with potassium iodide (KI). Since iodide anions are located in the aqueous phase due to their hydrophilicity, they are more likely to collide with water-soluble photosensitizer molecules of sulfonated aluminum phthalocyanine, causing fluorescence quenching, than with lipophilic chlorin molecules submerged into the membrane. Figure 5 presents the dependences of relative values of fluorescence intensity for photosensitizers on KI concentrations, approximated according to the Stern–Volmer equation: F0/F = 1 + Kq[KI], where F0 is the fluorescence in the absence of a quencher, and F is the fluorescence in the presence of a certain concentration of potassium iodide. The quenching constants Kq measured were 0.733, 0.564, and 0.225 M–1 for AlPcS3, chlorin e6, and amide of chlorin e6 dimethyl ester, respectively. The data we obtained indicated that chlorin e6 localization depth in the membrane is greater than that of AlPcS3, but less than the membrane immersion depth for amide of chlorin e6 dimethyl ester.
Fig. 5. Iodide fluorescence quenching plots for AlPcS3 (triangles, 1), chlorin e6 (circles, 2), amide of chlorin e6 dimethyl ester (squares, 3) in a suspension of 0.1 mg/ml liposomes from egg phosphatidylcholine in Stern–Volmer coordinates. Photosensitizer concentration was 200 nM; λex = 407 nm, λem = 672 nm (chlorin e6), 669 nm (amide of chlorin e6 dimethyl ester), or 681 nm (AlPcS3). The straight lines are the results of data fitting.
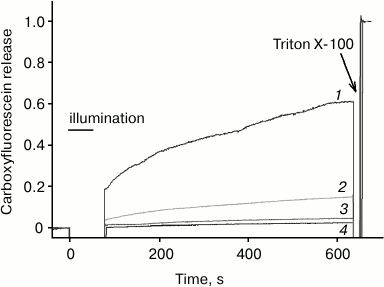
According to [4, 5], the efficacy of photodynamic damage of membrane targets by a photosensitizer depends significantly on its membrane immersion depth. This may explain the fact that chlorin e6, as compared to AlPcS3, exhibits a much greater photodynamic liposome permeabilization activity [27, 28], which is caused by oxidation of double bonds in fatty acid lipid chains [27, 29]. To compare this activity of the photosensitizers studied in this work with the relative depth of their membrane localization, we measured liposome photodynamic permeabilization by photosensitized leakage of carboxyfluorescein from them. As shown in Fig. 6, in this system chlorin e6 exhibits intermediate activity between the smallest for AlPcS3 and the highest for amide of chlorin e6 dimethyl ester, which is in agreement with data on fluorescence quenching by iodide. It should be taken into account that the studied photosensitizers are characterized by different quantum yields of singlet oxygen generation, which, according to [24, 30], are 61% for amide of chlorin e6 dimethyl ester, 76% for chlorin e6, and 38% for AlPcS3; however, these differences are significantly less than the observed difference in the ability of these compounds to sensitize photodynamic liposome permeabilization.
Fig. 6. Carboxyfluorescein leakage from liposomes as measured by an increase in its fluorescence under photodynamic action in the presence of amide of chlorin e6 dimethyl ester (1), chlorin e6 (2), AlPcS3 (3) and in the absence of photosensitizer (4).
DISCUSSION
We found earlier that unsaturated lipids decrease photodynamic damage to gramicidin A peptide [23] sensitized by a boronated derivative of chlorin e6 – BACE. Such protective effect is caused by the antioxidant action of double bonds in unsaturated lipids. It is significant that binding of BACE to a BLM did not depend on the degree of lipid saturation. By analogy, we suppose that the adsorption of other photosensitizers on lipid membranes does not change with substitution of a saturated lipid DPhPC with unsaturated DOPC. The photosensitizers used in this study have different hydrophobicity, and therefore their membrane localization depth varies. Indeed, AlPcS3 has been shown earlier [20] to bind to lipid membrane surface by a coordination bond of the central aluminum atom with the lipid phosphate group, and the negatively charged sulfonic groups, which are located on different sides of the macrocycle, do not allow the AlPcS3 molecule to immerse into the lipid environment. Unlike AlPcS3, three carboxyl groups of chlorin e6 are located on one side of the macrocycle (under our conditions, all of them are negatively charged at pH 7.4) and face the aqueous phase, wherein the uncharged part of the molecule is located in the hydrophobic layer of a membrane [31]. Depth of chlorin e6 immersion has also been shown earlier to strongly depend on pH, which is explained by protonation of carboxyl groups at low pH [31]. Carboxyl groups in a molecule of the amide of chlorin e6 dimethyl ester are methylated, and only one positive charge is present on the free amino group of ethylene diamine. It can therefore be assumed that the amide of chlorin e6 dimethyl ester is more hydrophobic and is localized deeper in lipid membrane than chlorin e6. These assumptions are confirmed by measurements of photosensitizer fluorescence quenching. The constant of fluorescence quenching by iodide for chlorin e6 turned out to be significantly higher than Kq for amide of chlorin e6 dimethyl ester.
The relative amplitude of gramicidin channel photoinactivation decreased in the case of membranes formed from DOPC in the presence of all of the used photosensitizers (Fig. 3, empty symbols). The decrease in amplitude was the most pronounced for the amide of chlorin e6 dimethyl ester, which is submerged in the membrane deeper than the other compounds, and the least for the surface-bound phthalocyanine. Apparently, close mutual positioning of photosensitizer molecules and hydrocarbon chains of unsaturated lipids increases the efficacy of quenching of photosensitizer-generated singlet oxygen by double bonds, leading to a decrease in the relative gramicidin photoinactivation amplitude. In the literature, there is growing evidence for the necessity of close arrangement of the photosensitizer and the target molecule for effective singlet oxygen quenching [4, 6, 32]. In particular, Sokolov et al. found the damage to target molecules by singlet oxygen in model lipid membranes during PDA of water-soluble aluminum phthalocyanine occurring on the both sides of the membrane; however, the damage rate was higher on the side of a membrane where the photosensitizer was added [33, 34].
The fact that double bonds of unsaturated lipids suppress oxidation of tryptophan residues in gramicidin molecules so much is surprising, as the deactivation rate constant of singlet oxygen by tryptophan is greater by three orders of magnitude than that of unsaturated fatty acids [1, 35]. However, the number of lipids containing double bounds per area unit of a membrane is significantly higher than the number of gramicidin molecules. This can be seen by estimating the surface density of gramicidin molecules in lipid membrane for our experiments, given that the conductivity was 8 ± 5 µS, the membrane surface was about 0.5 mm2 and the equilibrium constant between the monomeric and dimeric forms of gramicidin K ~ 1013-1014 cm2/mol [26, 36]. Hence, we find that one gramicidin molecule accounts for, on average, 5·104 nm2. At the same time, one lipid molecule in a bilayer membrane is known to occupy 0.7-1 nm2 [37]. Therefore, the surface density of lipid molecules is more than four orders of magnitude greater than that of the peptide.
In the case of using the water-soluble quencher ascorbate, the maximum decrease in gramicidin inactivation is observed in the presence of chlorin e6 and AlPcS3, and the minimal decrease is observed in the presence of the amide of chlorin e6 dimethyl ester. However, these differences are expressed not as strongly as in the case of the antioxidant effect of unsaturated fatty acids. This may be due to the ability of ascorbic acid bind to or even immerse into lipid membranes. It may be indirectly indicated by a distribution coefficient, which is significantly high for hydrophilic ascorbic acid (~0.01 [38]).
The significant influence of photosensitizer immersion depth in a lipid bilayer on the efficacy of protection by unsaturated lipids against singlet oxygen-mediated damage of a peptide embedded in the membrane indicates that the mean free path of singlet oxygen under these conditions is comparable with the thickness of a bilayer, i.e. close to the values recorded for natural membranes [1, 39, 40].
This study was supported by the Russian Foundation for Basic Research (grants 15-04-01755 and 15-04-01688).
REFERENCES
1.Krasnovsky, A. A., Jr. (1998) Singlet molecular
oxygen in photobiochemical systems: IR phosphorescence studies,
Membr. Cell Biol., 12, 665-690.
2.Ehrenberg, B., Anderson, J. L., and Foote, C. S.
(1998) Kinetics and yield of singlet oxygen photosensitized by
hypericin in organic and biological media, Photochem.
Photobiol., 68, 135-140.
3.Kearns, D. R. (1971) Physical and chemical
properties of singlet molecular oxygen, Chem. Rev., 71,
395-427.
4.Lavi, A., Weitman, H., Holmes, R. T., Smith, K. M.,
and Ehrenberg, B. (2002) The depth of porphyrin in a membrane and the
membraneʼs physical properties affect the photosensitizing
efficiency, Biophys. J., 82, 2101-2110.
5.Bronshtein, I., Afri, M., Weitman, H., Frimer, A.
A., Smith, K. M., and Ehrenberg, B. (2004) Porphyrin depth in lipid
bilayers as determined by iodide and parallax fluorescence quenching
methods and its effect on photosensitizing efficiency, Biophys.
J., 87, 1155-1164.
6.Dror, S. B., Bronshtein, I., Garini, Y.,
OʼNeal, W. G., Jacobi, P. A., and Ehrenberg, B. (2009) The
localization and photosensitization of modified chlorin
photosensitizers in artificial membranes, Photochem. Photobiol.
Sci., 8, 354-361.
7.Lissi, E. A., Encinas, M. V., Lemp, E., and Rubio,
M. A. (1993) Singlet oxygen O2(1-Delta-G) bimolecular
processes – solvent and compartmentalization effects,
Chem. Rev., 93, 699-723.
8.Wilkinson, F., Helman, W. P., and Ross, A. B.
(1995) Rate constants for the decay and reactions of the lowest
electronically excited singlet-state of molecular-oxygen in
solution – an expanded and revised compilation, J. Phys.
Chem. Ref. Data, 24, 663-1021.
9.Davies, M. J. (2005) The oxidative environment and
protein damage, Biochim. Biophys. Acta, 1703, 93-109.
10.Giulivi, C., Sarcansky, M., Rosenfeld, E., and
Boveris, A. (1990) The photodynamic effect of rose bengal on proteins
of the mitochondrial inner membrane, Photochem. Photobiol.,
52, 745-751.
11.Moreno, G., Poussin, K., Ricchelli, F., and
Salet, C. (2001) The effects of singlet oxygen produced by photodynamic
action on the mitochondrial permeability transition differ in
accordance with the localization of the sensitizer, Arch. Biochem.
Biophys., 386, 243-250.
12.Rodrigues, T., De Franca, L. P., Kawai, C., De
Faria, P. A., Mugnol, K. C., Braga, F. M., Tersariol, I. L., Smaili, S.
S., and Nantes, I. L. (2007) Protective role of mitochondrial
unsaturated lipids on the preservation of the apoptotic ability of
cytochrome c exposed to singlet oxygen, J. Biol. Chem.,
282, 25577-25587.
13.Strassle, M., and Stark, G. (1992) Photodynamic
inactivation of an ion channel: gramicidin A, Photochem.
Photobiol., 55, 461-463.
14.Rokitskaya, T. I., Antonenko, Y. N., and Kotova,
E. A. (1993) The interaction of phthalocyanine with planar lipid
bilayers – photodynamic inactivation of gramicidin channels,
FEBS Lett., 329, 332-335.
15.Kunz, L., Zeidler, U., Haegele, K., Przybylski,
M., and Stark, G. (1995) Photodynamic and radiolytic inactivation of
ion channels formed by gramicidin A: oxidation and fragmentation,
Biochemistry, 34, 11895-11903.
16.Rokitskaya, T. I., Antonenko, Y. N., and Kotova,
E. A. (1997) Effect of the dipole potential of a bilayer lipid membrane
on gramicidin channel dissociation kinetics, Biophys. J.,
73, 850-854.
17.Rokitskaya, T. I., Block, M., Antonenko, Y. N.,
Kotova, E. A., and Pohl, P. (2000) Photosensitizer binding to lipid
bilayers as a precondition for the photoinactivation of membrane
channels, Biophys. J., 78, 2572-2580.
18.Shapovalov, V. L., Rokitskaya, T. I., Kotova, E.
A., Krokhin, O. V., and Antonenko, Y. N. (2001) Effect of fluoride
anions on gramicidin photoinactivation sensitized by sulfonated
aluminum phthalocyanines, Photochem. Photobiol., 74,
1-7.
19.Antonenko, Yu. N., Kotova, E. A., and Rokitskaya,
T. I. (2005) Photodynamic action as a basis for relaxation method of
the study of gramicidin channels, Biol. Membr. (Moscow),
22, 275-289.
20.Pashkovskaya, A. A., Sokolenko, E. A., Sokolov,
V. S., Kotova, E. A., and Antonenko, Y. N. (2007) Photodynamic activity
and binding of sulfonated metallophthalocyanines to phospholipid
membranes: contribution of metal-phosphate coordination, Biochim.
Biophys. Acta, 1768, 2459-2465.
21.Pashkovskaya, A. A., Maizlish, V. E.,
Shaposhnikov, G. P., Kotova, E. A., and Antonenko, Y. N. (2008) Role of
electrostatics in the binding of charged metallophthalocyanines to
neutral and charged phospholipid membranes, Biochim. Biophys.
Acta, 1778, 541-548.
22.Antonenko, Y. N., Kotova, E. A., Omarova, E. O.,
Rokitskaya, T. I., Olʼshevskaya, V. A., Kalinin, V. N., Nikitina,
R. G., Osipchuk, J. S., Kaplan, M. A., Ramonova, A. A., Moisenovich, M.
M., Agapov, I. I., and Kirpichnikov, M. P. (2014) Photodynamic activity
of the boronated chlorin e6 amide in artificial and cellular
membranes, Biochim. Biophys. Acta, 1838, 793-801.
23.Rokitskaya, T. I., Kotova, E. A., Agapov, I. I.,
Moisenovich, M. M., and Antonenko, Y. N. (2014) Unsaturated lipids
protect the integral membrane peptide gramicidin A from singlet oxygen,
FEBS Lett., 588, 1590-1595.
24.Moisenovich, M. M., Olʼshevskaya, V. A.,
Rokitskaya, T. I., Ramonova, A. A., Nikitina, R. G., Savchenko, A. N.,
Tatarskiy, V. V., Jr., Kaplan, M. A., Kalinin, V. N., Kotova, E. A.,
Uvarov, O. V., Agapov, I. I., Antonenko, Y. N., and Shtil, A. A. (2010)
Novel photosensitizers trigger rapid death of malignant human cells and
rodent tumor transplants via lipid photodamage and membrane
permeabilization, PLoS One, 5, E12717.
25.Mueller, P., Rudin, D. O., Tien, H. T., and
Wescott, W. C. (1963) Methods for the formation of single bimolecular
lipid membranes in aqueous solution, J. Phys. Chem., 67,
534-535.
26.Rokitskaya, T. I., Antonenko, Y. N., and Kotova,
E. A. (1996) Photodynamic inactivation of gramicidin channels: a
flash-photolysis study, Biochim. Biophys. Acta, 1275,
221-226.
27.Pashkovskaya, A., Kotova, E., Zorlu, Y.,
Dumoulin, F., Ahsen, V., Agapov, I., and Antonenko, Y. (2010)
Light-triggered liposomal release: membrane permeabilization by
photodynamic action, Langmuir, 26, 5726-5733.
28.Kotova, E. A., Kuzevanov, A. V., Pashkovskaya, A.
A., and Antonenko, Y. N. (2011) Selective permeabilization of lipid
membranes by photodynamic action via formation of hydrophobic defects
or pre-pores, Biochim. Biophys. Acta, 1808,
2252-2257.
29.Ytzhak, S., and Ehrenberg, B. (2014) The effect
of photodynamic action on leakage of ions through liposomal membranes
that contain oxidatively modified lipids, Photochem. Photobiol.,
90, 796-800.
30.Kuznetsova, N. A., Gretsova, N. S., Derkacheva,
V. M., Kaliya, O. L., and Lukyanets, E. A. (2003) Sulfonated
phthalocyanines: aggregation and singlet oxygen quantum yield in
aqueous solutions, J. Porphyr. Phthalocyanines, 7,
147-154.
31.Zorin, V., Michalovsky, I., Zorina, T., and
Khludeyev, I. (1996) The distribution of chlorin e6
derivatives in biological systems – investigation of
pH-effect, Proc. SPIE, 2625, 146-155.
32.Vilensky, A., and Feitelson, J. (1999) Reactivity
of singlet oxygen with tryptophan residues and with melittin in
liposome systems, Photochem. Photobiol., 70, 841-846.
33.Sokolov, V. S., Block, M., Stozhkova, I. N., and
Pohl, P. (2000) Membrane photopotential generation by interfacial
differences in the turnover of a photodynamic reaction, Biophys.
J., 79, 2121-2131.
34.Sokolov, V. S., and Pohl, P. (2009) Membrane
transport of singlet oxygen monitored by dipole potential measurements,
Biophys. J., 96, 77-85.
35.Krasnovsky, A. A., Kagan, V. E., and Minin, A. A.
(1983) Quenching of singlet oxygen luminescence by fatty acids and
lipids – contribution of physical and chemical mechanisms,
FEBS Lett., 155, 233-236.
36.Bamberg, E., and Lauger, P. (1973) Channel
formation kinetics of gramicidin A in lipid bilayer membranes, J.
Membr. Biol., 11, 177-194.
37.Nagle, J. F., and Tristram-Nagle, S. (2000)
Structure of lipid bilayers, Biochim. Biophys. Acta,
1469, 159-195.
38.Rose, R. C. (1987) Solubility properties of
reduced and oxidized ascorbate as determinants of membrane permeation,
Biochim. Biophys. Acta, 924, 254-256.
39.Moan, J., and Berg, K. (1991) The
photodegradation of porphyrins in cells can be used to estimate the
lifetime of singlet oxygen, Photochem. Photobiol., 53,
549-553.
40.Krasnovsky, A. A., Jr. (2006) Photodynamic
regulation of biological processes: primary mechanisms, in Problems
of Regulation in Biological Systems (Rubin, A. B., ed.)
Moscow-Izhevsk.