Mini-REVIEW: On the Mechanism of Erythrocyte Hemolysis Induced by Photooxidized Psoralen*
E. V. Nevezhin1,2, N. V. Vlasova1, I. A. Pyatnitskiy1, E. P. Lysenko1, and M. V. Malakhov1,2**
1Pirogov Russian National Research Medical University, Department of Physics and Mathematics, 117997 Moscow, Russia; E-mail: frost88.07@mail.ru2Pirogov Russian National Research Medical University, Medicinal Chemistry and Toxicology Unit, 117997 Moscow, Russia; E-mail: malakhov.mikhail@gmail.com
* This mini-review is dedicated to the blessed memory of Professor Alexander Yakovlevich Potapenko (1944-2014), who was the Head of Photomedicine Research Laboratory at the N. I. Pirogov Russian National Research Medical University for nearly four decades, and it is mostly based on data obtained in this laboratory.
** To whom correspondence should be addressed.
Received January 13, 2015; Revision received March 16, 2015
Contemporary concepts on a possible mechanism of erythrocyte hemolysis induced by photooxidized psoralen – the medicinal photosensitizing furocoumarin – are reviewed. The hypothesis on the mechanochemical mechanism of hemolysis is considered in view of recent data on photoinduced aggregation in photooxidized psoralen solutions. Appropriate chemical structures of photoproduct hemolysins and aggregating photoproducts are discussed.
KEY WORDS: psoralen, photooxidized psoralen, photoproducts, erythrocyte hemolysis, photoinduced aggregation, chemical regulation of hemolysisDOI: 10.1134/S0006297915060115
Abbreviations: GSH, reduced glutathione; GSSG, oxidized glutathione; HI-PUVA-hemolysis, high-intensity PUVA-hemolysis (UV-A fluence rate >120 W/m2); LI-PUVA-hemolysis, low-intensity PUVA-hemolysis (UV-A fluence rate <40 W/m2); POP, photooxidized psoralen; PUVA, psoralen + UV-A; RLS, resonance light scattering; UV, ultraviolet; UV-A, ultraviolet A (315-400 nm).
Psoralens are tricyclic natural or synthetic UV-photosensitizers
combined of furans and coumarins that are successfully used for the
treatment of a variety of pathologies associated with a
hyperresponsiveness of T-cell immunity (vitiligo, psoriasis, allergic
contact dermatitis, etc.) [1]. This treatment can
be performed by means of PUVA-therapy [1] or
photopheresis [2], wherein the skin of a patient or
the leukapack derived therefrom are exposed to PUVA-treatment,
respectively. Presently, PUVA-therapy is deemed to be realized via
antiproliferative and proapoptotic effects on keratinocytes and
immunocytes, as well as via induction of immunosuppression [1].
In many studies carried out in our laboratory, it has been shown that photooxidized psoralen (POP) obtained in vitro also possesses therapeutic efficacy [3-5]. This fact forced us to reconsider all the previous concepts on photochemical mechanisms underlying PUVA-therapy and photopheresis and to focus on studies of POP photochemistry and photobiology [6-9]. Quite a number of interesting effects in line with our results were also attained by other researchers [10-13]. POP is a complex mixture of psoralen photoproducts that are formed during photooxidation of psoralen solutions, but only few of them have been chemically identified and tested in models in vitro and in vivo [14]. This is governed by the extreme difficulty of separation and identification of single photoproducts due to their low yields and instability during analytical procedures [14-16]. For the same reason, studies of the photochemical patterns of the formation of single biologically active photoproducts are possible only using their complex mixtures.
Both PUVA- and POP-treatments can damage erythrocyte membranes and induce hemolysis of erythrocytes in diluted suspensions (107 cells/ml) [5, 17-24]. Erythrocytes are not targeted by PUVA-therapy and photopheresis, and psoralen concentrations used during their courses are about two orders of magnitude lower than those used in studies on the induction of hemolysis. However, the model of erythrocyte hemolysis induced by PUVA- or POP-treatment can serve as a simple and easily reproducible model for studies on photochemical mechanisms of formation of biologically active photoproducts, as well as the influence of certain physicochemical factors (UV-A fluence rate, psoralen concentration, etc.) on their formation during photochemical reactions. Furthermore, studies on chemical regulation of hemolysis can provide information concerning the appropriate chemical structure of biologically active photoproducts.
Further, contemporary concepts on a possible mechanism of erythrocyte hemolysis induced by POP and the appropriate chemical structure of hemolysin photoproducts will be considered.
LOW-INTENSITY PUVA-HEMOLYSIS (LI-PUVA-HEMOLYSIS)
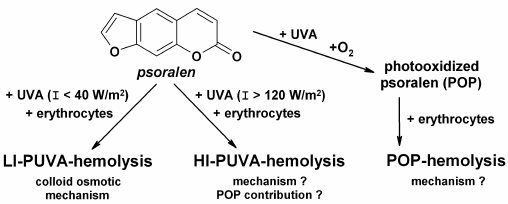
LI-PUVA-hemolysis is induced by low intensity (I < 40 W/m2) UV-A irradiation of erythrocytes in the presence of psoralen, and it has the same features and characteristics as the other types of photohemolysis, e.g. UV-hemolysis and hemolysis induced by other photosensitizers [25]: (i) all the cells in the suspension undergo the hemolysis; (ii) a threshold fluence, i.e. a fluence under which hemolysis is not induced, is absent; (iii) the hemolysis curve is sigmoid; (iv) LI-PUVA-hemolysis rate is directly proportional to the square of the fluence. LI-PUVA-hemolysis proceeds by a colloid osmotic mechanism (Fig. 1) resulting in formation of channels permeable for cations but at the same time impermeable for large osmotic molecules (e.g. sucrose), in erythrocyte membranes [19]. The characteristic feature of LI-PUVA-hemolysis is that it is a thermally activated process, i.e. hemolysis is induced only in cases when LI-PUVA-treated erythrocytes are further incubated at 37°C. Band 3 anion-exchange protein of erythrocyte membranes composed of two subunits and damaged on exposure to a variety of photosensitizers [26, 27] is seemed to be the most probable target of LI-PUVA hemolysis.
Fig. 1. Main types of hemolytic damage of erythrocyte membranes induced by photodynamic treatment (PUVA-hemolysis) or photooxidized psoralen (POP-hemolysis).
HIGH-INTENSITY PUVA-HEMOLYSIS (HI-PUVA-HEMOLYSIS)
On increase in UV-A fluence rate, the nature of hemolysis is changed, and at fluence rates above 120 W/m2 features which are not characteristic for LI-PUVA-hemolysis could be observed, as follows: (i) threshold fluence appears; (ii) at a short range of fluencies above the threshold, incomplete hemolysis is observed (only a portion of cells is lysed in suspension), and upon further increase in fluence, hemolysis became complete (all cells in suspension are lysed); (iii) the rate of complete hemolysis paradoxically slows with increase in fluence; (iv) channels formed in membranes are larger than the molecular dimensions of sucrose [19]. Thus, the HI-PUVA-hemolysis mechanism contrasts strikingly with colloid osmotic hemolysis and is reminiscent of hemolysis induced by specific detergents (e.g. digitonin) [18]. The temperature dependence of post-irradiation incubation on HI-PUVA-hemolysis is complex: increase in temperature shifts the fluence dependence towards larger fluencies, with the rate of hemolysis being raised. This suggested that the number of permeable channels decreases with increase in temperature of post-irradiation incubation, whilst the rate of hemolysis rises due to membrane viscosity reduction and acceleration of lateral diffusion, hence, due to enhancement of formation of large permeable channels. In view of the above, it is not a coincidence that the Bunsen–Roscoe law (reciprocity law of fluence rate and duration of irradiation) is not fulfilled for PUVA-hemolysis [20]. In an effort to explain the difference between the two types of PUVA-hemolysis, it was suggested that psoralen photooxidation products, which are formed and realize their hemolytic effects in situ during PUVA-treatment, considerably contribute to HI-PUVA-hemolysis induction (Fig. 1) [28].
HEMOLYSIS INDUCED BY PHOTOOXIDIZED PSORALEN
(POP-HEMOLYSIS)
A series of experiments allowed us to reveal that characteristic features described above for HI-PUVA-hemolysis are equally characteristic for POP-hemolysis [5, 21-24]. Of prime importance is the fact that formation of POP-hemolysins is possible only in the presence of oxygen in solution during UV-A irradiation (Fig. 1), i.e. just psoralen photooxidation products would be POP-hemolysins [21]. Furthermore, it was found that formation of POP-hemolysins occurred more efficiently with increase in UV-A fluence rate and/or psoralen concentration during preparation of POP [5]. Also, it was found that POP solutions comprised several hemolysins differing in their stability during storage at various temperatures (varying from 4 to 45°C) and their hemolytic efficacy [23, 24, 29]. However, neither of the effects observed gives any insight in the paradoxical slowdown of the hemolysis rate with increase in fluence.
Previously, we found erythrocyte hemolysis induced by butylated hydroxytoluene (2,6-di-tert-butyl-4-methylphenol) commonly used as an antioxidant [30]. At high concentrations (16-100 µM), butylated hydroxytoluene was capable of inducing erythrocyte hemolysis, where the rate of hemolysis slowed with increase in fluence as in the case of POP-hemolysis. In efforts to explain the phenomenological similarity of these two processes, we suggested a hypothesis of a mechanochemical mechanism of POP-hemolysis.
HYPOTHESIS ON MECHANOCHEMICAL MECHANISM OF POP-HEMOLYSIS
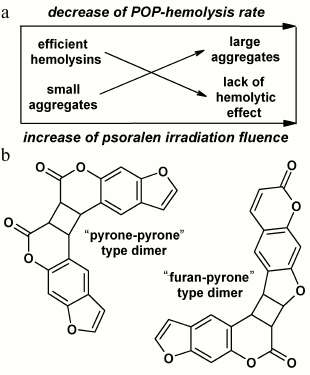
The hypothesis suggests that POP-hemolysins formed during photooxidation of psoralen aggregate in solutions, these aggregates mechanically damaging erythrocyte membranes and causing hemolysis (Fig. 2a). At low doses under threshold, the number of aggregates is so small that hemolysis is not observed. At doses above the threshold, the number of aggregates grows quickly with increase in fluence; therewith, the number of lysed cells rapidly increases. In parallel, the aggregates become larger in solution and become incapable of inducing hemolysis, and that could be exactly the reason for the slowing of the hemolysis rate with increase in fluence.
Fig. 2. Schematic illustration of mechanochemical mechanism of POP-hemolysis (a) and the structures of psoralen dimers formed in solutions during photolysis (b).
Using the resonance light scattering (RLS) technique [31], we have found the photoinduced aggregation in POP solutions [32]. Considering the patterns of formation of POP-hemolysins previously obtained and described in the chapter on POP-hemolysis, we analyzed the photoinduced aggregation in POP solutions more closely using the RLS technique.
We found that the dependences of aggregate and hemolysin formation on UV-A fluence rate and psoralen concentration are very similar. However, a fundamental difference exists: as compared to formation of hemolysins, aggregate formation is independent of the presence of oxygen during irradiation. This observation represents a crucial argument against the hypothesis of the mechanochemical mechanism of POP-hemolysis. At the same time, the experimental data allowed us to suggest that psoralen photoproducts responsible for RLS signal formation might be C4-cyclobutane dimers of psoralen (Fig. 2b) well-known from the literature [14, 33]. Indeed, all the physicochemical patterns of their formation resemble closely those characteristic for aggregates: dimer formation was not only independent of the presence of oxygen during irradiation, but was even quenched by it, and preferably occurred with increase in psoralen concentration and/or UV-A fluence rate [14-16, 33]. In view of relative stability of aggregates over time (that also differs them from the extremely unstable hemolysins), it is planned for the future to compare the kinetics of aggregate (by means of RLS monitoring) and dimer (e.g. by means of mass spectroscopy) formation along with sizing of aggregates (by means of dynamic light scattering).
PSORALEN PHOTOOXIDATION MECHANISMS AND MAIN TYPES OF
POP-PRODUCTS
In this section, the mechanisms of formation of some POP-products during psoralen photooxidation in aqueous phase will be considered. All photooxidation reactions resulting in POP formation can be conveniently classified into three groups: (i) reactions with furan ring splitting; (ii) reactions with pyrone ring splitting, and (iii) deep psoralen photolysis (Fig. 3).
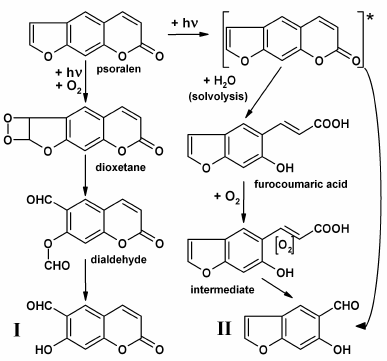
A mechanism for the formation of POP-products with split furan ring is associated with singlet oxygen, which is formed during psoralen photooxidation in solutions, and it attacks the furan ring double bond forming intermediate dioxetane [34, 35]. The simultaneous cleavage of O–O and C–C bonds of dioxetane results in dialdehyde production with further hydrolysis of the ether bond to yield the end-product, 6-formyl-7-hydroxycoumarin (Fig. 3, I). It is expected that photooxidative furan ring opening for 5-methoxypsoralen and 8-methoxypsoralen occurs similarly [14, 34, 35].
Fig. 3. Supposed photochemical mechanisms which result in formation of aldehydic POP-products (6-formyl-7-hydroxycoumarin, I; 5-formyl-6-hydroxybenzofuran, II).
POP-products with split pyrone rings can be formed according to two mechanisms: (i) upon photon absorption, an electronically excited psoralen molecule can undergo solvolysis with water to give furocoumaric acid [14-16]. Further oxidation of the opened pyrone ring double bond with oxygen dissolved in water (potentially, through the stage of intermediate production) can lead in 5-formyl-6-hydroxybenzofuran formation (Fig. 3, II); (ii) the same product could result from the attack of water or oxygen at the pyrone ring double bond in the electronically excited molecule of psoralen and the hydrolysis of the ester bond [15].
Photoproducts with both split furan and pyrone rings, classified as benzaldehydes and benzodialdehydes, as well as a small subset of aldehydes (formaldehyde and acetaldehyde) and corresponding carboxylic acids yielded during photooxidation, are counted as products of deep psoralen photolysis [15, 16]. Furthermore, formation and accumulation of hydrogen peroxide (H2O2) proceeds in POP solutions, with H2O2 being accumulated in solutions even after the end of irradiation, which may result from the autooxidation of aldehydic photoproducts of psoralen [36]. Aldehydic photoproducts absorb UV-A radiation, which may trigger autooxidation, with further processes progressing like a chain reaction [16, 36].
CHEMICAL REGULATION OF POP-HEMOLYSIS
Two aspects seem to be significant in the context of chemical regulation of POP-hemolysis. The first is attributed to POP-hemolysis modulation with Fe(II) ions: addition of Fe(II) to POP in the presence of erythrocytes leads to activation of POP-hemolysis, whereas incubation of POP with Fe(II) ions for 20 min completely cancelled it [37]. This may suggest that more efficient but short-lived hemolysins are formed upon interaction of POP with Fe(II) ions. In light of earlier described data on chemiluminescence induced by addition of Fe(II) ions to POP solutions, a peroxide nature of POP-hemolysins could be assumed [29, 37, 38]. At the same time, using catalase we found that hydrogen peroxide formed during psoralen photooxidation is not a hemolysin and is not involved in its formation [22]. Thus, it can be assumed that short-lived peroxides could serve as intermediates in the process of aldehydes formation, e.g. during oxidation of furocoumaric acid (Fig. 3). The fact that POP-hemolysins are more stable at lower (about 4°C) than at higher (about 45°C) temperatures may also indirectly indicate their peroxide nature [24, 29].
The second aspect is modulation of both PUVA-hemolysis [39] and POP-hemolysis with glutathione [22, 29]. Activation of PUVA-hemolysis with reduced glutathione (GSH) was attributed to possible formation of free radicals during irradiation. As for POP-hemolysis, a more complicated pattern was observed. Incubation of POP prepared at 25°C with either reduced or oxidized (GSSG) glutathione activated POP-hemolysis [22]. This suggests more efficient formation of hemolysins on interaction of POP and glutathione, as confirmed by data known from the literature [40-42]. At the same time, the hemolytic effect of POP prepared at 4°C was cancelled upon incubation with either GSH or GSSG [29]. Considering that primary photochemical processes are independent of temperature, it can be assumed that temperature governs post-irradiation conversion of one type of POP-products to another. With that, the similar reactivity of GSH and GSSG suggests that the thiol group may not be involved in chemical modification of POP-products, and reaction targets amine groups. This puts the aldehydic POP-products to the forefront, since interaction of aldehydes with amines forming a Schiff base is commonly known.
The POP-hemolysis mechanism remains elusive, while the set of experiments on photoinduced aggregation in POP solutions allowed us to recognize the invalidity of the hypothesis of the mechanochemical mechanism of POP-hemolysis. POP presents a mixture of products varying in their chemical nature, as well as in photochemical mechanisms of their formation. High reactivity of these photoproducts towards both biological targets and one another provides a wide field to interpret the results. In further studies, attention will be particularly focused on the mechanisms of formation and activity of aldehydic photoproducts, since their methods of preparation are known from the literature [15, 43]. Furthermore, the mechanisms of formation and chemical structure of peroxidic photoproducts are of interest, since the promoting role of peroxides in aldehyde-induced hemolysis is also known [44].
This work was supported by the Russian Foundation for Basic Research (grant 12-02-00629).
REFERENCES
1.Racz, E., and Prens, E. P. (2015) Phototherapy and
photochemotherapy for psoriasis, Dermatol. Clin., 33,
79-89.
2.Trautinger, F., Just, U., and Knobler, R. (2013)
Photopheresis (extracorporeal photochemotherapy), Photochem.
Photobiol. Sci., 12, 22-28.
3.Potapenko, A. Ya., Kyagova, A. A., Bezdetnaya, L.
N., Lysenko, E. P., Chernyakhovskaya, I. Yu., Bekhalo, V. A.,
Nagurskaya, E. V., Nesterenko, V. A., Korotky, N. G., Akhtyamov, S. N.,
and Lanshchikova, T. M. (1994) Products of psoralen photooxidation
possess immunomodulative and antileukemic effects, Photochem.
Photobiol., 60, 171-174.
4.Kyagova, A. A., Nagurskaya, E. N., Bekhalo, V. A.,
Chernyakhovskaya, I. Y., Belichenko, I. V., and Potapenko, A. Ya.
(1996) The attenuation of effectors and induction of suppressors of
delayed type hypersensitivity reaction under the treatment with
psoralen photooxidation products, Russ. J. Immunol., 1,
61-68.
5.Kyagova, A. A., Zhuravel, N. N., Malakhov, M. V.,
Lysenko, E. P., Adam, W., Saha-Moller, C. R., and Potapenko, A. Ya.
(1997) Suppression of delayed-type hypersensitivity and hemolysis
induced by previously photooxidized psoralen: effect of fluence rate
and psoralen concentration, Photochem. Photobiol., 65,
694-700.
6.Potapenko, A. Ia., Butov, Iu. S., Levinzon, E. S.,
Andina, E. S., Iurikova, N. A., Nekliukova, M. B., Mamedov, I. S.,
Lysenko, E. P., Bezdetnaia L. N., and Kiagova, A. A. (1999)
Photooxidative reactions of psoralens and their role in therapy of
dermatoses, Vestnik Ros. Akad. Med. Nauk, 2, 32-38.
7.Kyagova, A. A., Malakhov, M. V., and Potapenko, A.
Ya. (2009) in Immunosuppression: New Research (Taylor, C. B.,
ed.) Nova Science Publishers, N. Y., pp. 167-183.
8.Pyatnitskiy, I. A., Pavlova, S. I., Albegova, D.
Z., Kozir, L. A., Kozlov, I. G., Potapenko, A. Y., and Kyagova, A. A.
(2012) The mechanisms of the suppressive action of photooxidized
psoralen on the afferent phase of contact hypersensitivity in mice,
Russ. J. Immunol., 6, 139-146.
9.Pyatnitskiy, I. A., Pavlova, S. I., Albegova, D.
Z., Kozlov, I. G., Potapenko, A. Y., and Kyagova, A. A. (2013)
Suppressive effects of psoralene photooxidation products on contact
sensitivity reaction in mice: lymphocyte proliferation inhibition and
apoptosis induction, Ros. Zh. Kozhn. Vener. Bol., 6,
59-63.
10.Canton, M., Caffieri, S., Dall’Acqua, F.,
and Di Lisa, F. (2002) PUVA-induced apoptosis involves mitochondrial
dysfunction caused by the opening of the permeability transition pore,
FEBS Lett., 522, 168-172.
11.Caffieri, S., Di Lisa, F., Bolesani, F., Facco,
M., Semenzato, G., Dall’Acqua, F., and Canton, M. (2007) The
mitochondrial effects of novel apoptogenic molecules generated by
psoralen photolysis as a crucial mechanism in PUVA therapy,
Blood, 109, 4988-4994.
12.Viola, G., Vedaldi, D., Dall’Acqua, F.,
Lampronti, I., Bianchi, N., Zuccato, C., Borgatti, M., and Gambari, R.
(2008) Furocoumarins photolysis products induce differentiation of
human erythroid cells, J. Photochem. Photobiol. B, 92,
24-28.
13.Salvador, A., Dall’Acqua, S., Sardo, M. S.,
Caffieri, S., Vedaldi, D., Dall’Acqua, F., Borgatti, M., Zuccato,
C., Bianchi, N., and Gambari, R. (2010) Erythroid induction of chronic
myelogenous leukemia K562 cells following treatment with a photoproduct
derived from the UV-A irradiation of 5-methoxypsoralen, Chem. Med.
Chem., 5, 1506-1512.
14.Caffieri, S. (2002) Furocoumarin photolysis:
chemical and biological aspects, Photochem. Photobiol. Sci.,
1, 149-157.
15.Marley, K. A., and Larson, R. A. (1994) A new
photoproduct from furocoumarin photolysis in dilute aqueous solution:
5-formyl-6-hydroxybenzofuran, Photochem. Photobiol., 59,
503-505.
16.Marley, K. A., Larson, R. A., and Davenport, R.
(1995) Redox mechanisms of furocoumarin phototoxicity, The
Spectrum, 8, 9-14.
17.Potapenko, A. Y., Wunderlich, S., Pliquett, F.,
Bezdetnaya, L. N., and Sukhorukov, V. L. (1986) Photosensitized
modification of erythrocyte membranes induces by furocoumarins,
Photobiochem. Photobiophys., 10, 175-180.
18.Potapenko, A. Y., Bezdetnaya, L. N., Lysenko, E.
P., Sukhorukov, V. L., Remisov, A. N., and Vladimirov, Y. A. (1986)
Mechanisms of furocoumarin sensitized damage to biological membranes,
Stud. Biophys., 114, 159-170.
19.Bezdetnaya, L. N., Potapenko, A. Ya., Perkhova,
O. Yu., Nagiev, A. I., Sukhorukov, V. L., and Vladimirov, Yu. A. (1987)
Photodamage of erythrocyte membranes sensitized by psoralen: two
mechanisms, Biol. Membr. (Moscow), 4, 270-279.
20.Potapenko, A. Y., Agamalieva, M. A., Nagiev, A.
I., Lysenko, E. P., Bezdetnaya, L. N., and Sukhorukov, V. L. (1990)
Photohemolysis sensitised by psoralen: reciprocity law is not
fulfilled, J. Photochem. Photobiol. B, 54, 375-379.
21.Potapenko, A. (1991) Mechanisms of photodynamic
effects of furocoumarins, J. Photochem. Photobiol. B, 9,
1-33.
22.Lysenko, E. P., Melnikova, V. O., Andina, E. S.,
Wunderlich, S., Pliquett, F., and Potapenko, A. Y. (2000) Effects of
glutathione peroxidase and catalase on hemolysis and methemoglobin
modifications induced by photooxidized psoralen, J. Photochem.
Photobiol. B, 56, 187-195.
23.Kyagova, A. A., Ismailova, M. I., Malakhov, M.
V., and Potapenko, A. Y. (2004) Hemolysis induced by psoralen
previously photooxidized in ethanol or aqueous solutions, Proc.
SPIE, 5474, 272-280.
24.Potapenko, A. Ya., Malakhov, M. V., and Kyagova,
A. A. (2004) Photobiophysics of furocoumarins, Biophysics,
49, 307-324.
25.Pooler, J. P. (1985) The kinetics of colloid
osmotic hemolysis. II. Photochemolysis, Biochim. Biophys. Acta,
812, 199-205.
26.Pooler, J. P. (1986) A new hypothesis for the
target in photohemolysis: dimers of the band 3 protein, Photochem.
Photobiol., 43, 263-266.
27.Pooler, J. P., and Girotti, A. W. (1986)
Photohemolysis of human erythrocytes labeled in band 3 with
eosin–isothiocyanate, Photochem. Photobiol., 44,
495-499.
28.Potapenko, A. Y., Bezdetnaya, L. N., Lysenko, E.
P., Akhtyamov, S. N., Tomashaeva, S. K., and Sukhorukov, V. L. (1988)
Hypothesis of the induction of psoralen phototoxic effects through the
stage of photooxidized psoralen formation. Model studies of
erythrocytes, Stud. Biophys., 124, 205-223.
29.Malakhov, M. V. (2003) Investigation on the
Formation Mechanism and Stability of Biologically Active Psoralen
Photooxidation Products: Ph. D. thesis [in Russian], RSMU,
Moscow.
30.Belichenko, I. V., Lysenko, E. P., Zhuravel, N.
N., Kyagova, A. A., Malakhov, M. V., Bezdetnaya, L. N., and Potapenko,
A. Ya. (1995) Comparison of haemolytic effects of butylated
hydroxytoluene and previously photooxidized psoralen, Phys. Chem.
Biol. Med., 2, 159-164.
31.Pasternack, R. F., and Collings, P. J. (1995)
Resonance light scattering: a new technique for studying chromophore
aggregation, Science, 269, 935-939.
32.Pyatnitskiy, I. A., and Vlasova, N. V. (2011)
Investigation of aggregation of psoralen photooxidation products by the
method of resonance light scattering, Vestnik RGMU, 1,
69-73.
33.Caffieri, S., and Dall’Acqua, F. (1987)
C4-cyclodimers of psoralen engaging the
4′,5′-double bond, Photochem. Photobiol., 45,
13-18.
34.Logani, M. K., Austin, W. A., Shah, B., and
Davies, R. E. (1982) Photooxidation of 8-MOP with singlet oxygen,
Photochem. Photobiol., 35, 569-573.
35.Wasserman, H. H., and Berdahl, D. R. (1982) The
photooxidation of 8-methoxypsoralen, Photochem. Photobiol.,
35, 565-567.
36.Maslov, S. A., and Blyumberg, E. A. (1976)
Liquid-phase oxidation of aldehydes, Russ. Chem. Rev.,
45, 155-167.
37.Zhuravel, N. N., Belichenko, I. V., Kyagova, A.
A., Lysenko, E. P., Khalilov, E. M., and Potapenko, A. Y. (1996)
Activation of hemolysis induced by photooxidized psoralen (POP) by
Fe2+ ions. The role of Fe2+ reactions with POP
and erythrocytes, Membr. Cell Biol., 10, 381-387.
38.Rodenko, I. N., Osipov, A. N., Lysenko, E. P.,
and Potapenko, A. Y. (1993) Degradation of psoralen photooxidation
products induced by ferrous ions, J. Photochem. Photobiol. B,
19, 39-48.
39.Potapenko, A. Y., Saparov, S. M., Agamalieva, M.
A., Lysenko, E. P., Bezdetnaya, L. N., and Sukhorukov, V. L. (1993)
Fe2+-ions and reduced glutathione – chemical
activators of psoralen-sensitized photohaemolysis, J. Photochem.
Photobiol. B, 17, 69-75.
40.Uchida, K., Hasui, Y., and Osawa, T. (1997)
Covalent attachment of 4-hydroxy-2-nonenal to erythrocyte proteins,
J. Biochem., 6, 1246-1251.
41.Meacher, D. M., and Menzel, D. B. (1999)
Glutathione depletion in lung cells by low-molecular-weight aldehydes,
Cell Biol. Toxicol., 15, 163-171.
42.Ichihashi, K., Osawa, T., Toyokuni, S., and
Ushida, K. (2001) Endogenous formation of protein adducts with
carcinogenic aldehydes, J. Biol. Chem., 276,
23903-23913.
43.Malakhov, M. V., Dubinnyi, M. A., Vlasova, N. V.,
Zgoda, V. G., Efremov, R. G., and Boldyrev, I. A. (2014) End-group
differentiating ozonolysis of furocoumarins, RSC Adv., 4,
61277-61280.
44.Pryor, W. A., Miki, M., Das, B., and Church, D.
F. (1991) The mixture of aldehydes and hydrogen peroxide produced in
the ozonation of dioleoyl phosphatidylcholine causes hemolysis of human
red blood cells, Chem. Biol. Interact., 1, 41-52.