REVIEW: Gangliosides in Breast Cancer: New Perspectives
S. Groux-Degroote, Y. Guérardel, S. Julien, and P. Delannoy*
Structural and Functional Glycobiology Unit, UMR CNRS 8576, University of Lille, 59655 Villeneuve d’Ascq, France; E-mail: sophie.groux-degroote@univ-lille1.fr; yann.guerardel@univ-lille1.fr; sylvain.julien@univ-lille1.fr; philippe.delannoy@univ-lille1.fr* To whom correspondence should be addressed.
Received January 16, 2015; Revision received April 1, 2015
Gangliosides are essential compounds of the plasma membrane involved in cell adhesion, proliferation, and recognition processes, as well as in the modulation of signal transduction pathways. These functions are mainly supported by the glycan moiety, and changes in the structure of gangliosides occur under pathological conditions including cancers. With progress in mass spectrometric analysis of gangliosides, the role of gangliosides in breast cancer progression was recently demonstrated. In this review, we summarize current knowledge on the biosynthesis of gangliosides and of the role of disialogangliosides in triple-negative breast cancer progression and metastasis. New perspectives in breast cancer therapy targeting gangliosides are also discussed.
KEY WORDS: gangliosides, breast cancer, mass spectrometry, metastasis, c-Met, GD3 synthaseDOI: 10.1134/S0006297915070020
Abbreviations: DHB, 2,5-dihydroxybenzoic acid; ER, estrogen receptor; ESI, electrospray ionization; GSLs, glycosphingolipids; GTs, glycosyltransferases; HPLC, high-performance liquid chromatography; IMS, imaging mass spectrometry; mAb, monoclonal antibodies; MALDI, matrix-associated laser desorption-ionization; MS, mass spectrometry; shRNA, short hairpin RNA; siRNA, small interfering RNA; TLC, thin layer chromatography.
Gangliosides define a subclass of glycosphingolipids (GSLs)
characterized by the presence of one or several sialic acid residues in
the carbohydrate moiety. In mammals, they are essential compounds of
the outer leaflet of the plasma membrane, where they interact with
other sphingolipids, cholesterol, and transmembrane proteins including
receptors or signal transducers, forming lipid rafts. Gangliosides were
demonstrated to be central molecules in the plasma membrane involved in
cell adhesion, proliferation, and recognition processes, as well as in
the modulation of signal transduction pathways [1,
2]. These different functions are mainly supported
by the glycan moiety, and changes in the structure of gangliosides can
occur under pathological conditions, including atherosclerosis,
neurodegenerative disorders, and cancers [3-5]. In particular, the neo-expression of
disialogangliosides has been demonstrated in several
neuroectoderm-derived tumors in which they play a key role in invasion
and metastasis [6], making disialogangliosides
attractive target molecules for cancer immunotherapy [7, 8].
GANGLIOSIDES STRUCTURE AND BIOSYNTHESIS
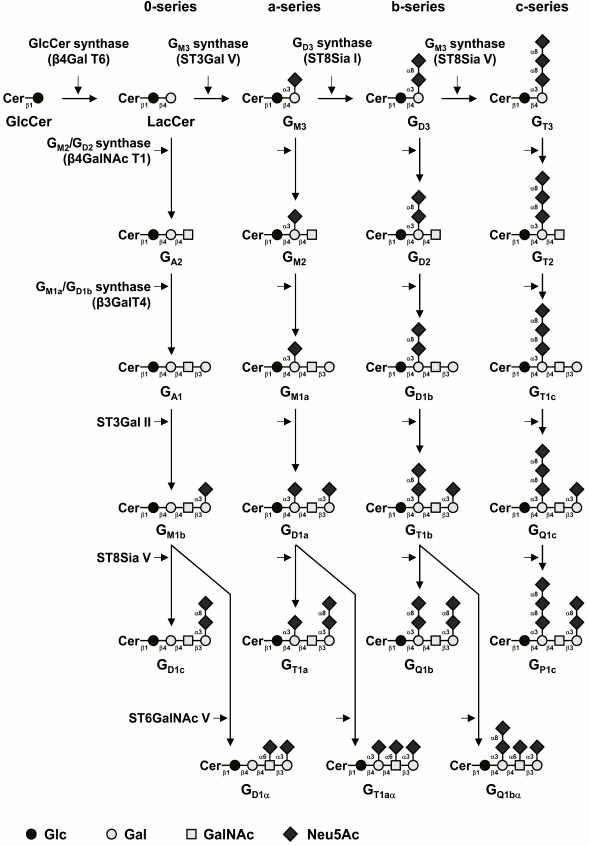
Most of the structural variability of GSLs is borne by the carbohydrate domain that exhibits staggering structural diversity. As a result, GSLs are classified in a number of series defined by their monosaccharide sequences that include (iso)globo, (iso)ganglio, (neo)lacto, (neo)gala, and muco for the most abundant series in vertebrates (table) [9]. Although some gangliosides derive from lacto-, neolacto-, and globo-series, most belong to the ganglio-series GSLs. Gangliosides display carbohydrate core sequence of variable length that can be differently sialylated quantitatively (number of sialic acid residues) and qualitatively (position of sialic acid residues) (Fig. 1). The main core sequence is the tetrasaccharide Galβ1-3GalNAcβ1-4Galβ1-4Glcβ (Gg4), but the di-, tri-, and tetrasaccharide versions usually coexist within a single cell type, whereas the total number of substituting sialic acids varies from 0 (which should formally not qualify as gangliosides) to 5.
Major series of glycosphingolipids in animals
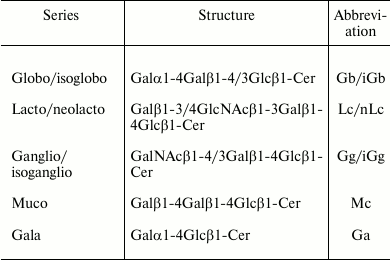
Fig. 1. Biosynthesis pathways for gangliosides. Gangliosides are synthesized by stepwise addition of monosaccharides to ceramide. Ceramide (Cer) is the acceptor for UDP-Glc:ceramide β-d-glucosyltransferase. Extension of GlcCer occurs by the action of UDP-Gal:GlcCer β1,4-galactosyltransferase to make lactosylceramide (GA3). The action of ST3Gal V (GM3 synthase), ST8Sia I (GD3 synthase), and ST8Sia V (GT3 synthase) leads to the biosynthesis of the precursors of a-, b-, and c-series gangliosides, respectively. The 0-series gangliosides are directly synthesized from lactosylceramide. Elongation is performed by the sequential action of N-acetyl-galactosaminyltransferase (β4GalNAc T1), galactosyltransferase (β3Gal T4), and sialyltransferases (ST3Gal II and ST8Sia V). α-Series gangliosides derive from the action of ST6GalNAc V on GM1b, GD1a, or GT1b. The code names of gangliosides are according to Svennerholm [19]. Cer, ceramide; LacCer, lactosylceramide.
The biosynthesis of gangliosides starts by the transfer in the cis-Golgi of a glucose residue onto ceramide (Cer) by the UDP-Glc:N-acylsphingosine β-D-glucosyltransferase (GlcCer synthase) encoded by the UGCG gene [10]. The glucosylceramide synthase is highly specific for ceramide and can be inhibited by d,l-threo-1-phenyl-2-decanoylamino-3-morpholino-1-propanol (PDMP) or d,l-threo-1-phenyl-2-palmitoylamino-3-morpholino-1-propanol (PPMP), blocking the synthesis of almost all GSLs [11]. The next step is the galactosylation of glucosylceramide (GlcCer) to lactosylceramide (LacCer) by the UDP-Gal:GlcCer β1,4-galactosyltransferase (LacCer synthase) [12, 13]. The transfer of sialic acid residues to LacCer is then catalyzed by the sialyltransferases ST3Gal V (GM3 synthase), ST8Sia I (GD3 synthase), and ST8Sia V (GT3 synthase), all showing high specificity toward glycolipid substrates [14]. The human ST3Gal V was shown to use only LacCer as an acceptor substrate to synthesize GM3 (II3Neu5Ac1-Gg2Cer) [15]. The GD3 synthase (GD3S) ST8Sia I is also highly specific to GM3 [16], but the human enzyme was also shown to use GD3 (II3Neu5Ac2-Gg2Cer) to synthesize GT3 (II3Neu5Ac3-Gg2Cer) [17]. The human ST8Sia V exhibits broader enzymatic activity toward gangliosides, using GD3, but also GM1b (IV3Neu5Ac1-Gg4Cer), GD1a (IV3Neu5Ac1II3Neu5Ac1-Gg4Cer), or GT1b (IV3Neu5Ac1II3Neu5Ac2-Gg4Cer) as acceptors [18]. Thus, LacCer, GM3, GD3, and GT3 are the precursors for the 0-, a-, b-, and c-series gangliosides, respectively [19], and the biosynthesis of these compounds determines the relative proportion of gangliosides in each series (Fig. 1).
Afterwards, GalNAc, Gal, and Neu5Ac residues can be transferred in a stepwise manner by the β1,4-N-acetyl-galactosaminyltransferase I (GM2/GD2 synthase) [20], the β1,3-galactosyltransferase IV [21], and different sialyltransferases (Fig. 1). The β1,4-N-acetyl-galactosaminyltransferase I is active on the four series of gangliosides and converts LacCer, GM3, GD3, and GT3 into GA2 (asialo-GM2), GM2, GD2, and GT2, respectively [22, 23]. Similarly, the β1,3-galactosyltransferase IV equally uses GA2, GM2, GD2, and GT2 as acceptor substrates [22]. The terminal Gal residue can be further used as acceptor substrate by an α2,3-sialyltransferase. Both ST3Gal I and ST3Gal II were shown to transfer a sialic acid residue onto Galβ1-3GalNAc disaccharide sequence [24, 25]. However, based on kinetic parameters and tissue distribution, it appears that ST3Gal II is largely responsible for ganglioside terminal α2,3-sialylation [26]. Finally, the terminal trisaccharide Neu5Acα2-3Galβ1-3GalNAc can be further substituted by another sialic acid residue in α2,8-linkage by ST8Sia V [18] or in α2,6-linkage to the GalNAc residue of GM1b, GD1a, or GT1b (Fig. 1) to form α-series gangliosides. Three members of the CMP-Neu5Ac:β-N-acetylgalactosaminide α2,6-sialyltransferase family (ST6GalNAc III, V, and VI) were shown to catalyze in vitro the transfer of a sialic acid residue onto GM1b (IV3Neu5Ac1Gg4-Cer) to form GD1α [27]. However, according to its substrate specificity and expression pattern, ST6GalNAc V is generally considered as the main enzyme forming the α-series gangliosides [28].
Normal human tissues mainly express “simple” gangliosides from the 0- and a-series, whereas “complex” gangliosides from the b- and c-series are essentially restricted to the nervous system of healthy adults but can be re-expressed in several types of cancer, including melanoma and brain tumors [29]. The enzymes of ganglioside biosynthesis are typical type II membrane-anchored glycosyltransferases (GTs) showing a gradient distribution within the Golgi apparatus and forming functional complexes, such as those shown for LacCer synthase, GM3 synthase, and GD3 synthase that are associated in multi-enzymatic complexes in the cis-Golgi [30]. These complexes are thought to act without releasing intermediate structures, ensuring the biosynthesis of a clearly defined ganglioside end-product. The regulation of GTs involved in the synthesis of gangliosides is mainly achieved at the transcriptional level, and GT gene expression is also tissue-specific. For example, the B4GALNACT1 gene is essentially expressed in human brain, lung, and testis, whereas ST3GAL5 is expressed in almost all human tissues [15, 31].
RECENT PROGRESS IN MASS SPECTROMETRIC ANALYSIS OF GSLs
Despite their intrinsic structural variability, GSLs can be easily extracted and separated by a wide panel of chromatographic techniques. Most of the purification protocols developed through the years since the isolation of homogenous ganglioside fractions in the forties are based on organic solvent extractions, and they have not significantly evolved recently. They have been comprehensively presented by R. L. Schnaar and by S. I. Hakomori [32, 33]. The majority of protocols used today, such as the Svennerholm and Fredman method [34], take advantage of the amphiphilic nature of glycosphingolipids through the extraction of tissues or cells by monophasic or diphasic mixtures of chloroform, methanol, and water. Other solvents such as tetrahydrofuran, ether, or butanol have also been used. To these, salts (KCl or phosphate salts usually) can be added to improve the extraction yield of the most polar compounds such as sulfated or sialylated glycolipids [35]. In addition, a number of chromatography media can be used to further purify and separate bulk glycosphingolipids, including anion-exchange (DEAE-Sephadex, Q-Sepharose, etc.), salicylic acid, or reverse-phase. Bulk separation can be coupled to mild-alkaline saponification to clean up triglycerides, but at the expense of alkali-labile functions such as acetyl groups.
Most of the recent progress has been observed in the analytical field and has been driven by the rapid pace of development and dissemination of mass spectrometry (MS)-associated technologies in research laboratories. Not only that, the constant improvement of mass spectrometers in terms of resolution, sensitivity, and analysis speed has dramatically improved the quality of robustness of the structural analyses, but it has also provided novel opportunities. The main technical shortcomings of MS for the analysis of GSLs are their intrinsic diversity borne by both the lipid and the sugar moieties and their amphiphilic properties, which render their handling difficult. Within the scope of this review, we will focus on recent technical advances that have helped to tackle these two aspects.
MALDI-MS. Compared with liquid sources, MALDI sources neutralized all the problems linked to the handling of organic solvents by co-crystallization of the compounds with a matrix, and thus they rapidly appeared as a method of choice for the analysis of GSLs. However, severe losses of sialic acid during the laser-induced desorption from gangliosides and other sialylated GSLs has been a nagging problem for analysts. One solution is to stabilize the sialic acids by specific carboxy-methyl esterification [36, 37] or permethylation of the molecules [38]. In particular, permethylation not only provides robust simultaneous semiquantitative profiles of both neutral and negatively charged sialylated species without loss of sialic acid [39], but also yields more sequence-informative fragmentation patterns in CID MS/MS than non-permethylated glycans. In parallel, intense efforts have been made to minimize the cleavage of very labile glycosidic bonds without chemically modifying GSLs by testing a vast panel of so-called “cold” matrixes. Doing this, numerous dry droplet and ionic liquid matrixes were shown to produce less fragmentation for ganglioside than 2,5-dihydroxybenzoic acid (DHB) does. This is the case for 5-methoxysalicylic acid (MSA), matrix preparation of 2,9-dihydroxyacetophenone (DHA)/ammonium sulfate/heptafluorobutyric acid (HFBA), or 2,5-dihydroxybenzoic acid butylamine (DHBB) that appear to generate better quality spectra of gangliosides than DHB [40-42].
Tissue imaging. Imaging mass spectrometry (IMS) using MALDI-MS technology has recently become a powerful tool for label-free visualization of biomolecules, including glycosphingolipids, on tissue slides. Most MALDI imaging mass spectrometers presently exhibit lateral resolution between 10 and 50 µm. Compared with the classical histochemical approach, it not only distinguishes gangliosides by their oligosaccharide moieties, but also by the nature of their ceramides. Furthermore, the use of tandem mass spectrometry can provide detailed information on the ganglioside structures. As for classical MALDI-MS, experimental efforts have been made to substitute the initially used DHB [43] by colder matrixes to minimize the loss of sialic acids from gangliosides [41, 44, 45]. Alternatively, a softer ionization method than MALDI, the newly developed Laserspray Ionization Inlet (LSII) demonstrated its usefulness in the field of IMS by providing imaging of disialylated GD1 without any visible fragmentation event [46]. These technological developments enabled several groups to convincingly demonstrate that gangliosides were differentially distributed in mouse brain in diverse pathological or environmental conditions [43, 47]. Finally, further studies recently demonstrated that the lateral resolution of ganglioside MALDI imaging could be enhanced down to 10 µm by using an oversampling method, which will open the possibility to achieve cellular resolution in the near future [48, 49].
TLC-MS coupling. No analytical technique other than thin-layer chromatography has had such long-lasting success in the field of structural analysis of GSLs. Compared with HPLC, it shows many advantages such as cost effectiveness, simplicity, single-run comparison of samples, and quantification by the colorimetric method. Indeed, although very simple to put into practice, neutral and sialylated GSLs can be reproductively separated with high resolution on silica gel 60 HPTLC plates after development in chloroform–methanol–water (e.g. 65 : 25 : 4 v/v) or chloroform–methanol–0.2% CaCl2 (e.g. 60 : 42 : 10 v/v). Thus, for decades it has been used both for analytical purposes using standard GSLs and for preparative purposes. Considering the wide distribution of this method, ways to combine TLC and MS analysis were developed early, but the technical challenge lies in the efficient extraction of GSLs from the stationary phase to introduce them into the mass spectrometer. The readers can refer to a recent publication that summaries most of the technological development of TLC-MS interfacing [50].
The earlier process for collecting GSLs for MS analysis relies on scraping the stationary phase (most often silica gel for GSLs) and eluting the molecules with organic solvents. Although presenting many drawbacks (low yield, labor intensiveness, restriction to major components), this technique is still effectively used to produce high quality structural data [51]. Direct sampling of GSLs from TLC in the MALDI source was developed early but was mostly restricted to neutral GSLs [52, 53] because of the high risk of sialic acid loss during laser-induced desorption. An alternative procedure using blotted gangliosides on PVDF membranes following TLC separation, although promising, exhibited similar desialylation [54]. Loss of sialic acids could, however, be minimized when using specific devices such as orthogonal or vibrational cooling MALDI sources [55, 56]. However, these high-end equipment requirements drastically reduce the widespread application of this method for the analysis of gangliosides to a handful of very specialized laboratories.
What seems to be the most promising recent development of TLC-MS interfacing in the field of gangliosides was achieved by coupling commercially available liquid extraction surface automated devices to an electrospray ionization MS source [57]. Indeed, the two surface extraction systems, namely the TLC-MS interface from CAMAG and the Liquid Extraction Surface Analysis (LESA®; CAMAG, Switzerland), showed reliable semi-automated extraction abilities toward gangliosides that could be injected online into a Q-q-TOF MS spectrometer to be analyzed by MS and MS/MS. Owing to the softer ionization procedure than MALDI-MS, gangliosides could be observed with minimal fragmentation, which opens up broad application for TLC-ESI-MS analysis of gangliosides.
LC-ESI-MS. As mentioned above, ESI-MS appears as the method of choice for ionizing gangliosides under soft conditions, and it has been heavily used for structural study of GSLs. Although it offers improved sensitivity and selectivity over TLC-based technologies, the application of LC-ESI-MS to the study of gangliosides lagged behind other biomolecules (peptides, oligosaccharides, etc.) because of the lack of resolution of liquid chromatography columns for gangliosides. Nonetheless, several studies have reported the use of LC-MS for the identification and quantification of gangliosides from complex biological matrixes such as dairy products [58], human plasma [59], or rat brains [60] using either reverse- or normal-phase columns. Recent developments in LC-MS analysis of gangliosides have focused on the use of nano-HPLC chips that can deliver high retention time and abundance reproducibility [61] and on rapid ultra-performance liquid chromatography tandem mass spectrometry (UPLC-MS) [62].
ST8Sia I IS THE KEY ENZYME FOR BIOSYNTHESIS OF b- AND c-SERIES
GANGLIOSIDES
ST8Sia I is the only sialyltransferase catalyzing the transfer of a sialic acid residue onto GM3 through an α2,8-linkage to synthesize GD3. Whereas ST8Sia I mainly sialylates GM3, its ability to synthesize GT3 from GD3, as well as the unusual tetra- and pentasialylated lactosylceramide derivatives GQ3 (II3Neu5Ac4-Gg2Cer) and GP3 (II3Neu5Ac5-Gg2Cer) has also been demonstrated [17, 63]. ST8Sia I was also shown to use GM1b, GD1a, or GT1b as acceptor substrates to synthesize GD1c, GT1a, or GQ1b, respectively, both in vitro and in vivo [64]. However, the α2,8-sialyltransferase ST8Sia V is a much better candidate for GT1a/GQ1b synthase activity [18], and no ST8Sia V activity was detected toward GM3. ST8Sia I is therefore considered as the only GD3S that controls the biosynthesis of b- and c-series gangliosides.
The human GD3S cDNA was simultaneously isolated by expression cloning by three research groups [16, 65, 66]. The ST8SIA1 gene is located on chromosome 12, in p12.1-p11.2, and consists of five coding exons (E1 to E5) spanning 135 kb of genomic DNA [67]. The ST8SIA1 gene encodes a typical type II 341 amino-acid protein having a 12-amino-acid cytoplasmic tail, a transmembrane domain of about 20 residues, and a Golgi catalytic domain containing the conserved sialyl motifs involved in substrate binding and transfer [27]. GD3S is expressed in fetal brain at an early developmental stage, where gangliosides play a key role in cell–cell interactions, cell differentiation, and proliferation [68, 69]. In adult human tissues, GD3S transcripts are significantly detected in brain [17]. GD3S has also been shown to be overexpressed in neuroectoderm-derived malignant tumors such as melanoma, glioblastoma, and neuroblastoma [29]. The promoter region controlling ST8SIA1 gene expression lacks a TATA or CCAAT box, as commonly observed for GT genes, but contains several SP1 sites. Electrophoretic mobility shift assay (EMSA) and mutagenesis experiments have demonstrated the key role of NFκB in activating the expression of ST8SIA1 in melanoma cells [70], and the essential role of AREB6 and Elk-1 transcription factors was also demonstrated for in ST8SIA1 expression in glioblastoma cells [71].
DISIALOGANGLIOSIDES AND BREAST CANCER
Breast tumor tissues were shown to be distinct from normal mammary tissues in terms of ganglioside composition. The gangliosides GM3, GD3, and their derivatives 9-O-acetyl-GD3 (CDw60 antigen) and 9-O-acetyl-GT3, which show a very restricted expression in normal breast tissues, are overexpressed in about 50% of invasive ductal carcinoma [72]. N-Glycolyl-GM3 is also detected in 100% of stage II breast cancers [73]. Neu5Gc is normally absent in humans due to the irreversible inactivation of the CMAH gene encoding the CMP-Neu5Ac hydroxylase. The lack of the enzyme responsible for the conversion of CMP-Neu5Ac into CMP-Neu5Gc results in the total absence of Neu5Gc in healthy human tissues. In contrast, Neu5Gc was shown to be expressed on glycoproteins and gangliosides in melanoma and colon, retinoblastoma, and breast cancers.
The expression of GTs implicated in ganglioside biosynthesis is also altered in breast cancer tumors. Microarray analysis of more than one thousand tumor samples in different subtypes of breast cancer has shown that GD3S displayed higher expression among estrogen receptor (ER)-negative breast cancer tumors [74]. ST8SIA1 overexpression was associated with poor pathohistological grading in ER-negative tumors and lower survival rate of patients [75]. In contrast, better prognosis for ER-positive samples exhibiting high expression of ST8SIA1 was described [75]. GD3S was also shown to be five-fold increased in a variant of MDA-MB-231 breast cancer cell line that colonizes bone [76]. It has been shown that GM3 synthase silencing in mammary murine 4T1 cells significantly inhibited cell migration, invasion, and anchorage-independent growth in vitro, as well as lung metastasis in vivo. In parallel, overexpression of GM3 synthase in non-metastatic 67NR cells significantly restored the malignant phenotype [77].
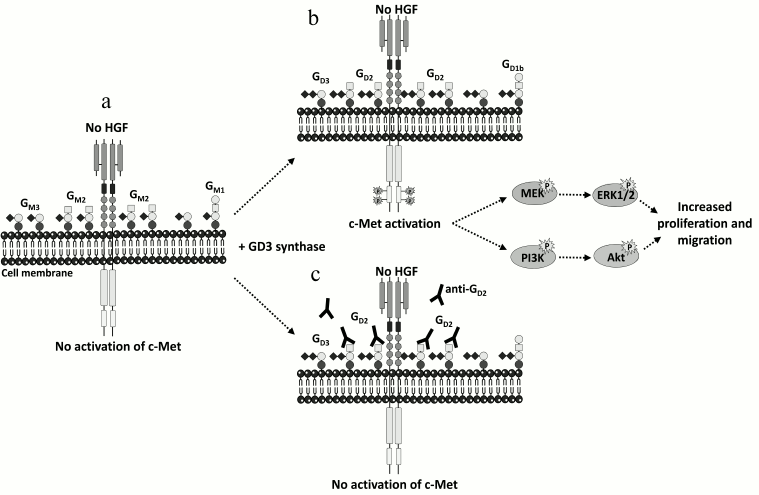
Recent data have shed light on the important role of disialoganglioside GD2 in breast cancer. The expression of the GD3S in MDA-MB-231 breast cancer cells induced the accumulation of b- and c-series gangliosides (GD3, GD2, and GT3) at the cell surface together with the acquisition of a proliferative phenotype in the absence of serum or exogenous growth factors [78]. GD3S expressing cells bypass the need for growth factors for cell growth by specific and constitutive activation of the c-Met receptor in the absence of its ligand, hepatocyte growth factor/scatter factor (HGF/SF), and the activation of the phosphoinositide 3-kinase (PI3K)/Akt and extracellular signal-regulated kinase (Erk)/mitogen-activated protein kinase (MAPK) pathways [79]. GD3S expression also enhanced tumor growth in severe combined immunodeficient (SCID) mice, and a higher expression of ST8SIA1 and MET in the “basal-like” subtype of human breast tumors was observed [79]. The decrease of GD2 expression by small interfering RNA (siRNA) silencing of the GM2/GD2 synthase reversed the proliferative phenotype as well as c-Met phosphorylation, and competition assays using anti-GD2 monoclonal antibodies also inhibited cell proliferation and c-Met phosphorylation [80] (Fig. 2), demonstrating the involvement of the disialoganglioside GD2 in MDA-MB-231 cell proliferation via the constitutive activation of c-Met. The accumulation of GD2 in c-Met expressing cells could therefore reinforce the tumorigenicity and aggressiveness of breast cancer tumors.
Fig. 2. Activation of c-Met by GD2 ganglioside. a) MDA-MB-231 breast cancer cells express mainly GM3 and GM2. b) The expression of GD3S induces the accumulation of b- and c-series gangliosides, mainly GD2. This leads to the activation of c-Met in the absence of HGF and increases proliferation and migration through the PI3K/Akt and MEK/Erk pathways. c) Anti-GD2 mAb used in competition assays inhibits c-Met phosphorylation and cell proliferation [79, 80].
Ganglioside GD2 was recently identified as a new specific cell surface marker of CD44hiCD24lo breast cancer stem cells (CSC) from human breast cancer cell lines and patient samples that are capable of forming mammospheres and initiating tumors [81]. Gene expression analysis revealed that several GT genes involved in GD2 biosynthesis (ST3GAL5, B4GALNT1, and ST8SIA1) are highly expressed in CSC [81, 82]. The reduction of GD2 expression by ST8SIA1 knockdown reduced mammosphere formation and cell motility and completely abrogated tumor formation in vivo, changing the phenotype from CSC to non-CSC [81, 82]. Moreover, the induction of epithelial–mesenchymal transition (EMT) in transformed human mammary epithelial cells dramatically increased GD3S as well as GD2 expression, whereas the inhibition of GD3S compromised EMT initiation and maintenance and prevented metastasis [83]. Since GD3S expression correlated with the constitutive activation of the c-Met signaling pathway leading to increased stem cell properties and metastatic competence, the GD3S/c-Met axis could serve as an effective target for the treatment of metastatic breast cancer.
ALPHA-SERIES GANGLIOSIDES IN BREAST CANCER
Alpha-series gangliosides define a particular sub-class of GSL containing Neu5Ac residue α2,6-linked to the GalNAc of the gangliopentaosyl backbone Neu5Acα2-3Galβ1-3GalNAcβ1-4Galβ1-4Glcβ (IV3Neu5Ac1Gg4). The typical α-series ganglioside GD1α (IV3Neu5Ac1, III6Neu5Ac1Gg4-Cer) was first isolated as a minor compound from rat ascites hepatoma AH 7974F cells [84] and from bovine brain [85], with expression restricted to particular cell populations of the forebrain, the midbrain, and the cerebellum [86]. Amongst the ST6GalNAc sialyltransferases, ST6GalNAc V is generally considered as the main GD1α synthase catalyzing the transfer of a sialic acid residue onto GM1b (IV3Neu5Ac1Gg4-Cer) to form GD1α. ST6GalNAc V cDNA was cloned from mouse brain [28, 87], and the st6galnac5 gene is specifically expressed in brain tissues, mostly in forebrain and cerebellum [87]. When expressed as a soluble recombinant protein, the mouse ST6GalNAc V showed α2,6-sialyltransferase activity almost exclusively for GM1b, while being inactive toward glycoproteins [28]. However, it was shown that transfection of human ST6GalNAc V into U373MG glioma cells produced the unusual α2,6-monosialoganglioside GM2α (Neu5Acα2-6GalNAcβ1-4Galβ1-4Glcβ1-Cer, III6Neu5Ac1Gg3-Cer) instead of GD1α [88].
To date, little is known concerning the specific function of α-series gangliosides, but recent evidence indicates a possible function in the adhesion of cancer cells to endothelium. In highly metastatic murine lymphosarcoma cell line RAW117-H10, GD1α serves as an adhesion molecule to hepatic sinusoidal endothelial cells (HSE) [89]. Incubation with GD1α oligosaccharide or with anti-GD1α monoclonal antibody inhibited the adhesion between the RAW117-H10 and HSE cells. Recently, ST6GALNAC5 was identified as one of the genes overexpressed in breast cancer cell populations selected for their ability to produce brain metastasis [90]. Short hairpin RNA (shRNA) inhibition of ST6GALNAC5 decreased the brain metastatic capacity of breast cancer cells, whereas the expression of ST6GALNAC5 in parental cell lines mediated brain metastasis [90]. It was also demonstrated that ST6GalNAc V expression enhanced the adhesion of breast cancer cells to brain endothelium and passage through the blood–brain barrier, Moreover, ST6GALNAC5 is the only gene specifically correlated with brain metastasis of breast cancer and is upregulated in human brain metastasis samples. However, the capacity of human breast cancer cells expressing ST6GALNAC5 to produce α-series gangliosides remains to be clearly demonstrated.
THERAPEUTIC PERSPECTIVES
Innovative therapeutic tools based on the use of antitumor-associated ganglioside mAbs have been developed and are currently under investigation in preclinical or clinical studies, especially in the field of neuroectoderm-related cancers (melanoma, neuroblastoma, small cell lung cancer) [7, 8]. As an example, anti-GD2 clinical trials for neuroblastoma have confirmed the efficacy of anti-GD2 antibody immunotherapy for this rare but often lethal childhood cancer [91]. Racotumomab, an anti-idiotypic antibody registered under the trade name Vaxira [92], and Neu5Gc-GM3/VSSP, a Neu5Gc-GM3 ganglioside conjugated to proteoliposomes, have shown efficacy for patients with advanced melanoma [93] and non-small-cell lung cancer [94]. To date, no clinical trial has addressed the possible use of anti-ganglioside antibodies in breast cancer treatment. Only a phase I clinical trial has shown that the 14F7 mAb specific for Neu5Gc-GM3 could target human breast carcinoma [73]. The recently demonstrated involvement of the GD2/Met axis in ER-negative breast cancer aggressiveness strongly supports that anti-GD2 immunotherapeutic approaches could be useful in breast cancer treatment. In parallel, recent findings strongly suggest that c-Met itself may be a valuable therapeutic target in triple negative breast cancers, since high expression of c-Met in breast cancer was correlated with poor overall survival (p = 0.001) and disease-free survival (p = 0.01) [95]. In addition, c-Met expression was relatively high in triple negative breast cancers cell lines, and its silencing using siRNA reduced cell proliferation and migration [95]. Other results demonstrated that miR-185, a micro RNA whose expression is downregulated in breast cancer tissues, inhibited the proliferation of breast cancer cells by regulating the expression of c-Met, confirming its potential as a therapeutic target for breast cancer [96]. As mentioned before, GD3S expression and generated GD2 ganglioside correlated with the constitutive activation of the c-Met signaling pathway leading to increased metastatic competence of breast cancer cells. GD2 overexpression enhanced stem cell properties and might be responsible for the aggressive nature of triple negative breast cancers and the poor clinical outcome observed, suggesting that GD3S and the c-Met axis could be synergically targeted for the treatment of metastatic breast cancer [83] using, for example, anti-Met and anti-GD2 mAbs. Because of its restricted normal tissue distribution, ganglioside GD2 has been proven safe for mAb targeting, and anti-GD2 mAb treatment is now part of the standard care for the treatment of high-risk metastatic neuroblastoma [97]. Since both GD2 and c-Met are independently expressed in a range of healthy cells with few overlaps in the body, one can consider their combination to be a cancer-specific event. Pending appropriate demonstration of this, the GD2/c-Met couple might therefore also qualify as an interesting target for anticancer bispecific mAb [98]. Another strategy to target the GD3S/c-Met axis could be the use of specific inhibitors of both targets.
Finally, pharmacological approaches targeting ganglioside biosynthesis could also be applied for breast cancer therapy. As an example, Triptolide is an inhibitor of GD3S expression that exhibits a strong cytotoxicity against in human breast cancer stem cells and primary breast cancer cells in vitro and in vivo, suggesting that this natural diterpenoid triepoxide compound from Tripterygium wilfordii might have clinical applications for the suppression of breast tumor growth [99]. In addition, recent data by Sarkar and coworkers showed that inhibition of GD3S, using shRNA or Triptolide, reduced metastatic burden in mice and primary tumor growth, suggesting again that this molecule could be of great interest for the treatment of triple negative breast cancers and for targeting cancer stem cell-enriched tumors [83]. The ongoing clinical trials for Triptolide and the development of new specific inhibitors of GD3S seem highly promising for breast cancer therapy.
This work was supported by the University of Sciences and Technologies of Lille, the CNRS, and the “Comité du Pas-de-Calais de La Ligue contre le Cancer”.
REFERENCES
1.Hakomori, S. I. (2002) The glycosynapse, Proc.
Natl. Acad. Sci. USA, 99, 225-232.
2.Regina Todeschini, A., and Hakomori, S. I. (2008)
Functional role of glycosphingolipids and gangliosides in control of
cell adhesion, motility, and growth, through glycosynaptic
microdomains, Biochim. Biophys. Acta, 1780, 421-433.
3.Birkle, S., Zeng, G., Gao, L., Yu, R. K., and
Aubry, J. (2003) Role of tumor-associated gangliosides in cancer
progression, Biochimie, 85, 455-463.
4.Prokazova, N. V., and Bergelson, L. D. (1994)
Gangliosides and atherosclerosis, Lipids, 29, 1-5.
5.Ariga, T., McDonald, M. P., and Yu, R. K. (2008)
Role of ganglioside metabolism in the pathogenesis of Alzheimer’s
disease – a review, J. Lipid Res., 49,
1157-1175.
6.Furukawa, K., Hamamura, K., Aixinjueluo, W., and
Furukawa, K. (2006) Biosignals modulated by tumor-associated
carbohydrate antigens: novel targets for cancer therapy, Ann. N. Y.
Acad. Sci., 1086, 185-198.
7.Krengel, U., and Bousquet, P. A. (2014) Molecular
recognition of gangliosides and their potential for cancer
immunotherapies, Front. Immunol., 5, 325.
8.Rabu, C., McIntosh, R., Jurasova, Z., and Durrant,
L. (2012) Glycans as targets for therapeutic antitumor antibodies,
Future Oncol., 8, 943-960.
9.Yu, R. K., Yanagisawa, M., and Ariga, T. (2007)
Glycosphingolipid structures, in Comprehensive Glycoscience
(Kamerling, H., ed.) Elsevier, Oxford, UK, pp. 73-122.
10.Ichikawa, S., Sakiyama, H., Suzuki, G., Hidari,
K. I., and Hirabayashi, Y. (1996) Expression cloning of a cDNA for
human ceramide glucosyltransferase that catalyzes the first
glycosylation step of glycosphingolipid synthesis, Proc. Natl. Acad.
Sci. USA, 93, 4638-4643.
11.Abe, A., Inokuchi, J., Jimbo, M., Shimeno, H.,
Nagamatsu, A., Shayman, J. A., Shukla, G. S., and Radin, N. S. (1992)
Improved inhibitors of glucosylceramide synthase, J. Biochem.,
111, 191-196.
12.Nomura, T., Takizawa, M., Aoki, J., Arai, H.,
Inoue, K., Wakisaka, E., Yoshizuka, N., Imokawa, G., Dohmae, N., Takio,
K., Hattori, M., and Matsuo, N. (1998) J. Biol. Chem.,
273, 13570-13577.
13.Takizawa, M., Nomura, T., Wakisaka, E.,
Yoshizuka, N., Aoki, J., Arai, H., Inoue, K., Hattori, M., and Matsuo,
N. (1999) cDNA cloning and expression of human lactosylceramide
synthase, Biochim. Biophys. Acta, 1438, 301-304.
14.Zeng, G., and Yu, R. K. (2008) Cloning and
transcriptional regulation of genes responsible for synthesis of
gangliosides, Curr. Drug Targets, 9, 317-324.
15.Ishii, A., Ohta, M., Watanabe, Y., Matsuda, K.,
Ishiyama, K., Sakoe, K., Nakamura, M., Inokuchi, J., Sanai, Y., and
Saito, M. (1998) Expression cloning and functional characterization of
human cDNA for ganglioside GM3 synthase, J. Biol. Chem.,
273, 31652-31655.
16.Haraguchi, M., Yamashiro, S., Yamamoto, A.,
Furukawa, K., Takamiya, K., Lloyd, K. O., Shiku, H., and Furukawa, K.
(1994) Isolation of GD3 synthase gene by expression cloning of GM3
alpha-2,8-sialyltransferase cDNA using anti-GD2 monoclonal antibody,
Proc. Natl. Acad. Sci. USA, 91, 10455-10459.
17.Nakayama, J., Fukuda, M. N., Hirabayashi, Y.,
Kanamori, A., Sasaki, K., Nishi, T., and Fukuda, M. (1996) Expression
cloning of a human GT3 synthase. GD3 and GT3 are synthesized by a
single enzyme, J. Biol. Chem., 271, 3684-3691.
18.Kim, Y. J., Kim, K. S., Do, S., Kim, C. H., Kim,
S. K., and Lee, Y. C. (1997) Molecular cloning and expression of human
alpha2,8-sialyltransferase (hST8Sia V), Biochem. Biophys. Res.
Commun., 235, 327-330.
19.Svennerholm, L. (1980) Ganglioside designation,
Adv. Exp. Med. Biol., 125, 11.
20.Nagata, Y., Yamashiro, S., Yodoi, J., Lloyd, K.
O., Shiku, H., and Furukawa, K. (1992) Expression cloning of
beta-1,4-N-acetylgalactosaminyltransferase cDNAs that determine the
expression of GM2 and GD2 gangliosides, J. Biol. Chem.,
267, 12082-12089.
21.Amado, M., Almeida, R., Carneiro, F., Levery, S.
B., Holmes, E. H., Nomoto, M., Hollingsworth, M. A., Hassan, H.,
Schwientek, T., Nielsen, P. A., Bennett, E. P., and Clausen, H. (1998)
A family of human beta3-galactosyltransferases. Characterization of
four members of a
UDP-galactose:beta-N-acetyl-glucosamine/beta-N-acetyl-galactosamine/beta-1,3-galactosyltransferase
family, J. Biol. Chem., 273, 12770-12778.
22.Iber, H., Zacharias, C., and Sandhoff, K. (1992)
The c-series gangliosides GT3, GT2, and GP1c are formed in rat liver
Golgi by the same set of glycosyltransferases that catalyze the
biosynthesis of asialo-, a- and b-series gangliosides,
Glycobiology, 2, 137-142.
23.Yamashiro, S., Haraguchi, M., Furukawa, K.,
Takamiya, K., Yamamoto, A., Nagata, Y., Lloyd, K. O., Shiku, H., and
Furukawa, K. (1995) Substrate specificity of beta
1,4-N-acetylgalactosaminyltransferase in vitro and in
cDNA-transfected cells. GM2/GD2 synthase efficiently generates
asialo-GM2 in certain cells, J. Biol. Chem., 270,
6149-6155.
24.Kitagawa, H., and Paulson, J. C. (1994) Cloning
of a novel alpha 2,3-sialyltransferase that sialylates glycoprotein and
glycolipid carbohydrate groups, J. Biol. Chem., 269,
1394-1401.
25.Giordanengo, V., Bannwarth, S., Laffont, C., Van
Miegem, V., Harduin-Lepers, A., Delannoy, P., and Lefebvre, J. C.
(1997) Cloning and expression of cDNA for a human Gal(beta1-3)GalNAc
alpha2,3-sialyltransferase from the CEM T-cell line, Eur. J.
Biochem., 247, 558-566.
26.Sturgill, E. R., Aoki, K., Lopez, P. H.,
Colacurcio, D., Vajn, K., Lorenzini, I., Majic, S., Yang, W. H.,
Heffer, M., Tiemeyer, M., Marth, J. D., and Schnaar, R. L. (2012)
Biosynthesis of the major brain gangliosides GD1a and GT1b,
Glycobiology, 22, 1289-1301.
27.Harduin-Lepers, A. (2013) Vertebrate
sialyltransferases, in Sialobiology: Structure, Biosynthesis and
Function of Sialic Acid Glycoconjugates in Health and Disease
(Tiralongo, J., and Martinez-Duncker, J. T., eds.) Bentham Science
Publishers, pp. 139-187.
28.Okajima, T., Fukumoto, S., Ito, H., Kiso, M.,
Hirabayashi, Y., Urano, T., and Furukawa, K. (1999) Molecular cloning
of brain-specific GD1alpha synthase (ST6GalNAc V) containing
CAG/Glutamine repeats, J. Biol. Chem., 274,
30557-30562.
29.Bobowski, M., Cazet, A., Steenackers, A., and
Delannoy, P. (2012) Role of complex gangliosides in cancer progression,
Carbohydr. Chem., 37, 1-20.
30.Giraudo, C. G., and Maccioni, H. J. (2003)
Ganglioside glycosyltransferases organize in distinct multienzyme
complexes in CHO-K1 cells, J. Biol. Chem., 278,
40262-40271.
31.Hidari, J. K., Ichikawa, S., Furukawa, K.,
Yamasaki, M., and Hirabayashi, Y. (1994)
beta-1-4-N-acetylgalactosaminyltransferase can synthesize both
asialoglycosphingolipid GM2 and glycosphingolipid GM2 in vitro
and in vivo: isolation and characterization of a
beta-1-4-N-acetylgalactosaminyltransferase cDNA clone from rat ascites
hepatoma cell line AH7974F, Biochem. J., 303,
957-965.
32.Schnaar, R. L. (1994) Isolation of
glycosphingolipids, Methods Enzymol., 230, 348-370.
33.Hakomori, S. I. (1983) Chemistry of
glycosphingolipids, in Sphingolipid Biochemistry – Handbook of
Lipid Research (Kanfer, J. N., and Hakomori, S. I., eds.) Plenum
Press, New York, pp. 1-165.
34.Svennerholm, L., and Fredman, P. (1980) A
procedure for the quantitative isolation of brain gangliosides,
Biochim. Biophys. Acta, 617, 97-109.
35.Ijuin, T., Kitajima, K., Song, Y., Kitazume, S.,
Inoue, S., Haslam, S. M., Morris, H. R., Dell, A., and Inoue, Y. (1996)
Isolation and identification of novel sulfated and nonsulfated
oligosialylglycosphingolipids from sea urchin sperm, Glycoconj.
J., 13, 401-413.
36.Powell, A. K., and Harvey, D. J. (1996)
Stabilization of sialic acids in N-linked oligosaccharides and
gangliosides for analysis by positive ion matrix-assisted laser
desorption/ionization mass spectrometry, Rapid Commun. Mass
Spectrom., 10, 1027-1032.
37.Wheeler, S. F., Domann, P., and Harvey, D. J.
(2009) Derivatization of sialic acids for stabilization in
matrix-assisted laser desorption/ionization mass spectrometry and
concomitant differentiation of alpha(2→3)- and
alpha(2→)-isomers, Rapid Commun. Mass Spectrom.,
23, 303-312.
38.Liang, Y. J., Kuo, H. H., Lin, C. H., Chen, Y.
Y., Yang, B. C., Cheng, Y. Y., Yu, A. L., Khoo, K. H., and Yu, J.
(2010) Switching of the core structures of glycosphingolipids from
globo- and lacto- to ganglio-series upon human embryonic stem cell
differentiation, Proc. Natl. Acad. Sci. USA, 107,
22564-22569.
39.Boccuto, L., Aoki, K., Flanagan-Steet, H., Chen,
C. F., Fan, X., Bartel, F., Petukh, M., Pittman, A., Saul, R., Chaubey,
A., Alexov, E., Tiemeyer, M., Steet, R., and Schwartz, C. E. (2014) A
mutation in a ganglioside biosynthetic enzyme, ST3GAL5, results in salt
and pepper syndrome, a neurocutaneous disorder with altered glycolipid
and glycoprotein glycosylation, Hum. Mol. Genet., 23,
418-433.
40.Lee, D., and Cha, S. (2014) 5-Methoxysalicylic
acid matrix for ganglioside analysis with matrix-assisted laser
desorption/ionization mass spectrometry, J. Am. Soc. Mass
Spectrom., Dec 11, Epub ahead of print.
41.Colsch, B., and Woods, A. S. (2010) Localization
and imaging of sialylated glycosphingolipids in brain tissue sections
by MALDI mass spectrometry, Glycobiology, 20,
661-667.
42.Mank, M., Stahl, B., and Boehm, G. (2004)
2,5-Dihydroxybenzoic acid butylamine and other ionic liquid matrixes
for enhanced MALDI-MS analysis of biomolecules, Anal. Chem.,
76, 2938-2950.
43.Sugiura, Y., Shimma, S., Konishi, Y., Yamada, M.
K., and Setou, M. (2008) Imaging mass spectrometry technology and
application on ganglioside study; visualization of age-dependent
accumulation of C20-ganglioside molecular species in the mouse
hippocampus, PLoS One, 3, e3232.
44.Chan, K., Lanthier, P., Liu, X., Sandhu, J. K.,
Stanimirovic, D., and Li, J. (2009) MALDI mass spectrometry imaging of
gangliosides in mouse brain using ionic liquid matrix, Anal. Chim.
Acta, 639, 57-61.
45.Goto-Inoue, N., Hayasaka, T., Zaima, N.,
Kashiwagi, Y., Yamamoto, M., Nakamoto, M., and Setou, M. (2010) The
detection of glycosphingolipids in brain tissue sections by imaging
mass spectrometry using gold nanoparticles, J. Am. Soc. Mass
Spectrom., 21, 1940-1943.
46.Richards, A. L., Lietz, C. B., Wager-Miller, J.,
Mackie, K., and Trimpin, S. (2012) Localization and imaging of
gangliosides in mouse brain tissue sections by laserspray ionization
inlet, J. Lipid Res., 53, 1390-1398.
47.Whitehead, S. N., Chan, K. H., Gangaraju, S.,
Slinn, J., Li, J., and Hou, S. T. (2011) Imaging mass spectrometry
detection of gangliosides species in the mouse brain following
transient focal cerebral ischemia and long-term recovery, PLoS
One, 6, e20808.
48.Snel, M. F., and Fuller, M. (2010) High-spatial
resolution matrix-assisted laser desorption ionization imaging analysis
of glucosylceramide in spleen sections from a mouse model of Gaucher
disease, Anal. Chem., 82, 3664-3670.
49.Kettling, H., Vens-Cappell, S., Soltwisch, J.,
Pirkl, A., Haier, J., Müthing, J., and Dreisewerd, K. (2014) MALDI
mass spectrometry imaging of bioactive lipids in mouse brain with a
Synapt G2-S mass spectrometer operated at elevated pressure: improving
the analytical sensitivity and the lateral resolution to ten
micrometers, Anal. Chem., 86, 7798-7805.
50.Cheng, S. C., Huang, M. Z., and Shiea, J. (2011)
Thin layer chromatography/mass spectrometry, J. Chromatogr. A,
1218, 2700-2711.
51.Mlinac, K., Fabris, D., Vukelic, Z., Rozman, M.,
Heffer, M., and Bognar, S. K. (2013) Structural analysis of brain
ganglioside acetylation patterns in mice with altered ganglioside
biosynthesis, Carbohydr. Res., 382, 1-8.
52.Suzuki, A., Miyazaki, M., Matsuda, J., and
Yoneshige, A. (2011) High-performance thin-layer chromatography/mass
spectrometry for the analysis of neutral glycosphingolipids,
Biochim. Biophys. Acta, 1811, 861-874.
53.Miyazaki, M., Yonesige, A., Matsuda, J., Kuroda,
Y., Kojima, N., and Suzuki, A. (2008) High-performance thin-layer
chromatography/mass spectrometry for rapid analysis of neutral
glycosphingolipids, J. AOAC Int., 91, 1218-1226.
54.Valdes-Gonzalez, T., Goto-Inoue, N., Hirano, W.,
Ishiyama, H., Hayasaka, T., Setou, M., and Taki, T. (2011) New approach
for glyco- and lipidomics – molecular scanning of human brain
gangliosides by TLC-Blot and MALDI-QIT-TOF MS, J. Neurochem.,
116, 678-683.
55.Ivleva, V. B., Elkin, Y. N., Budnik, B. A.,
Moyer, S. C., O’Connor, P. B., and Costello, C. E. (2004)
Coupling thin-layer chromatography with vibrational cooling
matrix-assisted laser desorption/ionization Fourier transform mass
spectrometry for the analysis of ganglioside mixtures, Anal.
Chem., 76, 6484-6491.
56.Ivleva, V. B., Sapp, L. M., O’Connor, P.
B., and Costello, C. E. (2005) Ganglioside analysis by thin-layer
chromatography matrix-assisted laser desorption/ionization orthogonal
time-of-flight mass spectrometry, J. Am. Soc. Mass Spectrom.,
16, 1552-1560.
57.Park, H., Zhou, Y., and Costello, C. E. (2014)
Direct analysis of sialylated or sulfated glycosphingolipids and other
polar and neutral lipids using TLC-MS interfaces, J. Lipid Res.,
55, 773-781.
58.Sorensen, L. K. (2006) A liquid
chromatography/tandem mass spectrometric approach for the determination
of gangliosides GD3 and GM3 in bovine milk and infant formulae,
Rapid Commun. Mass Spectrom., 20, 3625-3633.
59.Fuller, M., Duplock, S., Hein, L. K., Rigat, B.
A., and Mahuran, D. J. (2014) Liquid chromatography/electrospray
ionization-tandem mass spectrometry quantification of GM2 gangliosides
in human peripheral cells and plasma, Anal. Biochem.,
458, 20-26.
60.Fong, B., Norris, C., Lowe, E., and McJarrow, P.
(2009) Liquid chromatography-high-resolution mass spectrometry for
quantitative analysis of gangliosides, Lipids, 44,
867-874.
61.Lee, H., Lerno, L. A., Jr., Choe, Y., Chu, C. S.,
Gillies, L. A., Grimm, R., Lebrilla, C. B., and German, J. B. (2012)
Multiple precursor ion scanning of gangliosides and sulfatides with a
reversed-phase microfluidic chip and quadrupole time-of-flight mass
spectrometry, Anal. Chem., 84, 5905-5912.
62.Zhang, J., Ren, Y., Huang, B., Tao, B., Pedersen,
M. R., and Li, D. (2012) Determination of disialoganglioside GD3 and
monosialoganglioside GM3 in infant formulas and whey protein
concentrates by ultra-performance liquid chromatography/electrospray
ionization tandem mass spectrometry, J. Sep. Sci., 35,
937-946.
63.Steenackers, A., Vanbeselaere, J., Cazet, A.,
Bobowski, M., Rombouts, Y., Colomb, F., Le Bourhis, X., Guerardel, Y.,
and Delannoy, P. (2012) Accumulation of unusual gangliosides G(Q3) and
G(P3) in breast cancer cells expressing the G(D3) synthase,
Molecules, 17, 9559-9572.
64.Nara, K., Watanabe, Y., Kawashima, I., Tai, T.,
Nagai, Y., and Sanai, Y. (1996) Acceptor substrate specificity of a
cloned GD3 synthase that catalyzes the biosynthesis of both GD3 and
GD1c/GT1a/GQ1b, Eur. J. Biochem., 238, 647-652.
65.Nara, K., Watanabe, Y., Maruyama, K., Kasahara,
K., Nagai Y., and Sanai, Y. (1994) Expression cloning of a
CMP-NeuAc:NeuAc alpha 2-3Gal beta 1-4Glc beta 1-1′Cer alpha
2,8-sialyltransferase (GD3 synthase) from human melanoma cells,
Proc. Natl. Acad. Sci. USA, 91, 7952-7956.
66.Sasaki, K., Kurata, K., Kojima, N., Kurosawa, N.,
Ohta, S., Hanai, N., Tsuji, S., and Nishi, T. (1994) Expression cloning
of a GM3-specific alpha-2,8-sialyltransferase (GD3 synthase), J.
Biol. Chem., 269, 15950-15956.
67.Furukawa, K., Horie, M., Okutomi, K., Sugano, S.,
and Furukawa, K. (2003) Isolation and functional analysis of the
melanoma specific promoter region of human GD3 synthase gene,
Biochim. Biophys. Acta, 1627, 71-78.
68.Yamamoto, A., Haraguchi, M., Yamashiro, S.,
Fukumoto, S., Furukawa, K., Takamiya, K., Atsuta, M., Shiku, H., and
Furukawa, K. (1996) Heterogeneity in the expression pattern of two
ganglioside synthase genes during mouse brain development, J.
Neurochem., 66, 26-34.
69.Yu, R. K., Macala, L. J., Taki, T., Weinfield, H.
M., and Yu, F. S. (1988) Developmental changes in ganglioside
composition and synthesis in embryonic rat brain, J. Neurochem.,
50, 1825-1829.
70.Kang, N. Y., Kim, C. H., Kim, K. S., Ko, J. H.,
Lee, J. H., Jeong, Y. K., and Lee, Y. C. (2007) Expression of the human
CMP-NeuAc:GM3 alpha2,8-sialyltransferase (GD3 synthase) gene through
the NF-kappaB activation in human melanoma SK-MEL-2 cells, Biochim.
Biophys. Acta, 1769, 622-630.
71.Dae, H. M., Kwon, H. Y., Kang, N. Y., Song, N.
R., Kim, K. S., Kim, C. H., Lee, J. H., and Lee, Y. C. (2009) Isolation
and functional analysis of the human glioblastoma-specific promoter
region of the human GD3 synthase (hST8Sia I) gene, Acta Biochim.
Biophys. Sin. (Shanghai), 41, 237-245.
72.Marquina, G., Waki, H., Fernandez, L. E., Kon,
K., Carr, A., Valiente, O., Perez, R., and Ando, S. (1996) Gangliosides
expressed in human breast cancer, Cancer Res., 56,
5165-5171.
73.Oliva, J. P., Valdes, Z., Casaco, A., Pimentel,
G., Gonzalez, J., Alvarez, I., Osorio, M., Velazco, M., Figueroa, M.,
Ortiz, R., Escobar, X., Orozco, M., Cruz, J., Franco, S., Diaz, M.,
Roque, L., Carr, A., Vazquez, A. M., Mateos, C., Rubio, M. C., Perez,
R., and Fernandez, L. E. (2006) Clinical evidences of GM3 (NeuGc)
ganglioside expression in human breast cancer using the 14F7 monoclonal
antibody labelled with (99m)Tc, Breast Cancer Res. Treat.,
96, 115-121.
74.Ruckhaberle, E., Rody, A., Engels, K., Gaetje,
R., von Minckwitz, G., Schiffmann, S., Grosch, S., Geisslinger, G.,
Holtrich, U., Karn, T., and Kaufmann, M. (2008) Microarray analysis of
altered sphingolipid metabolism reveals prognostic significance of
sphingosine kinase 1 in breast cancer, Breast Cancer Res.
Treat., 112, 41-52.
75.Ruckhaberle, E., Karn, T., Rody, A., Hanker, L.,
Gatje, R., Metzler, D., Holtrich, U., and Kaufmann, M. (2009) Gene
expression of ceramide kinase, galactosyl ceramide synthase and
ganglioside GD3 synthase is associated with prognosis in breast cancer,
J. Cancer Res. Clin. Oncol., 135, 1005-1013.
76.Carcel-Trullols, J., Stanley, J. S., Saha, R.,
Shaaf, S., Bendre, M. S., Monzavi-Karbassi, B., Suva, L. J., and
Kieber-Emmons, T. (2006) Characterization of the glycosylation profile
of the human breast cancer cell line, MDA-231, and a bone colonizing
variant, Int. J. Oncol., 28, 1173-1183.
77.Gu, Y., Zhang, J., Mi, W., Yang, J., Han, F., Lu,
X., and Yu, W. (2008) Silencing of GM3 synthase suppresses lung
metastasis of murine breast cancer cells, Breast Cancer Res.,
10, R1.
78.Cazet, A., Groux-Degroote, S., Teylaert, B.,
Kwon, K. M., Lehoux, S., Slomianny, C., Kim, C. H., Le Bourhis, X., and
Delannoy, P. (2009) GD3 synthase overexpression enhances proliferation
and migration of MDA-MB-231 breast cancer cells, Biol. Chem.,
390, 601-609.
79.Cazet, A., Lefebvre, J., Adriaenssens, E.,
Julien, S., Bobowski, M., Grigoriadis, A., Tutt, A., Tulasne, D., Le
Bourhis, X., and Delannoy, P. (2010) GD₃ synthase expression
enhances proliferation and tumor growth of MDA-MB-231 breast cancer
cells through c-Met activation, Mol. Cancer Res., 8,
1526-1535.
80.Cazet, A., Bobowski, M., Rombouts, Y., Lefebvre,
J., Steenackers, A., Popa, I., Guerardel, Y., Le Bourhis, X., Tulasne,
D., and Delannoy, P. (2012) The ganglioside G(D2) induces the
constitutive activation of c-Met in MDA-MB-231 breast cancer cells
expressing the G(D3) synthase, Glycobiology, 22,
806-816.
81.Battula, V. L., Shi, Y., Evans, K. W., Wang, R.
Y., Spaeth, E. L., Jacamo, R. O., Guerra, R., Sahin, A. A., Marini, F.
C., Hortobagyi, G., Mani, S. A., and Andreeff, M. (2012) Ganglioside
GD2 identifies breast cancer stem cells and promotes tumorigenesis,
J. Clin. Invest., 122, 2066-2078.
82.Liang, Y. J., Ding, Y., Levery, S. B., Lobaton,
M., Handa, K., and Hakomori, S. I. (2013) Differential expression
profiles of glycosphingolipids in human breast cancer stem cells vs.
cancer non-stem cells, Proc. Natl. Acad. Sci. USA, 110,
4968-4973.
83.Sarkar, T. R., Battula, V. L., Werden, S. J.,
Vijay, G. V., Ramirez-Pena, E. Q., Taube, J. H., Chang, J. T., Miura,
N., Porter, W., Sphyris, N., Andreeff, M., and Mani, S. A. (2015) GD3
synthase regulates epithelial-mesenchymal transition and metastasis in
breast cancer, Oncogene, 34, 2958-2967.
84.Taki, T., Hirabayashi, Y., Ishikawa, H., Ando,
S., Kon, K., Tanaka, Y., and Matsumoto, M. (1986) A ganglioside of rat
ascites hepatoma AH 7974F cells. Occurrence of a novel
disialoganglioside (GD1 alpha) with a unique N-acetylneuraminosyl(alpha
2-6)-N-acetylgalactosamine structure, J. Biol. Chem.,
261, 3075-3078.
85.Hirabayashi, Y., Hyogo, A., Nakao, T., Tsuchiya,
K., Suzuki, Y., Matsumoto, M., Kon, K., and Ando, S. (1990) Isolation
and characterization of extremely minor gangliosides, GM1b and GD1
alpha, in adult bovine brains as developmentally regulated antigens,
J. Biol. Chem., 265, 8144-8151.
86.Furuya, S., Irie, F., Hashikawa, T., Nakazawa,
K., Kozakai, A., Hasegawa, A., Sudo, K., and Hirabayashi, Y. (1994)
Ganglioside GD1 alpha in cerebellar Purkinje cells. Its specific
absence in mouse mutants with Purkinje cell abnormality and altered
immunoreactivity in response to conjunctive stimuli causing long-term
desensitization, J. Biol. Chem., 269, 32418-32425.
87.Ikehara, Y., Shimizu, N., Kono, M., Nishihara,
S., Nakanishi, H., Kitamura, T., Narimatsu, H., Tsuji, S., and
Tatematsu, M. (1999) A novel glycosyltransferase with a polyglutamine
repeat; a new candidate for GD1alpha synthase (ST6GalNAc V), FEBS
Lett., 463, 92-96.
88.Kroes, R. A., He, H., Emmett, M. R., Nilsson, C.
L., Leach, F. E., 3rd, Amster, I. J., Marshall, A. G., and Moskal, J.
R. (2010) Overexpression of ST6GalNAcV, a ganglioside-specific
alpha2,6-sialyltransferase, inhibits glioma growth in vivo,
Proc. Natl. Acad. Sci. USA, 107, 12646-12651.
89.Taki, T., Ishikawa, D., Ogura, M., Nakajima, M.,
and Handa, S. (1997) Ganglioside GD1alpha functions in the adhesion of
metastatic tumor cells to endothelial cells of the target tissue,
Cancer Res., 57, 1882-1888.
90.Bos, P. D., Zhang, X. H., Nadal, C., Shu, W.,
Gomis, R. R., Nguyen, D. X., Minn, A. J., van de Vijver, M. J., Gerald,
W. L., Foekens, J. A., and Massague, J. (2009) Genes that mediate
breast cancer metastasis to the brain, Nature, 459,
1005-1009.
91.Modak, S., and Cheung, N. K. (2007)
Disialoganglioside directed immunotherapy of neuroblastoma, Cancer
Invest., 25, 67-77.
92.Gajdosik, Z. (2014) Racotumomab – a novel
anti-idiotype monoclonal antibody vaccine for the treatment of cancer,
Drugs Today (Barc.), 50, 301-307.
93.Osorio, M., Gracia, E., Reigosa, E., Hernandez,
J., de la Torre, A., Saurez, G., Perez, K., Viada, C., Cepeda, M.,
Carr, A., Avila, Y., Rodriguez, M., and Fernandez, L. E. (2012) Effect
of vaccination with N-glycolyl GM3/VSSP vaccine by subcutaneous
injection in patients with advanced cutaneous melanoma, Cancer
Manag. Res., 4, 341-345.
94.Alfonso, S., Valdes-Zayas, A., Santiesteban, E.
R., Flores, Y. I., Areces, F., Hernandez, M., Viada, C. E., Mendoza, I.
C., Guerra, P. P., Garcia, E., Ortiz, R. A., de la Torre, A. V.,
Cepeda, M., Perez, K., Chong, E., Hernandez, A. M., Toledo, D.,
Gonzalez, Z., Mazorra, Z., Crombet, T., Perez, R., Vazquez, A. M., and
Macias, A. E. (2014) A randomized, multicenter, placebo-controlled
clinical trial of racotumomab-alum vaccine as switch maintenance
therapy in advanced non-small cell lung cancer patients, Clin.
Cancer Res., 20, 3660-3671.
95.Kim, Y. J., Choi, J. S., Seo, J., Song, J. Y.,
Lee, S. E., Kwon, M. J., Kwon, M. J., Kundu, J., Jung, K., Oh, E.,
Shin, Y. K., and Choi, Y. L. (2014) MET is a potential target for use
in combination therapy with EGFR inhibition in
triple-negative/basal-like breast cancer, Int. J. Cancer,
134, 2424-2436.
96.Fu, P., Du, F., Yao, M., Lv, K., and Liu, Y.
(2014) MicroRNA-185 inhibits proliferation by targeting c-Met in human
breast cancer cells, Exp. Ther. Med., 8, 1879-1883.
97.Dobrenkov, K., and Cheung, N. K. (2014)
GD2-targeted immunotherapy and radioimmunotherapy, Semin.
Oncol., 41, 589-612.
98.Chames, P., and Baty, D. (2009) Bispecific
antibodies for cancer therapy: the light at the end of the tunnel?
MAbs, 1, 539-547.
99.Li, J., Liu, R., Yang, Y., Huang, Y., Li, X.,
Liu, R., and Shen, X. (2014) Triptolide-induced in vitro and in vivo
cytotoxicity in human breast cancer stem cells and primary breast
cancer cells, Oncol. Rep., 31, 2181-2186.