REVIEW: Hypotheses of the Origin of Natural Antibodies: A Glycobiologist’s Opinion
N. R. Khasbiullina and N. V. Bovin*
Shemyakin–Ovchinnikov Institute of Bioorganic Chemistry, Russian Academy of Sciences, 117997 Moscow, Russia; fax: +7 (495) 330-5592; E-mail: professorbovin@yandex.ru* To whom correspondence should be addressed.
Received March 27, 2015; Revision received April 6, 2015
It is generally accepted that the generation of antibodies proceeds due to immunization of an organism by alien antigens, and the level and affinity of antibodies are directly correlated to the presence of immunogen. At the same time, vast experimental material has been obtained providing evidence of antibodies whose level remains unchanged and affinity is constant during a lifetime. In contrast to the first, adaptive immunoglobulins, the latter are named natural antibodies (nAbs). The nAbs are produced by B1 cells, whereas adaptive Abs are produced by B2. This review summarizes general data on nAbs and presents in more detail data on antigens of carbohydrate origin. Hypotheses on the origin of nAbs and their activation mechanisms are discussed. We present our thoughts on this matter supported by our experimental data on nAbs to glycans.
KEY WORDS: natural antibodies, B1 cells, bacterial antigens, autoantibodies, polysaccharides, molecular patterns, DAMPDOI: 10.1134/S0006297915070032
Abbreviations: DAMP, damage-associated molecular patterns; mAbs, monoclonal antibodies; MAMP, microorganism-associated molecular patterns; nAbs, natural antibodies; TACA, tumor-associated carbohydrate antigens.
The first hypothesis on development of immunoglobulin specificity was
proposed only a half century after the discovery of antibodies.
According to a theory by Ehrlich, who discovered humoral immunity,
antibodies preexist in the body and are required for response to
infectious agents [1-3]. The
first serious contradiction to this concept was a study by Landsteiner,
who considered antigens as a template for synthesis of corresponding
(cognate) antibodies and demonstrated that antibodies were generated
de novo in response to an antigen, which the organism had not
encountered previously [4-6].
Burnet was the first to draw attention to a fact that antibodies not
only distinguish “self” from “alien”, but also
provide long-term immunological memory [7, 8]. Later, it became generally accepted that:
1) antibodies preexist in the body, but only in very small amounts, and they can be found as receptors on the surface of B lymphocytes;
2) the immune system operates on the principle of “one B cell, one antibody”; upon entering the body, an antigen finds its B cell receptor and stimulates proliferation of the cell capable of producing antibodies;
3) specificity of antibodies improves in the course of immune response via fast selection of higher-affinity B cell clones;
4) tolerance (lack of autoantibodies) is explained by elimination of potentially autoreactive B cell clones, which come into contact with antigens from their own cells during embryogenesis.
Clonal selection theory has earned the right to be considered the dominant one in immunology, but a common model of induction of antibody production by immunization does not explain the existence of antibodies that are present in a healthy body throughout its lifetime, so-called natural antibodies (nAbs). Recently, the term “naturally occurring antibodies” has been popularized. One of the examples is alloantibodies. This article discusses this, apparently the most controversial, branch of humoral immunity.
There are several points of view on the functional role and origin of nAbs. We will consider them from a perspective of the nature of cognate antigens. The first hypothesis suggests that the crucial role here is played by specific stimulation of B lymphocytes by bacterial microflora. Antibodies that are generated as a result of this process function as the first line of defense in case of pathogen invasion. The diverse repertoire of nAbs is attributed to high diversity of the microbiota, which acts as a source of stimulating antigens. The second hypothesis is based on the concept of endogenous stimulation; corresponding B1 cells are already formed in utero in response to antigens, which are degradation products of the fetus’ own cells. Later, over a lifetime of an individual, these nAbs perform the same function of elimination of waste material, playing an important role in maintenance of homeostasis. According to the third hypothesis, antigens that stimulate B1 cells originate from molecular patterns, a group of molecules (both exogenous and endogenous), that form regular and commonly occurring combinations at the membrane, which are specific for pathological conditions. Next, we will discuss the three above-mentioned hypotheses on the origin of nAbs and will examine the differences between nAbs and adaptive antibodies in more detail.
95 vs. 5 (FROM ADAPTIVE IMMUNOGLOBULINS TO NATURAL
ANTIBODIES)
Highly specific adaptive antibodies are produced by B2 lymphocytes in response to a contact with an alien antigen. According to the clonal selection theory, antibodies and lymphocytes with desired specificity exist in the body even before its exposure to the antigen [7, 9]. After sensitization from a dormant state by an antigen, a B lymphocyte enters active proliferation phase and is converted into a plasma cell that produces antibodies with particular specificity. According to the logic of this theory, it is impossible and even meaningless to store in the genome the information on antibodies to a virtually infinite variety of antigens, because microorganisms, if we consider them the main source of danger, are evolving far too fast. The basic mechanism for increasing the diversity of antibodies is somatic recombination (V(D)J-recombination) of two types of genes, which have inbuilt high frequency of mutations, accompanied by gradual selection of B cell variants with the highest affinity to the antigen. Some B lymphocytes are transformed into memory cells, which are required for fast reaction to re-emergence of the antigen in the body. B cell proliferation requires not only external stimulation, i.e. by an antigen, but also an internal one by other cells participating in immune response, such as T-helper cells and regulatory cells [2, 10-13]. The clonal selection theory works for ~95% of all produced antibodies present in the blood.
The remaining 5% are exceptions, and their repertoire is generated by another way. These are natural antibodies that are present in the blood even in the absence of explicit antigenic stimulation [12-16]. The fact that they should be distinguished from adaptive antibodies and considered as a single group became clear already in the early twentieth century when the AB0 blood group system was discovered. Anti-A and anti-B antibodies are highly reactive with erythrocytes of donors from the other group; noteworthy a person from one group did not come into antigenic contact with a person from the other; thus immunization in its classical form is excluded. Later, other alloantibodies were discovered, including those targeting blood group antigens Rh, Lewis, Ii, P (overall, there are 33 known blood group systems), xenoantigens, and tumor-associated antigens [17]. Finally, there is the most mysterious type of antibodies to components of normal cells, i.e. autoantibodies [18].
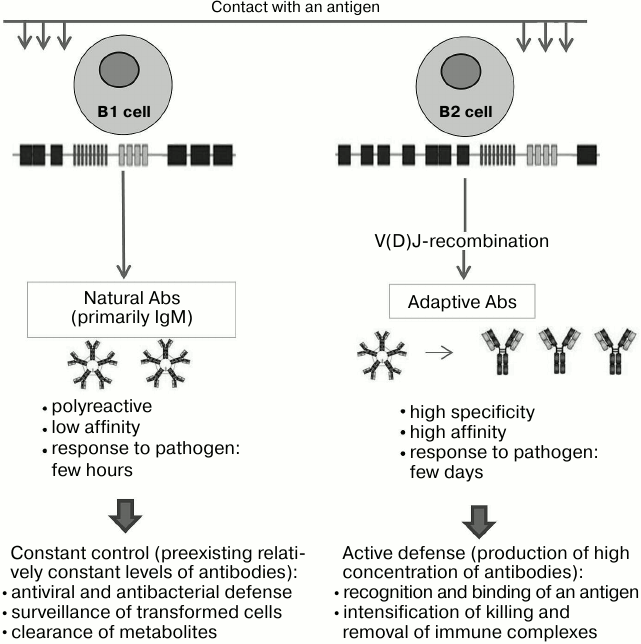
Properties. The nAbs are primarily class M immunoglobulins, they can be polyreactive (i.e. bind to not only chemically related, but even to quite different antigens); their affinity is lower than that of typical adaptive immunoglobulins. nAb levels remain relatively constant throughout a lifetime, and their repertoire displays certain conservatism across the entire population (or a part of it, in the case of alloantibodies), it does not depend on gender and almost does not depend on race [12, 19-21].
Functions. According to integrated modern concepts, the first of nAb functions is protection, which is expressed through a rapid response to an invasion by a pathogen. It gives the host organism time to initiate the slower mechanism of development of highly specific adaptive antibodies that requires at least 1-2 days; in the absence of “rapid response team”, such as nAbs constantly present in the blood, this time would be enough for critical spread of infection. The total content of immunoglobulins in blood is limited, and therefore the expansion of a preexisting repertoire of antibodies implies reduction in concentration of each of them; as a compromise, nAbs are polyreactive, i.e. one protein can recognize multiple antigens. Polyreactivity leads to lower affinity of the given immunoglobulin for each particular antigen; in compensation, nAbs are mostly IgM that increase their avidity for multivalent antigens typical for microorganisms [12, 20-25].
The second important function of nAbs is the disposal of aged and dead cells and molecules that are products of their metabolism, whose level should not exceed a critical threshold [12, 26-29].
Their third function is to serve as a barrier to interspecies transmission of viruses. Xenoantibodies against the “alpha-Gal” epitope in Galα1-3Galβ1-4GlcNAc minimize the chance of a human being infected with monkey’s and domestic animals’ membrane viruses (including HIV), since the virus does not have its own glycosylation machinery, and glycosylation of mammalian hosts (but not humans) leads to biosynthesis of glycoproteins with terminal Galα1-3Galβ1-4GlcNAc host glycans [12, 30].
Finally, nAbs to tumor-associated antigens, especially those of glycopeptide and glycolipid nature (see more detailed discussion below), are detected in healthy people. As common for all nAbs, the antibodies against tumor-associated antigens are present in all individuals, and their concentration is of the order of 10 µg/ml [12] (Fig. 1).
Fig. 1. Differences between adaptive and natural antibodies. Producing cells, classes of immunoglobulins, and their properties determine the functional significance of these two groups of antibodies.
It should be emphasized that a contact of adult B1 cells with a target pathogen does not lead to a dramatic increase in their proliferation and synthesis of immunoglobulins [35]. Upon coming into contact with a diverse array of antigens, B1 cells can have similar reaction to early immunologically naive stages (before birth and immediately after birth). In our view, such stimulation should be more correctly referred to as activation of B1 lymphocytes, in which a genetically possible process becomes a reality. It is appropriate to question why stimulation of B1 cells is required, why they (at least most of them) do not begin to function “automatically”. Apparently, the main reason is the presence during in utero development of the fetus of “non-standard” embryonic antigens, antibodies to which would be required later, but whose presence at the time could be deleterious. In particular, there are so-called oncoembryonic glycoantigens that are expressed at the embryonic stage of development and later reappear in epithelial tumors. Even though they are completely absent from normal adult tissues, an adult human has nAbs to oncoembryonic glycans [36, 37]. Thus, it is advisable to activate nAbs necessary for oncosurveillance only after birth. Two indirect evidences supports this assumption: the level of antibodies falls during pregnancy [38, 39], and class M antibodies (nAbs are mainly IgM) do not penetrate the placental barrier [40, 41].
Despite the wealth of accumulated data, the issue of stimuli responsible for activation of only these and no other B1 lymphocytes and related nAbs remains open for discussion. The existing hypotheses, as will be shown below, are not able to explain all experimental observations. Nevertheless, each is valuable and deserves attention, because it not only helps to interpret experimental data, but also to critically evaluate them.
BACTERIAL STIMULATION HYPOTHESIS
From the moment of birth, the gastrointestinal tract and respiratory system in mammals are actively colonized by bacteria [42, 43]. About 500 species of nonpathogenic bacteria form the basis of normal intestinal microflora; transient flora is represented by all other types of microorganisms that occur in the environment, but usually do not linger in the body [44, 45]. The number of bacteria ranges from 109 per gram of the content of the small intestine to 1011 per gram in its lowest section [45, 46]. The role of the microflora is not limited to participation in digestion of food [47-51]; upon entering a macroorganism, it transforms its environment by production of biofilms, which are necessary for attachment and stable reproduction and are beneficial for the macroorganism via protection of the adjacent epithelial layer of the intestinal wall [52, 53]. Furthermore, bacteria can actively influence adjacent epithelial cells, stimulating them to synthesize substances necessary for bacteria [54-58]. In some cases the commensal relationship become symbiotic, for example, bacteria can synthesize vitamins [51]. The diverse microbial community in the gut ensures a competition for adhesion sites and resources, and normally it is difficult for pathogenic microorganisms to win this competition against commensal and symbiotic bacteria [45, 59, 60].
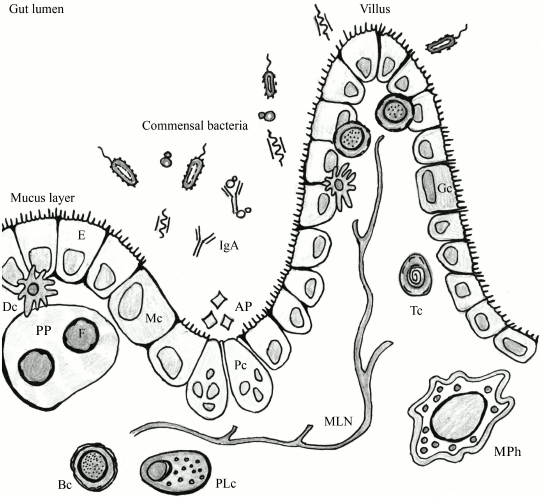
Numerous bacteria and an even greater number of viruses (including bacteriophages) are involved in constant interaction with the immune system of macroorganisms. Such terms as “physiological inflammation” can be found in the literature to describe the constantly active state of the mucosal immune system [61, 62]. Indeed, the underlying layer of the intestine is an area of “border control” (Fig. 2). During their transfer through the intestinal epithelium, microorganisms are recognized by the host immune system. A small number of them is perceived as training materials for the immune system [64, 65], but intensification of the transfer can result in bacteremia with associated negative consequences.
Fig. 2. The lumen of the intestinal tube: an area of active interaction between components of microflora and host cells, including immune cells. F, follicle; PP, Peyer’s patch; E, enterocyte; Dc, dendritic cell; Bc, B cell; PLc, plasma cell; MPh, macrophage; Tc, T cell; Mc, M cell; Gc, goblet cell; AP, antimicrobial peptides; MLN, mesenteric lymph node; Pc, Paneth cell (adapted from Abreu [63]).
In the mid-twentieth century, Springer et al. described generation of anti-B alloantibodies in human blood (AB0 blood group system) as a result of contact with a bacterium, whose polysaccharide was structurally similar to the blood group B antigen. Based on these and other data, Springer suggested that nAbs appear in response to stimulation by enterobacteria. In the works by Springer et al., the concept of “stimulation” is similar to “immunization”, when antibodies with appropriate specificity appear in response to recognition of bacterial antigen [66, 67].
Springer’s concept does not explain why classical immunization featuring B2 cells, i.e. activation of the complement system, development of inflammation, and destruction of antigen source does not occur in parallel with activation of B1 cells. There are several possible answers to this question. First, the nature of a microbe: under normal circumstances, commensals do not attempt to get into the bloodstream and cause bacteremia, but remain within the biofilm that they form themselves. Bacterial translocation of commensal bacteria is a rare event, which does not lead to damage to the mucus layer [61, 68-70]. In contrast, single cells, which get through the layer of epithelium, become the “training material” for the immune system, which allows it to be in active state. Second, in the first months of life, which Springer was focused on in his studies, are unique in human life due to temporary immunosuppression. Third, B1 cells secrete antibodies that are different from adaptive antibodies (low affinity and polyreactivity).
Springer’s hypothesis is supported by model experiments [50, 71, 72] that showed in particular that once sterile experimental animals come into contact with opportunistic microorganisms, the diversity of antibodies and their titers increase. The contradiction is that even in the absence of interaction with the environment, sterile animals still have a certain set of preexisting antibodies. As shown in 2000 by Butler et al., the same peripheral B cells are active in both sterile animals and those exposed to microorganisms [73]. In addition, Springer’s hypothesis does not fully explain the “peaceful” existence of natural autoantibodies.
NATURAL ANTIBODIES = AUTOANTIBODIES? (INTERNAL STIMULATION
HYPOTHESIS)
While Springer considers external factors (microorganisms) to be stimuli for the production of natural antibodies by B cells, another, internal stimulation, hypothesis assigns the leading role to antigens of the body’s own cells.
Accumulated data [74-79] leave no room for doubt that healthy people do have a wide range of antibodies capable of binding its own (mostly polypeptide) antigens. “Housekeeping” is the term used in the literature, which implies that nAbs are responsible for internal control by maintaining “order and cleanliness in the house”. nAbs are involved in clearance of metabolites, senescent (including erythrocytes) and transformed cells. During apoptosis, cell fragments undergo changes and acquire new antigenic properties as a result (neoantigen and alternatively presented native antigens), which make them available for recognition by antibodies. Removal of waste material occurs mainly through phagocytosis [2, 80].
Previously, it was thought that autoantibodies are a sign of a pathology. However, research conducted in the past two decades confirmed that autoantibodies are present in healthy individuals as well. These include, among others, autoantibodies to cytoskeletal proteins, DNA, enzymes, and structural components of the cell membrane. We would like to stress that in this case we are talking about nAbs not to neoantigens, but to components of normal cells [77, 79, 81-84]. Their existence is paradoxical at first sight, but can be explained if we consider this phenomenon not from a quantitative, but from a qualitative point of view. Indeed, antigen–antibody reaction follows the law of mass action, i.e. the amount of Ag–Ab complex depends on the concentrations of both Ab and Ag.
[Ag–Ab] = [Ag] × [Ab]
This means that to eliminate an undesired self-antigen, there is no need to increase the level of an antibody (Ab component) – it happens automatically due to the increased concentration of Ag component. Undoubtedly, other factors affect the implementation of this simple law in bloodstream, such as non-equilibrium state and interconnection between formation of Ag–Ab complex and other processes. Nonetheless, it gives us reason to suggest that there is a threshold concentration of Ag, below which there is no autoimmune elimination of the body’s own normal components [12]. A case in point is the so-called accommodation phenomenon, when an AB0-incompatible organ is successfully transplanted (in the long-term). Before and after such operations, recipient’s blood is treated with A- or B-antigen specific sorbent to remove corresponding antibodies [85]. Accommodation refers to the fact that the level of antibodies that cause rejection falls sharply, but their synthesis does not stop completely.
It should be noted that the distinctive feature of nAbs – the constancy of their levels – is consistent with the reasoning given above. Moreover, sharp increase in antibody levels typical for B2 cells is unnecessary and even counterproductive in case of B1 cells, since it leads to initiation of chronic autoimmune process. In other words, the appearance of autoaggressive antibodies damaging its own organs, apparently, is a consequence of erroneous high production of normal natural antibodies [24, 86, 87]. The internal stimulation hypothesis does not answer the question why a contact of B2 cells with self-antigens results in tolerance (elimination of the clone), whereas in case of B1 cells it leads to their activation.
MOLECULAR PATTERNS
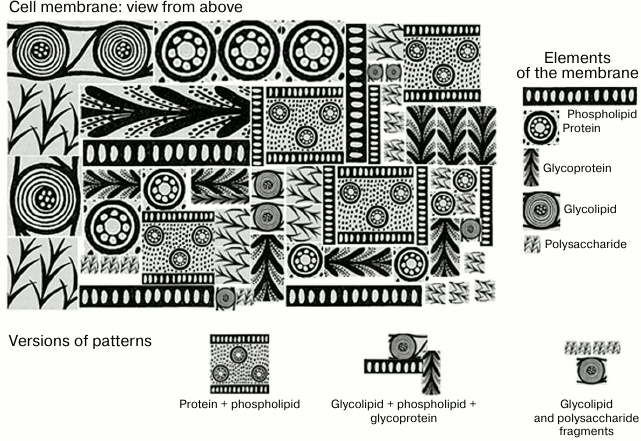
In the early stages of development of immunology, the term “antigen” was poorly correlated with a concept of a “molecule”. Development of molecular immunology gave us deep understanding of mechanisms of antigen/antibody interaction at the molecular level, but at the same time it led to a skewed perspective in which the essence of an antigen, its antigenic determinants, started to be perceived in overtly mechanistic terms, i.e. as a fragment of an individual molecule. Significant paradigm shift occurred when it was discovered that antigenic determinants of a protein might be composed of fragments that are far apart from each other on a linearly recorded primary sequence. It was even harder to accept that antigenic determinants can be composed of altogether different molecules on the cell membrane located close to each other in space due to various factors. This may not only be identical adjacent molecules (e.g. gangliosides cluster), not only different molecules of the same class (e.g. two polysaccharides), but also molecules from various classes (protein + phospholipid) [88, 89] (Fig. 3).
Fig. 3. Schematic representation of molecular patterns presented as Greek ornamental elements. If you visualize a cell membrane (top view), it is a complex mosaic of orderly and regular repeating structures, phospholipids, proteins, glycoproteins, polysaccharides (if it is a bacterial cell), and glycolipids. The combination of several molecules of the same or different classes forms patterns that are presented on the surface of cells as numerous repeats.
The MAMP concept unites various molecules typical for microorganisms, primarily bacteria. Repetitive motifs are common in bacterial structures, in particular in structures of most lipids A and a large portion of lipopolysaccharide cores. However, the most important “material” built of repeating molecular patterns are polysaccharides. Capsular polysaccharides and lipopolysaccharide glycans are built of repeating oligosaccharide units. Most polysaccharides tend to assume helical conformation, and the number of archetypes of these helices is very limited and depends mostly not on the structure of monosaccharide units, but on the type of linkage between them (1–2, 1–4, etc.) [93]. In other words, an almost infinite variety of primary structures of bacterial polysaccharides paradoxically results in only a few common motifs at the level of secondary and tertiary structures, which provide the basis for recognition by nAbs surveillance. A useful analogy would be the existence of only ~200 crystallographic groups for millions of known crystalline substances, as well as the presence of only a few dozens of protein folds for tens of thousands of proteins with known tertiary structure. Moreover, repeating molecular patterns can be formed by adjacent polysaccharides densely packed at the bacterial wall [92, 93]. In addition to polysaccharides, it is also necessary to pay attention to repeated motifs of peptidoglycans of both Gram-negative and Gram-positive bacteria, to which, according to experimental data, humans generate several types of nAbs [94]. This statement is indirectly confirmed by specificities of many lectins, which can bind to a variety of glycan classes, which are, at the first glance, completely different [95]. Several studies by Hakomori [96-98] describe the so-called glycosynapses, stable molecular complexes enriched with glycosphingolipids. These supramolecular entities have several functions, such as transmission of a signal into a cell during its interaction with external stimuli, including antiglycan antibodies. According to Hakomori, glycosynapses are modified in pathologies, and therefore they can be considered as a type of DAMP that got another common name.
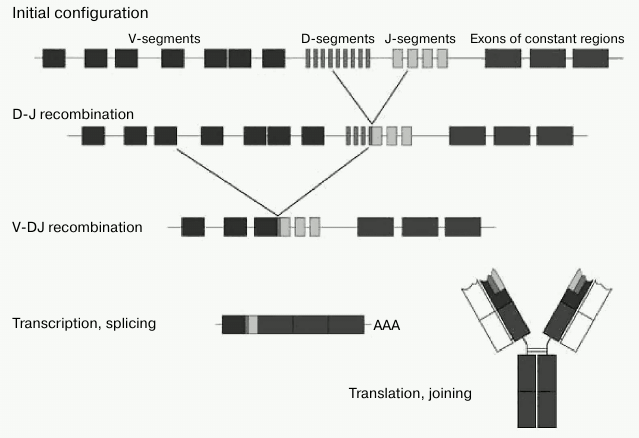
From the standpoint of classical B2 cell response, T-dependent antigen presentation by molecular pattern looks difficult, if not altogether impossible. At the same time, immunoglobulins generated by B1 cells have no such limitation, since they are not selected by V-gene recombination, but rather by long-term evolution of the B1 cell component of the immunity. That it is to say that the B1 cell mechanism and repertoire of nAbs is more suitable for recognition of molecular patterns. The fact that for nAbs there is no restriction associated with the mechanism of antigen presentation, in our view, is the key feature of these antibodies. It should be noted that DAMPs and MAMPs are recognized not only by antibodies, but also by other classes of proteins, such as lectins, Toll- and NOD-receptors [99, 100], but nAbs have a distinct advantage as they are by far more abundant. Their abundance can be estimated as follows: since the typical contents of individual monospecific nAbs in the blood is of the order of a few mg/liter [94], and the total content of IgM is 103 mg/liter, highly represented nAbs should therefore be of the order of 103 specificities. We would like to emphasize again that nAbs are mainly immunoglobulins of M class, which, thanks to its ten valences, is the most suited to meet the requirements for the avidity of interaction with repeated patterns of regular molecular patterns. IgM polyvalency allows them to be less specific on the level of one immunoglobulin subunit [22]. In other words, it is more reasonable to have a loose-fitting wrench, which “barely” unscrews several similar nuts, than to have a perfect complementary tool to a single nut. Furthermore, it is advantageous to store in the genome the information on the recognition of patterns that are common across many bacteria, rather than on recognition of individual molecules. The diversity of V-genes is limited because, in contrast to B2 cells, B1 cells are not involved in somatic hypermutation, and therefore they do not contain any N-terminal inserts [31-34]. Adaptive antibodies should provide effective immune response to a potentially infinite number of antigen variants. Upon contact of antigen with B2 cells, DNA rearrangement and translocation of one of the V-genes to one of the J-segments are initiated; recombination between the D- and J-regions occurs in heavy chain genes (Fig. 4).
Fig. 4. V(D)J-recombination of immunoglobulin genes (adapted from Janeway et al. [10]).
CAN THREE HYPOTHESES BE COMBINED?
We believe that this question must be answered in the affirmative, especially if one re-evaluates Springer’s ideas in terms of modern concepts of B1 cells. Common to all three hypotheses is the idea that there is a population of special antibodies whose synthesis is activated in the early stages of development, and which thereafter remains almost unchanged. The main difference between them is associated with the structure of the antigens in question and, subsequently, the functions of nAbs, but that is not a contradiction. The concept of molecular patterns (MAMPs) complements not only Springer’s hypothesis of polysaccharides, but also the hypothesis of autoantibodies against self-proteins (i.e. DAMPs), while simultaneously imbuing purely immunological concepts with immunogenetic content.
We believe that in its purest form the second hypothesis has a serious flaw in the part that requires explanation for the activation of B1 lymphocyte clones responsible for recognition of self-proteins. Indeed, since B lymphocytes can meet an antigen immediately after its emergence on the cells in the embryo, nAbs to self-proteins should be produced much earlier than it is the case in reality. Can the activation of anti-protein nAbs (potential autoantibodies) occur without the participation of protein antigens? We believe it can, and to explain it we would like to consider the phenomenon of molecular mimicry. There are many examples where a peptide immunogen induces the generation of highly specific antibodies to a carbohydrate antigen, and vice versa [102]. It can be assumed that B1 cells that synthesize antibodies to self-proteins are activated not by these proteins, but rather by numerous bacteria encountered by a newborn organism. Recent experiments on stimulation of sterile mice by bacterial antigens demonstrated appearance of antibodies to glycans that were not a part of the bacterial activator; a study of children in the first year of life have shown that peptides in infant feeding formulas activate antiglycan antibodies (N. R. Khasbiullina et al., manuscript in preparation). The estimated number of human nAbs is close to 103 (see above), and the published [77, 103-105] estimate of the number of proteins to which autoantibodies were found has the same order of magnitude, i.e. the number of autoantibodies that can be found in a healthy human is relatively limited. On the other hand, the aforementioned [44, 45] number of major bacteria in microbiota, ca. 500 species, suggests one or two orders of magnitude higher number of potential antigenic determinants, both classical ones and those of MAMP-type, of polysaccharide (and other) nature. Therefore, bacteria of the microbiota are an inexhaustible reservoir for activation of B cells of any (genetically programmed) specificity. We would like to mention that the long history of designing molecular vaccines showed that whole bacteria are much more actively immunogenic than single molecules or their conjugates. Without going into the details and considering possible causes of this phenomenon, let us assume that in addition to BCR, B1 cells also have coreceptors (TLR?) for recognition of bacteria, which result in activation of B1 clones, specific for autoproteins, even though by themselves these proteins are not immunogenic. Such paradoxical “bypass” mechanism would allow involving B1 cells at precisely the right time to avoid autoimmune reaction during the embryonic stage of development (see above).
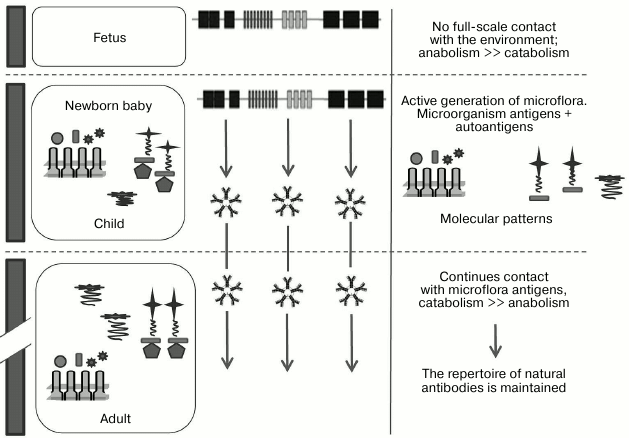
Therefore, by combining all three hypotheses and supplementing them, we can propose the following scenario for generation of nAbs. In the period before birth, the fetus normally has very limited capacity for bacterial activation of B cells, and therefore it has very few of its own nAbs; nAbs are primarily class M immunoglobulins, and normally only IgG have the ability to pass through utero–placental barrier. After birth, microorganisms start to actively occupy the intestines of a newborn, initiating the generation of a repertoire of nAbs to classic bacterial polysaccharide and MAMP epitopes, self-proteins, allo- and xenoantigens, DAMPs, and tumor-associated glycopeptide epitopes, anionic polymers (DNA, GAG), and others. We would like to once again emphasize that according to this hypothesis the diversity of nAbs is generated by activation by microorganisms that are a part of the normal microflora (Fig. 5).
Fig. 5. Generation of nAbs repertoire. The emergence of the immune system is observed at different stages of ontogenesis, which is affected by endogenous (autoantigens) and exogenous (environmental antigens, primarily microorganisms) factors and responds by production of antibodies directed against the patterns (adapted from Janeway et al. [10]).
A GLYCOBIOLOGIST’S OPINION
Looking at the nAbs from a position of glycobiology, some aspects of the topic need to be presented more prominently. The first of these aspects was discussed in the previous section; it is the assumption that B1 cells are activated (stimulated) by polysaccharide glyco-MAMPs of commensal bacteria, regardless of the chemical nature of target antigens. Commensal bacteria represent such a rich reservoir of activating epitopes that their diversity by far exceeds necessary requirements for activation of both antiglycan and antiprotein antibodies, the latter via molecular mimicry [106].
The second aspect follows from the detailed analysis of antiglycan nAbs specificity [107]. The cited study demonstrated that there are no compelling arguments in favor of considering at least one of many antiglycan nAbs as true autoantibodies, even though they do target glycans typical for normal human cells. In particular, these are antibodies to disaccharides LeC, Fucα1-3GlcNAc, Fucα1-2Gal, GalNAcα1-3Gal, as well as fragments of core glycans such as –Galβ1-4Glc and –GalNAcα. Even though ELISA and other methods of analysis, wherein carbohydrate hapten is immobilized on a solid phase (or acts as an inhibitor), clearly indicate the presence and even high titers of antibodies to these determinants of natural glycans, these nAbs are, nonetheless, unable to bind to the same glycoprotein or glycolipid epitopes in vivo, as part of normal living cells. The explanation can be summarized as follows: epitope specificity of these antibodies is such that they do not bind to short (di- or trisaccharide) fragments, which are spatially masked in longer chains of native natural glycans. Therefore, the analysis of human antibodies using routine analytical approaches that are based on the use of small oligosaccharides should be interpreted with caution, keeping in mind real, natural context of the glycan.
Here is only one, but a typical example. During the analysis of the repertoire of antiglycan antibodies from healthy donors, one of the highest titers was observed for antibodies to LeC disaccharide. A detailed examination, that included affinity isolation, revealed that the disaccharide interacts with IgM, which is insensitive to the nature of the R substituent in 3-O-R-{{anchor|OLELINK3}} {{anchor|OLELINK4}} Galβ1-3GlcNAc-X [108]. At the same time, their binding was strongly dependent on the nature of the X fragment; any long-chain carbohydrate with Galβ1-3GlcNAc moiety on the non-reducing end, for example, Galβ1-3GlcNAcβ1-3Galβ1-4GlcNAc, is completely non-reactive to these nAbs. These results make it clear that “anti-LeC” are unable to interact with normal natural carbohydrate chains, where the LeC fragment is always linked to lactosamine core. However, according to the data of cytometry and histochemical studies, these antibodies bind to aberrant cells, namely, to breast tumor cells with unknown structure of the carbohydrate chains [109]. We would like to emphasize that a similar argument turned out to be true as an explanation of tolerance for dozens of other antiglycan nAbs, especially anticore ones, for which the role of masking (constraining) of X groups is performed by the surface of the cell or polypeptide chain of protein. Therefore, it was concluded [107] that there are no true antiglycan autoantibodies in healthy individuals; however, antibodies to tumor-associated epitopes (TF, Tn, SiaTn, etc., which will be discussed below) can be considered to be autoantibodies to some extent.
It seems, therefore, unlikely that other products of B1 cells, such as anti-polypeptides, are true autoantibodies, even though a healthy person has a lot of such proteins [103-105]. Is it possible to extend the glycobiological concept of antiglycan antibodies to the interpretation of data on antiprotein nAbs? Can we be certain that the proteins (and especially synthetic peptides) that are used in solid-phase assay systems for identification of potential antiprotein autoantibodies are truly representative of the native protein as part of a normal cell? We believe that the following explanation of data accumulated on the presence of autoantibodies to proteins in healthy individuals is reasonable: nAbs recognize cognate peptide epitopes only in aberrant situations, such as: (i) intracellular protein outside a cell; (ii) previously hidden epitope of membrane-bound protein unmasked by the action of metalloproteinases; (iii) previously hidden epitope unmasked as a result of incomplete glycosylation or there is a conformational rearrangement, which leads to the formation of a neoepitope; (iv) a complex with another molecule is formed that constitutes a DAMP. These considerations contradict the discussion in the “Natural antibodies = autoantibodies?” section, which referred to quantification of interactions between nAbs and proteins, because too high concentration of a protein is in itself indicative of an aberrant situation.
Another idea, which is well developed in glycobiology and deserves to be more widespread, is the concept of tumor-associated carbohydrate antigens (TACA) [110-112]. More than 50 TACAs are mentioned in the literature and identified in various tumors either chemically or immunochemically; the most studied ones are glycopeptides, O-glycosylation sites and glycosphingolipids; their cognate antibodies are in fact probably directed against DAMP, and glycomolecules are only a part of the DAMP. Natural antibodies to some TACAs were discovered, often in high titers, especially to core fragments of glycoprotein O-glycans, primarily nAbs to TF, Tn, and SiaTn, which are not generated in response to the presence of a tumor, but are present over a lifetime, i.e. must be considered as nAbs [12, 113]. As discussed above, in Springer’s concept these antibodies play an important role. Springer believes that they are, just like the other nAbs, “turned on” (in our terminology are “activated”) by intestinal microflora. Recent studies that were performed using glycoarrays [114] revealed a broader and generalized group of nAbs to core motifs of glycans, i.e. not only to glycan–protein moiety, but also to lactose fragment of glycosphingolipids (as we have already suggested above, directed against glycolipid-containing DAMP), and, apparently, also to a GPI-anchor. Some anticore nAbs were included in diagnostic signatures, because their level differs significantly in cancer patients [115-117]. Notably, the level of nAbs to TF disaccharide and some other TACAs is lower in oncopatients than in healthy donors [113, 118], which once again suggests the surveillance function of these antibodies, such as tracking and elimination of cells with abnormal antigens (including tumor ones). Therefore, individuals with genetically programmed low level of nAbs are exposed to high-risk of oncotransformation by default. We believe that the concept of surveillance by nAbs fits well with the idea of DAMPs, i.e. not only antiglycan antibodies, but also those aimed at peptide epitopes [77, 103-105], and especially DAMPs (complex composite epitopes) perform a vital function, regular recognition of constantly forming transformed blood and endothelium cells.
Glycobiological research provides one more observation that runs counter to the generally accepted ideas about nAbs, namely their low specificity/polyspecificity. A study of several dozens of antiglycan antibodies isolated by hapten-specific affinity chromatography (glycan was immobilized on Sepharose) showed that many of thus obtained monospecific polyclonal antibodies are actually highly specific. There are only few examples of natural antibodies with dual specificity, one of them is nAbs capable of binding to both DNA and xenoantigen Galα1-3Galβ1-4GlcNAc [119]. At the same time anti-Galα1-3Galβ1-4GlcNAc from blood group B donors (but not blood group A [30]) do not react with blood group B antigens, even in high titers, including tetrasaccharide Galα1-3(Fucα1-2)Galβ1-4GlcNAc, which differs from Galα1-3Galβ1-4GlcNAc trisaccharide in the presence of an additional residue of L-fucose only, indicating their high specificity. Thus, antibodies that were evolutionarily selected for destruction of animal viruses can interact with multiple versions of “alpha-Gal” epitope, but simultaneously are unable to bind to a large variety of closely related antigens, such as blood group B and “histo-blood group B”. Therefore, the concepts of “polyreactivity” and “high specificity” are not necessarily mutually exclusive.
In the context of this chapter, we should discuss the therapeutic aspect of antibody therapy (primarily, anti-tumor therapy). Most antibody-based drugs are obtained by the traditional scheme: target antigen is used to obtain hybridomas, from which only one with a therapeutic effect is selected, followed by “humanization” of the selected monoclonal antibody (mAb). Therapies by mAbs pose two questions: 1) why their doses are so high; 2) why only some of the specific mAbs have therapeutic properties, whereas others, which also bind well to the target antigen, are not drugs. The DAMP hypothesis provides an easy answer: in reality, the effective mAbs do not bind to any particular protein, but to a tumor-specific pattern. Therefore, another route to obtain therapeutic mAbs seems to be more promising: immortalization of those B1 human lymphocytes that are specific to glycopatterns [120], precisely those that have been evolutionarily selected to oversee tumor cells. Of course, this route will only become therapeutically promising if we develop an efficient algorithm for discovery and extraction of such cells among millions of other B lymphocytes. Application of this methodology seems appropriate for the treatment of some other pathologies, such as autoimmune diseases and birth defects in B cell-mediated immunity, as long as these disorders are based on disruptions in the production of antibodies.
Despite significant progress in the study of natural antibodies and lymphocytes that produce them, it is too early to call the relevant area of immunology classical and established. Many questions remain unanswered or are still actively debated. We would like to highlight some of them that in our opinion deserve more attention.
1) Discrimination between natural and adaptive antibodies with similar specificity is challenging. B1 lymphocytes differ from B2 ones in a specific set of clusters of differentiation, but immunoglobulins produced by them are either indistinguishable in terms of structure at all, or any currently employed methods do not allow to see the differences. It is possible that nAbs and adaptive immunoglobulins differ in glycosylation [121, 122], but the issue has not been investigated. In addition, nAbs are primarily class M immunoglobulins, and it is much more difficult to study carbohydrate chains of these antibodies than those of IgG.
2) It is obvious that nAbs are generated at an early age, during the first year of life, but the “starting point” remains unknown. Does the activation of B cells begin only after birth, or does it happen already during fetal development? The question of whether the placental barrier provides complete protection of the fetus against the penetration of maternal bacteria remains open and is constantly augmented with new, often conflicting data [123, 124].
3) Limited available data on the repertoire of nAbs in animals indicate a significant difference between human nAbs and antibodies of even evolutionarily close mammals. It would be interesting to trace changes in nAbs repertoire over the course of evolution (if it occurs), or, on the contrary, to convincingly demonstrate that the repertoires of antibodies follow laws that are not driven by evolution.
4) Detailed and long-term observation of the production of the repertoire of antibodies in the same individual look promising: (a) nAbs repertoire (by specificity); (b) intestinal microflora of the same individual using data of functional and comparative genomics; (c) colonization of certain sections of the intestine by specific communities of bacteria in the same individual; (d) polysaccharide structures in identified bacteria; (e) repertoire of bacteriophages and their lytic specificity (ability to cleave a particular bacterial polysaccharide). Systemic analysis of these data would help to understand the mutual conditionality of the mentioned factors, as well as the plasticity of the production of nAbs repertoire, if the plasticity exists.
5) The question of the mechanism responsible for the tolerance of B1 cells to autologous antigens remains open. The mechanisms discussed in this article are for the most part only speculative. The fate of related B2 cells after their binding to an antigen is well known: either they are “banned” (anergy, apoptosis) or they are activated and proliferate. However, in the case of B1 cells, the molecular mechanism of stimulation and conditions of clone selection are poorly understood. In other words, it is unclear why a contact of B2 cells with autologous antigens results in the elimination of the clone, whereas for B1 cells it results in activation.
6) From the medical point of view, it is very important to find a place for nAbs in the pathogenesis of autoimmunity, i.e. to understand when nAbs cause pathology and when, on the contrary, they are a factor of protection against autoimmunity.
The authors are grateful to N. V. Shilova for critical reading of the manuscript.
This study was supported by the Russian Science Foundation (project No. 14-14-00579).
REFERENCES
1.Winau, F., Westphal, O., and Winau, R. (2004) Paul
Ehrlich – in search of the magic bullet, Microbes
Infect., 68, 786-789.
2.Roit, A. (1991) Fundamentals of Immunology
[Russian translation], Mir, Moscow.
3.Abelev, G. I. (1996) Fundamentals of immunity,
Soros Educat. J., 5, 4-10.
4.Landsteiner, K., and Philip Miller, C. Ph., Jr.
(1925) Serological studies on the blood of the primates. II. The blood
groups in anthropoids apes, J. Exp. Med., 42,
853-862.
5.Landsteiner, K., and Levine, P. (1927) Further
observations on individual differences of human blood, Proc.
Soc. Exp. Biol., 24, 941-942.
6.Burnet, F. M. (1976) A modification of
Jerne’s theory of antibody production using the concept of clonal
selection, CA Cancer J. Clin., 26, 119-121.
7.Silverstein, A. M. (2009) A History of
Immunology, Academic Press, N. Y., 2nd Edn.
8.Burnet, F. M. (1978) Clonal selection and after, in
Theoretical Immunology (Bell, G. I., Perelson, A. S., and
Pimbley, G. H., Jr., eds.) Marcel Dekker Inc., pp. 63-85.
9.Yarilin, A. A. (2010) Immunology [in
Russian], GEOTAR-Media, Moscow.
10.Janeway, Ch. A., Travers, P., Jr., Walport, M.,
and Shlomchik, M. J. (2001) The Immune System in Health and Disease.
Immunobiology, 5th Edn., Garland Science, N. Y.
11.Schatz, D. G., Oettinger, M. A., and Baltimore,
D. (1989) The V(D)J recombination activating gene, RAG-1,
Cell, 59, 1035-1048.
12.Lutz, H. U. (2012) Naturally occurring antibodies
(nAbs), Adv. Exp. Med. Biol., 750, vii-x, p. 267.
13.Hayakawa, K., and Hardy, R. R. (2000) Development
and function of B-1 cells, Curr. Opin. Immunol., 12,
346-353.
14.Guilbert, B., Digheiro, G., and Avrameas, S.
(1982) Naturally occurring antibodies against nine common antigens in
human sera, J. Immunol., 128, 2779-2787.
15.Zhou, Z-H., Tzioufas, G. A., and Notkins, A. L.
(2007) Properties and function of polyreactive antibodies and
polyreactive antigen-binding B cells, J. Autoimmun., 29,
219-228.
16.Cohen, I. (2013) Autoantibody repertoires,
natural biomarkers, and system controllers, Trends Immunol.,
34, 620-625.
17.Daniels, G. (2003) Human Blood Groups, 3rd
Edn., Blackwell Science, Oxford.
18.Gershvin, M. E., Meroni, P. L., and Shoenfeld, Y.
(2006) Autoantibodies, 2nd Edn., Elsevier Science.
19.Avrameas, S. (1991) Natural autoantibodies: from
“horror autotoxicus” to “gnothiseauton”,
Immunol. Today, 12, 154-159.
20.Boes, M. (2000) Role of natural and immune IgM
antibodies in immune responses, Mol. Immunol., 37,
1141-1149.
21.Zhou, Z-H., Zhang, Y., Hu, Y-F., Wahl, L. M.,
Cisar, J. O., and Notkins, A. L. (2007) The broad antibacterial
activity of the natural antibody repertoire is due to polyreactive
antibodies, Cell Host Microbe, 1, 51-61.
22.Racine, R., and Winslow, G. M. (2009) IgM in
microbial infections: taken for granted? Immunol. Lett.,
125, 79-85.
23.Ochsenbein, A. F., Fehr, T., Lutz, C., Suter, M.,
Brombacher, F., Hengartner, H., and Zinkernagel, R. M. (1999) Control
of early viral and bacterial distribution and disease by natural
antibodies, Science, 286, 2156-2159.
24.Ochsenbein, A. F., and Zinkernagel, R. M. (2000)
Natural antibodies and complement link innate and acquired immunity,
Immunol. Today, 21, 624-630.
25.Baumgarth, N., Tung, J. W., and Herzenberg, L. A.
(2005) Inherent specificities in natural antibodies: a key to immune
defense against pathogen invasion, Springer Semin. Immun.,
26, 347-362.
26.Chou, M.-Y., Fogelstrand, L., Hartvigsen,
K., Hansen, L. F., Woelkers, D., Shaw, P. X., Choi, J., Perkmann, T.,
Backhed, F., Miller, Y. I., Horkko, S., Corr, M., Witztum, J. L., and
Binder, C. J. (2009) Oxidation-specific epitopes are dominant targets
of innate natural antibodies in mice and humans, J. Clin.
Invest., 199, 1335-1349.
27.Lutz, H. U. (2007) Homeostatic roles of naturally
occurring antibodies: an overview, J. Autoimmun., 29,
287-294.
28.Binder, C. J., Shaw, P. X., Chang, M.-K.,
Boullier, A., Hartvigsen, K., Horkko, S., Miller, Y. I., Woelkers, D.
A., Corr, M., and Witztum, J. L. (2005) The role of natural antibodies
in atherogenesis, J. Lipid Res., 46, 1353-1363.
29.Tsiantoulas, D., Gruber, S., and Binder, C. J.
(2013) B-1 cell immunoglobulin directed against
oxidation-specific epitopes, Front. Immunol., 9,
415.
30.Galili, U. (2004) Immune response, accommodation,
and tolerance to transplantation carbohydrate antigens,
Transplantation, 78, 1093-1098.
31.Hayakawa, K., Hardy, R. R., and Herzenberg, L. A.
(1986) Peritoneal Ly-1 B cells: genetic control, autoantibody
production, increased lambda light chain expression, Eur. J.
Immunol., 16, 450-456.
32.Bendelac, A., Bonneville, M., and Kearney, J. F.
(2001) Autoreactivity by design: innate B and T lymphocytes, Nature
Rev. Immunol., 1, 177-186.
33.Hayakawa, K., Asano, M., Shinton, S. A., Gui, M.,
Allman, D., Stewart, C. L., Silver, J., and Hardy, R. R. (1999)
Positive selection of natural autoreactive B cells, Science,
285, 113-116.
34.Hao, Z., and Rajewsky, K. (2001) Homeostasis of
peripheral B cells in the absence of B cell influx from the bone
marrow, J. Exp. Med., 194, 1151-1163.
35.Itakura, A., Szczepanik, M., Campos, R. A.,
Paliwal, V., Majewska, M., Matsuda, H., Takatsu, K., and Askenase, P.
W. (2005) An hour after immunization peritoneal B-1 cells are activated
to migrate to lymphoid organs where within 1 day they produce IgM
antibodies that initiate elicitation of contact sensitivity, J.
Immunol., 175, 7170-7178.
36.Abelev, G. L. (1971) Alpha-fetoprotein in
ontogenesis and its association with malignant tumors, Adv. Cancer
Res., 14, 295-358.
37.Barak, V. (2006) Tumor Biology. Tumor Markers,
Tumor Targeting and Translational Cancer Research (Stigbrand, T.,
ed.) Karger Medical and Scientific Publishers, N.Y., p. 116.
38.Armenti, V. T., Moritz, M. J., Cardonick, E. H.,
and Davison, J. M. (2002) Immunosuppression in pregnancy: choices for
infant and maternal health, Drugs, 62, 2361-2375.
39.Elliott, A. B., and Chakravarty, E. F. (2010)
Immunosuppressive medications during pregnancy and lactation in women
with autoimmune diseases, Womens Health (London), 6,
431-440.
40.Badami, K. G., Vanhecke, C., and Bingham, J.
(2009) Maternal IgM anti-D, borderline foetal Doppler middle cerebral
artery velocities and absent neonatal hemolysis, Transfus. Med.,
19, 146-147.
41.Alberts, B., Johnson, A., Lewis, J., Raff, M.,
Roberts, K., and Walter, P. (2002) Molecular Biology of the
Cell, 4th Edn., Garland Science, N.Y.
42.Mackie, R. I., Sghir, A., and Gaskins, H. R.
(1999) Developmental microbial ecology of the neonatal gastrointestinal
tract, Am. J. Clin. Nutr., 69, 1035-1045.
43.Mariat, D., Firmesse, O., Levenez, F., Guimaraes,
V. D., Sokol, H., Dore, J., Corthier, G., and Furet, J.-P. (2009) The
Firmicutes/Bacteroidetes ratio of the human microbiota changes with
age, BMC Microbiol., 9, 1-6.
44.Ley, R. E., Lozupone, C. A., Hamady, M., Knight,
R., and Gordon, J. I. (2008) Worlds within worlds: evolution of the
vertebrate gut microbiota, Nature Rev. Microbiol., 6,
776-788.
45.Dethlefsen, L., Eckburg, P. B., Bik, E. M., and
Relman, D. A. (2006) Assembly of the human intestinal microbiota,
Trends Ecol. Evol., 21, 517-523.
46.Lanning, D. K., Rhee, K. J., and Knight, K. L.
(2005) Intestinal bacteria and development of the B-lymphocyte
repertoire, Trends Immunol., 26, 419-425.
47.Bulatova, E. M., and Bogdanova, N. M. (2010) The
importance of the intestinal microbiota and probiotics in generation of
immune response and health of a child, Vorp. Sovrem. Pediatr.,
6, 37-44.
48.Berg, R. D. (1999) Bacterial translocation from
the gastrointestinal tract, Adv. Exp. Med. Biol., 473,
11-30.
49.Kelly, D., King, T., and Aminov, R. (2007)
Importance of microbial colonization of the gut in early life to the
development of immunity, Mutat. Res., 622, 58-69.
50.Cash, H. L., and Hooper, L. V. (2005) Commensal
bacteria shape intestinal immune system development, ASM News,
71, 77-83.
51.Kau, A. L., Ahern, P. P., Griffin, N. W.,
Goodman, A. L., and Gordon, J. I. (2011) Human nutrition, the gut
microbiome and the immune system, Nature, 474,
327-336.
52.Otter, J. A., Vickery, K., Walker, J. T., de
Lancey, P. E., Stoodley, P., Goldenberg, S. D., Salkeld, J. A.,
Chewins, J., Yezli, S., and Edgeworth, J. D. J. (2015) Surface-attached
cells, biofilms and biocide susceptibility: implications for hospital
cleaning and disinfection, Hosp. Infect., 89, 16-27.
53.Leid, J. G., Willson, C. J., Shirtliff, M. E.,
Hassett, D. J., Parsek, M. R., and Jeffers, A. K. (2005) The
exopolysaccharide alginate protects Pseudomonas aeruginosa
biofilm bacteria from IFN-gamma-mediated macrophage killing, J.
Immunol., 175, 7512-7518.
54.Suzuki, K., Ha, S-A., Tsuji, M., and Fagarasan,
S. (2007) Intestinal IgA synthesis: a primitive form of adaptive
immunity that regulates microbial communities in the gut, Semin.
Immunol., 19, 127-135.
55.Cerutti, A., and Rescigno, M. (2008) The biology
of intestinal immunoglobulin A responses, Immunity, 28,
740-750.
56.Tsuji, M., Suzuki, K., Kinoshita, K., and
Fagarasan, S. (2008) Dynamic interactions between bacteria and immune
cells leading to intestinal IgA synthesis, Semin. Immunol.,
20, 59-66.
57.Deplancke, B., and Gaskins, H. R. (2001)
Microbial modulation of innate defense: goblet cells and the intestinal
mucus layer, Am. J. Clin. Nutr., 73, 1131-1141.
58.Frederiksen, R. F., Paspaliari, D. K., Larsen,
T., Storgaard, B. G., Larsen, M. H., Ingmer, H., Palcic, M. M., and
Leisner, J. J. (2013) Bacterial chitinases and chitin-binding proteins
as virulence factors, Microbiology, 159, 833-847.
59.Langhendries, J. P. (2005) Early bacterial
colonization of the intestine: why it matters, Ital. J.
Pediatr., 31, 360-369.
60.Wold, A. E., and Adlerberth, I. (2000) Breast
feeding and the intestinal microflora of the infant-implications for
protection against infectious diseases, Adv. Exp. Med. Biol.,
478, 77-93.
61.Lu, L., and Walker, W. A. (2001) Pathologic and
physiologic interactions of bacteria with the gastrointestinal
epithelium, Am. J. Clin. Nutr., 73, 1124-1130.
62.Panigrahi, P., Parida, S., Pradhan, L.,
Mohapatra, S. S., Misra, P. R., Johnson, J. A., Chaudhry, R., Taylor,
S., Hansen, N. I., and Gewolb, I. H. (2008) Long-term colonization of a
Lactobacillus plantarum synbiotic preparation in the neonatal
gut, J. Pediatr. Gastroenterol. Nutr., 47, 45-53.
63.Abreu, M. T. (2010) Toll-like receptor signaling
in the intestinal epithelium: how bacterial recognition shapes
intestinal function, Nature Rev. Immunol., 10,
131-144.
64.Guarner, F., and Malagelada, J.-R. (2003) Gut
flora in health and disease, Lancet, 361, 512-519.
65.Balzan, S., Almeida Quadros, A., de Cleva, R.,
Zilberstein, B., and Cecconello, I. (2007) Bacterial translocation:
overview of mechanisms and clinical impact, J. Gastroenterol.
Hepatol., 22, 464-471.
66.Springer, G. F., Horton, R. E., and Forbes, M.
(1959) Origin of antihuman blood group B agglutinins in germfree
chicks, J. Exp. Med., 110, 221-244.
67.Springer, G. F. (1971) Blood-group and Forssman
antigenic determinants shared between microbes and mammalian cells,
Prog. Allergy, 15, 9-77.
68.Wagner, R. D. (2008) Effects of microbiota on GI
health: gnotobiotic research, Adv. Exp. Med. Biol., 635,
41-56.
69.De Filippo, C., Cavalieri, D., Di Paola, M.,
Ramazzotti, M., Poullet, J. B., Massart, S., Collini, S., Pieraccini,
G., and Lionetti, P. (2010) Impact of diet in shaping gut microbiota
revealed by a comparative study in children from Europe and rural
Africa, Proc. Natl. Acad. Sci. USA, 107, 14691-14696.
70.Bischof, S. C. (2011) “Gut health”: a
new objective in medicine? BMC Medicine, 9, 1-14.
71.Bos, N. A., Kimura, H., Meeuwsen, C. G., De
Visser, H., Hazenberg, M. P., Wostmannn, B. S., Pleasants, J. R.,
Benner, B., and Marcus, D. M. (1980) Serum immunoglobulin levels and
naturally occurring antibodies against carbohydrate antigens in
germ-free BALB/c mice fed chemically defined ultrafiltered diet,
Eur. J. Immunol., 19, 2335-2339.
72.Kozakova, H., Kolinska, J., Lojda, Z., Rehakova,
Z., Sinkora, J., Zakostelecka, M., Splichal, I., and
Tlaskalova-Hogenova, E. (2006) Effect of bacterial monoassociation on
brush-border enzyme activities in ex-germ-free piglets: comparison of
commensal and pathogenic Escherichia coli strains, Microbes
Infect., 8, 2629-2639.
73.Butler, J. E., Sun, J., Weber, P., Navarro, P.,
and Francis, D. (2000) Antibody repertoire development in fetal and
newborn piglets. III. Colonization of the gastrointestinal tract
selectively diversifies the preimmune repertoire in mucosal lymphoid
tissues, Immunology, 100, 119-130.
74.Coutinho, A., Kazatchkine, M. D., and Avrameas,
S. (1995) Natural autoantibodies, Curr. Opin. Immunol.,
7, 812-818.
75.Nores, G. A., Lardone, R. D., Comin, R., Alaniz,
M. E., Moyano, A. L., and Irazoqui, F. J. (2008) Anti-GM1 antibodies as
a model of the immune response to self-glycan, BBA, 1780,
538-545.
76.Danussi, C., Coslovi, A., Campa, C., Mucignat, M.
T., Spessotto, P., Uggeri, F., Paoletti, S., and Colombatti, A. (2009)
A newly generated functional antibody identifies Tn antigen as a
novel determinant in the cancer cell–lymphatic endothelium
interaction, Glycobiology, 19, 1056-1067.
77.Mouthon, L., Haury, M., Lacroix-Desmazes, S.,
Barreau, C., Coutinho, A., and Kazatchkine, M. D. (1995) Analysis of
the normal human IgG antibody repertoire, J. Immunol.,
154, 5769-5778.
78.Hayakawa, K., and Hardy, R. R. (2005) Development
of B cells producing natural autoantibodies to thymocytes and senescent
erythrocytes, Springer Semin. Immun., 26, 363-375.
79.Lacroix-Desmazes, S., Srini, U., Kaveri, V.,
Mouthon, L., Ayouba, A., Malanchere, E., Coutinho, A., and Kazatchkine,
M. D. (1998) Self-reactive antibodies natural autoantibodies in healthy
individuals, J. Immunol. Methods, 216, 117-137.
80.Pancer, Z., and Cooper, M. D. (2006) The
evolution of adaptive immunity, Annu. Rev. Immunol., 24,
497-518.
81.Servettaz, A., Guilpain, P., Tamas, N., Kaveri,
S. V., Camoin, L., and Mouthon, L. (2008) Natural anti-endothelial cell
antibodies, Autoimmun. Rev., 7, 426-430.
82.Ronda, N., Haury, M., Nobrega, A., Kaveri, S. V.,
Coutlnho, A., and Kazatchkine, M. D. (1994) Analysis of natural and
disease-associated autoantibody repertoires: anti-endothelial cell IgG
autoantibody activity in the serum of healthy individuals and patients
with systemic lupus erythematosus, Int. Immunol., 6,
1651-1660.
83.Pashov, A., Kenderov, A., Kyurkchiev, S.,
Kehayov, I., Hristova, S., Lacroix-Desmazes, S., Giltiay, N.,
Varamballi, S., Kazatchkine, M. D., and Kaveri, S. V. (2002)
Autoantibodies to heat shock protein 90 in the human natural antibody
repertoire, Int. Immunol., 14, 453-461.
84.Yadin, O., Sarov, B., Naggan, L., Slor, H., and
Shoenfeld, Y. (1989) Natural autoantibodies in the serum of healthy
women – a five-year follow-up, Clin. Exp. Immunol.,
75, 402-406.
85.Pierson, R. N., Loyd, J. E., Goodwin, A., Majors,
D., Dummer, J. S., Mohacsi, P., Wheeler, A., Bovin, N., Miller, G. G.,
Olson, S., Johnson, J., Rieben, R., and Azimzadeh, A. (2002) Successful
management of an ABO-mismatched lung allograft using antigen-specific
immunoadsorption, complement inhibition, and immunomodulatory therapy,
Transplantation, 15, 79-84.
86.Wardemann, H., Yurasov, S., Schaefer, A., Young,
J. W., Meffre, E., and Nussenzweig, M. C. (2003) Predominant
autoantibody production by early human B cell precursors,
Science, 301, 1374-1377.
87.Quintana, F. J., and Cohen, I. R. (2004) The
natural autoantibody repertoire and autoimmune disease, Biomed.
Pharmacother., 58, 276-281.
88.Oka, Y., Hirabayashi, Y., Ikeda, T., Fujii, H.,
Ishii, T., and Harigae, H. (2011) A single-stranded DNA-cross-reactive
immunogenic epitope of human homocysteine-inducible endoplasmic
reticulum protein, Scand. J. Immunol., 74, 296-303.
89.Allos, B. M., Lippy, F. T., Carlsen, A.,
Washburn, R. G., and Blaser, M. J. (1998) Campylobacter jejuni strains
from patients with Guillain–Barre syndrome, Emerg. Infect.
Dis., 4, 263-268.
90.Thornton, C. A., and Morgan, G. (2009) Innate and
adaptive immune pathways to tolerance, Nestle Nutr. Workshop Ser.
Pediatr. Program., 64, 45-57.
91.Janeway, C. A., and Medzhitov, R. (2002) Innate
immune recognition, Annu. Rev. Immunol., 20, 197-216.
92.Baldus, S. E., Hanisch, F. G., Kotlarek, G. M.,
Zirbes, T. K., Thiele, J., Isenberg, J., Karsten, U. R., Devine, P. L.,
and Dienes, H. P. (1998) Coexpression of MUC-1 mucin peptide core and
the Thomsen–Friedenreich antigen in colorectal neoplasms,
Cancer, 82, 1019-1027.
93.Gorshkova, T. A. (2007) The Plant Cell Wall as
a Dynamic System [in Russian], Nauka, Moscow, p. 426.
94.Bovin, N. V. (2013) Natural antibodies to
glycans, Biochemistry (Moscow), 78, 786-797.
95.Knirel, Y. A., Gabius, H.-J., Blixt, O.,
Rapoport, E. M., Khasbiullina, N. R., Shilova, N. V., and Bovin, N. V.
(2014) Human tandem-repeat-type galectins bind bacterial non-βGal
polysaccharides, Glycoconj. J., 31, 7-12.
96.Hakomori, S. (2001) The glycosynapse,
PNAS, 99, 225-232.
97.Hakomori, S., and Handa, K. (2015) GM3 and
cancer, Glycoconj. J., 32, 1-8.
98.Todeschini, A. R., Dos Santos, J. N., Handa, K.,
and Hakomori, S. (2008) Ganglioside GM2/GM3 complex affixed on silica
nanospheres strongly inhibits cell motility through CD82/cMet-mediated
pathway, Proc. Natl. Acad. Sci. USA, 105, 1925-1930.
99.Di Virgilio, F. (2013) The therapeutic potential
of modifying inflammasomes and NOD-like receptors,
Pharmacological, 65, 872-905.
100.Mills, K. H. (2011) TLR-dependent T cell
activation in autoimmunity, Nature Rev. Immunol., 11,
807-822.
101.Gellert, M. (1997) Recent advances in
understanding V(D)J recombination, Adv. Immunol.,
64, 39-64.
102.Hennings, L., Artaud, C., Jousheghany, F.,
Monzavi-Karbassi, B., Pashov, A., and Kieber-Emmons, T. (2011)
Carbohydrate mimetic peptides augment carbohydrate-reactive immune
responses in the absence of immune pathology, Cancers (Basel),
3, 4151-4169.
103.Avrameas, S., Dighiero, G., Lymberi, P., and
Guilbert, B. (1983) Studies on natural antibodies and autoantibodies,
Ann. Immunol. (Paris), 134, 103-113.
104.Stahl, D., Lacroix-Desmazes, S., Mouthon, L.,
Kaveri, S. V., and Kazatchkine, M. D. (2000) Analysis of human
self-reactive antibody repertoires by quantitative immunoblotting,
J. Immunol. Methods, 240, 1-14.
105.Madi, A., Kenett, D. Y., Bransburg-Zabary, S.,
Merbl, Y., Quintana, F. J., Boccaletti, S., Tauber, A. I., Cohen, I.
R., and Ben-Jacob, E. (2011) Analyses of antigen dependency networks
unveil immune system reorganization between birth and adulthood,
Chaos, 21, 1-11.
106.Springer, G. F. (1971) Blood-group and Forssman
antigenic determinants shared between microbes and mammalian cells,
Prog. Allergy, 15, 9-77.
107.Bovin, N., Obukhova, P., Shilova, N., Rapoport,
E., Popova, I., Navakouski, M., Unverzagt, C., Vuskovic, M., and
Huflejt, M. (2012) Repertoire of human natural anti-glycan
immunoglobulins. Do we have auto-antibodies? Biochim. Biophys.
Acta, 1820, 1373-1382.
108.Obukhova, P., Piskarev, V., Severov, V.,
Pazynina, G., Tuzikov, A., Navakouski, M., Shilova, N., and Bovin, N.
(2011) Profiling of serum antibodies with printed glycan array: room
for data misinterpretation, Glycocon. J., 28,
501-505.
109.Tupitsyn, N. N., Udalova, Y. A., Galanina, O.
E., Kadagidze, Z. G., Borovkova, N. B., Podolsky, V. V., Shinkarev, S.
A., Gadetskaya, N. A., Letyagin, V. P., Obukhova, P. S., Shilova, N.
V., Subbotina, A. A., and Bovin, N. V. (2009) Tumor-associated glycan
Lewis C in breast cancer, Hematopoiesis Immunol., 2,
45-54.
110.Hakomori, S. (1984) Tumor-associated
carbohydrate antigens, Annu. Rev. Immunol., 2,
103-126.
111.Lloyd, K. O. (1991) Humoral immune responses to
tumor-associated carbohydrate antigens, Semin. Cancer Biol.,
2, 421-431.
112.Livingston, P. O. (1995) Augmenting the
immunogenicity of carbohydrate tumor antigens, Semin. Cancer
Biol., 2, 357-366.
113.Springer, G. F. (1984) T and Tn, general
carcinoma autoantigens, Science, 224, 1198-1206.
114.Huflejt, M. E., Vuskovic, M., Vasiliu, D., Xu,
H., Obukhova, P., Shilova, N., Tuzikov, A., Galanina, O., Arun, B., Lu,
K., and Bovin, N. V. (2009) Anti-carbohydrate antibodies of normal
sera: findings, surprises and challenges, Mol. Immunol.,
46, 3037-3049.
115.Jacob, F., Goldstein, D. R., Huflejt, M.,
Bovin, N., Pochechueva, T., Spengler, M., Caduff, R., Fink, D., and
Heinzelmann-Schwarz, V. (2012) Serum antiglycan antibody detection of
nonmucinous ovarian cancers by using a printed glycan array, Int. J.
Cancer, 130, 138-146.
116.Bovin, N. V., and Huflejt, M. E. (2008)
Unlimited glycochip, Trends Glycosci. Glycotechnol., 20,
245-258.
117.Cheng, H., Yang, Z., Estabrook, M. M., John, C.
M., Jarvis, G. A., McLaughlin, S., and Griffiss, M. (2011) Human
lipopolysaccharide IgG that prevents endemic meningococcal disease
recognizes an internal lacto-N-neotetraose structure, J. Biol.
Chem., 286, 43622-43633.
118.Kurtenkov, O., Miljukhina, L., Smorodin, J.,
Klaamas, K., Bovin, N., Ellamaa, M., and Chuzmarov, V. (1999) Natural
IgM and IgG antibodies to Thomsen–Friedenreich (T) antigen in
serum of patients with gastric cancer and blood donors, Acta
Oncol., 38, 939-943.
119.Lekakh, I. V., Bovin, N. V., Bezyaeva, G. P.,
and Poverenny, A. M. (2001) Natural hidden autoantibodies react with
negatively charged carbohydrates and xenoantigen Bdi,
Biochemistry (Moscow), 66, 205-210.
120.Krenn, V., von Landenberg, P., Wozniak, E.,
Kissler, C., Hermelink, H. K., Zimmermann, U., and Vollmers, H. P.
(1995) Efficient immortalization of rheumatoid synovial tissue
B-lymphocytes. A comparison between the techniques of electric
field-induced and PEG fusion, Hum. Antibodies Hybridomas,
6, 47-51.
121.Anthony, R. M., and Ravetch, J. V. (2010) A
novel role for the IgG Fc glycan: the anti-inflammatory activity of
sialylated IgG Fcs, J. Clin. Immunol., 30, 9-14.
122.Stadlmann, J., Pabst, M., and Altmann, F.
(2010) Analytical and functional aspects of antibody sialylation, J.
Clin. Immunol., 30, 15-19.
123.Wassenaar, T. M., and Panigrahi, P. (2014) Is a
fetus developing in a sterile environment? Lett. Appl.
Microbiol., 59, 572-579.
124.Aagaard, K. M. (2014) Author response to
comment on “the placenta harbors a unique microbiome”,
Sci. Transl. Med., 6, 254-256.