REVIEW: Specificity of Human Galectins on Cell Surfaces
E. M. Rapoport and N. V. Bovin*
Shemyakin and Ovchinnikov Institute of Bioorganic Chemistry, Russian Academy of Sciences, 117997 Moscow, Russia; fax: +7 (495) 330-5592; E-mail: professorbovin@yandex.ru* To whom correspondence should be addressed.
Received March 5, 2015; Revision received April 13, 2015
Galectins are β-galactoside-binding proteins sharing homology in amino acid sequence of their carbohydrate-recognition domain. Their carbohydrate specificity outside cells has been studied previously. The main conclusion of these studies was that several levels of glycan ligand recognition exist for galectins: (i) disaccharide Galβ1-4GlcNAc (LN, N-acetyllactosamine) binds stronger than β-galactopyranose; (ii) substitution at O-2 and O-3 of galactose residue as well as core fragments (“right” from GlcNAc) provides significant increase in affinity; (iii) similarly glycosylated proteins can differ significantly in affinity to galectins. Information about the natural cellular receptors of galectins is limited. Until recently, it was impossible to study specificity of cell-bound galectins. A model based on controlled incorporation of a single protein into glycocalyx of cells and subsequent interaction of loaded cells with synthetic glycoprobes measured by flow cytometry made this possible recently. In this review, data about glycan specificity of proto-, chimera-, and tandem-repeat type galectins on the cell surface are systematized, and comparative analysis of the results with data on specificity of galectins in artificial systems was performed. The following conclusions from these studies were made: (i) cellular galectins have practically no ability to bind disaccharide LNn, but display affinity to 3′-substituted oligolactosamines and oligomers LNn; (ii) tandem-repeat type galectins recognize another disaccharide, namely Galβ1-3GlcNAc (Lec); (iii) on the cell surface, tandem-repeat type galectins conserve the ability to display high affinity to blood group antigens of ABH system; (iv) in general, when galectins are immersed into glycocalyx, they are more selective regarding glycan interactions. Thus, we conclude that competitive interaction of galectins with cell microenvironment (endogenous cell glycans) is the main factor providing selectivity of galectins in vivo.
KEY WORDS: galectins, glycans, cells, carbohydrate specificity, carbohydrate-recognition domainDOI: 10.1134/S0006297915070056
Abbreviations: CRD, carbohydrate-recognition domain; fluo, fluorescein; gal, galectin; Glyc, glycan residue; PAA, polyacrylamide.
Galectins are β-galactoside-binding lectins defined by shared
consensus amino acid sequence of carbohydrate-recognition domain (CRD)
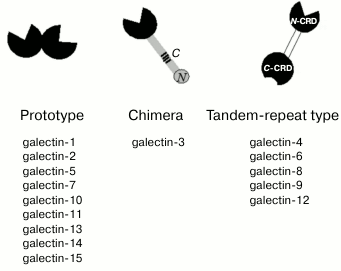
[1, 2]. Currently, 15 proteins
of this family have been identified in mammals. Based on the structural
organization of CRDs, galectins are divided into proto-, chimera- and
tandem-repeat types [3]. Prototype galectins have
one CRD and usually form noncovalent dimers (Fig. 1); tandem-repeat type galectins contain two
carbohydrate-recognition domains (N-CRD and C-CRD) connected by a short
linker peptide. The chimera-type is presented by galectin-3 that has
two distinct domains, i.e. C-terminal CRD and N-terminal regulatory
domain that contains collagen-like repetitive regions [4].
Fig. 1. Structure of galectins. The CRDs are highlighted in black, the tandem-repeat type galectins linker is shown in white. The regulatory domain of galectin-3 is shown in gray. C- and N-, collagen- and N-terminal regions of the regulatory domain.
Despite the vast number of studies, information on real (functioning, rather than potential) galectin cell ligands (i.e. glycans) is extremely limited. It is, however, in high demand as it provides the key to identification of their natural receptors (i.e. glycoproteins and, possibly, glycosphingolipids).
The carbohydrate specificity of galectins has been studied by numerous extracellular methods, including solid-phase assays [8-11], surface plasmon resonance [12], fluorescence polarization [11], frontal affinity chromatography [3, 13], and calorimetry [14]. The development of microarray technology has made it possible to apply several hundreds of different glycans to a single chip to get information on carbohydrate-recognition profile of all currently known mammalian galectins [15, 16].
It should be noted that data on specificity obtained for the galectin using different methods might not be consistent. For example, gal-3 binds to lactosamine (LN) in isothermal calorimetry [14], but no interaction was observed for the same disaccharide on a glycochip [16]. Another example is GM1, which was identified as a gal-1 ligand in SK-N-MC neuroblastoma cells using a cellular variant of enzyme immunoassay [17]; frontal affinity chromatography and glycochip technology did not display binding to glycan which is part of the ganglioside [3, 16]. Increase in a number of LN units in the oligolactosamine chain expands binding of gal-1 and gal-8 in solid-phase assays, but not in the study by frontal affinity chromatography [3, 8, 11]. Discrepancies in the literature seem to be associated with particular features of various test system designs, in particular with limited spatial accessibility of the ligands.
The analysis of data obtained in cell-free systems reveals the following regularities:
1) galectins bind to oligolactosamines; for most of galectins, the affinity for glycans increases in the LN → (LN)2 → (LN)3 → (LN)5 sequence;
2) galectins bind to sulfated and sialylated glycans; the presence of negatively-charged groups substantially increases the affinity of galectins for glycans;
3) fucosylation of the LN-core increases affinity of galectins for glycans; the binding of all investigated galectins to Fucα1-2LN-LN was stronger than to the corresponding dilactosamine (LN)2;
4) except for gal-1, all galectins recognize glycans containing Galα/GalNAcα.
Obviously, the ability to recognize such a wide range of glycans implies that galectins have a sufficiently wide repertoire of receptors. Indeed, galectin receptors have been identified in virtually all organs – they are represented by glycoproteins of extracellular matrix and immune cells, integrins, lysosomal integral membrane proteins LAMP-1 and -2, and glycosphingolipids (the data are systematized in reviews [6, 18-21]). It should be noted, that available information on cellular receptors is primarily based on data obtained in cell-free models where: 1) galectins are precipitated with extracts of cell membrane or extracellular matrix proteins; 2) glycoproteins are isolated by cell lysate chromatography on a sorbent with immobilized galectin; 3) binding of recombinant glycoproteins to galectins is investigated using solid-phase assays. For example, chromatography of T-cell lysate on Sepharose with immobilized gal-3 followed by electrophoresis and Western blot analysis of proteins using corresponding antibodies showed that gal-3 binds to glycans of CD29, CD43, CD45, and CD71 on T-lymphocytes [22]. Another example, co-immunoprecipitation of gal-8 with lysate of CHO cells (Chinese hamster ovarian cells) transfected with podoplanin, showed that the latter is a receptor for galectin on endothelial cells [23]. The results of study of the binding of gal-1 immobilized on plastic to proteins from HUVEC (endothelial cells from umbilical vein) lysate indicate that neuropilin is the receptor for galectin [24]. However, there are many examples demonstrating that in vivo galectins are more selective, i.e. bind only to few ligands. For example, gal-9 does not bind directly to TIM-3 on endothelial cells [25], even though glycoprotein–lectin complex has been crystallized previously [26]. Gal-4 mediates the adhesion of only those cells that have sulfated glycolipids exposed on their surface [27]; induction of cell adhesion to extracellular matrix by gal-1 is associated with selective binding of galectin to oligolactosamine chains of certain proteins only, namely of laminin or fibronectin [28, 29]. Obviously, cell-free assays do not reproduce correctly the interaction of galectins with cognate glycans. In this regard, it is unknown whether cell-free systems can correctly reflect the nature setting, i.e. when galectins are anchored in the glycocalyx. Except for gal-3 (which presumably exists as a pentamer [30]), galectins are bivalent, and they do not have other functional domains beside CRDs. If both CRDs are bound to ligands exposed on a cell (cis-ligands), the protein is unable to recognize “external” (trans-) glycans. It is obviously impossible to construct a cell-free model that simulates both the effect of cis-glycans and the cell–cell interactions.
Studies on specificity of other cell bound lectins are commonly based on the analysis of binding of cells transfected with corresponding proteins [31-34] to glycoprobes by flow-cytometry or by cell version of solid-phase assay. These approaches yielded data on carbohydrate specificity of siglecs [31], macrophage galactose-specific lectin (MGL) [33], and dendritic cell lectin (DC-SIGN) [34]. In some cases, studies revealed discrepancies with data obtained in the cell-free systems. For example, most siglecs bound to glycoprobes (identified as affine in the solid-phase assays) better after desialylation of the cells [31], indicating that the CRD of lectins is masked by cis-sialosides.
Galectins have no signaling sequence and transmembrane domain, making it difficult to obtain stable transfected lines [35]; therefore, the approach described above is not applicable. The data on cellular ligands of galectins are mainly obtained using a “reverse” approach based on investigation of the binding of an exogenic galectin to cell surface glycans that are either native or altered using glycosidases or glycosylation inhibitors [16, 36-38].
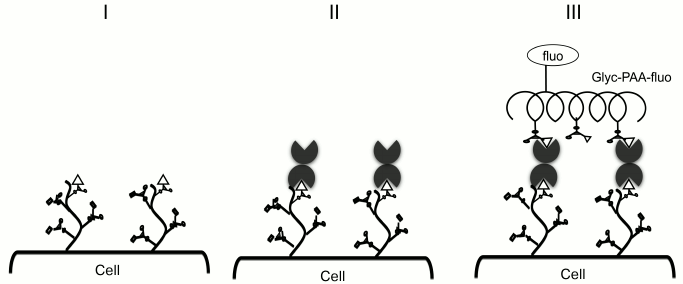
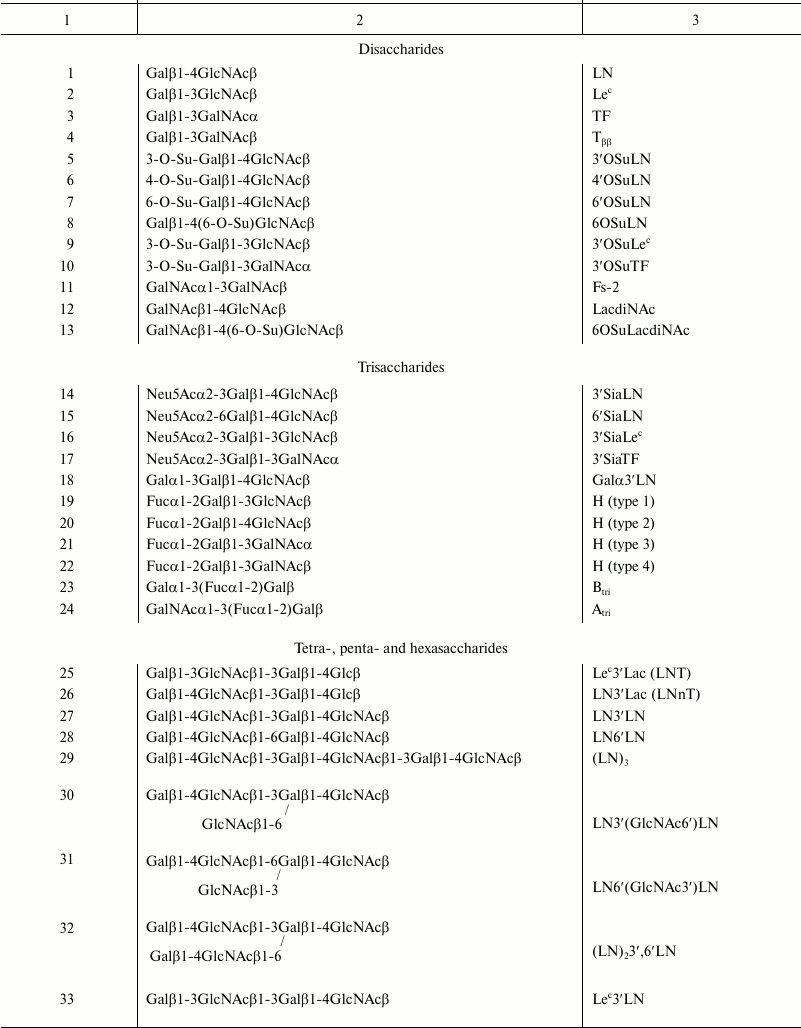
In 2008, a model was proposed to study galectin specificity within cellular glycocalyx, in which galectin is loaded onto Raji cells (B lymphocytes that do not express endogenous galectins; Fig. 2, I and II), and the binding of cells to fluorescein polyacrylamide-labeled glycoconjugates (Glyc-PAA-fluo glycoprobes) is studied by flow cytometry (Fig. 2, III) [37-39]. Glyc are regular glycans (usually terminal fragments) that form glycocalyx of animal cells: oligolactosamines, sulfated and sialylated glycans, oligosaccharides of the ABH system, and others (presented in the table).
Fig. 2. Methodology of cell-bound galectin specificity studies: galectin are loaded onto Raji cells and probed with Glyc-PAA-fluo. PAA, polyacrylamide; Glyc, carbohydrate moiety; fluo, fluorescein residue [40, 41].
Glycans in Glyc-PAA-fluo

PROTOTYPE GALECTINS
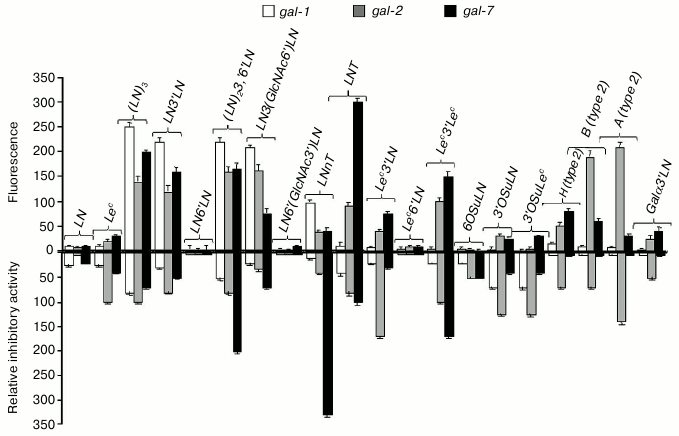
Prototype galectins -1, -2, and -7 did not bind LN disaccharide in the cellular assay, but showed an affinity to oligolactosamines with β1-3 linkage between LN-repeats (Fig. 3, top panel). In general, galectins bound to the linear (table, Nos. 27 and 29) and branched (table, Nos. 30 and 32) oligolactosamines at the same level. In contrast to published data [8, 16], higher number of LN-units in the chain did not result in stronger binding. Though prototype galectins did not bind to Lec-disaccharide, gal-2 and gal-7 displayed affinity to more complex glycans that contain this disaccharide, namely to Lec3′Lac, Lec3′LN, and Lec3′Lec (table, Nos. 25, 33, and 34). These lectins also bound to the ABH-glycans (table, Nos. 19, 20, 36-39).
Fig. 3. Comparison of specificity profiles for prototype galectins in cellular and cell-free assays. Top panel: cell assay, flow cytometry; the ordinate shows increase in fluorescence; values <20 were indicative of the lack of binding. Bottom panel: solid-phase inhibition assay [42]; the ordinate shows relative inhibitory potency.
When loaded onto cells, however, prototype galectins did not exhibit any affinity to 3′-O-sulfated and 3′-sialylated derivatives of LN (table, Nos. 5, 8, and 14) and Lec (table, Nos. 9 and 16) (Fig. 3, top panel) despite the binding observed in solid-phase assays [44] (Fig. 3, bottom panel). Remarkably, in both cases, Glyc-PAA glycoprobes that interacted with galectins had the same molecular weight and the same concentration of the Glyc component; therefore, the only substantial difference was the presence of glycocalyx in the cellular assay.
GALECTIN-3
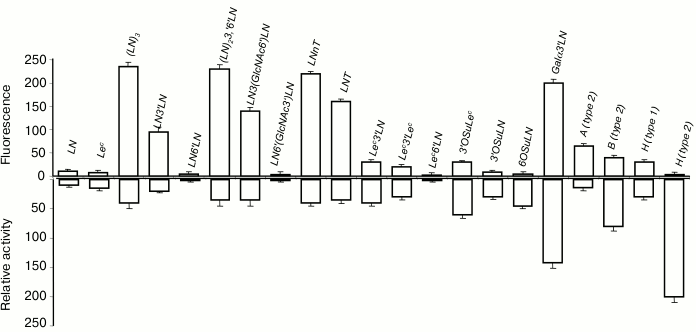
In cellular assay, gal-3 did not bind to LN disaccharide (Fig. 4, top panel) but interacted with oligomeric forms of LN (table, Nos. 27, 29, 30, and 32), the increase of LN units in the chain enhancing the affinity. Among all glycans containing a terminal Lec-fragment, the lectin bound only to Lec3′Lac, although the binding was somewhat weaker than in the case of LN3′Lac. Furthermore, gal-3 showed affinity to Galα3′LN trisaccharide and A- and B-tetrasaccharides (types 1 and 2). The binding to B (type 2), which features Fucα at the O-2 position of Gal residue (table, No. 39), was weaker than that to Galα3′LN (table, No. 18), implying that the contribution of the fucose residue in this case was negative.
Fig. 4. Comparison of specificity profiles for gal-3 in cellular and cell-free assays. Top panel: cytometry. Bottom panel: solid-phase assay (for details see caption to Fig. 3).
TANDEM-REPEAT TYPE GALECTINS
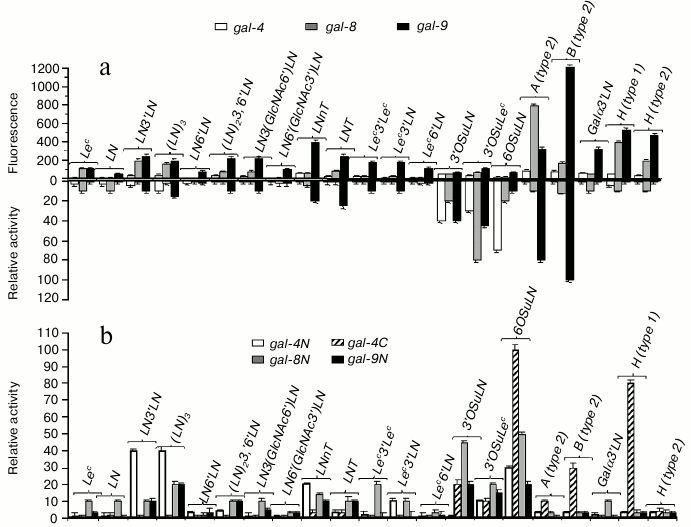
Just like prototype and chimera-type galectins, tandem-repeat galectins did not (gal-4, gal-8) or weakly (gal-9) bind LN (Fig. 5a, top panel); however, they displayed affinity for linear and branched oligolactosamines. Increasing the number of LN units in the chain did not affect the binding. Unlike other galectins, gal-9 bound not only to LN3′LN3 and LN3′(GlcNAc6)′LN; (table, Nos. 27 and 30), but also to oligosaccharides in which LN units were β1-6 linked, namely LN6′LN and LN6′(GlcNAc3′)LN (table, Nos. 28 and 31), although the affinity to these glycans was lower than to the β1-3 versions (Fig. 5a, top panel). The ability of gal-9 to bind to β1-6 oligo-LN is a unique feature of this particular galectin, rather than of the cellular assay [15].
Fig. 5. Comparison of specificity profiles for tandem-repeat type galectins in cellular and cell-free assay systems. a) Full-length galectin – top panel: cytofluorimetry; bottom panel: solid-phase assay. b) Single-domain galectin – solid-phase assay (for details, see caption to Fig. 3).
All three investigated tandem-repeat type galectins displayed affinity for sulfated glycans, namely 3′OSuLN, 6OSuLN, 3′OSuLec, and 3′OSuTF, to some extent. However, in the case of gal-8 and gal-9, the binding to these glycans was comparable with that to their neutral analogs, LN, Lec, and TF, and the impact of a negatively charged group was observed only for gal-4 (Fig. 5a, top panel).
The most affine ligands for tandem-repeat type galectins were blood group specific ABH-antigens – the binding to them was significantly stronger than to oligolactosamines (Fig. 5a, top panel); gal-4 and gal-8 preferably bound to A glycans, and gal-9 to B glycans.
In solid-phase assays, the highest affinity ligands for full-size galectins were negatively charged glycans; only gal-9 displayed affinity to the A- and B-blood group glycans (Fig. 5a, bottom panel). In the same assay, N-domain galectins displayed affinity to oligolactosamines, whereas C-domain of gal-4 bound to ABH glycans (Fig. 5b). Both C- and N-single-domain galectins bound to negatively charged glycans, although N-forms displayed higher affinity. The data obtained in the solid phase assays suggest that the N-CRD of tandem-repeat type galectins is responsible for binding to oligolactosamines and negatively charged glycans, whereas the C-CRD displays affinity to ABH glycans. If this is true, the N-CRD plays the key role in galectin anchoring cells, whereas the C-domain is involved in recognition of exogenous (trans-) glycans.
COMMON FEATURES OF LIGAND RECOGNITION BY GALECTINS ON THE CELL
SURFACE
Binding to short disaccharide ligands. Except for gal-9, all galectins anchored the cell surface did not bind LN, though this disaccharide is considered the “canonical” ligand for all galectins and significantly bound to them in various cell-free assays [14, 45]. Other β-galactose terminated disaccharides, such as Lec (Galβ1-3GlcNAcβ), TF (Galβ1-3GalNAcα), and Tββ (Galβ1-3GalNAcβ) as a part of glycoconjugates, were also not recognized by galectins, except for tandem-repeat type galectins (-8 and -9).
Binding to oligolactosamines and Lec-terminated glycans. All investigated galectins displayed affinity to oligolactosamines, in which LN fragments were β1-3 linked. Increasing the number of LN units in the chain from 2 to 3 did not affect significantly the binding of lectins to these glycans; binding to linear and branched trilactosamines was practically identical, except for gal-8, which displayed weaker binding to the branched variants [38]. Though only tandem-repeat type galectins interacted with Lec disaccharide, all the others (except for gal-1) also exhibited affinity for Lec-terminated oligosaccharides to some extent.
Binding to glycans containing Fucα, Galα, GalNAcα, Neu5Acα, and HSO–3 residues. The presence of Fucα at O-2 or/and Galα/GalNAcα at O-3 of galactose residue of the Galβ1-4GlcNAc disaccharide transforms inert glycan into a galectin ligand. This elongation of the structure also positively impacted the affinity of other disaccharides that displayed weak binding (see above). The affinity for the blood group antigens was most pronounced in tandem-repeat type galectins. All galectins (except for gal-1) exhibited affinity for ABH-glycans of type 1 or 2, whereas only tandem-repeat type galectins bound to glycans containing type 3 (Galβ1-3GalNAcα; table, Nos. 21 and 40) and type 4 (Galβ 1-3GalNAcβ; table, Nos. 22 and 41) glycans.
While the presence of neutral substituents at O-3 of Gal residue (see above) positively affected the binding to glycans, the presence of a sulfate group at the same position had no effect on the interaction. This is true for disaccharides, which do not bind to proto- and chimera-type galectins, whereas in case of tandem-repeat type galectins the effect was as negligible as in the case of neutral disaccharides. Despite the fact that in cell-free assays 3′-sialylation results in increased binding of galectins to oligolactosamines [9], in cellular system the positive effect was reported for gal-8, whose binding is decreased after desialylation of the cells [38]. Meanwhile, the treatment of cells with a neuraminidase resulted in the increased binding of gal-2, -3 [16], -4, and -9 [38]. These results indicate that 3′-sialylation interferes rather than promotes the binding. This was confirmed by the fact that interaction of gal-1, -2, and -3 with CHO cells defective by biosynthesis of 3′-sialylated glycans was stronger than that with parent mock transfected cells [36].
REASONS FOR SELECTIVITY OF GALECTINS ON CELLS
The study including more than a dozen galectins and 40 glycoprobes brings us to the following conclusion. Though the specificity pattern of galectins loaded onto cells is similar to that observed in cell-free assays, galectins are more selective regarding the binding to the cognate ligands on the cell surface. For example, galectins do not bind to certain ligands at all, including canonical LN. We are sure that the reason of that is not steric inaccessibility of short disaccharide ligands, since some of the disaccharides still display affinity. The observed phenomenon is not associated with the charge of the ligands either. There is no reason to consider weak interactions with disaccharides (including sulfated ones) as artefacts, since the results of substantially different approaches are consistent overall.
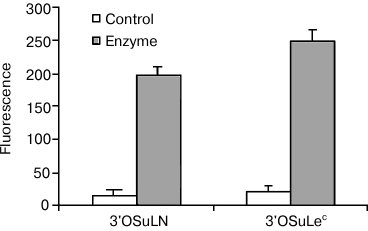
If ligands are ranked according to their affinity in cell-free assays, those with the lowest affinity are completely inactive in a cellular system, i.e. cell refutes weak interactions, but does not affect strong ones, which appears to be similar to inhibitory effect. The cell glycocalyx most likely acts as an inhibitor, i.e. glycans of the same cells, which we call cis-ligands (as opposed to trans-ligands of a glycoprobe or a second cell, which participates in galectin-mediated adhesion). It has been shown that galectin CRDs contact with a larger moiety in glycans than a disaccharide; therefore, it is not surprising that natural glycans easily win the competition. In addition, if a cis-ligand is in close proximity to an anchored galectin, in terms of entropy it takes precedence over a free-moving trans-competitor. Several experiments support the hypothesis of cis-influence, including treatment of galectin-loaded cells with β-galactosidase, which results in loss of the selectivity, observed for native cell galectins, as shown in Fig. 6.
Fig. 6. Gal-1 on Raji cells did not bind to low-affine ligands in the form of 3′OSuLN-PAA-fluo and 3′OSuLec-PAA-fluo probes. However, β-galactosidase treatment results in interactions, with intensity typical for ligands of medium or even high rank.
It should be noted, however, that though masking of weak interactions in a cellular system appeared to be a common phenomenon, sometimes the opposite effect is observed. In particular, gal-4 and gal-7 essentially do not interact with ABH-glycans in a solid-phase assay but display significant binding to them in the cellular one. Therefore, the hypothesis of cis-regulation does not reflect full complexity and versatility of galectin-mediated cell–cell adhesion.
GALECTINS OF OTHER ANIMALS
Due to the limited published data, the comparison of galectin profiles in humans and other animals was out of scope of this review.
The specificity profiles established for avian and human galectins loaded onto cells were quite similar [39, 44]. However, there are some differences as well. For example, the glycan-binding profile of rodent galectins differs from that of their human orthologs; interestingly, the differences were observed in cellular but not in solid-phase assay (E. M. Rapoport, unpublished data). The available data suggest conservative rather than evolving specificity profile of animal galectins.
UNANSWERED QUESTIONS
As mentioned above, there are reasons to believe that galectins have “natural” receptors, i.e. glycoproteins or other glycoconjugates whose affinity, for unknown cause, is orders of magnitude higher than affinity of glycans as such. They may be, e.g. yet unidentified minor glycans. This hypothesis is indirectly supported by preference of avian tandem-repeat type galectins to ABH-glycans not found in birds. This may suggest that their natural receptors are related glycans, which have not been discovered yet. It should not be excluded that high-affinity ligands of galectins are not glycans, per se, but molecular patterns (microbe-associated (MAMP) or damage-associated molecular patterns (DAMP) [46, 47]), which contain glycans. This version is supported by several papers describing nonclassical interactions of galectins, in particular, (1) human tandem-repeat galectins bind to bacterial E. coli O86 polysaccharide related to blood group B antigenic determinant [48, 49], (2) tandem-repeat type galectins bind to rhamnopolysaccharide of E. coli 19ab, which contains no “classical” galectin-binding motifs like Galβ1-4GlcNAc [49]; (3) gal-3 interacts with β1,2-mannosides from Candida albicans [50]; (4) gal-3, -7, and -9 bind to partially desulfated glycosaminoglycans [51].
At the same time, there are many arguments against the existence of high-affinity ligands. In particular, the available information on the experiments with galectins (four studied proteins) loaded onto cells (seven studied cell lines) did not prove that the galectins might bind with endogenous glycans so strongly that it could prevent their detection by glycoprobes.
The question of localization of galectins within glycocalyx remains unanswered. It is not clear whether they are immersed into glycocalyx (that would be consistent with a mechanism of release from a cell) or are accumulated at its periphery (that would be expected for their adhesion function); whether they are distributed rather uniformly, concentrated in rafts, or deployed in a completely different manner. We do not have information about the dynamics of distribution of galectins on glycocalyx. Studies of galectin localization in glycocalyx are hampered because of limited information about 3D glycome of the glycocalyx and, consequently, distribution of galectin ligands. Confocal microscopy combined with an experimental model discussed here allows resolving these questions at least in part.
The authors express their sincere gratitude to Prof. H.-J. Gabius and S. Andre for providing galectins and to E. Y. Korchagina and G. V. Pazynina for their comments and fruitful discussion of the material.
This work was financially supported by the Russian Foundation for Basic Research (grants 07-04-00969 and 13-04-00096, E. M. Rapoport) and the Russian Science Foundation (project 14-50-00131, N. V. Bovin).
REFERENCES
1.Cooper, D. N. (2002) Galectinomics: finding themes
in complexity, Biochim. Biophys. Acta, 1572, 209-231.
2.Kaltner, H., and Gabius, H.-J. (2012) A toolbox of
lectins for translating the sugar code: the galectin network in
phylogenesis and tumors, Histol. Histopathol., 27,
397-416.
3.Hirabayashi, J., Hashidate, T., Arata, Y., Nishi,
N., Nakamura, T., Hirashima, M., Urashima, T., Oka, T., Futai, M.,
Muller, W. E. G., Yagi, F., and Kasai, K. (2002) Oligosaccharide
specificity of galectins: a search by frontal affinity chromatography,
Biochim. Biophys. Acta, 1572, 232-254.
4.Dumic, J., Dabelic, S., and Flogel, M. (2006)
Galectin-3: an open-ended story, Biochim. Biophys. Acta,
1760, 616-635.
5.Liu, Zh., Zhang, Q., Peng, H., and Zhang, W. Z.
(2012) Animal lectins: potential antitumor therapeutic targets in
apoptosis, Appl. Biochem. Biotechnol., 168, 629-637.
6.Di Lella, S., Sundblad, V., Cerliani, J. P.,
Guardia, C. M., Estrin, D. A., Vasta, G. R., and Rabinovich, G. A.
(2011) When galectins recognize glycans: from biochemistry to
physiology and back again, Biochemistry, 50, 7842-7857.
7.Viguier, M., Advedissian, T., Delacour, D.,
Poirier, F., and Deshayes, F. (2014) Galectins in epithelial functions,
Tissue Barriers, doi: 10.4161/tisb.29103.
8.Stowell, S. R., Dias-Baruffi, M., Penttila, L.,
Renkonen, O., Nyame, A. K., and Cummings, R. D. (2004) Human galectin-1
recognition of poly-N-acetyllactosamine and chimeric polysaccharides,
Glycobiology, 14, 157-167.
9.Leppanen, A., Stowell, S., Blixt, O., and Cummings,
R. D. (2005) Dimeric galectin-1 binds with high affinity to
α2,3-sialylated and non-sialylated terminal N-acetyllactosamine
units on surface-bound extended glycans, J. Biol. Chem.,
280, 5549-5562.
10.Wu, A. M., Wu, J. H., Singh, T., Andre, S.,
Kaltner, H., and Gabius, H.-J. (2004) Effects of polyvalency of
glycotopes and natural modifications of human blood group ABH/Lewis
sugars at the gal-beta1-terminated core saccharides on the binding of
domain-I of recombinant tandem-repeat-type galectin-4 from rat
gastrointestinal tract (G4-N), Biochimie, 86,
317-326.
11.Carlsson, S., Oberg, C. T., Carlsson, M. C.,
Sundin, A., Nilsson, U. J., Smith, D., Cummings, R. D., Almkvist, J.,
Karlsson, A., and Leffler, H. (2007) Affinity of galectin-8 and its
carbohydrate recognition domains for ligands in solution and at the
cell surface, Glycobiology, 17, 663-676.
12.Munoz, F. J., Santos, J. I., Arda, A., Andre, S.,
Gabius, H.-J., Sinisterra, J. V., Jimenez-Barbero, J., and Hernaiz, M.
J. (2010) Binding studies of adhesion/growth-regulatory galectins with
glycoconjugates monitored by surface plasmon resonance and NMR
spectroscopy, Org. Biomol. Chem., 28, 2986-2992.
13.Takeuchi, T., Tamura, M., Nishiyama, K., Iwaki,
J., Hirabayashi, J., Takahashi, H., Natsugari, H., Arata, Y., and
Kasai, K.-I. (2013) Mammalian galectins bind galactose β1–4
fucose disaccharide, a unique structural component of protostomial
N-type glycoproteins, Biochem. Biophys. Res. Commun.,
436, 509-513.
14.Brewer, C. F. (2004) Thermodynamic binding
studies of galectin-1, -3 and -7, Glycoconj. J., 19,
459-465.
15.CFG Functional Glycomix Gateway (http://www.functionalglycomics.org).
16.Stowell, S. R., Arthur, C. M., Mehta, P.,
Slanina, K. A., Blixt, O., Leffler, H., Smith, D. F., and Cummings, R.
D. (2008) Galectins-1, -2 and -3 exhibit differential recognition of
sialylated glycans and blood group antigens, J. Biol. Chem.,
283, 10109-10123.
17.Kopitz, J., von Reitzenstein, C.,
Burchert, M., Cantz, M., and Gabius, H.-J. (1998) Galectin-1
is a major receptor for ganglioside GM1, a product of the
growth-controlling activity of a cell surface ganglioside sialidase, on
human neuroblastoma cells in culture, J. Biol. Chem., 273,
11205-11211.
18.Elola, M. T., Chiesa, M. E., Alberti, A. F.,
Mordoh, J., and Fink, N. E. (2005) Galectin-1 receptors in different
cell types, J. Biomed. Sci., 12, 13-29.
19.Rapoport, E. M., Kurmyshkina, O. V., and Bovin,
N. V. (2008) Mammalian galectins: structure, carbohydrate specificity
and functions, Biochemistry (Moscow), 73, 483-497.
20.Gabius, H., and Wu, A. (2008) in Galectins
(Klyosov, A. A., Witczak, Z. J., and Platt, P., eds.) Wiley & Sons
Inc., New Jersey, USA, pp. 71-85.
21.Hauselmann, I., and Borsig, L. (2014) Altered
tumor-cells glycosylation promotes metastasis, Front. Oncol.,
doi: 10.3389/fonc.2014.00028.
22.Stillman, B. N., Hsu, D. K., Pang, M., Brewer, F.
C., Johnson, P., Liu, F.-T., and Baum, L. G. (2006) Galectin-3 and
galectin-1 bind distinct cell surface glycoprotein receptors to induce
T cell death, J. Immunol., 176, 778-789.
23.Cueni, L. N., and Detmar, M. (2009) Galectin-8
interacts with podoplanin and modulates lymphatic endothelial cell
functions, Exp. Cell Res., 315, 1715-1723.
24.Hsieh, S. H., Ying, N. W., Wu, M. H., Chiang, W.
F., Hsu, C. L., Wong, T. Y., Yin, Y. T., Hong, T. M., and Chen, Y. L.
(2008) Galectin-1, a novel ligand of neuropilin-1, activates VEGFR-2
signaling and modulates the migration of vascular endothelial cells,
Oncogene, 27, 3746-3753.
25.Wu, F. H., Yuan, Y., Li, D., Lei, Z., Song, C.
W., Liu, Y. Y., Li, B., Huang, B., Feng, Z. H., and Zhang, G. M.
(2010) Endothelial cell-expressed Tim-3 facilitates metastasis of
melanoma cells by activating the NF-kappa B pathway, Oncol. Rep.,
24, 693-699.
26.Cao, E., Zang, X., Ramagopal, U. A.,
Mukhopadhaya, A., Fedorov, A., Fedorov, E., Zencheck, W. D., Lary, J.
W., Cole, J. L., Deng, H., Xiao, H., Dilorenzo, T. P., Allison, J. P.,
Nathenson, S. G., and Almo, S. C. (2007) T cell immunoglobulin mucin-3
crystal structure reveals a galectin-9-independent ligand-binding
surface, Immunity, 26, 311-321.
27.Ideo, H., Seko, A., and Yamashita, K. (2005)
Galectin-4 binds to sulfated glycosphingolipids and carcinoembryonic
antigen in patches on the cell surface of human colon adenocarcinoma
cells, J. Biol. Chem., 280, 4730-4737.
28.Moiseeva, E. P., Williams, B., and Samani, N. J.
(2003) Galectin-1 interacts with beta-1 subunit of integrin,
Biochim. Biophys. Acta, 1619, 125-132.
29.Van den Brule, F., Calfice, S., Garnier, F.,
Fernandez, P. L., Berchuck, A., and Castronovo, V. (2003) Galectin-1
accumulation in the ovary carcinoma peritumoral stroma is induced by
ovary carcinoma cells and affects both cancer cell proliferation and
adhesion to laminin-1 and fibronectin, Lab. Invest., 83,
377-386.
30.Ahmad, N., Gabius, H.-J., Andre, S., Kaltner, H.,
Sabesan, S., Roy, R., Liu, B., Macaluso, F., and Brewer, C. F. (2004)
Galectin-3 precipitates as a pentamer with synthetic multivalent
carbohydrates and forms heterogeneous cross-linked complexes, J.
Biol. Chem., 279, 10841-10847.
31.Rapoport, E. M., Pazynina, G. V., Sablina, M. A.,
Kroker, P. R., and Bovin, N. V. (2006) Siglec interactions with
sulfated oligosaccharides, Biochemistry (Moscow),
71, 615-625.
32.Galanina, O. E., Tuzikov, A. B., Rapoport, E. M.,
Le Pendu, J., and Bovin, N. V. (1998) Carbohydrate-based probes for
detection of cellular lectins, Anal. Biochem., 265,
282-289.
33.Van Vliet, S. J., van Liempt, E., Saeland, E.,
Aarnoudse, C. A., Appelmelk, B., Irimura, T., Geijtenbeek, T. B. H.,
Blixt, O., Alvarez, R., van Die, I., and van Kooyk, Y. (2005)
Carbohydrate profiling reveals a distinctive role for the C-type lectin
MGL in the recognition of helminth parasites and tumor antigens by
dendritic cells, Int. Immunol., 17, 661-669.
34.Meyer, S., van Liempt, E., Imberty, A., van
Kooyk, Y., Geyer, H., Geyer, R., and van Die, I. (2005) DC-SIGN
mediates binding of dendritic cells to authentic pseudo-LewisY
glycolipids of Schistosoma mansoni cercariae, the first
parasite-specific ligand of DC-SIGN, J. Biol. Chem., 280,
37349-37359.
35.Hughes, R. C. (1999) Secretion of the galectin
family of mammalian carbohydrate-binding proteins, Biochim. Biophys.
Acta, 1473, 172-185.
36.Patnaik, S. K., Potvin, B., Carlsson, S., Sturm,
D., Leffler, H., and Stanley, P. (2006) Complex N-glycans are the major
ligands for galectin-1, -3 and -8 on Chinese hamster ovary cells,
Glycobiology, 16, 305-317.
37.Rapoport, E. M., Andre, S., Kurmyshkina, O. V.,
Pochechueva, T. V., Severov, V. V., Pazynina, G. V., Gabius, H.-J., and
Bovin, N. V. (2008) Galectin-loaded cells as platform for profiling of
lectin specificity by fluorescent neoglycoconjugates: case study on
galectins-1 and -3 and the impact of assay setting,
Glycobiology, 18, 315-324.
38.Vokhmyanina, O. A., Rapoport, E. M., Andre, S.,
Severov, V. V., Ryzhov, I. M., Pazynina, G. V., Korchagina, E. Ju.,
Gabius, H.-J., and Bovin, N. V. (2012) Comparative study of the glycan
specificities of cell-bound human tandem repeat-type galectins -4, -8
and -9, Glycobiology, 22, 1207-1217.
39.Vokhmyanina, O. A., Rapoport, E. M., Ryzhov, I.
M., Korchagina, E. Ju., Pazynina, G. V., Severov, V. V., Kaltner, G.,
Andre, S., Gabius, H.-J., and Bovin, N. V. (2011) Carbohydrate
specificity of chicken and human galectins-8 on cell surface,
Biochemistry (Moscow), 76, 1452-1460.
40.Bovin, N. V., Korchagina, E. Y., Zemlyanukhina,
T. V., Byramova, N. E., Galanina, O. E., Zemlyakov, A. E., Ivanov, A.
E., Zubov, V. P., and Mochalova, L. V. (1993) Synthesis of polymeric
neoglycoconjugates based on N-substituted polyacrylamides,
Glycoconj. J., 10, 142-151.
41.Rapoport, E. M., Kovalenko, E. I., Belyanchikov,
I. M., and Bovin, N. V. (2007) in Lectins: Analytical Technologies
(Nilsson, C. L., ed.) Elsevier B. V., San-Diego, USA, pp.
397-415.
42.Rapoport, E. M., Pochechueva, T. V., Kurmyshkina,
O. V., Pazynina, G. V., Severov, V. V., Gordeeva, E. A., Belyanchikov,
I. M., Andre, S., Gabius, H.-J., and Bovin, N. V. (2010) Solid-phase
assays for galectins specificity studies, Biochemistry
(Moscow), 75, 380-391.
43.Allen, H. J., Ahmed, H., and Matta, K. L. (1998)
Binding of synthetic sulfated ligands by human splenic galectin 1, a
beta-galactoside-binding lectin, Glycoconj. J., 15,
691-695.
44.Rapoport, E. M., Matveeva, V. K., Kaltner, H.,
Andre, S., Vokhmyanina, O. A., Pazynina, G. V., Severov, V. V., Ryzhov,
I. M., Korchagina, E. Ju., Belyanchikov, I. M., Gabius, H. J., and
Bovin, N. V. (2015) Comparative lectinology: delineating
glycan-specificity profiles of the chicken galectins using
neoglycoconjugates in a cell assay, Glycobiology, pii: cwv012
[Epub ahead of print].
45.Ahmad, N., Gabius, H.-J., Sabesan, S., Oscarson,
S., and Brewer, C. F. (2004) Thermodynamic binding studies of bivalent
oligosaccharides to galectin-1, galectin-3, and the carbohydrate
recognition domain of galectin-3, Glycobiology, 14,
817-825.
46.Vasta, G. R. (2012) Galectins as pattern
recognition receptors: structure, function, and evolution, Adv. Exp.
Med. Biol., 946, 21-36.
47.Sato, S., St-Pierre, Ch., Bhaumik, P., and
Nieminen, J. (2009) Galectins in innate immunity: dual functions of
host soluble β-galactoside-binding lectins as damage-associated
molecular patterns (DAMPS) and as receptors for pathogen-associated
molecular patterns, Immunol. Rev., 230, 172-187.
48.Stowell, S. R., Arthur, C. M., Dias-Baruffi, M.,
Rodrigues, L., Gourdine, J.-Ph., Heimburg-Molinaro, J., Ju, T.,
Molinaro, R. J., Rivera-Marrero, C., Xia, B., Smith, D. F., and
Cummings, R. D. (2010) Innate immune lectins kill bacteria expressing
blood group antigen, Nat. Med., 16, 295-301.
49.Knirel, Y. A., Gabius, H.-J., Blixt, O.,
Rapoport, E. M., Khasbiullina, N. R., Shilova, N. V., and Bovin, N. V.
(2014) Human tandem-repeat-type galectins bind bacterial non-βGal
polysaccharides, Glycoconj. J., 31, 7-12.
50.Fradin, C., Poulain, D., and Jouault, T. (2000)
b-1,2-Linked oligomannosides from Candida albicans bind to a
32-kilodalton macrophage membrane protein homologous to the mammalian
lectin galectin-3, Infect. Immun., 68, 4391-4398.
51.Iwaki, J., Minamisawa, T., Tateno, H., Kominami,
J., Suzuki, K., Nishi, N., Nakamura, N., and Hirabayashi, J. (2008)
Desulfated galactosaminoglycans are potential ligands for galectins:
evidence from frontal affinity chromatography, Biochem. Biophys.
Res. Commun., 373, 206-212.