REVIEW: Synthetic Glycolipid-Like Constructs as Tools for Glycobiology Research, Diagnostics, and as Potential Therapeutics
E. Y. Korchagina1 and S. M. Henry2*
1Shemyakin and Ovchinnikov Institute of Bioorganic Chemistry, Russian Academy of Sciences, 117997 Moscow, Russia; E-mail: korchagina@carb.ibch.ru2AUT Centre for KODE Technology Innovation, School of Engineering, Faculty of Design and Creative Technologies, Auckland University of Technology, Private Bag 92006, Auckland, 1142, New Zealand; E-mail: shenry@aut.ac.nz
* To whom correspondence should be addressed.
Received March 24, 2015; Revision received April 14, 2015
Function–spacer–lipid (FSL) constructs are amphiphilic molecules that are able to disperse in water and then self-assemble into cell membranes or onto solid surfaces. Modification of a biological or non-biological surface is very easy and achieved by simple contact of the surface with an appropriately buffered solution containing one or more FSLs. When the functional head group of the FSL is a glycan, glycan modified surfaces can be rapidly formed. Once cells, viruses, or solid surfaces are FSL modified with either simple or complex glycans, they can be used in vitro and/or in vivo to measure interactions with cells, viruses, antibodies, and lectins. FSLs have already been used in a variety of techniques including antibody specificity mapping, antibody/toxin neutralization, diagnostic assays, immune system manipulation, and animal modeling of transfusion reactions. FSLs offer the easiest and fastest method available to achieve a glycan-modified surface.
KEY WORDS: function–spacer–lipid, KODE technology, kodecyte, neoglycolipidDOI: 10.1134/S0006297915070068
Abbreviations: CMG, carboxymethylglycine spacer; DOPE, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine; F, functional head group of an FSL construct; FSL, function–spacer–lipid constructs; HA, hyaluronic acid; L, lipid tail of an FSL construct; NGL, neoglycolipid; S, spacer group of an FSL construct. Glycan abbreviations are as per Table 1.
Glycans at the cell surface being associated with the membrane exist in
essentially three forms, i.e. surface-associated non-anchored
glycoconjugates, immobile membrane-anchored glycans such as
glycoproteins, and mobile membrane-bound glycans such as glycolipids
and glycophosphatidylinositol (GPI)-linked proteins. Some glycans
associated with a cell membrane can be simultaneously present in all
three different forms; for example, blood group glycans can be present
at the cell membrane as surface-adhered mucin and present in the
membrane as both glycoproteins and glycolipids. Although all these
different types of cell-associated glycans are potentially biologically
important, and each can have different functions [1, 2], this review will only focus
on the synthesis and biological activity of mobile membrane-bound
glycans in the form of synthetic glycolipid-like constructs.
The lipid tail of the natural glycolipid facilitates association of the glycan with the extracellular membrane, with the lipid tail being inserted into, and anchoring, the glycan in the plasma membrane [3, 4]. Once in the membrane, the lipid tail allows the glycan to either distribute freely and homogenously, or to cluster and associate with other membrane components [5, 6]. However, the lipid membrane association of a glycolipid is not necessarily permanent, and glycolipids can be lost to the extracellular environment (namely into plasma) or, conversely, they can be acquired from extracellular fluids into plasma membranes [3, 4]. It was the “free-spirit” nature of glycolipids that made them an ideal template to design synthetic glycolipid-like constructs for modifying cells with glycans (and other non-glycan functional moieties). Several techniques are now in use to prepare synthetic glycolipids (neoglycolipids). One of the methods to generate neoglycolipids (NGLs) is conjugation of reducing oligosaccharides to aminophospholipids by reductive amination [7, 8]. This methodology involves ring opening of the reducing-end monosaccharide and can affect the biological activities of the glycan. Another approach is oxime ligation whereby the aldehyde group of a reducing sugar is conjugated to the aminooxy group of functionalized lipid without reduction [9]. These neoglycolipids usually have both ring-opened and ring-closed forms of reducing monosaccharide, which result in indeterminacy of NGL structure and chemical instability to hydrolysis. Nevertheless, such NGLs are applied in microarray systems to identify novel glycoligands of biological relevance [8, 9]. Synthetic analogs of natural glycosphingolipids were obtained by multistep total chemical synthesis and used to study carbohydrate–carbohydrate and carbohydrate–protein interactions to elucidate such biological events as cell adhesion, bacterial and viral infection, inflammatory response, etc. [10, 11]. The main drawback of these synthetic glycosphingolipids is the complex procedure to get the glycosyl-ceramide bond. Synthesis of glycolipid-like molecules with sterol, double-chain, or higher alcohol aglycons was also reported [12-14]. However, these NGL constructs were often not easily soluble in saline, and often required the support of solvents such as methanol and/or other lipids for their solubilization or insertion into membranes [12].
To overcome the above-mentioned limitations, we adopted an approach that was to create water-dispersible glycolipid-like constructs by attaching glycans to lipids via spacers that facilitated water dispersibility, to create so-called function–spacer–lipid (FSL) constructs [15]. Furthermore, the design of FSLs was constrained by other criteria (to enable in vivo use) including that the constructs must be able to spontaneously and stably insert into cell membranes, and the spacer and lipid per se must be biologically inert [15]. Serendipitously, it was later found that these FSLs were also able to spontaneously self-assemble in a few seconds on almost any non-biological surface including metals, plastics, and paper to form a glycan coating, which is stable (not washed off) in plasma and buffer solutions [16].
SYNTHETIC GLYCOLIPID-LIKE CONSTRUCTS (FSLs)
FSL structure. The function–spacer–lipid (FSL) construct, as implicit in the name, consists of three components – a functional head group, a spacer, and a lipid tail. Although the functional head is critical to the functionality of the construct, the spacer and lipids are equally important as they facilitate the attachment of the construct to a membrane or surface. Additionally, because the spacer and lipid components can be individually engineered, multiple variations can be created, each with the potential to influence bioactivity by effecting presentation, orientation, location, and adhesion properties to surfaces for each functional head.
Functional head. The functional head group is usually the bioactive component of the FSL construct and can be almost anything [17], but typically needs to be hydrophilic to allow the FSL to be amphiphilic, which in turn drives the self-assembling and spontaneous membrane insertion or surface coating processes. Functional groups that have been incorporated in FSL constructs include glycans, peptides, proteins, labels, fluorophores, metals, enzyme substrates, chelators, oligonucleotides, and reactive functional groups, but in the context of this review only glycans will be discussed. Glycans ranging from monosaccharides and up to about 100 residues have been created as FSL constructs [15-19] (Table 1). Although the limit on the size of the glycan has yet to be determined, if the functional head group becomes too large it has the potential to disrupt the insertion/self-assembly process. To avoid this issue, with larger functional heads a two-stage approach can be used, where the FSL functional head group is a non-glycan reactive functional group that is able to react secondarily with a suitably modified glycan. Typically, this would involve the use of an FSL with biotin as the functional head, which could then be used to capture a biotinylated glycan via an avidin bridge [15]. However, a variety of alternative approaches can be used, for example, FSLs with reactive functional groups such as maleimide, click chemistry, enzyme–substrate interactions, or chelators where appropriate secondarily labeled glycans are available.
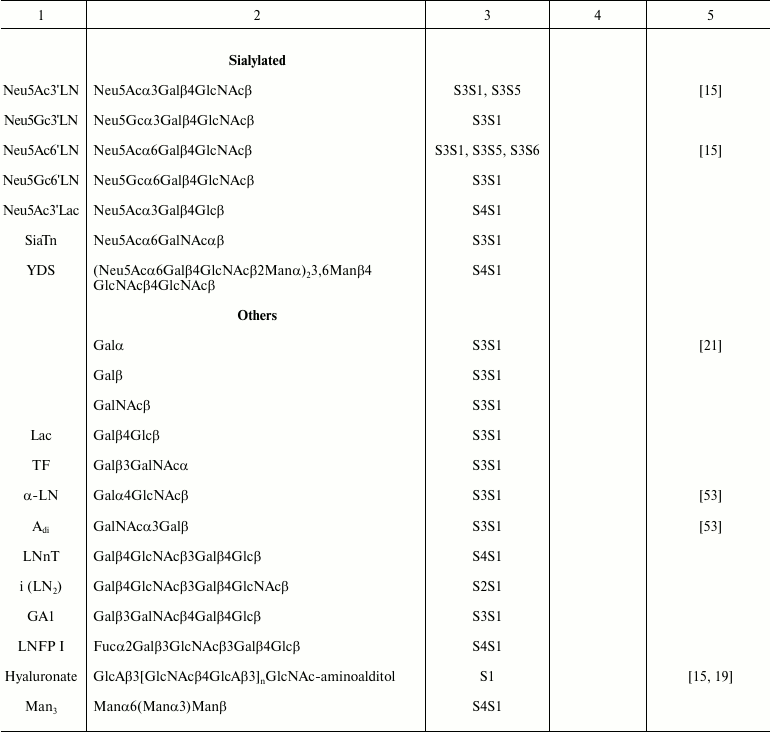
Table 1. Specific glycans synthesized as
FSL construct variations
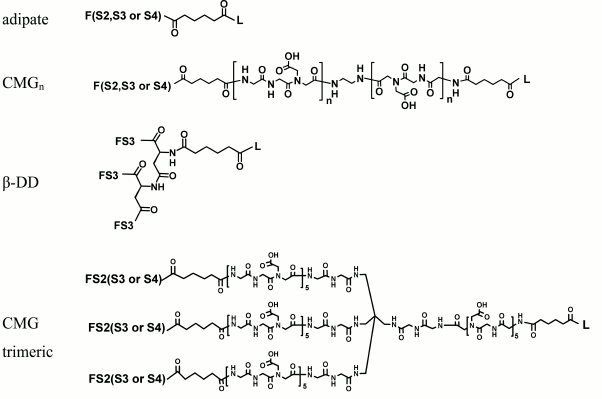
Spacer. The spacer is the core component of the FSL construct and also differentiates FSL constructs from natural glycolipids. Although FSL constructs can be considered as synthetic glycolipid-like constructs, the presence of a spacer brings additional and novel features to the glycan that are not necessarily present with natural glycolipids, including in some circumstances the ability to effect and modify biological activity. A primary feature of the spacer is to facilitate the chemistry to conjugate the lipid tail to the functional head, but it also ensures water dispersibility and spacing the glycan away from the membrane/surface, thereby reducing steric hindrance and increasing both sensitivity and specificity. To date, a variety of simple and complex spacers has been created (Fig. 1) including elongated, branched, and/or functionalized spacers to produce alternative or additional biological effects. Longer and multivalent spacers have the potential to improve sensitivity of glycans at the cell surface (unpublished observations), and as spacing of the glycans on multivalent structures can be controlled to some extent, optimization of ligand binding.
Fig. 1. Structural variations of spacers used to make FSL constructs. The possible functional head groups relate to each spacer (Table 1) and lipid tails (Fig. 2) are simply designated by letters F and L, respectively. See also Table 2 for spacer and pre-spacer IDs. The upper-most spacer is adipate, which gives (together with the glycans pre-spacer) a short 1.9 nm spacing of the glycan from the cell membrane. CMGn is representative of linear spacers based on partially carboxymethylated oligoglycine, which can be synthesized in longer lengths but is primarily used with a length of 7.2 nm (CMG2) in FSL construction (see further Fig. 3). The β-DD-cluster contains three glycan functional heads connected to a single lipid tail via a short (about 2 nm) spacer. The CMG trimeric spacer used to make FSL peptide constructs has not yet been utilized for glycans, although it is suitable for this.
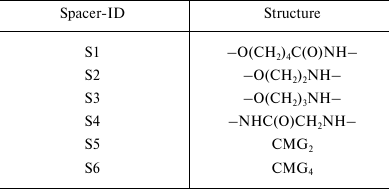
Table 2. Spacers incorporated in FSL
constructs. The different types of spacers used for specific FSL
constructs synthesized are identified by spacer-IDs and relate to
Table 1
A desirable feature to the design of the spacer is that it is biologically inert and does not react with undiluted serum, stimulate the immune system (including toll-like receptors and complement system), cause toxicity, or produce any apparent positive or negative biological effect [20-22]. To date, no undesired effects have been observed from the spacers used (Fig. 1) in both in vitro assays and in vivo testing of the constructs [20-22].
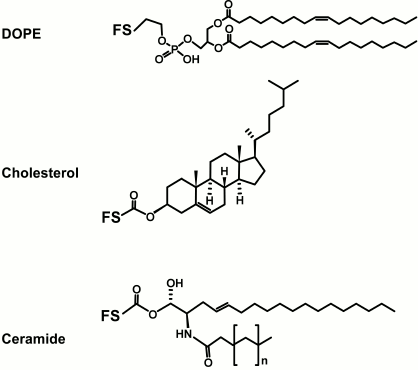
Lipid. The lipid tail of the natural glycolipid is known to anchor the glycan to the membrane surface [4], and for FSL constructs, the lipid tail can also undertake this same function. However, the lipid tail of the FSL is not necessarily an anchor on non-lipid surfaces. Instead, the lipid imparts on the FSL an amphiphilic character (which is also influenced by the spacer), and together with the hydrophilic functional head group, allows the FSL construct to disperse in water and spontaneously self-assemble onto or into surfaces. A variety of lipids have been used to create FSL constructs (Fig. 2), including 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE, L1; Table 1), cholesterol (L2; Table 1), and natural and synthetic ceramides. Other lipids such as 1,2-O-distearyl-sn-glycero-3-phosphatidylethanolamine (DSPE), rac-1,2-dioleoylglycerol (DOG), and monoacyl-octadecanoic acid have been evaluated but are not routinely used as they either gave no advantage or were inferior to DOPE with regards to ability to allow for rapid dispersion in water and spontaneously self-assembly onto or into surfaces.
Fig. 2. Structural variations of lipids commonly used to make FSL constructs. The possible functional head groups related to each spacer (Table 1) and spacers (Fig. 1) are simply designated by letters F and S, respectively. The ceramide lipid used to make FSL biotin constructs has not yet been utilized for glycans, although it is suitable for this.
Although the primary function of the lipid is to make the FSL construct amphiphilic, different lipids can influence FSL coating of a surface or its biological effect. In general, FSLs with ceramide and DOPE lipid tails are the best for insertion and membrane retention (unpublished observations). However, different lipid types can partition differently within a cell membrane, and it has been suggested that this is the basis for why FSL-Gb3 is able to prevent HIV infection of peripheral blood mononuclear cells [20].
SYNTHESIS OF GLYCOLIPID-LIKE CONSTRUCTS (FSLs)
Construction of FSLs is a complex procedure that includes synthesis of oligosaccharides (F), synthesis of appropriate spacers (S), and several approaches for conjugation of spacers (S) to lipids (L) and then spacer–lipids (SL) to functional groups (that can also have been pre-spacered to facilitate conjugation). Full descriptions of the preparation of FSLs with different spacers are given elsewhere [15]. Here we briefly overview the general steps.
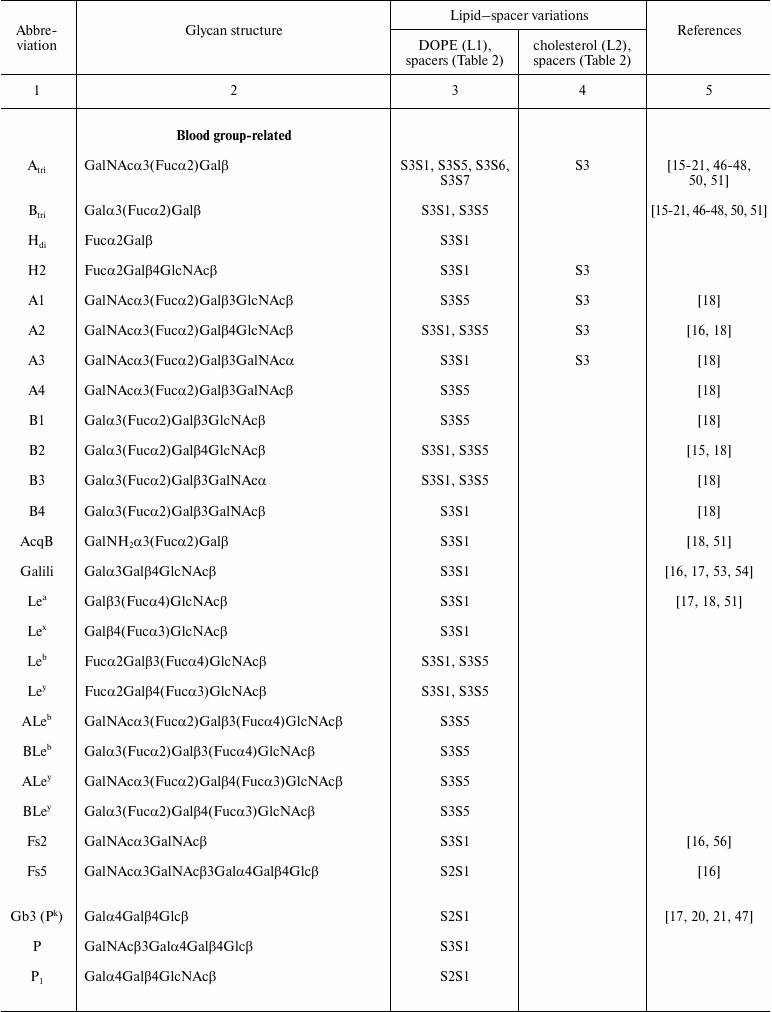
Glycans. All functional glycans (except hyaluronic acid) for FSL synthesis (Table 1) have pre-spacers (S2, S3, or S4; Table 2) with a terminal NH2-group to enable further modification and to allow for unification, to some extent, of FSL preparation. 2-Aminoethyl (S2) and 3-aminopropyl (S3) glycosides of glycans from Table 1 were obtained as reported [23-38]. To obtain N-glycyl glycosides (S4) from natural or synthetic reducing oligosaccharides (OS), we followed the methodology described in [39]. Reducing OS were transformed to glycosylamine-1-N-glycyl derivatives by sequential action of NH4HCO3, chloroacetic anhydride, and aqueous NH3 (Scheme 1).
Scheme 1. Synthesis of N-glycyl glycosides
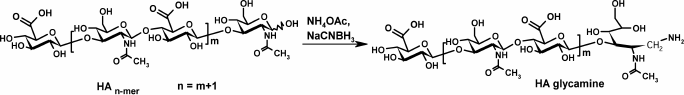
Hyaluronic acid (HA) oligomers (8-15 kDa) were converted to HA-glycamines by reductive amination with sodium cyanoborohydride and ammonium acetate (Scheme 2) [19].
Scheme 2. Synthesis of HA-glycamines
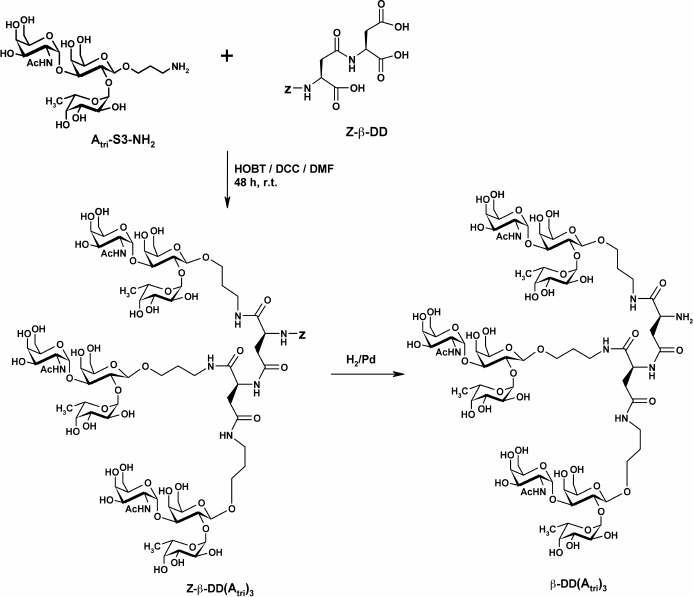
To study the effect of polyvalent ligand on the properties of FSLs, the Atri cluster (FSL-β-DD(Atri)3) was synthesized by attaching a 3-aminopropyl glycoside to each of the three carboxylic groups of β-L-aspartyl-L-aspartic acid (β-DD) following the methodology of [40] (Scheme 3).
Scheme 3. Synthesis of β-DD(Atri)3 cluster
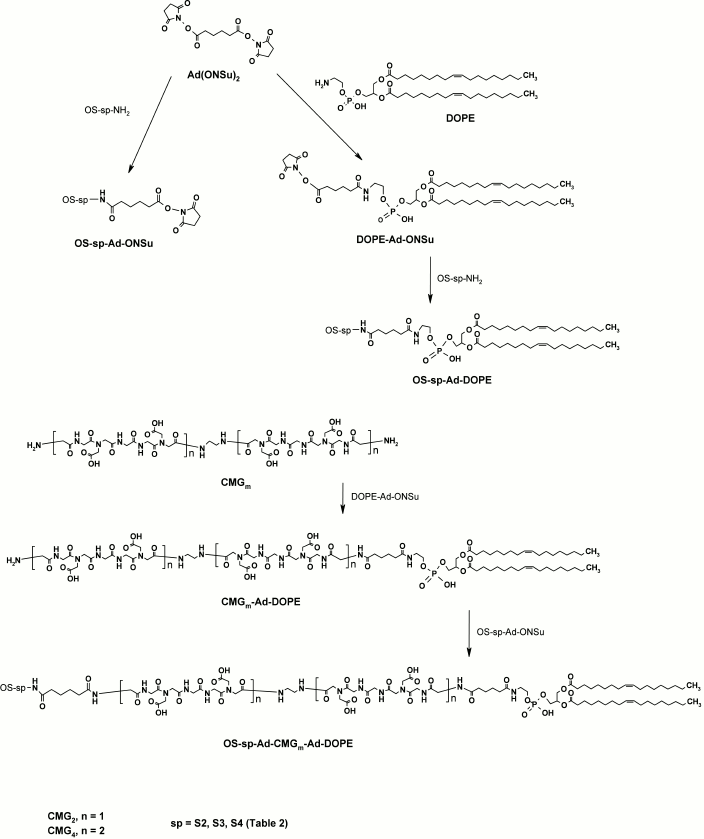
Spacer–lipids and FSL constructs. The general procedure for the synthesis of all FSL constructs is presented in Scheme 4. First, an activated spacer–lipid derivative of 1,2-di-O-oleoyl-sn-glycero-3-phosphoethanolamine (DOPE-Ad-ONSu) was obtained by conjugation of DOPE with bis(N-hydroxysuccinimidyl)adipate. This was used for coupling with amino-terminated glycans (OS-sp-NH2) to give FSLs with a short (adipate) spacer. FSL constructs with the polyvalent β-DD(Atri)3 cluster were obtained analogously. To prepare the FSL constructs based on the carboxymethylglycine spacer (Glyc-Ad-CMGn-DOPE, n = 2 or 4), we used a different approach. Coupling of CMG diamino-derivative with DOPE-Ad-ONSu resulted in amino-terminated CMGn-DOPE, which was subsequently condensed with activated glycans (OS-sp-Ad-ONSu) (Scheme 4).
Scheme 4. General procedure for synthesis of FSL constructs
SURFACE MODIFICATION WITH FSLs
To date, a range of FSLs has been prepared (Table 1) and some with a variety of spacer–lipid variations (Fig. 3). These FSLs once synthesized and freeze-dried are generally amorphous white powders that will dissolve in aqueous buffers and water. These constructs are generally stable and can be stored dry at room temperature in a normal atmosphere for many years without evidence of degradation. The FSLs are also stable when stored in solutions of buffers, and these constructs have been stored in sterile phosphate buffered saline for more than six years at 4°C with no evidence of degradation. However, despite their stability in buffers, FSL constructs will begin to degrade in deionized water, probably due to local pH changes in micelles leading to hydrolysis of the lipid ester bond. Overall, the FSL constructs will disperse as clear solutions at concentrations <50 mg/ml (although they will gel at higher concentrations) and can be used to create biologically active glycan modified cells/surfaces with concentrations as low as 5 µg/ml. Typical working concentrations of FSLs in solution are in the range of 10-100 µg/ml, but the actual concentration for any particular glycan, or purpose, needs to be experimentally determined.
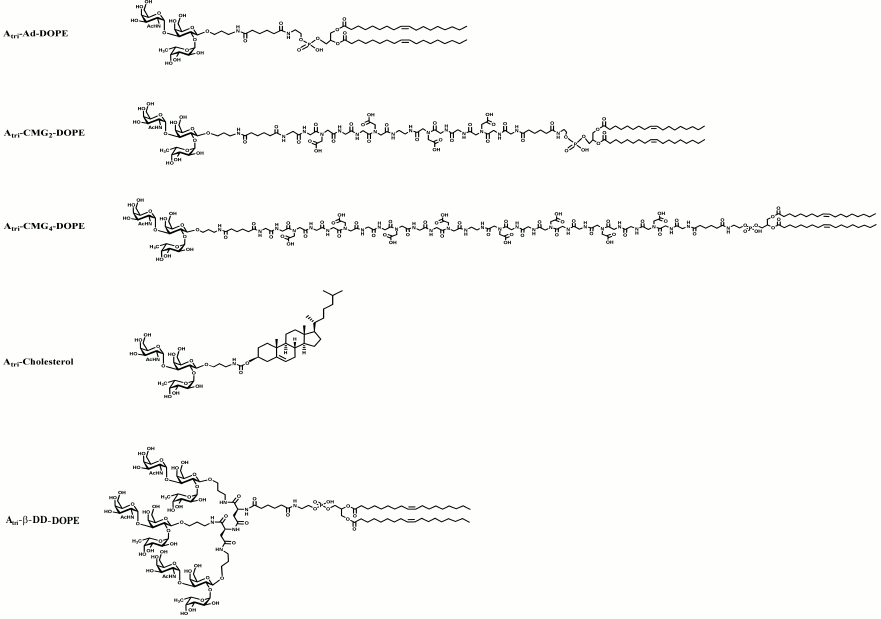
Fig. 3. Examples of variant blood group A trisaccharide FSL constructs that have been used in biological assays. The uppermost FSL construct designated Atri–Ad–DOPE represents blood group Atri on the short adipate spacer with a DOPE lipid tail. The next two FSL constructs (Atri–CMG2–DOPE and Atri–CMG4–DOPE) also based on DOPE lipid tail have two different length variations of the CMG spacers. The Atri–cholesterol construct utilizes cholesterol as its lipid moiety, and although less effective than DOPE-based constructs is still able to modify plasma membranes and materials surfaces. The final FSL construct, Atri–β-DD–DOPE, represents a short-spacer trimeric presentation of the A trisaccharide, which could alternatively be synthesized (not shown) with a CMG trimeric spacer (Fig. 1). In all instances, it is possible to replace Atri shown here with an alternative glycan such as those in Table 1.
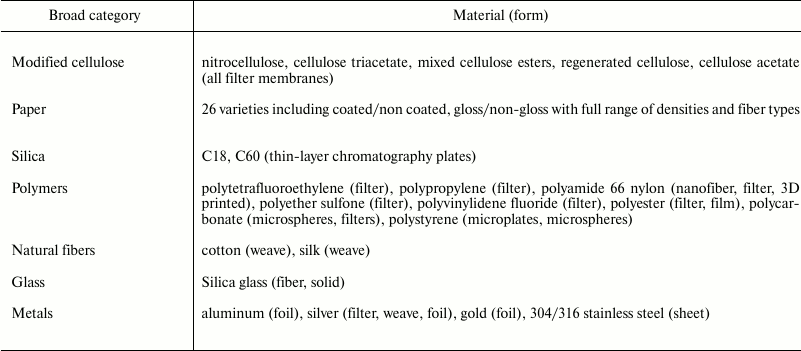
FSL constructs are able to modify most biological membranes (cells, viruses, bacteria, and whole organisms), liposomes, and non-biological surfaces (plastic, metal, rubber, paper, etc.) including hydrophobic and hydrophilic surfaces (Tables 3 and 4). To date, only a few surfaces with significant charged residues or those that are heavily glycosylated (such as on yeast) cannot be efficiently coated. Although it has been shown that coating of hydrophilic surfaces occurs as easily as the coating of hydrophobic surfaces, the mechanisms of action for FSL adherence to surfaces are not yet clearly established. It appears that FSL adhesion occurs via multiple mechanisms with all being essentially driven by the need to “exclude water” via layering, entrapment, encapsulation, and utilizing interactions including hydrogen bonding, van der Waals forces, electrostatic and ionic interactions, and combinations of all the above [41-45]. The role of the spacer in facilitating interaction with surfaces is probably minimal as there are no notable differences in adhesion of constructs with CMG (charged or uncharged) and adipate spacers.
Table 3. Published biologic uses of FSL
constructs
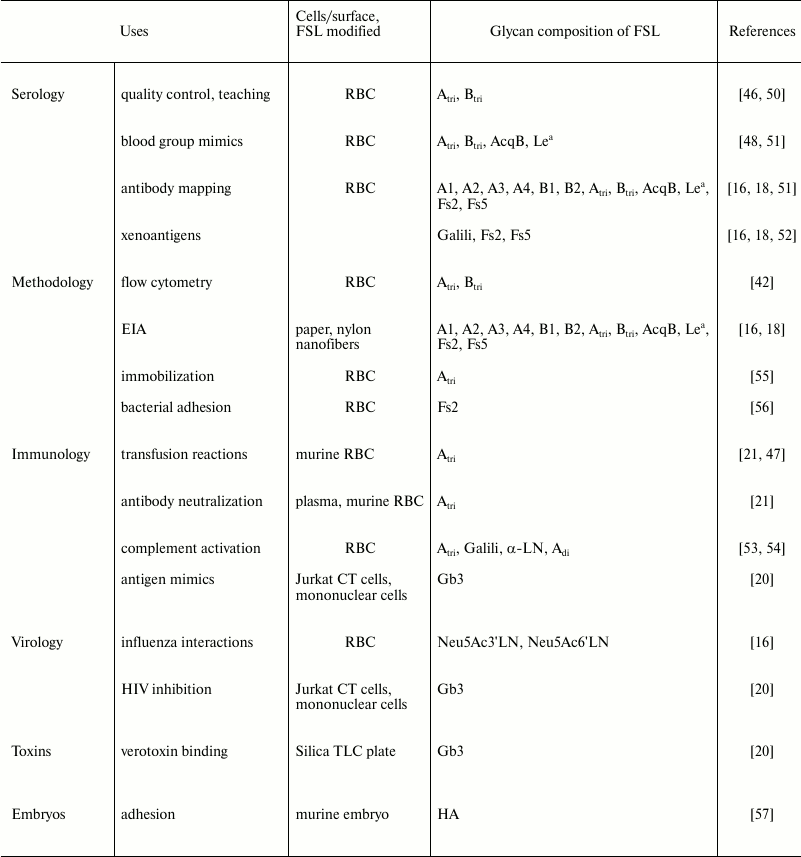
Note: RBC, red blood cells.
Table 4. Non-biological surfaces
successfully coated with FSL constructs.
The expected mechanism of coating lipid membranes of cells, viruses, or liposomes is via insertion of the FSL lipid tail into the lipid membrane. The mechanism of lipid tail integration into membrane is more complex compared to self-assembling of FSLs on non-biological surfaces. This is probably due to the relative stability of FSL micelles, which with its hydrophilic outer layer first needs to associate with glycocalyx of plasma membranes, and then to disassemble allowing the lipid tail of the FSL to integrate into the hydrophobic plasma membrane. This process takes significantly longer (up to an hour) than in case of non-biological surfaces (which usually takes from a few seconds to minutes).
Provided the concentrations of FSL in solution, ratios of cells, or surface area to FSL construct solution and experimental contact conditions (time, temperature, buffers, FSL mixture ratios, cell suspension concentrations, etc.) are kept constant, the process of FSL modification is extremely robust and controllable, with highly reproducible results being able to be produced for the same FSL construct at different times or locations [46]. The variation between the levels of surface coating prepared under controlled conditions is estimated to be less than 5% when prepared on different occasions. In general, most related FSL constructs will insert similarly, but because the nature of micelles will be determined in part by the combination of functional group, spacer, and lipid together with temperature, concentration, size, and hydrophobicity/hydrophilicity, subtle differences in ability to modify a surface can exist between different FSLs or when using FSL mixtures. FSL coating of biological surfaces generally requires incubation of at least 30 min, while non-biological surfaces will coat within seconds. Continued exposure to the FSL construct can increase the amount of FSL on a surface, and on non-biological surfaces FSL layering can occur. Once an FSL coating has formed, regardless of whether it is allowed to dry or not, the FSL is relatively resistant to removal by water, mild detergents, serum lipids, and to some extent solvents such as methanol. However, the choice of solvent (e.g. water or methanol) used to deliver the FSL to the surface is very important as it will affect the way the FSL self-assembles on different surfaces, and it can affect its subsequent resistance to removal with serum, water, or solvent. Although further research is required to resolve the various mechanisms of action, and the consequent limitations related to different FSLs, from a macroscopic perspective all surfaces appear to label similarly with FSLs.
In order to distinguish FSL construct modification of a surface from other forms of modification (which can also be used in conjugation with FSLs) and to accurately but simply describe the multiple variations of FSL-modified cells, viruses, and surfaces, specific terminology has been adopted [17]. The use of FSLs to modify surfaces has been termed KODE technology [17, 18, 47, 48], while FSL construct-modified cells are known as kodecytes [17, 18, 47, 48] and viruses as kodevirions [19, 49]. Surfaces modified with FSL constructs are simply termed koded.
Biological surface modification by FSLs. FSL constructs have been used to create novel glycan modifications of many different types of living cells, including blood cells, cell culture lines, embryos, spermatozoa (Table 3 and Fig. 4), and on a variety of other cells including stem cells (unpublished observations). In addition to modifying a variety of mammalian cells, other cells such as prokaryotic bacteria (unpublished) and viruses [49] have similarly been modified with FSL constructs. The process of labeling live cells does not appear to cause any apparent harm to the modified cell (provided the insertion conditions are mild, that is, with physiological buffers and FSL concentrations <500 µg/ml), with the resultant kodecytes having normal functionality and vitality, but also a modified biologically active glycan surface. In addition to the novel glycan(s) added to the kodecyte, it is usual to also simultaneously add a second FSL in the form of label (such as a fluorophore, 125I, or biotin) to enable visualization and/or recovery of the kodecyte from an in vivo/in vitro environment [21, 47, 49].
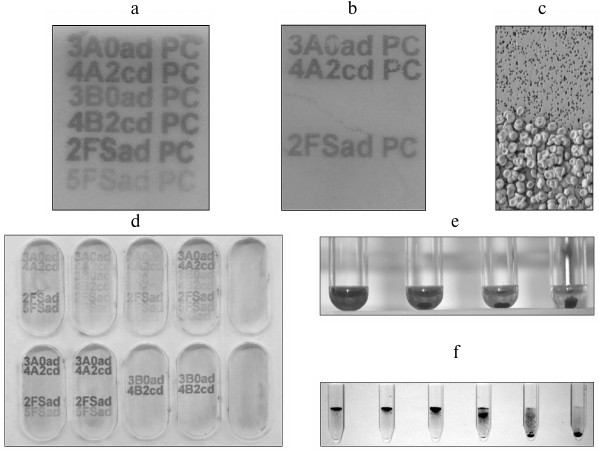
Fig. 4. Examples illustrating some uses for FSL constructs. a) EIA mapping of anti-glycan antibodies. In this example, six different ABO-related FSL constructs are printed with an inkjet printer onto polycarbonate and used to map the specificity of a monoclonal anti-AB reagent. Activity is determined by the appearance of alphanumeric characters following EIA development (details as [16, 18]). The polycarbonate surface used in this example can be replaced with most other surfaces listed in Table 4, with similar results. b) Capture of blood group A red cells, via anti-A, onto blood group A reactive FSL constructs. This plate is prepared identically to the EIA plate (panel (a)), except this time, visualization of activity is via the capture of blood group A red cells instead of EIA. This antibody cell-capture variation is useful, as some EIA substrates may not adhere to all surfaces, or the method could be used instead to capture specific cells. c) Scanning electron microscope magnification of red cells captured by antibodies reacting with FSLs. This image is a 500× enlargement of an area on panel (b). The upper region where no cells are bound is unprinted, while the lower region is FSL printed and therefore able to specifically capture blood group A red cells via interaction with anti-A bound to the printed FSL. d) EIA of glycan mapping [16, 18] the specificity of different polyclonal serum samples against FSL-glycans that had been inkjet printed onto paper. Each well represents a different serum sample, and the characters that have become visible identify the antibody specificities present in that sample. Some semiquantitative variance in results can also be seen. e) Controlling antibody-complement mediated hemolysis with variable levels of FSL constructs in kodecytes. In this example, reducing levels of FSL-Galili (Table 1) were used to make human red cell Galili-kodecytes, and then used to induce antibody–complement-mediated hemolysis. Hemolysis can be controlled from 100% (left most reaction) through to 0% by altering the level of FSL in the kodecytes. f) Determining antibody levels with standardized kodecytes. In this example, Galili-kodecytes were made with a single level of FSL-Galili and human red cells, and then used to determine the titer of anti-Galili in polyclonal serum samples. This figure (contrast enhanced) shows kodecyte agglutinates trapped in a gel matrix (gel card technology) relative to the serial dilution of the antibody. The left-most reaction is a strong positive (undiluted serum), while the reaction on the right is negative (1 : 32 dilution), thus indicating an IgM anti-Galili titer of 16. Anti-IgG gel cards can be additionally used to determine IgG titers.
The in vitro process of labeling cells is the same for all cells; simple incubation of cells with FSLs dispersed in lipid-free media (e.g. saline) for a period ranging from 30-120 min at 37°C [15-19, 21, 22, 46-48, 50, 51]. However, the temperature range of insertion into plasma membranes can be varied between 4-37°C, although more time is required for insertion at lower temperatures. As a rule, 1-2 h at 37°C is equivalent to 4-6 h at 22°C, which is equivalent to 18-24 h at 4°C. The usual concentration of cells is up to 100% mixed in a 1 : 1 ratio with FSL constructs. The concentration of the FSL solution determines the amount of FSL inserted into the cell membrane for a given period of time. If the FSL concentration is relatively low (<100 µg/ml), after about 1 h at 37°C >95% of the FSLs in solution will have inserted into the cell membrane (of a >70% cell suspension). Higher concentrations of FSL (up to 1000 µg/ml) in contact with cells for lesser times can achieve the same level of membrane modification, but unutilized FSL remaining in solution will need to be washed away to stop the insertion process. Once modified with FSLs, kodecytes can be reintroduced to media containing serum or other lipids.
Unlike genetic engineering approaches, FSL modification of cells is temporary, with the rate of FSL loss determined by activity of the membrane, such as endocytosis and cell division, and the environment and temperature in which the cells are held. However, inactive cells (e.g. red cells/fixed cells [17]) will retain FSL constructs indefinitely when stored in lipid-free media. When kodecytes are in the presence of serum lipids, their FSLs will slowly exchange into the plasma at a rate of about 1% per hour at 37°C (but not detectably reinsert into other cells) [21, 47]. Depending on cell type, in general, after 24 h the amount of FSL present in the kodecyte membrane will have reduced significantly, and within 3 days will be undetectable. There is no evidence that cells that were once kodecytes will not survive normally once the FSLs have been lost from their membranes [21, 22].
Kodecytes can also be formed in vivo with infusions of large doses of FSL constructs [21, 22]. In vivo modification of circulating cells is untargeted, with all circulating cells becoming F-moiety positive and requires significantly larger amount of material (about 40 times more than if in saline) as circulating plasma lipids preferentially acquire the constructs [21].
Non-biological surface modification with FSLs. The ability to easily modify non-biological surfaces with glycans creates opportunities within solid phase assays, for example, enzyme-linked immunoassays (EIA), biosensors, microarray screening, microsphere technologies, surface plasmon resonance, etc. The process of modification of non-biological surfaces with FSL constructs only involves contact of a surface with a solution containing one or more FSLs [16-18]. Methods for applying an FSL to a non-biological surface include immersion, flooding, spraying, painting, or inkjet printing [16-18]. Upon contact with a micellar surface, FSLs will rapidly spontaneously self-assemble onto or into the surface (seconds) by a variety of mechanisms [41-45]. The most important consideration is the nature of the surface being modified, but as very few surfaces are homogenous, multiple different factors (e.g. hydrophilicity, charge, shape, absorbency area, etc.) and combinations thereof, and the choice of FSL solvent, will all affect the mechanisms of action of how and where the FSL is able to bind and assemble onto the surface and its subsequent resistance to removal.
A large variety of dissimilar surfaces has all been successfully koded with FSLs (Table 4 and Fig. 4). A note of caution, when studying FSL interactions with surfaces the detection system used to observe the FSL coating must also be able to remain at the surface – for example, EIA enzyme precipitates may not necessarily adhere to the surface despite the presence of a significant FSL layer. Additionally, because FSL constructs will within seconds adhere to any non-biological surface they come in contact with, including glass, plastics, metal, etc. (regardless of whether they are rough or smooth), they will be easily contaminated with an FSL coating, and small-to-negligible amounts of FSL construct will also being lost during experimental handling (depending on surface area and time of exposure).
Once applied, koded surfaces are relatively resistant to FSL removal by subsequent exposure to buffers and serum lipids [16, 18], with serum/plasma at 37°C having only negligible effect after 30 h of in vitro contact. FSL-modified surfaces have been used to determine interactions with antibodies (agglutination, aggregation, EIA) [16, 18] and for capturing cells; however, despite the apparent simplicity of creating a modified surface, experimental conditions need to be optimized and standardized to create robust and reproducible results.
FSL constructs where F is glycan have been used in a variety of applications (Table 3) and are very suitable tools for glycobiology research. FSL constructs with either simple or complex glycans can be easily attached to a cell, virus, or solid surfaces and then used in vitro and/or in vivo to measure interactions with cells, viruses, antibodies, and lectins. Because FSL constructs per se are biologically inert and the modification process harmless, consequences observed with modified cells/viruses are more likely to be due to the glycan introduced by the FSL, rather than the process of the modification, which with other techniques can affect vitality and/or functionality. The ability to use exactly the same FSL constructs to modify biological and non-biological surfaces is useful in that in vitro assays can be developed to closely mimic or monitor the biological consequences of in vivo modifications. Furthermore, as the technique is robust and controllable (with the same results each time if identical parameters are used), and it allows for multiple constructs to be simultaneously added to the same surface including non-glycans, and it can be used additively with alternative existing modification techniques, FSLs have the potential to extend and enhance experimental observations. However, FSL construct modification has some limitations, primarily being that the modification of cells is temporary, with the labeling decreasing over time due to loss to the environment and/or dilution/consumption from active membranes. All the same, if a modification of a cell/virus surface with novel glycan(s) is desired, and its effect can be observed in <24 h, FSL modification is probably the easiest and most controllable method available to achieve a modified glycan surface (when glycans are readily available).
The number of applications and uses for this technology is steadily expanding, and today FSLs have been used in techniques including quality control, antibody mapping, antibody/toxin neutralization, diagnostic assays, immune modification, and in vivo animal modeling, thus making it an extremely versatile tool for glycobiology research.
The authors are grateful to N. V. Bovin, E. C. Williams, K. Barr, D. A. Blake, E. H. Perry, I. M. Belyanchikov, and A. B. Tuzikov for their help in preparing this article.
This work was supported by the Russian Science Foundation (project No. 14-50-00131).
REFERENCES
1.Varki, A., Cummings, R. D., Esko, J. D., Freeze, H.
H., Stanley, P., Bertozzi, C. R., Hart, G. W., and Etzler, M. E. (2009)
Essentials of Glycobiology, 2nd Edn., Cold Spring Harbor
Laboratory Press, N.Y.
2.Tailor, M. E., and Drickamer, K. (2006)
Introduction to Glycobiology, Oxford University Press, N.Y.
3.Marcus, D. M., and Cass, L. E. (1969)
Glycosphingolipids with Lewis blood group activity: uptake by human
erythrocytes, Science, 164, 553-555.
4.Sneath, J. S., and Sneath, P. H. A. (1955)
Transformation of the Lewis groups of human red cells, Nature,
176, 172.
5.Simons, K., and Ikonen, E. (1997) Functional rafts
in cell membranes, Nature, 387, 569-572.
6.Simons, K., and Ikonen, E. (2000) How cells handle
cholesterol, Science, 290, 1721-1726.
7.Tang, P. W., Gool, H. C., Hardy, M., Lee, Y.
C., and Feizi, T. (1985) Novel approach to the study of the
antigenicities and receptor functions of carbohydrate chains of
glycoproteins, Biochem. Biophys. Res. Commun., 132,
474-480.
8.Palma, A. S., Feizi, T., Childs, R. A., Chai, W.,
and Liu, Y. (2014) The neoglycolipid (NGL)-based oligosaccharide
microarray system poised to decipher the meta-glycome, Curr. Opt.
Chem. Biol., 18, 87-94.
9.Liu, Y., Feizi, T., Campanero-Rodes, M. Z., Childs,
R. A., Zhang, Y., Mullou, Y. B., Evans, Ph. G., Osborn, H. M. I., Otto,
D., Crocker, P. R., and Chai, W. (2007) Neoglycolipid probes prepared
via oxime ligation for microarray analysis of
oligosaccharide–protein interaction, Chem. Biol.,
14, 847-859.
10.Hasegawa, A., and Kiso, M. (1994) Synthesis of
sialyl Lewis X ganglioside and analogs, Methods Enzymol.,
242, 158-173.
11.Ando, T., Imamura, A., Ishida, H., and Kiso, M.
(2007) 1.20. Synthesis of glycolipids, Compr. Glycosci.,
1, 797-813.
12.Faivre, V., de Lourdes Costa, M., Boullanger, P.,
Baszkin, A., and Rosilio, V. (2003) Specific interaction of lectins
with liposomes and monolayers, Chem. Phys. Lipids, 125,
147-159.
13.Miyashita, H., Ikeda, T., and Nohara, T. (2007)
Synthesis of neosaponins and neoglycolipid containing a chacotriosyl
moiety, Carbohydr. Res., 342, 2182-2191.
14.Queneau, Y., Chambert, S., Besseta, S., and
Cheai, R. (2008) Recent progress in the synthesis of carbohydrate-based
amphiphilic materials: the examples of sucrose and isomaltulose,
Carbohydr. Res., 343, 1999-2009.
15.Korchagina, E., Tuzikov, A., Formanovsky, A.,
Popova, I., Henry, S., and Bovin, N. (2012) Toward creating cell
membrane glycolandscapes with glycan lipid constructs, Carbohydr.
Res., 356, 238-246.
16.Barr, K., Korchagina, E., Popova, I., Bovin, N.,
and Henry, S. (2015) Monoclonal anti-A activity against the FORS1
(Forssman) antigen, Transfusion, 55, 129-136.
17.Blake, D. A., Bovin, N. V., Bess, D., and Henry,
S. M. (2011) FSL constructs: a simple method for modifying cell/virion
surfaces with a range of biological markers without affecting their
viability, J. Vis. Exp., 54, e3289; DOI:
10.3791/3289.
18.Barr, K., Korchagina, E., Ryzhov, I., Bovin, N.,
and Henry, S. (2014) Mapping the fine specificity of ABO monoclonal
reagents with A and B type-specific FSL constructs in kodecytes and
inkjet printed on paper, Transfusion, 54, 2477-2484.
19.Carter, N. L., Blake, D. A., Bovin, N. V., Henry,
S. M., Korchagina, E. Y., Williams, E. C., and Tuzikov, A. B. (2006)
Cell Surface Coating with Hyaluronic Acid Oligomer Derivative,
US Patent WO/2007/035116.
20.Harrison, A. L., Olsson, M. L., Brad Jones, R.,
Ramkumar, S., Sakac, D., Binnington, B., Henry, S., Lingwood, C. A.,
and Branch, D. R. (2010) A synthetic globotriaosylceramide analogue
inhibits HIV-1 infection in vitro by two mechanisms,
Glycoconj. J., 27, 515-524.
21.Oliver, C., Blake, D., and Henry, S. (2011) In
vivo neutralization of anti-A and successful transfusion of A
antigen incompatible red cells in an animal model, Transfusion,
51, 2664-2675.
22.Lan, C.-C., Blake, D., Henry, S., and Love, D. R.
(2012) Fluorescent function-spacer-lipid construct labelling allows for
real-time in vivo imaging of cell migration and behavior in
zebrafish (Danio rerio), J. Fluoresc., 22,
1055-1063.
23.Bovin, N. V., and Khorlin, A. Ya. (1984)
Synthesis of the determinant oligosaccharides of ABH (type 1) blood
group antigens and Leb tetrasaccharide from the same
precursor, Bioorg. Khim., 10, 853-860.
24.Bovin, N. V., and Khorlin, A. Ya. (1985)
Convenient method for preparing the Le and ABH (type 1) determinant
oligosaccharides, Bioorg. Khim., 11, 826-829.
25.Bovin, N. V., Zemlyanukhina, T. V., Chagiashvili,
C. N., and Khorlin, A. Ya. (1988) Artificial antigens and affinity
sorbents of blood-group specificity Lea, Leb, and
Led, Khim. Prirod. Soedin., 6, 777-785.
26.Zemlyanukhina, T. V., and Bovin, N. V. (1990)
Synthesis of blood group oligosaccharides with A, B, and H (type 3)
specificity, Bioorg. Khim., 16, 1096-1104.
27.Korchagina, E. Yu., and Bovin, N. V. (1992)
Synthesis of spacered trisaccharides with blood group specificities A
and B, their fragments and structural analogs, Bioorg. Khim.,
18, 283-298.
28.Ovchinnikova, T. V., Ter-Grigoryan, A. G.,
Pazynina, G. V., and Bovin, N. V. (1997) Synthesis of terminal
disaccharide of Forssman antigen and some of its analogs as spacered
glycosides and free disaccharides, Rus. J. Bioorg. Chem.,
23, 55-68.
29.Simeoni, L. A., Byramova, N. E., and Bovin, N. V.
(1997) Synthesis of aminopropyl glycoside of the SiaTn
antigen Neu5Acα2-6GalNAcα, Russ. J. Bioorg. Chem.,
23, 683-691.
30.Simeoni, L. A., Byramova, N. E., and Bovin, N. V.
(2000) Neu5Gcα(2-6)GalNAcα as a spacer glycoside,
Russ. J. Bioorg. Chem., 26, 183-191.
31.Pazynina, G. V., Tyrtysh, T. V., and Bovin, N. V.
(2002) Synthesis of histo blood-group antigens A and B (type 2),
xenoantigen Galα1-3Galβ1-4GlcNAc and related type 2 backbone
oligosaccharides as haptens in spacered form, Mendeleev Commun.,
12, 143-145.
32.Pazynina, G., Tuzikov, A., Chinarev, A.,
Obukhova, P., and Bovin, N. (2002) Simple stereoselective synthesis of
α2-6 sialooligosaccharides, Tetrahedron Lett., 43,
8011-8013.
33.Korchagina, E. Y., Pochechueva, T. V., Obukhova,
P. S., Formanovsky, A. A., Imberty, A., Rieben, R., and Bovin, N. V.
(2005) Design of the blood group AB glycotopes, Glycoconj. J.,
22, 127-133.
34.Byramova, N. E., Tuzikov, A. B., Tyrtysh, T. V.,
and Bovin, N. V. (2007) 1,6-Anhydro-N-acetyl-β-D-glucosamine in
oligosaccharide synthesis: II. The synthesis of the spacered
LeY tetrasaccharides, Rus. J. Bioorg. Chem.,
33, 99-109.
35.Korchagina, E. Yu., Ryzhov, I. M., Byrgazov, K.
A., Popova, I. S., Pokrovsky, S. N., and Bovin, N. V. (2009) Block
synthesis of blood group tetrasaccharides B (types 1, 3 and 4),
Mendeleev Commun., 19, 152-154.
36.Ryzhov, I. M., Korchagina, E. Y., Popova, I. S.,
and Bovin, N. V. (2012) Block synthesis of A tetrasaccharides (types 1,
3, and 4) related to the human ABO blood group system, Carbohydr.
Res., 351, 17-25.
37.Pazynina, G., Tyrtysh, T., Nasonov, V.,
Belyanchikov, I., Paramonov, A., Malysheva, N., Zinin, A., Kononov, L.,
and Bovin, N. (2013) Divergent strategy for the synthesis of
α2-3-linked sialo-oligosaccharide libraries using a
Neu5TFA-(α2-3)-Gal building block, Synlett, 24,
226-230.
38.Pazynina, G. V., Chinarev, A. A., Tuzikov, A. B.,
Nasonov, V. V., Malysheva, N. N., and Bovin, N. V. (2013) Synthesis of
a spacer-armed 6′-sialyl-N-acetyllactosamine, in Carbohydrate
Chemistry: Proven Synthetic Methods, Vol. 2, Series: Carbohydrate
Chemistry, CRC Press, pp. 155-160.
39.Tuzikov, A. B., Gambaryan, A. S., Juneja, L. R.,
and Bovin, N. V. (2000) Conversion of complex sialooligosaccharides
into polymeric conjugates and their anti-influenza virus inhibitory
potency, J. Carbohydr. Chem., 19, 1191-1200.
40.Lee, R. T., and Lee, Y. C. (1997) Facile
synthesis of a high-affinity ligand for mammalian hepatic lectin
containing three terminal N-acetylgalactosamine residues, Bioconj.
Chem., 8, 762-765.
41.Cremer, P. S., and Boxer, S. G. (1999) Formation
and spreading of lipid bilayers on planar glass support, J. Phys.
Chem. B, 103, 2554-2559.
42.Nollert, P., Kiefer, H., and Jahnig, F. (1995)
Lipid vesicle adsorption versus formation of planar bilayers on solid
surfaces, Biophys. J., 69, 1447-1455.
43.Richter, R., Mukhopadhyay, A., and Brisson, A.
(2003) Pathways of lipid vesicle deposition on solid surfaces: a
combined QCM-D and AFM study, Biophys. J., 85,
3035-3047.
44.Radler, J., Strey, H., and Sackmann, E. (1995)
Phenomenology and kinetics of lipid bilayers spreading on hydrophilic
surfaces, Langmuir, 11, 4539-4548.
45.Whiles, J. A., Deems, R., Vold, R. R., and
Dennis, E. A. (2002) Bicelles in structure-function studies of
membrane-associated proteins, Bioorg. Chem., 30,
431-442.
46.Henry, S. (2009) Modification of red blood cells
for laboratory quality control use, Curr. Opin. Hematol.,
16, 467-472.
47.Oliver, C., Blake, D., and Henry, S. (2011)
Modeling transfusion reactions and predicting in vivo cell
survival with kodecytes, Transfusion, 51, 1723-1730.
48.Hult, A. K., Frame, T., Chesla, S., Henry, S.,
and Olsson, M. L. (2012) Flow cytometry evaluation of red blood cells
mimicking naturally-occurring ABO subgroups following modification with
variable amounts of FSL-A and B constructs, Transfusion,
52, 247-251.
49.Hadac, E. M., Federspiel, M. J., Chernyy, E.,
Tuzikov, A., Korchagina, E., Bovin, N. V., Russell, S., and Henry, S.
M. (2011) Fluorescein and radiolabeled function-spacer-lipid constructs
allow for simple in vitro and in vivo bioimaging of
enveloped virions, J. Virol. Methods, 176, 78-84.
50.Perry, H., and Henry, S. (2015) Training students
in serologic reaction grading increased perceptions of self-efficacy
and ability to recognize serologic reactions but decreased grading
accuracy, Transfusion, Jan. 7, DOI: 10.1111/trf.12985 [Epub
ahead of print].
51.Frame, T., Carroll, T., Korchagina, E., Bovin,
N., and Henry, S. (2007) Synthetic glycolipid modification of red blood
cell membranes, Transfusion, 47, 876-882.
52.Henry, S. M. (2005) Engineering the surface of
red cells with synthetic glycolipids (KODE CAE) to create ABO
analytical sensitivity controls and xeno-modified cells,
Xenotransplantation, 12, 356.
53.Georgakopoulos, T., Komarraju, S., Henry, S., and
Bertolini, J. (2012) An improved Fc function assay utilizing CMV
antigen coated red blood cells generated with synthetic
function-spacer-lipid constructs, Vox Sanguinis, 102,
72-78.
54.Perry, E. H., and Henry, S. M. (2013) Teaching
the recognition of hemolysis by controlling antibody mediated in
vitro hemolysis with kodecytes, Transfusion, 53
(Suppl.), 182A.
55.Henry, S. (2014) Magnetic Bead Coatings: Today
and Tomorrow. Chap. 5. Rapid biofunctionalization of magnetic beads
with function-spacer-lipid constructs, SepMag eBook (http://sepmag.eu/free-guide-magnetic-bead-coatings).
56.Svensson, L., Hult, A. K., Stamps, R., Angstrom,
J., Teneberg, S., Jorgensen, R., Rydberg, L., Henry, S. M., and Olsson,
M. L. (2013) Forssman expression on human erythrocytes: biochemical and
genetic evidence of a new histo-blood group system, Blood,
121, 1459-1468.
57.Henry, S., Korchagina, E., Tuzikov, A., and
Bovin, N. V. (2010) Glyco-landscaping with neo glycolipid constructs,
Glycobiology, 20, 1472.