REVIEW: Plant Oligosaccharides – Outsiders among Elicitors?
I. A. Larskaya and T. A. Gorshkova*
Kazan Institute of Biochemistry and Biophysics, Kazan Scientific Center, Russian Academy of Sciences, 420111 Kazan, Russia; fax: (843) 292-7347; E-mail: gorshkova@kibb.knc.ru* To whom correspondence should be addressed.
Received February 9, 2015; Revision received March 16, 2015
This review substantiates the need to study the plant oligoglycome. The available information on oligosaccharins – physiologically active fragments of plant cell wall polysaccharides – is summarized. The diversity of such compounds in chemical composition, origin, and proved biological activity is highlighted. At the same time, plant oligosaccharides can be considered as outsiders among elicitors of various natures in research intensity of recent decades. This review discusses the reasons for such attitude towards these regulators, which are largely connected with difficulties in isolation and identification. Together with that, approaches are suggested whose potentials can be used to study oligosaccharins. The topics of oligosaccharide metabolism in plants, including the ways of formation, transport, and inactivation are presented, together with data on biological activity and interaction with plant hormones. The current viewpoints on the mode of oligosaccharin action – perception, signal transduction, and possible “targets” – are considered. The potential uses of such compounds in medicine, food industry, agriculture, and biotechnology are discussed.
KEY WORDS: plant oligosaccharides, oligosaccharins, cell wall, oligoglycomeDOI: 10.1134/S0006297915070081
Abbreviations: ABA, abscisic acid; IAA, indole-3-acetic acid (auxin).
It has been more than 40 years since the discovery of the biological
activity of oligosaccharide fragments of plant cell wall
polysaccharides [1, 2].
Identification of the regulatory role of these compounds was associated
with the study of the interaction of plants with pathogens. The cell
wall polysaccharide fragments released by the action of hydrolases
secreted by microorganisms serve as elicitors that trigger response
reactions of the plant organism, in particular, phytoalexin formation
[2-4]. Then the participation
of plant oligosaccharides in other processes including morphogenesis
was revealed [5-7]. To
designate physiologically active oligosaccharide, the special term
“oligosaccharin” was introduced by R. Albersheim [8], who was the head of pioneering works on the
description and study of these compounds.
The first plant oligosaccharides with revealed physiological activity were oligouronides – fragments of polygalaсturonans [1, 2]. Later, elicitor properties were shown for oligomers that are present in almost all known plant cell wall polysaccharides: xyloglucan [9], xylan [10], galactoglucomannan [11], cellulose [12], rhamnogalacturonan I [13], and rhamnogalacturonan II [14]. It was established that the regulatory properties of plant oligosaccharins are pronounced in the range of very low concentrations: 10–9-10–8 M for oligogalacturonides [15] and for xyloglucan fragments [7]. There are numerous reviews devoted to the structure and the discovered effects of plant oligosaccharins [16-20]. There was a real boom of experimental works in this field in the 1980s and early 1990s, but then their intensity declined. Currently published reviews on the functional characterization of various cell wall components always contain a section about oligosaccharins, but, in fact, they are based on the results of two decades ago. On the background of the exceptional interest in the signaling processes in living organisms and intensive study of various regulators, oligosaccharins can be considered as outsiders among elicitors of various nature such as, for example, peptides produced, similarly to cell wall polymer fragments, during catabolic processes. In this review, we try to identify the causes of this situation and to indicate possible ways to change it, summarize the available data concerning both oligosaccharin characteristics and their mechanism of action, and highlight recently developed approaches for the study of complex carbohydrates that might be effective in the investigation of the plant oligoglycome.
PROBLEMS OF RESEARCH ON OLIGOSACCHARINS
Reduction in the number of studies devoted to plant oligosaccharides is partly explained by difficulties that researchers face in isolation of these compounds and characterization of their effects. There are two main problems when working with oligosaccharins: 1) obtaining an individual oligosaccharide with characterized structure, and 2) development of appropriate test systems for the analysis of its activity [21].
The complexity of the isolation of an individual oligosaccharide from plant tissue is associated with: (i) their low content; (ii) high diversity of endogenous oligosaccharides in plants; (iii) difficulty in separation of neutral oligosaccharides with the same degree of polymerization, and especially of isomers having similar total composition; (iv) necessity to provide evidence that the effect is not due to the presence of non-carbohydrate contaminants in the fractions, which are hard to avoid. These are the reasons that the data on the extraction of oligosaccharides directly from plant tissue are very limited (table). Studies of the team headed by O. A. Zabotina and A. I. Zabotin are among the few exceptions [36, 37, 43, 44]. Although with some caution, obtaining of active oligosaccharide fragments from cell culture medium could be considered in a similar way [46]. It should be noted that in all these studies, purification of endogenous oligosaccharides to individual compounds was not achieved; as a result, their structures have not been characterized. There are even fewer works revealing endogenous oligoglycans with known structure and biological activity: in aqueous extracts of tomato fruits (Lycopersicon esculentum Mill.), oligogalacturonides with a degree of polymerization of from 7 to 11 were identified [41], and biologically active fragments of xyloglucan were found in the cell culture medium of spinach (Spinacia oleracea L.) [47].
Plant oligosaccharins and their physiological effects
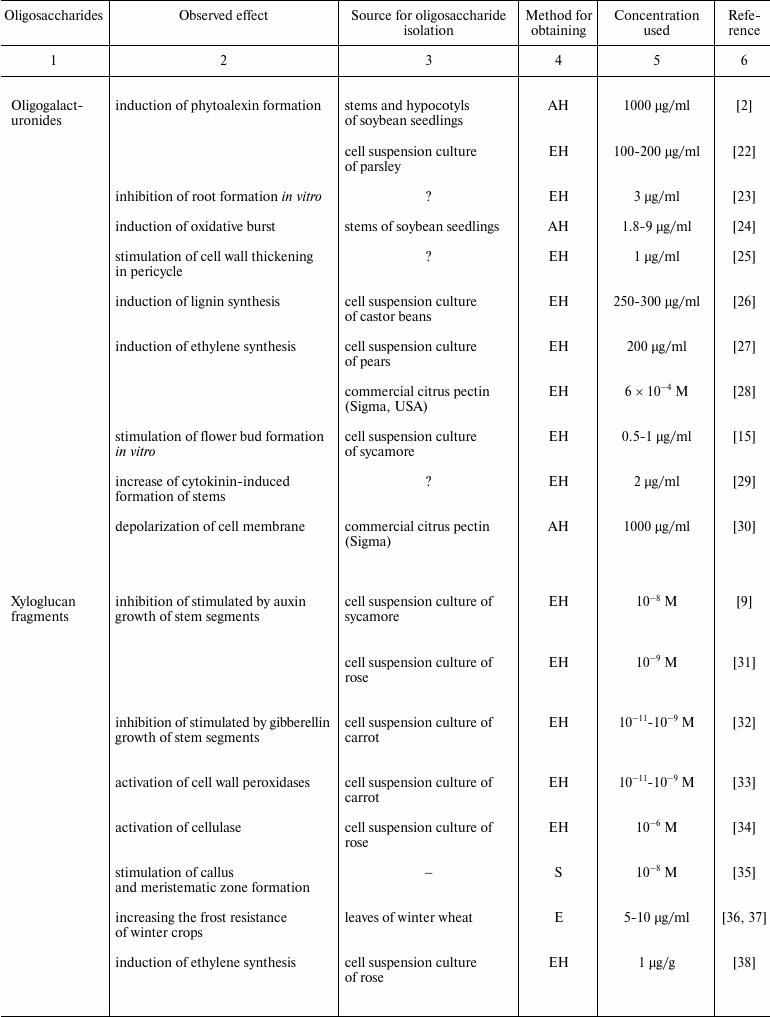
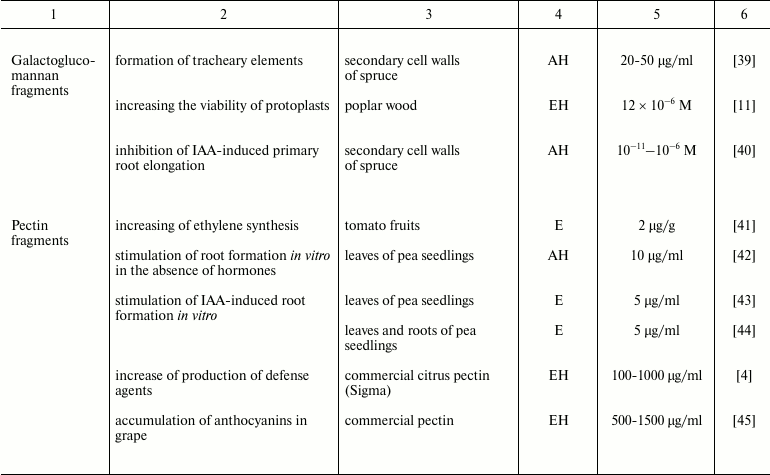
Note: EH, enzyme hydrolysis; AH, acid hydrolysis; E, extraction without
preliminary hydrolysis; S, chemical synthesis; ?, the cited paper does
not specify the source material for oligosaccharin isolation.
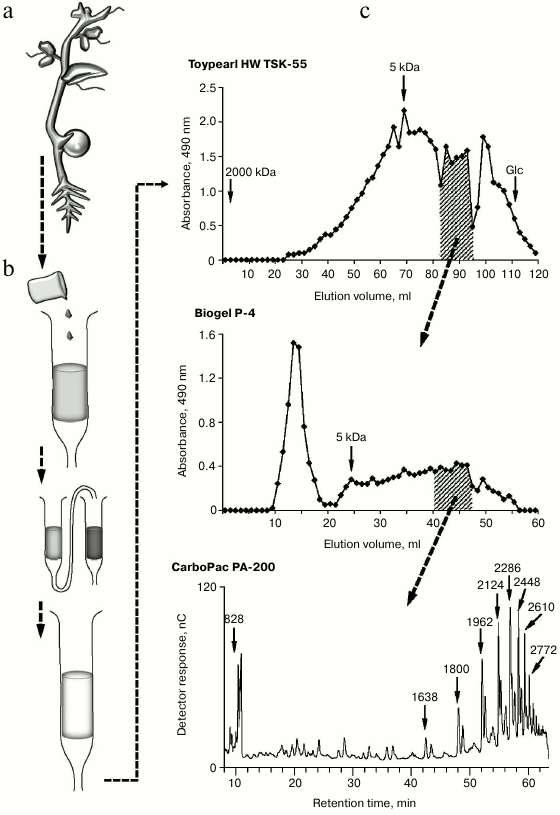
Such a limited number of studies on the characterization of endogenous oligoglycans is in dissonance with an extremely large variety of these compounds in plants. Multiple chromatographic steps divide oligomeric glycans isolated and concentrated from clarified homogenate of plant tissues into numerous subfractions, which can contain several individual compounds even after the final stages of separation using highly effective anion-exchange chromatography (Fig. 1); each compound individually or in interaction with others can have regulatory properties. That means a set of complex oligomeric glycans is present in plant tissue simultaneously.
Fig. 1. Steps for extraction of oligosaccharide from plants: a) plant material (pea seedlings); b) preliminary steps of purification of water-soluble oligosaccharides; c) separation of oligosaccharide mixtures and determination of polymerization degree of individual oligosaccharides in subfractions.
Monosaccharide analysis of the total oligosaccharide fraction reveals the monomers typical for most of the cell wall polysaccharides, both neutral and containing uronic acids. The total content of oligosaccharides in plant tissues is low, in the range 10–7-10–5 M [41]. According to our data, at least 25 kg of plant material is needed to obtain 1 mg of partially purified oligosaccharide fractions from pea seedlings (Pisum sativum L.). Taking into account that oligosaccharins cause physiological effect at extremely low concentrations, it can be assumed that the local concentration of the specific compound can be quite sufficient for the effect.
Challenges in endogenous oligosaccharide isolation and purification have forced researchers to use oligoglycans not from plant tissue extracts, but from chemical or enzymatic hydrolysis of polysaccharides in vitro [11, 12]. This makes purification of the individual compounds considerably easier, but it creates a skeptical attitude toward the results since it requires evidence that similar compounds are present in plant tissues, and observed effects are indeed controlled by the ones in the intact system.
Another approach is the use of synthetic oligosaccharides [18, 35]. Chemical synthesis allows both preparation of an amount of material required for establishing the mechanisms of oligosaccharide action and avoiding questions concerning the possible influence of non-carbohydrate contaminants with high activity. But in this case, the lack of developed methods for synthesis and provision of complex stereochemical characteristics of the majority of plant oligosaccharide (at least, with a degree of polymerization more than 7) imposes limits. Besides, both approaches leave open the question of similar molecules existing in vivo.
Even if the oligosaccharide would be purified to the individual compound level, or at least to a mixture of a small number of components, the establishment of its structure is not a trivial task due to the nonlinearity of molecules, variety of possible types of linkages, and number and location of modifying groups. However, there are established ways of solving the task by using various kinds of mass spectrometry [48-50] and NMR spectroscopy [51-53]. In such studies, the limiting factor is not the analysis as such, but difficulties in oligosaccharide purification and/or obtaining of its quantity sufficient for analysis.
Identification of biological activity of any compound requires an appropriate test system. A good example that resulted in oligosaccharin discovery was analysis of phytoalexin formation, which revealed the signaling role of the compounds of interest in plant defense reactions [8]. Investigation of other effects of oligosaccharides required different approaches. A diversity of potentially active fragments of cell wall polysaccharides made the development of test systems with high throughput capacity extremely important [9, 42]. Complete uncertainty in possible targets of action of these effectors forced checking their effects on integrated processes such as growth and morphogenesis [23, 54] or adaptation to external influences [37]. The majority of the identified oligosaccharins have been described as stimulators or inhibitors of stem segment elongation [9, 55, 56]. Significantly higher variety of responses is yielded in the study of morphogenesis in in vitro cultures [5, 29, 39], but this method is very time consuming. Definitely, the currently used test systems do not cover all possible varieties of plant cell responses to different oligosaccharins.
However, problems in the study of the oligosaccharide effects can be more systemic. Keeping in mind the enormous variety of oligosaccharides present in plant tissue, it is easy to assume that the simultaneous presence of not one, but several oligosaccharides, i.e. a specific combination of various fragments of cell wall polysaccharides, is necessary to achieve a certain physiological response. It is known, for example, that the effects of oligosaccharide elicitors that are fragments of fungal cell wall polymers, on one hand, and host plant on the other, had synergistic effect – plant defense reactions were initiated at lower concentrations of different oligosaccharide structures if they acted in combination [22]. The idea of a combined action of an oligosaccharide combination is relevant to the molecular pattern concept that has been developed for different types of organisms. This concept postulates that at different stages of development and under the influence of external factors, there are characteristic sets of low molecular mass compounds that serve as signals to implement defense response or programs of development [57-59]. In a plant organism, taking into account the special role of the cell wall, oligosaccharides could be the most important component of such patterns. For experimental testing of such hypotheses, there is a need to analyze the effect of oligosaccharides not “apiece”, but in certain combinations, the composition of which is only to be determined – the plant cell oligoglycome, its dynamics in different physiological process are not yet characterized. At best, change in the elution profile of oligomeric glycans isolated at different physiological stages has been shown. For example, it was demonstrated that on the elution profile of oligomeric compounds extracted in the first hours of cold acclimation of wheat plants, a special peak appeared that was absent on the elution profile of control plant extracts. By subfractionation of compounds eluted in this area, oligosaccharide fractions that increased frost resistance were discovered [60].
The nearly complete absence of data characterizing plant cell oligoglycome is explained primarily by the lack of appropriate methodical capacities. Methods of metabolomics used for the multi-component mixture analysis of low molecular mass noncarbohydrate compounds has not yet been properly developed for the characterization of complex sets of oligosaccharides. Marker ions, whose identification is the basis for detection of certain compounds, are still poorly identified for oligoglycans. Also, other glycomic approaches that recently came to light have not been implemented for plant tissues, but are used mainly in medical studies [61-64]. Therefore, information about plant oligosaccharins, discussed in the following chapters, is presented only for individual fragments of specific polysaccharides.
GENERAL INFORMATION ABOUT PHYSIOLOGICALLY ACTIVE OLIGOSACCHARIDES
OF PLANTS
The spectrum of detected effects of plant oligosaccharins is very wide (table). Activation of various protective effects is the most characterized; also, regulation of growth and differentiation was demonstrated on various explants. For example, oligosaccharides, primarily oligogalacturonides, activate the synthesis of phytoalexins [2], endo-β-1,3-glucanases and chitinases [22], proteinase inhibitors [65], and peroxidases [33], induce oxidative burst [24, 66], and stimulate lignin synthesis [26] and ethylene production [27, 28]. Inhibition of stem segment growth under the effect of various oligosaccharins, particularly of oligouronides [55], fragments of xyloglucan [9, 31, 54], and galactoglucomannan [11, 56] has been described repeatedly; stimulation of growth is rarely observed [34]. Oligosaccharides have various effects on morphogenetic processes in vitro, such as root [23, 35, 43, 44] and flower bud [15, 67] formation. Among other effects, we mention the stimulation of guard cell division and pericycle cell wall thickening [25], as well as increase in winter crop frost resistance [36, 37].
The fragments of polygalacturonic acid and xyloglucan are the best-characterized oligosaccharins produced by cell wall polysaccharide cleavage (table). Part of the reason for this is commercial availability both of these polysaccharides and of endoglycanases that hydrolyze them. Furthermore, homogeneous fractions of oligogalacturonides – linear molecules containing from two to twenty residues of α-(1→4)-D-GalA – are relatively easy to obtain because these molecules have no isomers, at least in the absence of modifying groups.
The dependence of a physiological effect on the structure of an oligosaccharide as well as the disappearance of the effect after appropriate glycosidase treatment of the sample serve as arguments in support of the view that oligoglucans themselves effect the physiological action, not their degradation products or contaminants present in the sample. For example, the activity of the oligosaccharide fractions affecting frost-resistance was not changed after proteinase treatment or boiling, but sharply decreased after treatment of the sample with glycosidase [60]. Similar approaches have been used for confirming the oligosaccharide nature of the factor isolated from zinnia (Zinnia elegans L.) cell culture medium, which stimulated cell growth, but inhibited differentiation of the tracheary elements [68].
During the analysis of biological activity, it was found that for the activity of oligouronide, the structure of the reducing end is essential: its modification resulted in a sharp decrease of oligosaccharin activity in a series of bioassays [69]. It was shown that most of the effects described for oligogalacturonides are caused by fragments with polymerization degree of 10-16 [20]. Other polyanions – polyglutamic acid, hyaluronic acid, dextran sulfate, oligomannouronides – do not exhibit activity similar to the oligogalacturonide effect [15]. To activate synthesis of proteinase inhibitor, the hemiacetal ring and a free carboxyl group at C-6 are required for uronides. The activity decreased, but did not disappear totally, when the hydroxyl group stoichiometry in C-3 position changed or the formation of a double bond at C-5 occurred [70]. Fully methylated oligogalacturonides as well as nonlinear isomers did not show activity [71].
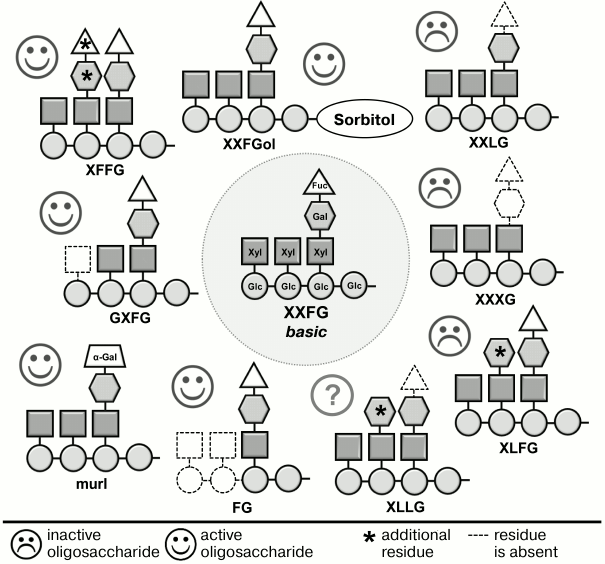
Xyloglucan fragments have a much more complex structure than oligouronides. The backbone of the canonical nonasaccharide fragment contains four residues of β-(1→4)-D-glucose; to three of them, α-D-xylose is attached at C-6, one of which, in turn, is linked at C-6 to β-D-galactose; the composition is completed by α-L-fucose attached to C-2 of galactose (Fig. 2). Detailed studies, including various modifications of this fragment and testing the activity of modified versions, have shown that the presence of the fucose residue is necessary for the best-known anti-auxin action of oligosaccharides (inhibition of elongation stimulated by auxin) [54, 72, 73], and for the effect on the immune reaction of the plant [74] – all oligosaccharides lacking fucose were inactive (Fig. 2). Fucose can be replaced by a structurally similar α-L-galactose residue. Such situation occurs in the mur1 mutant of arabidopsis (Arabidopsis thaliana L.), the primary cell wall of aboveground parts of which has less than 2% of normal α-fucose content. Xyloglucan fragments obtained from the cell walls of this mutant did not contain the terminal α-L-fucose residues characteristic for xyloglucan subunit of wild-type plants. Fucose was replaced by α-L-galactose residue, and the inhibition effect of the fragments on growth induced by auxin was not changed [73]. Some other elements of the active xyloglucan oligosaccharin structure also affected their activity but had lower importance than the fucose residue.
Fig. 2. Effect of changes in structure of XXFG xyloglucan oligosaccharide on its biological activity [54, 72, 73].
The structure of the most active fragments in different plant species is not always universal; for example, for the greatest induction of phytoalexin formation, the degree of polymerization of oligogalacturonides has to be 12 galacturonic residues for soybean (Glycine max L.), nine – for beans (Phaseolus vulgaris L.), and 13 – for castor bean (Ricinus communis L.) [3, 75]. Oligosaccharins have an effect in heterologous systems: oligosaccharins isolated from one plant species can induce a physiological response in another one [44, 76].
How are they produced? Mechanisms of oligosaccharide formation in plant tissues can be related, at least theoretically, with three types of processes. Some oligosaccharides are synthesized specially, as such, and are not fragments of cell wall polysaccharides. There are practically no oligomers among them with degree of polymerization over 3-4. Often it is a non-reducing carbohydrate formed for transport of assimilates. The best known of tetramers is stachyose. The content of such oligosaccharides in plant tissues is much higher than of the oligosaccharins discussed in this article.
One can consider various oligoglycans as “incomplete” polysaccharides that were secreted from the Golgi apparatus during synthesis. However, analysis of the kinetics of incorporation of labeled substrates into oligomeric and polymeric carbohydrates detected in cell suspension culture medium suggests that oligomeric fragments are formed later than polymeric ones [77]. Although this analysis was performed only for xyloglucan, it is generally accepted that the physiologically active oligosaccharides are produced by polysaccharide cleavage.
Formation of oligomers through cleavage of polysaccharides can occur both enzymatically, which is the main way, and non-enzymatically. Enzymatic cleavage of polysaccharides to oligomeric fragments requires the participation of endoglycanases, for example, polygalacturonase for the oligouronides and endo-(1→4)-β-glucanase for xyloglucan fragments. Endoglucanases are widely represented in plant cell wall; the enzymes active on specific polysaccharides are encoded by wide multigene families. However, there are no well-documented data that these enzymes actually release oligosaccharins at the time and in the place that correspond to their physiological activity [20]. Moreover, endoglycanases that can cleave the backbones of some is present in cell wall polysaccharide chains, for example, endogalactanases and endoarabinanases have still not been detected in plants [78, 79]. During symbiotic association, formation of oligosaccharins might be intensified due to endoglycanases of microorganisms.
Cell wall polysaccharides of living plant cells can also undergo nonenzymatic cleavage. For example, nonenzymatic degradation of various cell wall polysaccharides (xylan, polygalacturonan, arabinogalactan, and cellulose) occurs during incubation with 0.1-10 mM H2O2 [80]. In plant cell walls, during reaction of H2O2 with copper (II), free hydroxyl radicals are produced that can cleave plant polysaccharides such as xyloglucan [81] with high yield of low molecular mass fraction and good reproducibility. This nonenzymatic mechanism of polysaccharide depolymerization occurs during increase in cell size, fruit ripening, and organ abscission [82, 83].
Thus, one can state that the cell wall carbohydrate polymers, in addition to other functions, serve as keepers of “canned” signaling molecules [84, 85], and the oligosaccharin action is an example of the physiological activity of the catabolism products [86].
How are they transported? Oligosaccharin is transported in plants through the xylem. This conclusion is based on the distribution of label after incorporation into a plant of labeled oligogalacturonides [87], xyloglucan fragments [88], as well as N-glycan oligosaccharide fragments into a plant [89]. Experiments on the influence of oligosaccharides on low-temperature adaptation serves as another argument for the existence of upward transport of oligosaccharides [37, 60]: oligosaccharin added to the growth medium of winter wheat seedlings (Triticum aestivum L.) stimulated increase of resistance measured by the yield of electrolytes from leaves, which could be indirect evidence of its upward movement in the plant; however, labeled oligosaccharin were not studied in these works. Obviously, oligosaccharins are not transported through phloem, since after application of labeled pectin fragments onto mechanically damaged leaves, the label remained locally distributed for 20 h [90]. This experiment also demonstrated that apoplast oligosaccharide transport occurs only over short distances. This, however, is sufficient for passage of oligosaccharides formed in other tissues into xylem vessels. Thus, there is a system for oligosaccharin transport, and hence physiological effect can appear in tissues other than those where the oligosaccharin was formed.
Transported oligosaccharides can undergo significant modification or hydrolysis. For instance, oligogalacturonides can be cleaved into smaller oligomers or bind an unidentified alcohol [87]. Xyloglucan fragments are also modified and undergo degradation during transport, but some xyloglucan fragments (XXFG), added exogenously, and transported in pea seedlings over a distance of 5-6 cm, remained unaltered even 24 h after introduction into the stem [88].
How do they work? The structural diversity of oligosaccharins, as well as the dependence of the reaction type on concentrations and wide range of biological effects, suggest multiple mechanisms of the action of these substances [20]. Although a large number of plant physiological responses to oligosaccharins have been described, the mechanism of signal reception and transduction in most cases is still unknown. Oligosaccharide molecules do not penetrate well into the cell [91], so their action must be provided by structures localized either directly in the cell wall, or on the plasma membrane.
Complex stereochemistry and numerous hydroxyls typical for glycoside residues provide conditions for recognition of oligosaccharides by receptors, the search for which has been very active. These studies were developed most successfully for β-(1→6)-oligoglucans produced from the cell walls of fungi that infect plants. In the course of this work, a 70-kDa protein was revealed that bound hepta-β-glucan elicitor with high specificity, and the binding was characterized by saturation, reversibility, and affinity (Kd 3 nM), that was enough to exhibit elicitor activity at oligosaccharin concentration having a physiological effect [92]. However, specific and reversible binding are insufficient criteria to consider a protein as a receptor; proof of its involvement in the signal pathway transduction triggered by β-glucan oligosaccharins is needed. There are no such arguments to date despite intensive studies in this field.
For oligosaccharins that are fragments of plant cell wall polysaccharides, the situation with the search for receptor is less clear. For example, for xyloglucan fragments extremely low concentrations required for a variety of effects (10–9-10–8 M) occurred, and their dependence on the fragment structure (Fig. 2) indicate the presence of highly specific receptors for these oligosaccharins. At least some of these effects, such as increase of intracellular enzyme activity, were observed in the absence of cell wall – i.e. for isolated protoplasts [93]. Transcription and translation inhibitors do not remove the oligosaccharin effect, which is pronounced a few minutes after its addition to the medium. In sum, these data are arguments to support the existence of xyloglucan fragment receptors on the plasma membrane, as well as the existence of a cascade for signal transduction inside the cell, but no specific candidate that could serve as receptor has been identified.
To explain the mechanism of signal perception of oligouronides, a model was built considering as the receptors members of the WAK (wall-associated kinase) family – transmembrane proteins containing cytoplasmic Ser/Thr kinase domain, and the plasma membrane domain located outside and interacting with cell wall pectins. These enzymes are encoded in the arabidopsis genome by five genes (WAK1-WAK5), which are differentially expressed in various organs and also during stress. The presence of a covalent bond between such kinases and homogalacturonans in the cell wall is suggested [94]. In addition, the ability of WAK1 and WAK2 domains, located outside of the plasma membrane, to interact reversibly and noncovalently with de-etherified poly- and oligouronides was demonstrated in vitro [95, 96]. The study of wak1 mutants revealed that at least five specific amino acids are needed for this interaction [97], which apparently occurs with the participation of a charged uronic acid group. Binding specificity is determined not only by the presence of charge as other polyanions, including the structurally similar alginates, bound WAK1 with lower affinity [95].
Facts supporting that at least WAK1 and WAK2 are indeed oligouronide receptors and are involved in signal transduction have been obtained. Protoplasts isolated from leaves of wak2-1 mutants were not able to modify the expression of hundreds of genes in response to oligouronide treatment in a way similar to samples of control plants [96]. Expression in a heterologous system containing chimeric proteins located outside the plasma membrane domain of WAK1 and the cytoplasmic kinase domain of unrelated protein resulted in the appearance of sensitivity to the oligouronides in chimeric kinases [98]. It was shown that in the presence of some oligouronides some MAP kinases (particularly MAPK3) that are involved in signal transduction in plant cells are activated, and this effect is altered in wak2-1 mutants [96]. However, a clear interpretation of the results is difficult due to the lack of some expected effects [99] and the relatively small magnitude of the observed changes.
One of the first reactions to oligosaccharins is alteration of ionic flows and depolarization of the membrane potential of cells [20, 100]. For example, at an early stage of cell suspension culture response to oligogalacturonide presence, release of K+ and Cl– from the cytoplasm [101] and Ca2+ flow into the cell occurred [71, 100, 102]. Oligogalacturonides also induced “oxidative burst” – H2O2 formation was observed after their addition [24]. During the evaluation of the effect of oligouronides on an experimental system, it was demonstrated that the flow of Ca2+ into the cytoplasm preceded the appearance of the hydrogen peroxide, and the blocking of Ca2+ transport prevented the “oxidative burst”; thus the leading role in the chain of events was assigned to Ca2+ flows [71].
The flow of Ca2+ into the cytoplasm under the influence of oligouronide is blocked by tetrabromobenzotriazole – an inhibitor of serine/threonine kinases [71, 103]. This suggests that under the influence of oligosaccharin, the phosphorylation of a calcium channel component changes [103], but there is still no experimental data concerning this. There was a hypothesis that the oligouronide signal is perceived by WAKs, which perform the phosphorylation leading to a change in Ca2+ fluxes [71].
Proteins that are phosphorylated by WAK have not yet been identified [104]. However, the idea of participation of a specific kinase in signal perception of oligouronides is in good agreement with data on the differential phosphorylation of certain proteins after oligoglucan treatment. More than two decades ago, a 34-kDa plasma membrane protein was isolated, called remorin, which was identified as a protein that is specifically phosphorylated in the presence of oligogalacturonides [105, 106]. Remorins are specific for vascular plant hydrophilic proteins with unknown function, which are localized in the plasmodesmata area and in membrane rafts from the cytoplasmic side of the plasma membrane [107]. Remorins themselves are capable of binding with oligouronides and other polyanionic molecules and form oligomeric filament structure in vitro [105, 106, 108]. Proteins like remorin are found in many plant species [109], but their participation in any physiological effects provided by oligogalacturonides has not been established. Knockouts of one or more remorin genes had no significant phenotypic effect [106, 108].
Some lectin-like receptor with structural and catalytic properties similar to WAK-kinase might also be considered as oligosaccharin receptors. This assumption is based on their ability to recognize and bind carbohydrates [110]. All lectin receptor-like kinases (LecRLK) have an N-terminal lectin domain, a transmembrane domain, and a C-terminal kinase domain. It is believed that members of this family are involved in the interaction between the cell wall and the plasma membrane and play a key role in signaling processes involving carbohydrates, as well as in various stress responses [111]. Two receptor-like proteins (At3g15356, At1g78830) with lectin properties, which accumulate in response to oligouronide treatment, were found in the apoplast of arabidopsis plants [112]. These findings do not allow identifying them as the oligosaccharin receptor because their binding with oligosaccharin was not shown, but it is evidence that proteins with lectin properties might be involved in the signal transduction pathway induced by oligosaccharins. Perhaps they can be part of a multicomponent receptor complex; such an idea was proposed for chitin elicitor-binding protein-receptor (CEBiP). Due to the absence of an intracellular domain found in other receptors, it was suggested that CEBiP needs other protein components to form a functional receptor complex [113].
Interaction with a receptor is not the only discussed mechanism of oligosaccharin action. For example, xyloglucan fragments can have growth stimulating effect, which occurs at much higher concentrations (10–6-10–5 M), than the growth inhibitory one (10–9-10–8 M). In this case, the oligosaccharides do not act as signaling molecules, but as substrates for xyloglucan-endotransglycosylase [7]. Participation of this enzyme in the growth-inhibitory effects at lower oligosaccharin concentrations is unlikely since its KM for xyloglucan oligosaccharide is 2·10–5 M. In addition, differences in substrate specificity were noted [114].
Mechanisms of oligogalacturonide action are more diverse, because these molecules have a charge and are capable of forming covalent bonds in the cell wall. The degree of polymerization can also mediate the difference in the effects. Only low molecular mass oligogalacturonides (4-6 monomers) are capable of inducing ethylene formation [28]; to activate proteinase inhibitor synthesis, fragments of 2-3 galacturonides are effective [1], but for most other reactions the degree of polymerization has to be from 10 to 16 [20]. The kinetics and magnitude of the effect of plasma membrane depolarization differ in two groups of oligogalacturonide molecules of various sizes (1-7 and 10-20 monomers) [71]. It is believed that when the degree of oligouronide polymerization is below 10, it cannot take the conformation that will be recognized by the receptor – only oligogalacturonides with minimum length of 10 monomers are able to form (with participation of divalent cations) an “egg-box” structure, while when the length is over 16 monomers the possibility to reach or fit in the receptor is lost [15]. Thus, in plants at least two different oligogalacturonide recognition mechanisms are present.
In experiments using DNA microarrays containing the entire set of arabidopsis transcripts, the influence of exogenous oligogalacturonides with polymerization degree of 10-15 on gene expression in suspension cell culture was investigated [103]. During 2-h oligogalacturonide exposure, 1080 genes (4% of the entire genome!) changed their expression more than four-fold. Among the most significantly altered were mRNA levels of numerous kinases (which is additional evidence of the important role of posttranslational modifications in the effects of oligouronides) as well as cytochromes P450 and a wide group of proteins referred to as “providing resistance to disease”. Effects of oligouronides on transcription were subdivided into Ca2+-dependent and Ca2+-independent. Among the genes of the first group were genes of cell wall-modifying enzymes similar to those that are activated during defense reactions and jasmonate biosynthesis enzyme genes. The analysis of the promoter regions of these genes revealed specific cis-elements involved, presumably, in regulation of transcription.
Interrelation of oligosaccharin and hormone effects. The effects of oligosaccharins depends on their concentration, but also on the concentration and type of hormone present in the culture medium; this reveals the relationship between the mechanisms of action of these groups of physiologically active compounds [5, 15, 35, 37, 40, 115]. Most of the works demonstrating the interaction of oligosaccharins and hormones were focused on the analysis of their effect on plant growth and morphogenesis. Oligosaccharins mainly had the opposite effect to auxins in these processes. For instance, for xyloglucan oligosaccharides [9], oligogalacturonides [55], and galactoglucomannan oligosaccharides [56], inhibition of auxin-induced elongation of pea stem segments was shown. Later, for oligogalacturonides, inhibition of root formation induced by auxin was demonstrated on explants from tobacco (Nicotiana tabacum L.) and arabidopsis leaves [23, 116]. The inhibitory effect of oligosaccharins on root formation [113] and stem segment elongation [55] was abolished by increasing the auxin concentration, which served as evidence of their antagonistic interactions.
The close relationship of the actions of oligosaccharin and auxin was demonstrated also in other processes. Indole-3-acetic acid (IAA) (auxin) counteracted the protective response against Botrytis cinerea that was induced by oligosaccharin; this was shown in arabidopsis [116] and tobacco [99] explants. In the precursors of stomata guard cells, mitotic activity stimulated by oligogalacturonides decreased with the addition of exogenous hormone. And in phloem parenchyma cells, conversely, these oligosaccharins inhibited mitotic activity induced by auxin, while not having any effect on it in the absence of the hormone [25].
Studies on the molecular level demonstrated effect of oligosaccharin on activation of the auxin-regulated gene promoters Nt114 from Nicotiana tabacum L. and rolB from Agrobacterium rhizogenes, expressed in transgenic tobacco plants [117, 118]. Oligogalacturonide inhibition of the expression of the early response to auxin genes (IAA5, SAUR16, and SAUR-AC1) was observed in arabidopsis already within 30 min; this indicates that the induction of a cascade affecting the auxin signaling by oligosaccharin is very fast. This effect, according to the authors, was not mediated by changes in free auxin level, as it was only slightly altered during the experiment [116]. Moreover, modification or destruction of IAA was not observed in tissue explants from tobacco leaves that were treated with oligogalacturonides [117]. It has been suggested that oligogalacturonides bind to plasma membrane H+-ATPase that is involved in auxin responses [30]. The mechanism of action of galactomannan oligosaccharin is also associated with inactivation of the auxin receptor complex or interaction with plasma membrane proteins [56]. However, anti-auxin effect of a xyloglucan fragment is believed to be unrelated to the suppression of hydrogen ion release into the cell wall under the influence of auxin, but affects some common steps of auxin and the pH effect on cell growth [18].
Various elements of the auxin signaling pathway were analyzed as potential targets of the inhibitory effects mediated by oligosaccharins. It was shown that antagonism of oligosaccharins towards IAA does not include such mechanisms as stabilization of transcription repressors Aux/IAA (auxin/indole-3-acetic acid) or decrease in the level of auxin receptor transcripts [116]. It is possible that the inhibitory effect of oligosaccharin occurs later in the auxin-regulated signaling cascade, probably through posttranslational regulation of other than Aux/IAA elements or through inactivation of the transcription factor – ARF (auxin response factor).
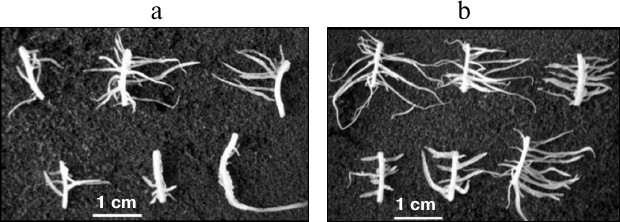
Among a large number of studies demonstrating the anti-auxin and inhibitory effects of oligosaccharins, works revealing their stimulating effect are of special interest. It was shown that galactoglucomannan oligosaccharides had a positive effect on the rate of division and cell viability in zinnia suspension culture. The effect was higher when they were added together with the hormone [39]. These oligosaccharides in combination with indole-3-butyric acid stimulated primary root elongation of mung bean (Vigna radiata L.) [119]. The oligosaccharide fraction isolated from pea seedlings had a stimulating effect on the IAA-induced formation of adventitious roots on maize root segments (Zea mays L.) (Fig. 3) and on buckwheat hypocotyl explants and tobacco leaves [44]. The stimulatory effect was significantly higher when the explants were treated with the oligosaccharin before addition of IAA into the medium, suggesting that the oligosaccharin action precedes hormone action at the early stages of root formation. It is assumed that this might be caused by the redistribution of auxin and formation of its gradient; alternatively, the interaction of two effectors occurs at the level of auxin reception, and oligosaccharin acts as a sensitizer of auxin receptors.
Fig. 3. Formation of adventitious roots on maize root segments on the 5th day of cultivation in medium containing IAA (a) and IAA + oligosaccharin (b).
Much less attention is devoted to oligosaccharin and hormone interaction during various stress responses than to the analysis of growth and morphogenetic reactions, despite the fact that it was a plant defense reaction study where the effect of oligosaccharins was originally demonstrated. Analysis of the relationship between the effects of oligosaccharide fraction and abscisic acid (ABA), which is considered as inducer of frost-resistance development, showed that the addition of the analyzed oligosaccharides to the medium increased the stimulating effect of the hormone [37]. With oligosaccharin treatment of winter wheat seedlings for ~15 h before the addition of ABA, synergism of their actions was observed, while treatment in the reverse order (first ABA, then oligosaccharin) or their simultaneous addition gave an additive effect [37, 120].
There are fragmentary data on the relationship between the actions of oligosaccharins and other hormones. Oligogalacturonides with degree of polymerization from 9 to 18 affected the signaling pathway mediated by gibberellic acid by suppressing the accumulation of α-amylase in barley embryos [115]. Xyloglucan fragments had inhibitory effect on elongation of etiolated pea seedlings, which was induced by gibberellic acid [32]. Oligogalacturonides enhanced the positive effect of cytokinin on the formation of tobacco shoot explant [29]. Low molecular mass oligogalacturonides (4-6 monomers) induced ethylene formation in tomatoes [28]. A sharp increase in ethylene content was also described in persimmon fruit (Diospyros kaki L.) when injected with xyloglucan oligosaccharins [38].
Thus, data demonstrating a close relationship between the effects of hormone and oligosaccharide have accumulated. It is known that hormones are involved in all vital processes including growth, morphogenesis, and defense from pathogens. These processes are accompanied by plant cell wall modification, causing formation of a set of active fragments with functions of positive and negative regulation of the hormonal signal. So, auxin induces expression of pectin degradation enzymes [38]. The enzymes in turn can release oligosaccharins to the apoplast for negative feedback regulation of auxin action. This means that there is a complex system of plant oligosaccharide and hormone interactions. Perhaps this explains, at least partially, the known pleiotropy of the effects of the latter. Participation of hormones in a variety of plant responses to external and internal signals can be mediated by the huge variety of oligosaccharins, providing conditions for hormones to make “point” impact.
How are they inactivated in vivo? Two main ways of oligosaccharin inactivation, needed to control their activity, are known:
1) enzymatic degradation of oligosaccharin with participation of glycosidases – enzymes that cleave single sugar residue from non-reducing end of oligo- or polymer. Fucosidase [121, 122] and xylosidase [123] inactivating xyloglucan fragments were identified. Analysis of the structure of products of degradation of mannose-containing oligosaccharides revealed the participation of α-mannosidase in their hydrolysis [89]. In rose suspension culture (Rosa sp.), 14C-labeled oligogalacturonides with degree of polymerization of 8-9 were quickly hydrolyzed to lower molecular mass fragments; “half-life time” (time interval for the hydrolysis of the half of oligogalacturonides) was from 2 to 4 h [124]. This process can be catalyzed by glycosidases and/or endoglucanases, the latter in the mentioned case being polygalacturonases widely represented in plants;
2) incorporation of oligosaccharin into the polymer by means of transglycosylation reactions, as occurs with xyloglucan fragments. Participation of xyloglucan-endotransglycosylase in xyloglucan fragment binding was demonstrated in experiments with labeled oligosaccharides; cleavage of fucose, which could have inactivated the XXFG fragment, was not detected [125].
ATTEMPTS FOR PRACTICAL APPLICATION OF PLANT
OLIGOSACCHARIDES
Establishing that plant cell wall oligosaccharides have biological activity naturally led to attempts to develop technologies for the use of these compounds for various purposes.
Use in plant biotechnology and agriculture. The proven ability of oligosaccharins to influence morphogenetic parameters of cultivated explants made perspective their application as growth-regulating agents in plant biotechnology. In particular, enhancement of root formation by oligosaccharins [43, 44, 119] opens the prospect for their use for plant vegetative reproduction in vitro. This is especially relevant for most fruit cultures, for which successful shoot rooting in vitro is a key stage of micropropagation. For example, there is a commercial oligosaccharide mixture stimulating root formation in various guava cultivars (Psidium guajava L.) [126]. Furthermore, oligosaccharins capable of enhancing the amount of adventitious roots can be used for increasing biomass and production of secondary metabolites for medicine [127], as in vitro cultivated roots can be sources of compounds used in the pharmaceutical industry [128, 129].
Xyloglucan oligosaccharides are promising as plant growth regulators due to their influence on cell cycle duration [130] and ability to manifest an anti-auxin effect [72]. Oligogalacturonides can be useful to intensify embryo development: it was shown that their addition to sugarcane cell suspension culture (Saccharum officinarum L.) reduced the time of transition from one embryogenesis stage to another [131].
An alternative application of beneficial properties of oligosaccharins might be their use as substances that cause various defense responses in plants instead of or in addition to currently used fungicides. This is especially significant for widely grown commercial plants such as grapes (Vitis vinifera L.), which are vulnerable to a variety of pathogenic fungi. It was shown that oligogalacturonides increased the resistance of vine branches to the powdery mildew pathogen Plasmopara viticola, inducing the production of reactive oxygen species [132]. Oligogalacturonides also induced antifungal chitinase and increased β-1,3-glucanase activity, which promoted the defense of plants against noble rot Botrytis cinerea [133].
{{Despite the fact that the majority of experiments demonstrating the effects of oligosaccharins were carried out in laboratories, the knowledge obtained using model systems has become the basis for development of technologies that improve the productivity and quality of agricultural products. Increase in tomato yield after spraying with two commercial oligosaccharin mixtures was 22 and 40%. Moreover, there was improvement in fruit quality [134]. Promising results were obtained for table grape cultivar Flame Seedless: treatment of plants before harvesting by oligosaccharides of pectin origin enhanced fruit coloring without affecting their firmness. Color improvement was achieved by increasing the anthocyanin content through stimulation of the propanoid pathway [45]. In studies on sugarcane treated with pectin oligomers, increase in internode length, number of shoots, and improvement in juice quality was demonstrated [135].
The small number of field experiments is insufficient to allow the wide use of oligosaccharins in agriculture, although their application as biostimulators or bioprotectors could be a good alternative to existing drugs, since it might contribute to the reduction in the amount of fertilizer and plant protection chemicals used. However, issues of the stability of oligosaccharides in complex ecosystems remain poorly defined. In particular, oligosaccharide biodegradability is both a shortcoming and a virtue. While oligosaccharides are rather stable compounds, weakly affected by environmental factors such as temperature and light, they are easily cleaved by enzymes. These enzymes are unlikely to come from the plant organism, since it is difficult for protein molecules to penetrate through the cell wall, and even more through a cell wall of surface plant tissues substantially strengthened due to cutin and various waxes. However, different carbohydrate-cleaving enzymes are secreted by various microorganisms, and their presence on plant surfaces can lead to the destruction of oligosaccharides and reduction of desired effects. An advantage of oligosaccharides is that they do not pollute the environment.
Use of oligosaccharins in medicine and food industry. There are numerous works characterizing immunomodulatory, prebiotic, and antioxidant properties of plant polysaccharides and their fragments. We will focus only on those that are obtained directly for the subject of this review – oligosaccharides that are fragments of higher plant cell walls.
Food components are classified as prebiotics if they neither undergo hydrolysis by human digestive enzymes nor are absorbed in the upper digestive tract, and are selective substrates for growth and/or metabolic activation of certain species or groups of microorganisms inhabiting the large intestine, resulting in normalization of their proportion. Breast milk oligosaccharides are the best natural prebiotics today. In breast milk, the proportion of carbohydrates with prebiotic properties is significant, comprising 12-15 g/liter, whereas in cow milk they are detected only in trace amounts [136]. The monomers of breast milk oligosaccharides are D-glucose, D-galactose, N-acetylglucosamine, L-fucose, and sialic acid. With few exceptions, all breast milk oligosaccharide structures have lactose as the base to form carbohydrate chains of different lengths and different degrees of branching by the addition of monosaccharides. The structures of about half of more than 150 breast milk oligosaccharides have been determined [137].
Plant oligosaccharides fully identical to breast milk components have not been found. But the possibility to be ascribed to the prebiotic class has been demonstrated for a number of oligoglycans that are fragments of higher plant cell wall polysaccharides. Their prebiotic potential has been confirmed both in vitro and in vivo [138-140]. The list of oligosaccharides with prebiotic properties includes galacto-, gluco-, and xylooligosaccharides [141, 142] and oligogalacturonides [143]; these oligosaccharides are usually obtained by controlled enzymatic hydrolysis of natural polysaccharides. Studied plant oligosaccharides have no toxic or mutagenic effects; therefore, they are suitable as additives for baby food from infancy [144]. Stimulation of Bifidobacterium and Lactobacillus growth in the intestinal flora under the influence of prebiotics promotes not only increase in useful bacteria, but it is also accompanied by other positive responses. Positive effects of plant oligosaccharides with proven prebiotic activity on the cardiovascular system [145] protecting colon cells from toxins [146], effecting bacterial translocation [147], and preventing bacterial adhesion in the intestine [148] are considered potential uses.
There are a limited number of studies directed towards understanding of the mechanisms of prebiotic activity. To identify the relationship between plant oligosaccharide structure and prebiotic properties, methylated and unmethylated pectin fragments of various degrees of polymerization as well as rhamnogalacturono-, arabino-, and galactooligosaccharides were investigated [149]. Acidic oligosaccharides did not show any bifidogenic activity, while a strong effect was observed during the first hours of fermentation with neutral fractions of low polymerization degree.
Immunomodulating properties of various compounds, including plant carbohydrates found within the digestive tract, are currently widely considered. A mixture of short-chain galactooligosaccharides and long-chain fructooligosaccharides diminished allergic asthma symptoms in mice [150]. Immunomodulatory as well as antioxidant and anticarcinogenic properties were shown for plant oligosaccharides that have not yet been assigned to the class of prebiotics. As such, wheat bran oligosaccharides, containing ferulic acid [151], and aloe (Aloe vera L.) glucans and mannans exhibited antioxidant activity [152]. The antioxidant activity of the latter, according to the authors, was provided by acetyl groups [153]. A wide range of immunomodulatory properties was shown by plant arabinogalactans and their fragments [154-158] as well as by plant acidic oligosaccharides [159].
The use of plant poly- and oligosaccharides as anticarcinogenic substances is also discussed. This idea originated from experiments demonstrating the binding of pectin oligosaccharides with Gal-3 – a member of the galectin family – evolutionarily conserved galactose-binding lectins distributed in a wide range of species from lower invertebrates to mammals [160, 161]. It is believed that Gal-3 plays an important role in the development and progression of cancer [162, 163], being one of the key biomarkers for certain types of cancer and a target for drug therapy. Gal-3 has a highly conserved carbohydrate-binding site for the galectin family, which “recognizes” β-galactosides, allowing galectin to bind a large number of different oligosaccharide ligands. Natural sources of ligands that inhibit biological functions Gal-3 include pectins containing arabinogalactan/galactan side chains [164]. The main commercially available plant-derived arabinogalactan is larch arabinogalactan [155]. There are several studies on the anticarcinogenic activity of arabinogalactans [154, 165, 166].
Other galactose-containing glycans that interact with Gal-1 include galactomannans that are especially enriched in legume seeds. The Galectin Therapeutics Company developed a technique for producing partially depolymerized galactomannans described in the literature as DavanatTM [167], which showed anticancer activity [168].
There are fragmentary data about the perspectives of plant oligogalacturonide use in cosmetics. This is based on their ability to stimulate the adhesion of epidermal cells (keratinocytes) with extracellular matrix proteins; its disruption causes autoimmune diseases of the skin and mucosae. This positive effect has been shown for oligouronides with degree of polymerization ≤5 [169].
Practical use of carbohydrate compounds, including plant oligosaccharides, is significantly limited by poor understanding of their action mechanisms and of the relationship between their structure and effect. This is largely due to the general problems of the study of complex carbohydrates, underdevelopment of theory that can identify and predict the determinants of certain properties associated with functional load in “native” organism and application in various fields.
NEW APPROACHES THAT CAN BE USED FOR OLIGOSACCHARIN
RESEARCH
In recent decades, approaches and facilities that made it possible to significantly broaden and deepen the investigations of plant oligosaccharides and their physiological action have been developed. These approaches can be divided into two major groups: the first group is related to carbohydrate analysis itself, while the second uses the possibilities of molecular genetic methods.
The first group includes both the improvement of methods of specific oligoglycan structure analysis and of plant tissue oligoglycome profiling in a changing environment. Structural studies of oligosaccharides employ mass spectrometry (MS/MSn, n > 2) as well as various types of NMR that have been used to characterize plant cell wall polysaccharide fragments [48-53]. Realization of oligoglycome profiling is more complicated. Metabolomics would have special perspectives for investigation of oligosaccharide metabolism [170, 171]. Based on mass spectrometry, metabolomics allows analyzing with high sensitivity complex sets of low molecular mass compounds. The water solubility of oligosaccharides together with rather low molecular mass (and its specific range, enabling to leave outside various monomers) allows relatively easy preparation of fractions suitable for analysis. However, a variety of endogenous oligosaccharides leads to a variety of marker ions that are necessary for evaluating the dynamics of contents of compounds in different conditions, but most oligosaccharides are not characterized in this respect.
Approaches for oligoglycome profiling have been developed and used mainly for glycoconjugate analysis, primarily of proteins, so that some works devoted in fact to carbohydrates were published in the journal Proteomics [61, 172]. Analysis of carbohydrate diversity of glycosylated proteins gave impressive results. For example, the study of the oligoglycan profiles typical for human glycoproteins in various physiological states led to the discovery of reliable markers of different physiological states, oncological diseases among them [63, 64, 173]. Oligosaccharide mixtures are so complex that preliminary separation using various types of chromatography or capillary electrophoresis is a necessary stage of their analysis. High sensitivity is mainly provided by a built-in online mass-spectrometric detector or by use of fluorescent labels [61, 62]. The developed approaches have not yet been applied for the analysis of the plant oligosaccharide ensemble.
New opportunities for studies on mechanisms of action of various regulators have emerged through the development of molecular genetic approaches. Their potential has not been fully realized for the development of ideas on plant oligosaccharide formation and function. Characteristics of gene expression for endoglycanases involved in cell wall oligosaccharide formation, for example, deserves more attention: systemic analysis from this point of view has not yet been done. Various methods for modification of gene expression, including use of mutants, has provided in some cases interesting information about mechanism of oligosaccharide action, in particular – in the study of kinases associated with the cell wall (WAK) [96, 97]. However, to apply such approaches there is the need to understand expression of which gene should be changed to achieve an effect; research in the case of oligosaccharides, in fact, is a problem from this point of view. Furthermore, such mutants often have poorly expressed phenotype. An interesting aspect could be the study of plant lectins. The majority of plant lectins and lectin-like proteins are located in the cell wall and/or on the surface of the plasma membrane [110-112] and thus available for interaction with oligosaccharides.
The development of methods of functional genomics opened significant additional opportunities for understanding the effects of oligosaccharides. The first works analyzing transcriptome changes under the influence of oligosaccharins have appeared [96, 103]. This powerful approach allows drawing a large-scale and objective picture of the processes. Its use in combination with information obtained in the course of the oligoglycome analysis could form a conceptual understanding of the mechanisms of oligosaccharide action and their place in the hierarchy of plant signaling systems.
CONCLUSION
Cell wall is a unique structure of the plant organism. In the course of evolution, it has been shaped after the acquisition of the ability to photosynthesize, as a result of the functioning of which there was need to dispose (or bind) metabolic and osmotically active monosaccharides formed in the light. This problem was solved by immobilization of monosaccharides into relatively inert polysaccharides and depositing them outside the plasma membrane. Formation of peculiar polysaccharide skeleton around each plant cell largely determined biological characteristics of plants, particular in such processes as growth, morphogenesis, and signal transduction. Moreover, these processes in plants are largely based on the properties of cell wall polymers. In the course of plant evolution, an exclusive variety of polysaccharide structures with tissue and functional specificity appeared. Higher plants are indisputable champions in the creation and use of complex and diverse polysaccharides.
The plant cell wall is a dynamic structure not only with deposition of newly synthesized layers, but also with constant modification of already deposited polysaccharides. These modifications result in formation of a large number of oligosaccharides, of which at least some have physiological activity, inducing or inhibiting various processes. Regulatory properties were revealed only in a small number of fragments produced by cleavage of any plant cell wall polymer, wherein fragments with the opposite effect can be obtained during the hydrolysis of certain polysaccharides. The set of physiologically active products of cell wall polysaccharide cleavage can lead to the formation of a kind of information field, fluctuations of which are important components of plant signaling systems. All this demonstrates the importance of the oligosaccharide set profiling in plant tissues, of its change under the influence of various factors, and of understanding of its functional role that can be especially important in plant organisms.
Today the picture of oligosaccharin action is still fragmented, especially due to the fact that some of its features were obtained from different plant species. While it is clear that the cell wall polysaccharide fragments are an essential component of branched networks of plant signaling systems [174], the plant tissue oligoglycome has never been investigated. Movement in this direction is necessary, combining works with individual compounds and their complex combinations present in specific physiological conditions. Methodological possibilities for this have already become apparent on the horizon, so probably oligoglycome study that has particular importance to the specific conditions of plant organism will be the next boom, which will rely on high-technology research methods involving modern ideology.
This work was partially supported by the Russian Foundation for Basic Research (project No. 14-04-01591).
REFERENCES
1.Bishop, P. D., Makus, D. J., Pearce, G., and Ryan,
C. (1981) Proteinase inhibitor inducing factor activity in tomato
leaves resides in oligosaccharides enzymically released from cell
walls, Proc. Nat. Acad. Sci. USA, 78,
3536-3540.
2.Hahn, M. G., Darvill, A. G., and Albersheim, P.
(1981) Host–pathogen interactions. XIX. The endogenous elicitor,
a fragment of a plant cell wall polysaccharide that elicits phytoalexin
accumulation in soybeans, Plant Physiol., 68,
1161-1169.
3.Nothnagel, E. A., McNeil, M., Ahbersheim, P., and
Dell, A. (1983) Host–pathogen interactions. XXII. A
galacturonic acid oligosaccharide from plant cell walls elicits
phytoalexins, Plant Physiol., 71, 916-926.
4.Jin, D. F., and West, C. A. (1984)
Characteristics of galacturonic acid oligomers as elicitors of casbene
synthetase activity in castor bean seedlings, Plant Physiol.,
74, 989-992.
5.Tran Than Van, K., Toubart, P., and Cousson, A.
(1985) Manipulation of the morphogenetic pathways of tobacco explants
by oligosaccharins, Nature, 314, 615-617.
6.Darvill, A., Augur, C., Bergmann, C., Carlson, R.
W., Cheong, J. J., Eberhard, S., Hahn, M. G., Lo, V. M., Marfa, V., and
Meyer, B. (1992) Oligosaccharins – oligosaccharides that
regulate growth, development and defense responses in plants,
Glycobiology, 2, 181-198.
7.Aldington, S., and Fry, S. C. (1993)
Oligosaccharins, Adv. Bot. Res., 19, 1-101.
8.Albersheim, P., Darvill, A. G., McNeil, M., Valent,
B. S., Sharp, J. K., Nothnagel, E. A., Davis, K. R., Yamazaki, N.,
Gollin, D. J., York, W. S., Dudman, W. F., Darvill, J. E., and Dell, A.
(1983) Oligosaccharins: naturally occurring carbohydrates with
biological regulatory functions, in Structure and Function of Plant
Genomes (Ciferri, O., and Dure, L., eds.) Plenum Publishing Corp.,
N.Y., pp. 293-312.
9.York, W. S., Darvill, A. G., and Albersheim, P.
(1984) Inhibition of 2,4-dichlorophenoxyacetic acid-stimulated
elongation of pea stem segments by a xyloglucan oligosaccharide,
Plant Physiol., 75, 295-297.
10.Ishii, T., and Saka, H. (1992) Inhibition
of auxin-stimulated elongation of cells in rice lamina joints by a
feruloylated arabinoxylan trisaccharide, Plant Cell Physiol.,
33, 321-324.
11.Liskova, O., Auxtova, O., Kakoniova, D.,
Kubackova, M., Karacsonyi, S., and Bilisics, L. (1995) Biological
activity of galactoglucomannan-derived oligosaccharides, Planta,
196, 425-429.
12.Lorences, E. P., McDougall, G. J., and Fry, S. C.
(1990) Xyloglucan — and cello-oligosaccharides: antogonists of
the growth-promoting effect of H+, Physiol. Plant.,
80, 109-113.
13.Dinand, E., Excoffier, G., Lienart, Y., and
Vignon, M. R. (1997) Two rhamnogalacturonide tetrasaccharides isolated
from semi-retted flax fibers are signaling molecules in Rubus
fruticosus L. cells, Plant Physiol., 115,
793-801.
14.Boudart, G., Dechamp-Guillaume, G., Lafitte, C.,
Ricart, G., Barthe, J.-P., Mazau, D., and Esquerre-Tugaye, M-T. (1995)
Elicitors and suppressors of hydroxyproline-rich glycoprotein
accumulation are solubilized from plant cell walls by
endopolygalacturonase, Biochem. J., 232, 449-457.
15.Marfa, V., Gollin, D. J., Eberhard, S., Mohnen,
D., Danill, A., and Albersheim, P. (1991) Oligogalacturonides are able
to induce flowers to form on tobacco explants, Plant J.,
1, 217-225.
16.Albersheim, P., and Darvill, A. G. (1985)
Oligosaccharins, Sci. Am., 253, 58-64.
17.Ryan, C. A. (1987) Oligosaccharide signaling in
plants, Annu. Rev. Cell Biol., 3, 295-317.
18.Usov, A. I. (1993) Oligosaccharins — a new
class of signaling molecules in plants, Russ. Chem. Rev.,
62, 1047-1071.
19.Ozeretskovskaya, O. L., and Romenskaya, I. G.
(1996) Oligosaccharins as regulatory molecules of plants, Russ. J.
Plant Physiol., 43, 648-655.
20.Ridley, B. L., O’Neill, M. A., and Mohnen,
D. (2001) Pectins: structure, biosynthesis, and
oligogalacturonide-related signaling, Phytochemistry, 57,
929-967.
21.Gorshkova, T. A. (2007) Plant Cell Wall as a
Dynamic System [in Russian], Nauka, Moscow.
22.Davis, K. R., and Hahlbrock, K. (1987) Induction
of plant defense responses in cultured parsley cells by plant cell wall
fragments, Plant Physiol., 85, 1286-1290.
23.Bellincampi, D., Salvi, G., De Lorenzo, G.,
Cervone, F., Marfa, V., Eberhard, S., Darvill, A., and Albersheim, P.
(1993) Oligogalacturonides inhibit the formation of roots on tobacco
explants, Plant J., 4, 207-213.
24.Legendre, L., Yueh, Y. G., Crain, R., Haddock,
N., Heinsteinll, P. F., and Low, P. S. (1993) Phospholipase C
activation during elicitation of the oxidative burst in cultured plant
cells, J. Biol. Chem., 268, 24559-24563.
25.Altamura, M. M., Zaghi, D., Salvi, G., De
Lorenzo, G., and Bellincampi, D. (1998) Oligogalacturonides stimulate
pericycle cell wall thickening and cell divisions leading to stoma
formation in tobacco leaf explants, Planta, 204,
429-436.
26.Bruce, R., and West, C. (1989) Elicitation of
lignin biosynthesis and isoperoxidase activity by pectic fragments in
suspension cultures of castor beans, Plant Physiol., 91,
889-897.
27.Tong, C., Labavitch, J., and Yang, S. (1986) The
induction of ethylene production from pear cell culture by cell wall
fragments, Plant Physiol., 81, 929-930.
28.Simpson, S. D., Ashford, D. A., Harvey, D. J.,
and Bowles, D. J. (1998) Short chain oligogalacturonides induce
ethylene production and expression of the gene encoding
aminocyclopropane 1-carboxylic acid oxidase in tomato plants,
Glycobiology, 8, 579-583.
29.Falasca, G., Capitani, F., Della Rovere, F.,
Zaghi, D., Franchin, C., Biondi, S., and Altamura, M. M. (2008)
Oligogalacturonides enhance cytokinin-induced vegetative shoot
formation in tobacco explants, inhibit polyamine biosynthetic gene
expression, and promote long-term remobilization of cell calcium,
Planta, 227, 835-852.
30.Thain, J. F., Gubb, J. K., and Wildon, D. C.
(1995) Depolarization of tomato leaf cells by oligogalacturonide
elicitors, Plant Cell Environ., 18, 211-214.
31.McDougall, G. J., and Fry, S. C. (1988)
Inhibition of auxin-stimulated growth of pea stem segments by a
specific nonasaccharide of xyloglucan, Planta, 175,
412-416.
32.Warneck, H., and Seitz, H. U. (1993) Inhibition
of gibberellic acid-induced elongation-growth of pea epicotyls by
xyloglucan oligosaccharides, J. Exp. Bot., 44,
1105-1109.
33.Warneck, H. M., Haug, T., and Seitz, H. U. (1996)
Activation of cell wall-associated peroxidase isoenzymes in pea
epicotyls by a xyloglucan-derived nonasaccharide, J. Exp. Bot.,
47, 1897-1904.
34.McDougall, G. J., and Fry, C. S. (1990)
Xyloglucan oligosaccharides promote growth and activate cellulase:
evidence for a role of cellulase in cell expansion, Plant
Physiol., 93, 1042-1048.
35.Pavlova, Z. N., Ash, A. O., Vnuckova, V. A.,
Babakov, A. V., Torgov, V. I., Nechaev, O. A., Usov, A. I., and
Shibaev, V. N. (1992) Biological activity of a synthetic
pentasaccharide fragment of xyloglucan, Plant Sci., 85,
131-134.
36.Zabotina, O. A., Ayupova, D. A., Larskaya, I. A.,
Nikolaeva, O. G., Petrovicheva, G. A., and Zabotin, A. I. (1998)
Physiologically active oligosaccharides accumulating in the roots of
winter wheat during adaptation to low temperature, Russ. J. Plant
Physiol., 45, 221-226.
37.Zabotin, A. I., Barisheva, T. S., Trofimova, O.
I., Torosсhina, T. E., Larskaya, I. A., and Zabotina, O. A.
(2009) Oligosaccharin and ABA synergistically affect the acquisition of
freezing tolerance in winter wheat, Plant Physiol. Biochem.,
47, 854-858.
38.Cutillas-Iturralde, A., Fulton, D. C., Fry, S.
C., and Lorences, E. P. (1998) Xyloglucan-derived oligosaccharides
induce ethylene synthesis in persimmon (Diospyros kaki L.)
fruit, J. Exp. Bot., 49, 701-706.
39.Kakosova, A., Digonnet, C., Goffner, D., and
Liskova, D. (2013) Galactoglucomannan oligosaccharides are assumed to
affect tracheary element formation via interaction with auxin in
Zinnia xylogenic cell culture, Plant Cell Rep.,
32, 479-487.
40.Kollarova, K., Vatehova, Z., Slovakova, L., and
Liskova, D. (2010) Interaction of galactoglucomannan oligosaccharides
with auxin in mung bean primary root, Plant Physiol. Biochem.,
48, 401-406.
41.Melotto, E., Greve, L. C., and Labavitch, J. M.
(1994) Cell wall metabolism in ripening fruit: biologically active
pectin oligomers in ripening tomato (Lycopersicon esculentum
Mill) fruits, Plant Physiol., 106, 575-581.
42.Lozovaya, V. V., Zabotina, O. A., Rumyantseva, N.
I., Malihov, R. G., and Zihareva, M. V. (1993) Stimulation of root
development on buckwheat thin cell-layer explants by pectic fragments
from pea stem cell walls, Plant Cell Rep., 12,
530-533.
43.Zabotina, O. A., Gurjanov, O. P., Ibragimova, N.
N., Ayupova, D. A., and Lozovaya, V. V. (1998) Rhizogenesis in
buckwheat thin-cell-layer explants: effect of plant oligosaccharides,
Plant Sci., 135, 195-201.
44.Larskaya, I. A., Barisheva, T. S., Zabotin, A.
I., and Gorshkova, T. A. (2015) Character of oligosaccharin OS-RG
participation in the IAA-induced formation of adventitious roots,
Russ. J. Plant Physiol., 62, 171-178.
45.Ochoa-Villarreal, M., Aispuro-Hernandez, E.,
Vargas-Arispuro, I., and Martinez-Tellez, M. A. (2012) Plant cell wall
polymers: function, structure and biological activity of their
derivatives, in Polymerizatin, Vol. 4 (De Souza Gomes, A., ed.)
InTech, pp. 63-86.
46.Schroder, R., and Knoop, B. (1995) An
oligosaccharide growth-factor in plant suspension-cultures – a
proposed structure, J. Plant Physiol., 146, 139-147.
47.Fry, S. C. (1986) In vivo formation of
xyloglucan nonasaccharide: a possible biologically-active cell-wall
fragment, Planta, 169, 443-453.
48.Kabel, M. A., Schols, H. A., and Voragen, A. G.
J. (2001) Mass determination of oligosaccharides by matrix-assisted
laser desorption/ionization time-of-flight mass spectrometry
following HPLC, assisted by on-line desalting and automated sample
handling, Carbohydr. Polym., 44, 161-165.
49.Matamoros Fernandez, L. E., Obel, N., Scheller,
H. V., and Roepstorff, P. (2003) Characterization of plant
oligosaccharides by matrix-assisted laser desorption/ionization and
electrospray mass spectrometry, J. Mass. Spectrom., 38,
427-437.
50.Bauer, S. (2012) Mass spectrometry for
characterizing plant cell wall polysaccharides, Front. Plant
Sci., 3, 45-50.
51.Schols, H. A., Voragen, A. G. J., and Colquhoun,
I. J. (1994) Isolation and characterization of rhamnogalacturonan
oligomers, liberated during degradation of pectic hairy regions by
rhamnogalacturonase, Carbohydr. Res., 256, 97-111.
52.Jia, Z., Cash, M., Darvill, A. G., and York, W.
S. (2005) NMR characterization of endogenously O-acetylated
oligosaccharides isolated from tomato (Lycopersicon esculentum)
xyloglucan, Carbohydr. Res., 340, 1818-1825.
53.Toukach, F. V., and Ananikov, V. P. (2013) Recent
advances in computational predictions of NMR parameters for the
structure elucidation of carbohydrates: methods and limitations,
Chem. Soc. Rev., 42, 8376-8415.
54.Augur, C., Yu, I., Sakai, K., Ogawa, T., Sina,
P., Darvill, A. G., and Albersheim, P. (1992) Further studies of the
ability of xyloglucan oligosaccharides to inhibition auxin-stimulated
growth, Plant Physiol., 99, 180-185.
55.Branca, C., De Lorenzo, G., and Cervone, F.
(1988) Competitive inhibition of the auxin-induced elongation by
α-D-oligogalacturonides in pea stem segments, Physiol.
Plant., 72, 499-504.
56.Auxtova-Samajova, O., Liskova, D., Kakoniova, D.,
Kubackova, M., Karacsonyi, S., and Bilisics, L. (1996) Inhibition of
auxin stimulated short-term elongation growth of pea stem segments by
galactoglucomannan-derived oligosaccharides, J. Plant
Physiol., 147, 611-613.
57.Boller, T., and Felix, G. (2009) A renaissance of
elicitors: perception of microbe-associated molecular patterns and
danger signals by pattern recognition receptors, Annu. Rev. Plant
Biol., 60, 379-400.
58.Tor, M., Lotze, M. T., and Holton, N. (2009)
Receptor-mediated signaling in plants: molecular patterns and
programmes, J. Exp. Bot., 60, 3645-3654.
59.Malinovsky, F. G., Fangel, J. U., and Willats, W.
G. T. (2014) The role of the cell wall in plant immunity, Front.
Plant Sci., 5, 1-11.
60.Zabotin, A. I., Barisheva, T. S., Larskaya, I.
A., Toroshina, T. E., Trofimova, O. I., Hahn, M. G., and Zabotina, O.
A. (2005) Oligosaccharin – a new systemic factor in the
acquisition of freeze tolerance in winter plants, Plant
Biosyst., 139, 36-41.
61.Domann, P. J., Pardos-Pardos, A. C., Fernandes,
D. L., Spencer, D. I. R., Radcliffe, C. M., Royle, L., Dwek, R. A., and
Rudd, P. M. (2007) Separation-based glycoprofiling approaches using
fluorescent labels, Pract. Proteom., 1, 70-76.
62.Wuhrer, M. (2013) Glycomics using mass
spectrometry, Glycoconj. J., 30, 11-22.
63.Kristic, J., Vuckovic, F., Menni, C., Klaric, L.,
Keser, T., Beceheli, I., Pucic-Bakovic, M., Novokmet, M., Mangino, M.,
Thaqi, K., Rudan, P., Novokmet, N., Sarac, J., Missoni, S., Kolcic, I.,
Polasek, O., Rudan, I., Campbell, H., Hayward, C., Aulchenko, Y.,
Valdes, A., Wilson, J. F., Gornik, O., Primorac, D., Zoldos, V.,
Spector, T., and Lauc, G. (2014) Glycans are a novel biomarker of
chronological and biological ages, J. Gerontol. Biol. Sci. Med.
Sci., 69, 779-789.
64.Hudak, J. E., and Bertozzi, C. R. (2014)
Glycotherapy: new advances inspire a reemergence of glycans in
medicine, Chem. Biol., 21, 16-37.
65.Moloshok, T., Pearce, G., and Ryan, C. A. (1992)
Oligouronide signaling of proteinase inhibitor genes in plants:
structure-activity relationships of di- and trigalacturonic acids and
their derivatives, Arch. Biochem. Biophys., 294,
731-734.
66.Low, P. S., and Merida, J. R. (1996) The
oxidative burst in plant defense: function and signal transduction,
Physiol. Plant., 96, 533-542.
67.Rodionova, N. A., Milyaeva, E. L., Nikiforova, V.
Yu., Martinovich, L. I., Zagustina, N. A., Mestechkina, N. M.,
Shcherbukhin, V. D., and Bezborodov, A. M. (1999) Effects of
oligogalacturonic acids and pectolytic enzymes on plant flowering,
Appl. Biochem. Microbiol., 35, 502-506.
68.Roberts, A. W., Donovan, S. G., and Haigler, C.
H. (1997) A secreted factor induces cell expansion and formation of
metaxylem-like tracheary elements in xylogenic suspension cultures of
Zinnia, Plant Physiol., 115, 683-692.
69.Spiro, M. D., Ridley, B. L., Eberhard, S., Kates,
K. A., Mathieu, Y., O’Neill, M. A., Mohnen, D., Guern, J.,
Darvill, A., and Albersheim, P. (1998) Biological activity of
reducing-end-derivatized oligogalacturonides in tobacco tissue
cultures, Plant Physiol., 116, 1289-1298.
70.Ryan, C. A. (1992) The search for the proteinase
inhibitor-inducing factor, Plant Mol. Biol., 19,
123-133.
71.Navazio, L., Moscatiello, R., Bellincampi, D.,
Baldan, B., Meggio, F., Brini, M., Bowler, C., and Mariani, P. (2002)
The role of calcium in oligogalacturonide-activated signaling in
soybean cells, Planta, 215, 596-605.
72.McDougall, G. J., and Fry, S. C. (1989)
Structure-activity relationships for xyloglucan oligosaccharides with
antiauxin activity, Plant Physiol., 189, 883-887.
73.Zablackis, E., York, W. S., Pauly, M., Hantus,
S., Reiter, W-D., Chapple, C. C. S., Albersheim, P., and Darvill, A.
(1996) Substitution of L-fucose by L-galactose in cell walls of
Arabidopsis mur1, Science, 272, 1808-1810.
74.Ash, O. A., Loskutova, N. A., Pavlova, Z. N.,
Abramycheva, N. Yu., Vnuchkova, V. A., Babakov, A. V., Muromtsev, G.
S., Melnikova, T. N., Nechaev, O. A., Torgov, V. I., Usov, A. I., and
Shibaev, V. N. (1995) New physiological effects of oligosaccharide
fragments of plant xyloglucan, Doklady RAN, 340,
427-429.
75.Dixon, R. A., Jennings, A. C., Davies, L. A.,
Gerrish, G., and Murthy, D. L. (1989) Elicitor-active components from
French bean hypocotyls, Physiol. Mol. Plant Pathol., 34,
99-115.
76.Gonzalez-Perez, L., Vazquez-Glaria, A., Perrotta,
L., Acosta, A., Scriven, S. A., Herbert, R., Cabrera, J. C., Francis,
D., and Rogers, H. J. (2012) Oligosaccharins and
Pectimorf® stimulate root elongation and shorten the
cell cycle in higher plants, Plant Growth Reg., 68,
211-221.
77.McDougall, G. J., and Fry, S. C. (1991)
Xyloglucan nonasaccharide, a naturally-occurring oligosaccharin, arises
in vivo by polysaccharide breakdown, Plant Physiol.,
137, 332-336.
78.Frankova, L., and Fry, S. C. (2013) Biochemistry
and physiological roles of enzymes that “cut and paste”
plant cell-wall polysaccharides, J. Exp. Bot., 64,
3519-3550.
79.Bonnin, E., Garnier, C., and Ralet, M-C. (2014)
Pectin-modifying enzymes and pectin-derived materials: applications and
impacts, Appl. Microbiol. Biotechnol., 98, 519-532.
80.Miller, A. R. (1989) Oxidation of cell wall
polysaccharides by hydrogen peroxide: a potential mechanism for cell
wall breakdown in plants, Biochem. Biophys. Res Commun.,
26, 238-244.
81.Tabbi, G., Fry, S. C., and Bonomo, R. P. (2001)
ESR study of the non-enzymic scission of xyloglucan by an
ascorbate-H2O2-copper system: the involvement of
the hydroxyl radical and the degradation of ascorbate, J. Inorg.
Biochem., 84, 179-187.
82.Dumville, J. C., and Fry, S. C. (2000) Uronic
acid-containing oligosaccharins: their biosynthesis, degradation and
signaling roles in non-diseased plant tissues, Plant Physiol.
Biochem., 38, 125-140.
83.Elboutachfaiti, R., Delattre, C., Michaudc, P.,
Courtois, B., and Courtois, J. (2008) Oligogalacturonans production by
free radical depolymerization of polygalacturonan, Int. J. Biol.
Macromol., 43, 257-261.
84.Bacic, A., Harris, P. J., and Stone, B. A. (1988)
Structure and function of plant cell walls, Biochem.
Plants, 14, 297-371.
85.Wolf, S., Hematy, K., and Heofte, H. (2012)
Growth control and cell wall signaling in plants, Annu. Rev. Plant
Biol., 63, 381-407.
86.Tarchevsky, I. A. (1993) Catabolism and stress in
plants, in 52nd Timiryazev’s Readings [in Russian], Nauka,
Moscow.
87.MacDougall, A. J., Rigby, N. M., Needs, P. W.,
and Selvendran, R. R. (1992) Movement and metabolism of
oligogalacturonide elicitors in tomato shoots, Planta,
188, 566-574.
88.Warneck, H. M., Fulton, D. C., Seitz, H. U., and
Fry, S. C. (1998) Transport, degradation and cell wall-integration of
XXFGol, a growth-regulating nonasaccharide of xyloglucan, in pea stems,
Planta, 204, 78-85.
89.Faugeron, C., Sakr, S., Lhernould, S., Michalski,
J. C., Delrot, S., and Morvan, H. (1999) Long-distance transport and
metabolism of unconjugated N-glycans in tomato plants, J. Exp.
Bot., 50, 1669-1675.
90.Baydoun, E. A. H., and Fry, S. C. (1985) The
immobility of pectic substances in injured tomato leaves and its
bearing on the identity of the wound hormone, Planta,
165, 269-276.
91.Smith, R. C., and Fry, S. C. (1991)
Endotransglycosylation of xyloglucans in plant cell suspension
cultures, Biochem. J., 279, 529-535.
92.Cosio, E. G., Frey, T., and Ebel, J. (1992)
Identification of a high-affinity binding protein for a
hepta-beta-glucoside phytoalexin elicitor in soybean, Eur. J.
Biochem., 204, 1115-1123.
93.Vargas-Rechia, C., Reicher, F., Sierakowski, M.
R., Heyraud, A., Driguez, H., and Lienart, Y. (1998) Xyloglucan
octasaccharide XXLgol derived from the seeds of Hymenaea
courbaril acts as a signaling molecule, Plant Physiol.,
116, 1013-1021.
94.He, Z-H., Fujiki, M., and Kohorn, B. D. (1996) A
cell wall-associated, receptor-like protein kinase, J. Biol.
Chem., 127, 19789-19793.
95.Decreux, A., and Messiaen, J. (2005)
Wall-associated kinase WAK1 interacts with cell wall pectins in a
calcium-induced conformation, Plant Cell Physiol.,
46, 268-278.
96.Kohorn, B. D., Johansen, S., Shishido, A.,
Todorova, T., Martinez, R., Defeo, E., and Obregon, P. (2009) Pectin
activation of MAP kinase and gene expression is WAK2 dependent,
Plant J., 60, 974-982.
97.Decreux, A., Thomas, A., Spies, B., Brasseur, R.,
van Cutsem, P., and Messiaen, J. (2006) In vitro
characterization of the homogalacturonan binding domain of the
wall-associated kinase WAK1 using site-directed mutagenesis,
Phytochemistry, 67, 1068-1079.
98.Brutus, A., Sicilia, F., Macone, A., Cervone, F.,
and De Lorenzo, G. (2010) A domain swap approach reveals a role of the
plant wall-associated kinase 1 (WAK1) as a receptor of
oligogalacturonides, Proc. Nat. Acad. Sci. USA, 107,
9452-9457.
99.Ferrari, S., Savatin, D. V., Sicilia, F.,
Gramegna, G., Cervone, F., and De Lorenzo, G. (2013)
Oligogalacturonides: plant damage-associated molecular patterns and
regulators of growth and development, Front. Plant Sci.,
4, 49-54.
100.Mathieu, Y., Kurkdijan, A., Xia, H., Guern, J.,
Koller, A., Spiro, M. D., O’Neil, M., Albersheim, P., and
Darvill, A. (1991) Membrane responses induced by oligogalacturonides in
suspension-cultured tobacco cells, Plant J., 1,
333-343.
101.Mathieu, Y., Guern, J., Spiro, M. D.,
O’Neill, M. A., Kates, K., Darvill, A. G., and Albersheim, P.
(1998) The transient nature of the oligogalacturonide-induced ion
fluxes of tobacco cells is not correlated with fragmentation of the
oligogalacturonides, Plant J., 16, 305-311.
102.Messiaen, J., and Van Cutsem, P. (1994) Pectic
signal transduction in carrot cells: membrane, cytosolic and nuclear
responses induced by oligogalacturonides, Plant Cell Physiol.,
35, 677-689.
103.Moscatiello, R., Mariani,
P., Sanders, D., and Maathuis, F. J. (2006) Transcriptional
analysis of calcium-dependent and calcium-independent signaling
pathways induced by oligogalacturonides, J. Exp. Bot., 57,
2847-2865.
104.Kohorn, B. D., and Kohorn, S. L. (2012) The
cell wall-associated kinases, WAKs, as pectin receptors, Front.
Plant Sci., 3, 1-5.
105.Farmer, E. E., Moloshok, T. D., Saxton, M. J.,
and Ryan, C. A. (1991) Oligosaccharide signaling in plants. Specificity
of oligouronide-enhanced plasma membrane protein phosphorylation, J.
Biol. Chem., 266, 3140-3145.
106.Reymond, P., Kunz, B., Paul-Pletzer, K., Grimm,
R., Eckerskorn, C., and Farmer, E. E. (1996) Cloning of a cDNA encoding
a plasma membrane associated, uronide binding phosphoprotein with
physical properties similar to viral movement proteins, Plant
Cell, 8, 2265-2276.
107.Raffaele, S., Bayer, E., Lafarge, D., Cluzet,
S., Retana, S. G., Boubekeur, T., Leborgne-Castel, N., Carde, J-P.,
Lherminier, J., Noirot, E., Satiat-Jeunemaitre, B., Laroche-Traineau,
J., Moreau, P., Otti, T., Maule, A. J., Reymond, P., Simon-Plas, F.,
Farmer, E. E., Bessoule, J-J., and Mongrand, S. (2009) Remorin, a
Solanaceae protein resident in membrane rafts and plasmodesmata,
impairs Potato virus X movement, Plant Cell,
21, 1541-1555.
108.Bariola, P. A., Retelska, D., Stasiak, A.,
Kammerer, R. A., Fleming, A., Hijri, M., Frank, S., and Farmer, E.
E. (2004) Remorins form a novel family of coiled coil-forming
oligomeric and filamentous proteins associated with apical, vascular
and embryonic tissues in plants, Plant Mol.
Biol., 55, 579-594.
109.Raffaele, S., Mongrand, S., Gamas, P., Niebel,
A., and Ott, T. (2007) Genome-wide annotation of remorins, a
plant-specific protein family: evolutionary and functional
perspectives, Plant
Physiol., 145, 593-600.
110.Vaid, N., Macovel, A., and Tuteja, N. (2013)
Knights in action: lectin receptor-like kinases in plant development
and stress responses, Mol. Plant., 6, 1405-1418.
111.Gouget, A., Senchou, V., Govers, F., Sanson,
A., Barre, A., Rouge, P., Pont-Lezica, R., and Canut, H. (2006) Lectin
receptor kinases participate in protein–protein interactions to
mediate plasma membrane–cell wall adhesions in
Arabidopsis, Plant Physiol., 140, 81-90.
112.Casasoli, M., Spadoni, S., Lilley, K. S.,
Cervone, F., De Lorenzo, G., and Mattei, B. (2008) Identification by
2-D DIGE of apoplastic proteins regulated by oligogalacturonides in
Arabidopsis thaliana, Proteomics, 8,
1042-1054.
113.Kaku, H., Nishizawa, Y., Ishii-Minami, N.,
Akimoto-Tomiyama, C., Dohmae, N., Takio, K., Minami, E., and Shibuya,
N. (2006) Plant cells recognize chitin fragments for defense signaling
through a plasma membrane receptor, Proc. Natl. Acad. Sci. USA,
103, 11086-11091.
114.Lorences, E., and Fry, S. (1993) Xyloglucan
oligosaccharides with at least two α-D-xylose residues act as
acceptor substrates for xyloglucan endotransglycosylase and promote the
depolymerization of xyloglucan, Physiol. Plant., 88,
105-112.
115.Loreti, E., Bellincampi, D., Millet, C., Alpi,
A., and Perata, P. (2002) Elicitors of defense responses repress a
gibberellin signaling pathway in barley embryos, J. Plant
Physiol., 159, 1383-1386.
116.Savatin, D. V., Ferrari, S., Sicilia, F., and
De Lorenzo, G. (2011) Oligogalacturonide auxin antagonism does not
require posttranscriptional gene silencing or stabilization of auxin
response repressors in Arabidopsis, Plant Physiol.,
157, 1163-1174.
117.Bellincampi, D., Cardarelli, M., Zaghi, D.,
Serino, G., Salvi, G., Gatz, C., Cervone, F., Altamura, M. M.,
Costantino, P., and Lorenzo, G. D. (1996) Oligogalacturonides prevent
rhizogenesis in rol B transformed tobacco explants by inhibiting
auxin-induced expression of the rolB gene, Plant Cell,
8, 477-487.
118.Mauro, M. L., De Lorenzo, G., Costantino, P.,
and Bellincampi, D. (2002) Oligogalacturonides inhibit the induction of
late but not of early auxin-responsive genes in
tobacco, Planta, 215, 494-501.
119.Richterova-Kucerova, D., Kollarova, K., Zelko,
I., Vatehova, Z., and Liskova, D. (2012) How do galactoglucomannan
oligosaccharides regulate cell growth in epidermal and cortical tissues
of mung bean seedlings? Plant Physiol. Biochem., 57,
154-158.
120.Zabotina, О. A., and Zabotin, A. I.
(2010) Biologically active oligosaccharide functions in plant cell:
updates and prospects, in Oligosaccharides: Sources, Properties and
Applications (Gordon, N. S., ed.) Nova Science Publishers, Inc.,
pp. 1-34.
121.Augur, C., Benhamou, N., Darvill, A., and
Albersheim, P. (1993) Purification, characterization and cell wall
localization of an α-fucosidase that inactivates a xyloglucan
oligosaccharin, Plant J., 3, 415-426.
122.De la Torre, F., Sampedro, J., Zarra, I., and
Revilla, G. (2002) AtFXG1, an Arabidopsis gene encoding
α-L-fucosidase active against fucosylated xyloglucan
oligosaccharides, Plant Physiol., 128, 247-255.
123.Sampedro, J., Sieiro, C., Revilla, G.,
Gonzalez-Villa, T., and Zarra, I. (2001) Cloning and expression pattern
of a gene encoding an α-xylosidase active against xyloglucan
oligosaccharides from Arabidopsis, Plant Physiol.,
126, 910-920.
124.Garcia-Romera, I., and Fry, S. C. (1995) The
longevity of biologically active oligosaccharide in rose cell cultures:
degradation by exopolygalacturonase, J. Exp. Bot., 46,
1853-1867.
125.Fry, S. C., Aldington, S., Hetherington, P. R.,
and Aitken, J. (1993) Oligosaccharides as signals and substrates in the
plant cell wall, Plant Physiol., 103, 1-5.
126.Ramirez, A., Cruz, N., and Franchialfaro, O.
(2003) Uso de bioestimuladores en la produccion de guayaba (P.
guajava L.) mediante el enraizamiento de esquejes, Cultivos
Tropicales, 24, 59-63.
127.Baque, M. A., Shiragi, M. H. K., Lee, E.-J.,
and Paek, K.-Y. (2012) Elicitor effect of chitosan and pectin on the
biosynthesis of anthraquinones, phenolics and flavonoids in
adventitious root suspension cultures of Morinda citrifolia
(L.), Austr. J. Crop Sci., 6, 1349-1355.
128.Nosov, A. M. (1991) Regulation of the
Secondary Compound Synthesis in the Plant Cell Culture in Biology of
Cultured Cells and Plant Biotechnology [in Russian], Nauka,
Moscow.
129.Praveen, N., and Murthy, H. N. (2010)
Production of withanolide-a from adventitious root cultures of
withania somnifera, Acta Physiol. Plant., 32,
1017-1022.
130.Kaida, R., Sugawara, S., Negoro, K., Maki, H.,
Hayashi, T., and Kaneko, T. S. (2010) Acceleration of cell growth by
xyloglucan oligosaccharides in suspension-cultured tobacco cells,
Mol. Plant., 3, 549-554.
131.Nieves, N., Poblete, A., Cid, M., Lezcano, Y.,
Gonzalez-Olmedo, J. L., and Cabrera, J. C. (2006) Evaluacion del
Pectimorf como complemento del 2,4-D en el proceso de embriogenesis
somatica en cana de azucar, Cultivos Tropicales, 27,
25-30.
132.Allegre, M., Heloir, M. C., Trouvelot, S.,
Daire, X., Pugin, A., Wendehenne, D., and Adrian, M. (2009) Are
grapevine stomata involved in the elicitor-induced protection against
downy mildew? Mol. Plant Microbe Interact., 22,
977-986.
133.Aziz, A., Heyraud, A., and Lambert, B. (2004)
Oligogalacturonide signal transduction, induction of defense-related
responses and protection of grapevine against Botrytis
cinerea, Planta, 218, 767-774.
134.Garcia-Sahagun, M. L., Martinez-Juarez, V.,
Avendaio-Lopez, A. N., Padilla-Sahagun, M. C., and Izquierdo-Oviedo, H.
(2009) Accion de oligosacaridos en el rendimiento y calidad de tomate,
Revista Fitotecnia Mexicana, 32, 295-301.
135.Marina-de la Huerta, C., Fernandez, L.,
Saborit, M., Castillo, P., and Nieto, M. (2005) Comportamiento de la
planta de cana de azucar tratada con ENERPLANT cultivada en suelos
vertisoles, Revista Electronica Granma Ciencia, 9,
1-6.
136.Jeurink, P. V., van Esch, B. C., Rijnierse, A.,
Garssen, J., and Knippels, L. M. J. (2013) Mechanisms underlying immune
effects of dietary oligosaccharides, Am. J. Clin. Nutr.,
98, 572S-577S.
137.Ninonuevo, M. R., and Lebrilla, C. B. (2009)
Mass spectrometric methods for analysis of oligosaccharides in human
milk, Nutr. Rev., 67, 216-226.
138.Roberford, M. (2007) Prebiotics: the concept
revisited, J. Nutr., 137, 830S-837S.
139.Casci, T., and Rastall, R. A. (2006)
Manufacture of prebiotic oligosaccharides, in Prebiotics:
Development and Application (Gibson, G. R., and Rastall, R. A.,
eds.) John Wiley & Sons Ltd, Chichester, pp. 29-56.
140.Valyshev, A. V., and Golovchenko, V. V. (2012)
Prebiotic activity of pectins and their derivatives, Byull.
Orenburg Nauch. Tsentra UrO RAN, 3, 1-8.
141.Rycroft, C. E., Jones, M. R., Gibson, G. R.,
and Rastall, R. A. (2001) A comparative in vitro evaluation of the
fermentation properties of prebiotic oligosaccharides, J. Appl.
Microbiol., 91, 878-887.
142.Hartemink, R., van Laere, K. M. J., Mertens, A.
K. C., and Rombouts, F. M. (1996) Fermentation of xyloglucan by
intestinal bacteria, Anaerobe, 2, 223-230.
143.Van Laere, K. M. J., Hartemink, R., Bosveld,
M., Schols, H. A., and Voragen, A. G. J. (2000) Fermentation of plant
cell wall derived polysaccharides and their corresponding
oligosaccharides by intestinal bacteria, J. Agricult. Food
Chem., 48, 1644-1652.
144.Garthoff, J. A., Heemskerk, S., Hempenius, R.
A., Lina, B. A. R., Krul, C. A. M., and Koeman, J. H. (2010) Safety
evaluation of pectin derived acidic oligosaccharides (pAOS):
genotoxicity and sub-chronic studies, Reg. Toxicol. Pharmacol.,
57, 31-42.
145.Li, T., Li, S., Du, L., Wang, N., Guo, M.,
Zhang, J., and Zhang, H. (2010) Effects of haw pectic oligosaccharide
on lipid metabolism and oxidative stress in experimental hyperlipidemia
mice induced by high-fat diet, Food Chem., 121,
1010-1013.
146.Olano-Martin, E., Williams, M. R., Gibson, G.
R., and Rastall, R. A. (2003) Pectins and pectic-oligosaccharides
inhibit Escherichia coli O157:H7 Shiga toxin as directed towards
the human colonic cell line HT29, FEMS Microbiol. Lett.,
218, 101-105.
147.Trevisi, P., De Filippi, S., Minieri, L.,
Mazzoni, M., Modesto, M., Biavati, B., and Bosi, P. (2008) Effect of
fructo-oligosaccharides and different doses of Bifidobacterium
animalis in a weaning diet on bacterial translocation and Toll-like
receptor gene expression in pig, Nutrition, 24,
1023-1029.
148.Guggenbichler, J. P., Bettignies-Dutz, A.,
Meissner, P., Schellmoser, S., and Jurenitsch, J. (1997) Acidic
oligosaccharides from natural sources block adherence of Escherichia
coli on uroepithelial cells, Pharmac. Pharmacol. Lett.,
7, 35-38.
149.Onumpai, C., Kolida, S., Bonnin, E., and
Rastall, R. (2011) Utilization and selectivity of pectin fractions with
various structures, Appl. Environ. Microbiol., 77,
5747-5754.
150.Vos, A. P., van Esch, E. C. A. M., Stahl, B.,
M’Rabet, L., Folkerts, G., Nijkamp, F. P., and Garssen, J. (2007)
Dietary supplementation with specific oligosaccharide mixtures
decreases parameters of allergic asthma in mice, Int.
Immunopharmacol., 7, 1582-1587.
151.Wang, J., Sun, B., Cao, Y., Song, H., and Tian,
Y. (2008) Inhibitory effect of wheat bran feruloyl oligosaccharides on
oxidative DNA damage in human lymphocytes, Food Chem.,
109, 129-136.
152.Wu, J. H., Xu, C., Shan, C. Y., and Tan, R. X.
(2006) Antioxidant properties and PC12 cell protective effects of APS-1
a polysaccharide from Aloe vera var. chinensis, Life
Sci., 78, 622-630.
153.Chun-hui, L., Chang-hai, W., Zhi-liang, X., and
Yi, W. (2007) Isolation, chemical characterization and antioxidant
activities of two polysaccharides from the gel and the skin of Aloe
barbadensis Miller irrigated with sea water, Process
Biochem., 42, 961-970.
154.Uhlenbruk, G., Beuth, J., Oette, K.,
Roszkowski, W., Ko, H. L., and Pulverer, G. (1986) Prevention of
experimental liver metastases by arabinogalactan,
Naturwissenschaften, 73, 626-627.
155.Medvedeva, E. N., Babkin, V. A., and
Ostroukhova, L. A. (2003) Larch arabinogalactan – properties and
prospects of using, Khim. Rast. Syr’ya, 1,
27-37.
156.Gronhaug, T. E., Ghildyal, P., Barsett, H.,
Michaelsen, T. E., Morris, G., and Diallo, D. (2010) Bioactive
arabinogalactans from the leaves of Opilia celtidifolia Endl. ex
Walp. (Opiliaceae), Glycobiology, 20, 1654-1664.
157.Popov, S. V., and Ovodov, Y. S. (2013)
Polypotency of the immunomodulatory effect of pectins, Biochemistry
(Moscow), 78, 823-835.
158.Popov, S. V., Ovodova, R. G., Golovchenko, V.
V., Khramova, D. S., Markov, P. A., Smirnov, V. V., Shashkov, A. S.,
and Ovodov, Y. S. (2014) Pectic polysaccharides of the fresh plum
Prunus domestica L. isolated with a simulated gastric fluid and
their anti-inflammatory and antioxidant activities, Food Chem.,
143, 106-113.
159.Vos, A. P., Haarman, M., van Ginkel, J.-W. H.,
Knol, J., Stahl, B., Boehm, G., M’Rabet, L., Nijkamp, F. P., and
Garssen, J. (2007) Dietary supplementation of neutral and acidic
oligosaccharides enhances Th1-dependent vaccination responses in
mice, Pediatr. Allergy Immunol., 18, 304-312.
160.Barondes, S. H., Castronovo, V., Cooper, D. N.
W., Cummings, R. D., Drickmer, K., and Feizi, T. (1994) Galectins
– a family of animal beta-galactoside-binding lectins,
Cell, 76, 597-598.
161.Rapoport, E. M., Kurmyshkina, O. V., and Bovin,
N. V. (2008) Mammalian galectins: structure, carbohydrate specificity,
and functions, Biochemistry (Moscow), 73, 393-405.
162.Glinsky, V. V., and Raz, A. (2009) Modified
pectin anti-metastatic properties: one bullet, multiple targets,
Carbohydr. Res., 344, 1788-1791.
163.Takenaka, Y., Fukumori, T., and Raz, A. (2002)
Galectin-3 and metastasis, Clycoconj. J., 19,
543-549.
164.Gunning, A. P., Bongaerts, R. J. M., and
Morris, V. J. (2009) Recognition of galactan components of pectin by
galectin-3, FASEB J., 23, 415-424.
165.Hagmar, B., Ryd, W., and Skomedal, H. (1991)
Arabinogalactan blockade of experimental metastases to liver by murine
hepatoma, Invasion and Metastasis, 11, 348-355.
166.Hauer, J., and Anderer, F. A. (1993) Mechanism
of stimulation of human natural killer cytotoxicity by arabinogalactan
from Larixoccidentalis, Cancer Immunol. Immunother.,
36, 237-244.
167.Miller, M. C., Klyosov, A., and Mayo, K. H.
(2009) The alpha-galactomannan Davanat binds galectin-1atasitedifferent
from the conventional galectin carbohydrate binding domain,
Glycobiology, 19, 1034-1045.
168.Tevyashova, A. N., Olsufyeva, E. N.,
Preobrazhenskaya, M. N., Klyosov, A. A., Zomer, E., and Platt, D.
(2007) New conjugates of antitumor antibiotic doxorubicin with
water-soluble galactomannan: synthesis and biological activity,
Russ. J. Bioorg. Chem., 33, 139-145.
169.Lubrano, C., Flavet, L., Saintigny, G., and
Robin, J. (2007) Methods of treating aging of skin with
oligosaccharides in cosmetic or dermatological compositions that
stimulate adhesion of keratinocytes to major proteins of the
dermoepidermal junction and restore epidermal cohesion, US
Patent, No. US 2007/0293433A1.
170.Fiehn, O. (2002) Metabolomics – the link
between genotypes and phenotypes, Plant Mol. Biol.,
48, 155-171.
171.Lokhov, P. G., and Archakov, A. I. (2009) Mass
spectrometry methods in metabolomics, Biochemistry (Moscow), Suppl.
Ser. B: Biomed. Chem., 3, 1-9.
172.Pabst, M., and Altmann, F. (2011) Glycan
analysis by modern instrumental methods, Proteomics, 11,
631-643.
173.Adamczyk, B., Tharmalingam, T., and Rudd, P. M.
(2011) Glycans as cancer biomarkers, Biochim. Biophys. Acta,
1820, 1347-1353.
174.Tarchevsky, I. A. (2002) Signal System of
Plant Cells [in Russian], Nauka, Moscow.