Influence of Fucoidans and Their Derivatives on Antitumor and Phagocytic Activity of Human Blood Leucocytes
N. Yu. Anisimova1, N. E. Ustyuzhanina2, F. V. Donenko1, M. I. Bilan2, N. A. Ushakova3, A. I. Usov2, N. E. Nifantiev2, and M. V. Kiselevskiy1*
1Blokhin Russian Cancer Research Center, 115478 Moscow, Russia; fax: +7 (499) 324-2794; E-mail: kisele@inbox.ru2Zelinsky Institute of Organic Chemistry, 119991 Moscow, Russia; fax: +7 (499) 135-5328; E-mail: nen@ioc.ac.ru
3Orekhovich Institute of Biomedical Chemistry, 119121 Moscow, Russia; fax: +7 (495) 245-0857
* To whom correspondence should be addressed.
Received December 26, 2014; Revision received March 28, 2015
The immunotropic activity of structurally different fucoidans and their derivatives towards isolated immune blood cells, effectors of innate immune system, was studied. The most potent effect was observed for high molecular weight fucoidan CF from the alga Chordaria flagelliformis, whose backbone is built of (1→3)-linked units of α-L-fucopyranose, and branches included residues of α-D-glucuronic acid and α-L-fucofuranose. This compound at the concentration of 0.05 mg/ml potentiated phagocytosis of Saccharomyces cerevisiae and Lactobacillus acidophilus by neutrophils, increasing relative quantity of phagocytes as well as their effectiveness. Along with this, 14% increase in the concentration of membrane-bound integrin CD11c molecules was observed. The systemic effect of CF at the dose of 0.01 mg/mouse i.p. led to potentiation of cytotoxic activity of spleen mononuclear leucocytes towards melanoma cells of line B16 by 1.9-fold and towards chronic myelogenous leukemia cells of line K-562 by 1.7-fold. These results indicate that fucoidan CF can stimulate anti-infective and antitumor activity of effectors of the innate immune system via CD11c integrins.
KEY WORDS: fucoidans, leucocytes, natural killers, phagocytosis, cytotoxicityDOI: 10.1134/S0006297915070111
Abbreviations: CF, high molecular weight fucoidan from alga Chordaria flagelliformis; DMSO, dimethyl sulfoxide; EC, effector cells; IC, index of cytotoxicity; MFI, mean fluorescence intensity; ML, mononuclear leukocytes; NBT, nitroblue tetrazolium; NK, natural killer cells; OS, synthetic fully sulfated octasaccharide; PI, phagocytic index; PN, phagocytic number; PPdX, dexylosylated low molecular weight fucoidan from alga Punctaria plantaginea; SL, high molecular weight fucoidan from alga Saccharina latissima; TC, target cells.
Sulfated polysaccharides fucoidans from brown algae are of interest
because they possess biological activities, such as anticoagulant,
antithrombotic, antiinflammatory, antitumor, antivirus activities, etc.
[1-4]. On the basis of such
biomolecular systems [5] such as fucoidans,
efficient pharmacological preparations for treatment of a number of
important diseases could be developed. At present, fucoidans are
considered one of the most promising classes of natural compounds for
immunomodulating drug development as they are characterized by a broad
spectrum of immunotropic properties along with relatively low
cytotoxicity. The immunomodulating action of fucoidans is thought to be
connected to their ability to activate phagocytes and to induce
production of such cytokines as tumor necrosis factor α,
interleukin-6, and interleukin-8 [1, 4, 6, 7].
These effects lead to stimulation of natural killer (NK) cells and
T-cells, which is accompanied by an increase in their cytotoxicity
towards transformed cells as well as by the release of
interferon-γ and interleukin-12. Besides, they cause activation
and maturation of antigen-presenting dendritic cells [8].
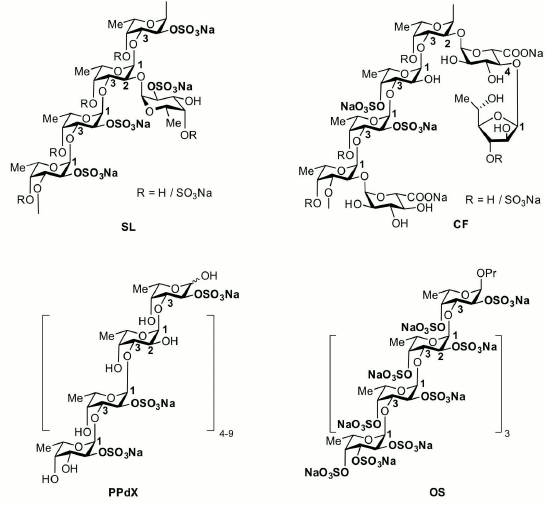
Fucoidans produced by different algal species differ in their structure and spectrum of biological activity [9-12]. The present study was aimed at estimation of the action of structurally different fucoidans and their derivatives on the activity of immunocompetent blood cells that are effectors of the innate immune response. We studied two high molecular weight fucoidans from the algae Saccharina latissima SL [13] and Chordaria flagelliformis CF [14], dexylosylated low molecular weight fucoidan PPdX [15] from the alga Punctaria plantaginea, and synthetic fully sulfated octasaccharide OS [16]. The main structural features of the studied saccharides are presented in Fig. 1, and monosaccharide and sulfate contents are listed in Table 1. Natural killer cells and neutrophils possessing antitumor and anti-infective activities, respectively, were chosen as biological materials.
Fig. 1. Main structural features of the studied saccharides and their derivatives.
Table 1. Compositiona of studied
poly- and oligosaccharides
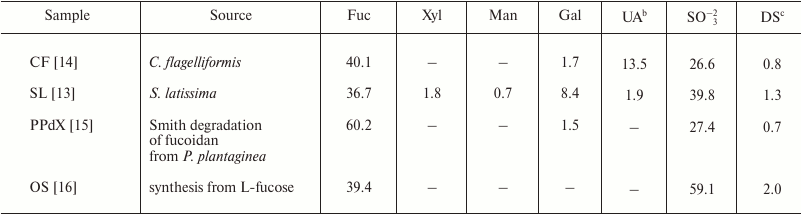
a Content (wt. %) of monosaccharides and sulfate.
b UA, uronic acids.
c Degree of sulfation (DS) calculated as sulfate to sum of
monosaccharides (Fuc + Xyl + Gal + UA) molar ratio.
We previously studied anticoagulant, antiinflammatory and antiangiogenic activities of a number of structurally different fucoidans [9]. The active concentration range for these polysaccharides was 10-100 µg/ml; therefore, we used similar quantities of saccharides in the present study.
MATERIALS AND METHODS
Analyzed poly- and oligosaccharides. High molecular weight fucoidans from algal species S. latissima (SL) and C. flagelliformis (CF) were purified as described earlier [13, 14]. Polysaccharide PPdX was obtained by dexylosylation of the native fucoidan from algae P. plantaginea [15]. Fully sulfated octasaccharide (OS) was synthesized from L-fucose [16]. Monosaccharide and sulfate content in the studied saccharides is indicated in Table 1. For preparation of stock solutions, 5 mg samples of poly- and oligosaccharides were first dissolved in 1 ml of dimethyl sulfoxide (DMSO) and then in 9 ml of sterile 0.9% sodium chloride solution.
Study of phagocytic activity of neutrophils. Blood from healthy donors, stabilized by heparin (20 units/ml) or 3.8% solution of sodium citrate (at the 9 : 1 ratio) were used for these studies. Blood (0.9 ml) was incubated for 3 h with solutions of the samples (0.1 ml) at 37°C and 250 rpm in a reciprocal shaker. Final concentration of the sulfated saccharides samples was 0.05 mg/ml. In the control series of experiments, 0.1 ml of 0.9% sodium chloride solution containing 10% DMSO was added to 0.9 ml of stabilized blood.
To estimate phagocytosis using light microscopy, we incubated suspensions of baker’s yeasts Saccharomyces cerevisiae and Lactobacillus acidophilus strain N.V.EP 317/402 “Narine” TNSi (Virion, Russia) with blood that was pre-stimulated with fucoidans for 45 min [17]. Smears were stained with Romanowsky–Giemsa dye and then analyzed by microscopy with oil immersion. About 100 neutrophiles were counted in each smear for calculation of phagocytic index (PI, percentage of cells containing phagocytized objects) and phagocytic number (PN, number of bacteria or yeasts engulfed per phagocyte) [18].
The phagocytic activity of blood neutrophils by oxygen-dependent extracellular killing of E. coli was studied using FagoFlowEx Kit (EXBIO Diagnostics, Czech Republic) by flow cytometry. The neutrophilic leukocyte population was isolated by gating using parameters of forward and side light scattering. Results were registered in the FL1 fluorescence channel (FITC) of BD Canto II cytofluorimeter (Becton Dickinson, USA). Estimation of the mean fluorescence intensity (MFI) of phagocytes that indicates activation level of a single neutrophil was made according to the manufacturer’s manual.
To dissect oxygen-dependent mechanisms of phagocytosis, we used a spectrophotometric variant of the nitroblue tetrazolium (NBT) reduction test. The method is based on the quantities of diformazan that was reduced from NBT by phagocytes. This chemical reaction occurs due to activation of oxygen-dependent biocidity of phagocytes. The NBT-test was carried out in two variants: spontaneous and induced [18]. Suspensions of the yeast S. cerevisiae and the bacterium L. acidophilus were used as inducer. Blood was incubated for 3 h with the studied fucoidans at 37°C and 200 rpm in a reciprocal shaker, and then 1.5 h with the microorganisms under the same conditions. The concentration of sulfated saccharide sample was 0.05 mg/ml. Then, we added NBT solution and incubated for an additional 45 min. The results were measured by optical density in a MS Multiscan microplate spectrophotometer (Multiscan, Finland) at 540 nm.
Calculation of concentration of CD45+CD66b+CD11+ leukocytes. Fluorochrome-conjugated antibodies (Becton Dickinson) against human CD45, CD11c, and CD66b were used. After incubation of blood with the antibodies, the erythrocytes were lysed by OptyLyse reagent (Immunotech, France). Then the cells were washed by phosphate buffered saline. Phenotype of leukocytes was analyzed using flow cytometry in the BD Canto II cytofluorimeter (Becton Dickinson). About 10,000 cells were analyzed in each sample. The population of neutrophiles was gated according to forward and side light scattering, and then CD45+ cells were isolated. The results are presented as percentage of fluorescing cell number to total cell number and as MFI – fluorescence intensity of a target marker on a single cell. Results were analyzed using FACSDiva 6.1.3 software.
Estimation of cytotoxicity of leukocytes ex vivo. Twenty C57Bl/6 line mice were divided into two equal groups – experimental and control. In the experimental group, CF solution was injected i.p. at dose of 0.01 mg/mouse five times, once per day (first injection – 24 h before tumor subinoculation). Mice from both groups were subinoculated with B16 melanoma (500,000 cells/mouse). After 20 days, the mouse spleens were collected under sterile conditions. Using Ficoll-Urografin gradients, mononuclear leukocytes (ML) were isolated from spleen homogenates that were twice washed by centrifugation in in RPMI-1640 medium (PanEco, Russia). NK-sensitive erythroblastic leukemia K-562 cells and B16 melanoma cells (cell line bank of the N. N. Blokhin Russian Cancer Research Center) were used as target cells (TC). Effector and tumor cells in suspensions were counted with trypan blue (Sigma, USA). For co-incubating cells with tested substances, 96-well plates (Nunc, USA or Costar, France) were used. The concentration of target cells in medium was 500,000 per ml. Target cell to ML effector cell (EC) ratio in medium was 1 : 2, 1 : 5, and 1 : 10. After 24-h co-incubation, the index of cytotoxicity (IC) of natural killer cells was calculated using colorimetric MTT assay that is based on the ability of dehydrogenases of live cells to reduce 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) to violet crystals of formazan soluble in DMSO [19].
Statistical analysis of primary data was done using Statistica 6.0 (StatSoft, Russia) software. Results of analysis are presented as follows: sample mean (χ) ± standard deviation (SD). To calculate the probability of difference from a control (p-value), we used Student’s t-test. The p-values less than 0.05 were considered as significant.
RESULTS
The backbone of studied carbohydrates is built up of repeated (1→3)-linked α-L-fucopyranose residues. SL and CF samples are high molecular weight polymers bearing side chains consisting of α-L-fucopyranose (SL), α-D-glucuronic acid (CF), and α-L-fucofuranose (CF). Degree of sulfation (sulfate/monosaccharide mass ratio) for SL and CF oligosaccharides was 1.3 and 0.8, respectively. The PPdX sample is a linear low molecular weight polysaccharide with degree of sulfation of 0.7. OS is synthetic low molecular weight octasaccharide with degree of sulfation of 2.0.
In Table 2, results are presented of determination of the influence of CF, SL, PPdX, and OS at 0.05 mg/ml concentration on activation of the lactic acid bacterium L. acidophilus and of yeast S. cerevisiae engulfment by the segmented neutrophils in heparin-stabilized blood.
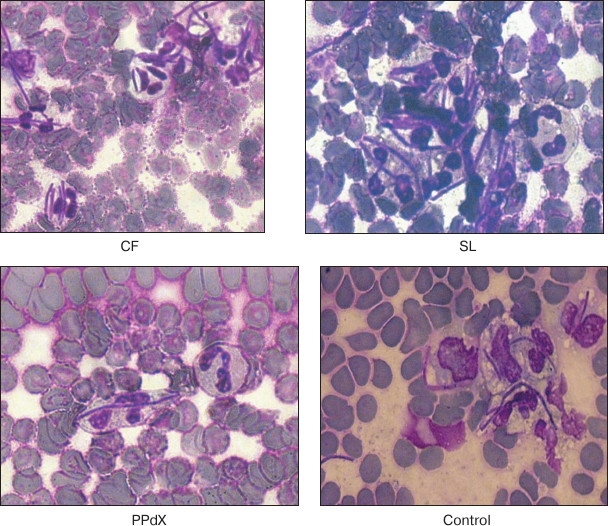
Data in Table 2 indicate that L. acidophilus engulfment by neutrophils is induced after stimulation by high molecular weight polysaccharides CF and SL. At the same time, we observed an increase in relative number of phagocytes (PI was 1.8 and 1.7 times higher, respectively, as compared to control) as well as tendency for PN increase after preincubation with these compounds. Also, in the case of yeast the compounds promoted 23% (p < 0.05) and 7% (p > 0.05) PI increase, respectively. Hence, after CF stimulation of neutrophils PN increased as much as twice compared to control (Fig. 2). Low molecular weight saccharides PPdX and OS were inactive in this assay.
Fig. 2. Intensity of L. acidophilus engulfment by neutrophils after stimulation with sulfated saccharides CF, SL, and PPdX in comparison with control.
Table 2. Phagocytic index (PI) and
phagocytic number (PN) for leukocytes from human blood after
stimulation with sulfated saccharides CF, SL, PPdX, and OS as compared
to control
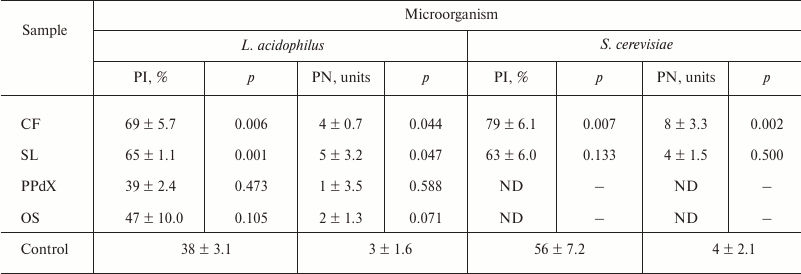
Note: ND, not determined.
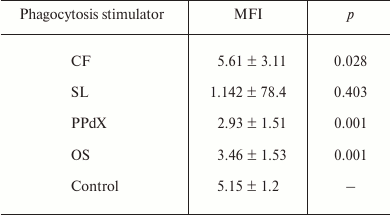
Similar results were obtained for oxygen-dependent E. coli killing after the preincubation of cells of heparin-stabilized blood with sulfated saccharides using flow cytometry (Table 3). Reliable increase in antibacterial phagocytic activity of blood neutrophils was observed after preliminary stimulation with CF. However, though after CF stimulation MFI value of phagocytizing neutrophils increased by 9%, in the case of PPdX and OS stimulation it decreased 1.8- and 1.5-fold, respectively (p < 0.05).
Table 3. Influence of CF, SL, PPdX, and OS
samples on oxygen-dependent biocidity of neutrophils from the healthy
donor blood towards E. coli
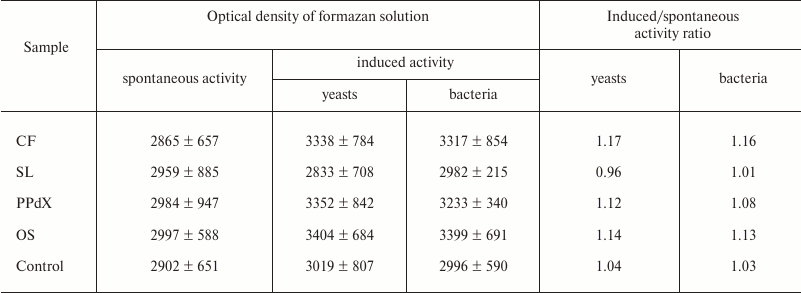
It is known that oxygen-dependent biocidity of phagocytes is mediated by active oxygen metabolites formed during so-called “respiratory burst”. Thus, quantitative analysis of this parameter after preincubation of the blood cells with sulfated saccharides was carried out using the NBT-assay: spontaneous and induced with S. cerevisiae and L. acidophilus. Experimental results are presented in Tables 4 and 5.
The data in Table 4 suggest that preliminary stimulation of cells in heparin-treated blood with CF promoted increase in oxygen-dependent phagocytic activity in vitro towards both yeasts and bacteria. The other samples had virtually no effect on activity of leukocytes in the NBT test. The same results were obtained during study of blood samples stabilized with sodium citrate (Table 5).
To study mechanisms of CF-mediated increase in phagocytic activity of neutrophils, we dissected the phenotype of neutrophils in heparin-stabilized blood after coincubation with fucoidan. In particular, we measured changes in relative concentration of CD45+CD66b+CD11c+ cells.
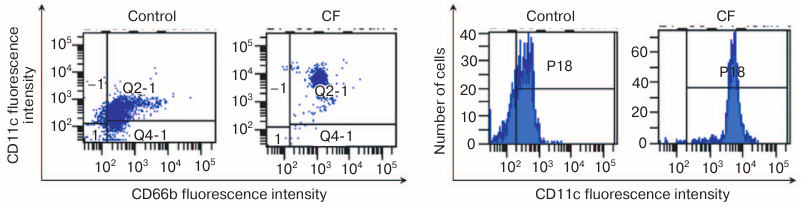
As a result, we established that after 4-h coincubation of human blood leukocytes with CF at a concentration of 0.05 mg/ml, reliable increase in relative concentration of CD45+CD66b+CD11c+ leukocytes from 72 ± 1.1% in the control to 86 ± 1.7% in the probe occurred with this fucoidan, p < 0.001; MFI value increased from 390 ± 298 to 586 ± 175 relative units, respectively, p < 0.001 (Fig. 3).
Fig. 3. Increase in relative concentration of CD11c+ neutrophils after preincubation with CF polysaccharide: a, b) distribution of CD66b+ and CD11c+ cells (abscissa axis, CD66b; ordinate axis, CD11c); c, d) CD11c+ cell content (abscissa axis, fluorescence intensity; ordinate axis, cell number).
Table 4. Change in activity of leukocytes in
NBT test modulated by CF, SL, PPdX, and OS samples (heparin-stabilized
blood)
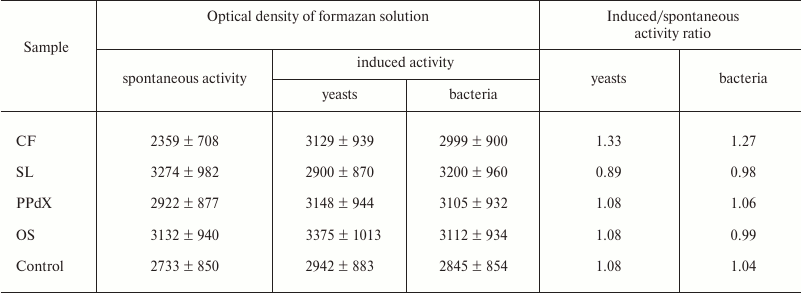
Table 5. Change in activity of leukocytes in
NBT test modulated by CF, SL, PPdX, and OS samples (sodium
citrate-stabilized blood)
The data indicate that CF-mediated increase in CD11c integrin concentration in membrane positively correlates with an increase in microorganism engulfment by phagocytes. At the same time, we could not reliably establish an increase in respiratory burst intensity in the effector cells that determine successful destruction of the object engulfed by a phagocyte.
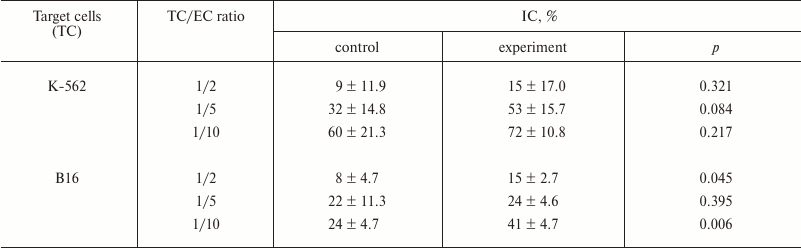
To estimate the effect of the most active fucoidan CF on functionality of innate antitumor immunity effectors, we studied change in cytotoxicity of ML in spleen of mice after a course of parenteral administration of CF. Change in antitumor cytotoxicity of ML was studied compared to control. NK-sensitive K-562 cells and B16 melanoma cells were used as target cells (TC). Target cell/effector cell of ML (EC) ratios in the medium were 1 : 2, 1 : 5, and 1 : 10. Results are presented in Table 6.
The data in Table 6 suggest that the systemic effect of CF on immunity of mice is realized through an increase in antitumor activity of immunocompetent cells. At the same time, we observed both a tendency for increase in NK-activity towards NK-sensitive K-562 line cells (IC of ML increased 1.2-1.7-fold as compared to control) and statistically reliable increase in cytotoxicity towards melanoma B16 line cells (IC of ML was 1.7-1.9-fold increased in comparison with control).
Table 6. Cytotoxicity of splenocytes after
systemic administration of CF (experimental group) in comparison with
control
DISCUSSION
The results of our study show that the composition of fucoidans substantially affects their immunotropic properties. High molecular weight fucoidan from the alga C. flagelliformis CF whose backbone is built of (1→3)-linked residues of α-L-fucopyranose and branches including residues of α-D-glucuronic acid and α-L-fucofuranose demonstrated the highest activity. High molecular weight fucoidan SL, having side chains built of α-L-fucopyranose residues, demonstrated moderate activity. Low molecular weight linear saccharides PPdX and OS were virtually inactive.
After coincubation of human blood leukocytes with CF fucoidan, 14% increase in relative quantity of CD11c was established alongside with MFI value augmentation from 390 ± 298 to 586 ± 175 relative units, p < 0.001. CD11c is known to be a glycoprotein of the integrin family, an α-subunit of αXβ2 integrin, the fibrinogen receptor. One of the previously described properties of CD11c is the ability to mediate phagocytosis of iC3b-opsonized particles [20]. This molecule is also a C4 complement receptor. CD11c is involved in phagocytosis of latex particles and bacteria in the absence of complement, cell migration, and cytokine production by monocytes/macrophages, and it is able to bind many ligands involved in cell adhesion, such as ICAM-1 and ICAM-4 as well as LPS [21]. This led many researchers to consider an increase in CD11c concentration in blood immune cells to be a marker of augmentation of their functional activity. In particular, we previously obtained analogical results confirming positive correlation between increase in levels of integrins and high phagocytic activity of innate immunity effectors [22, 23]. In the present study, this pattern in a series of tests on estimation of engulfment of bacteria (L. acidophilus and E. coli) and yeasts. Results were registered with both light microscopy and flow cytometry. In particular, after incubation of blood cells with CF, the PI for L. acidophilus and yeast engulfment was 1.8- and 1.4-fold increased, respectively (p < 0.05), along with increase in CD11+ neutrophil fraction and expression levels of this marker in the membrane. Thus, the data indicate fucoidan CF-mediated activation of the initial phase of phagocytosis – engulfment ability of neutrophiles. Heparin was used as the blood stabilizer. In the series of experiments, it was shown that substitution of heparin for sodium citrate did not affect cell response to the action of the fucoidans.
The last phase of phagocytosis includes destruction of corpuscular structures (bacterial and fungal pathogens, protozoal cells, and damaged cells and products of their decay) by means of oxygen-dependent and oxygen-independent mechanisms. In elimination of microorganisms, oxygen-dependent biocidity of phagocytes (respiratory burst), mediated by activation of NADPH-oxidase that catalyzes reduction of molecular oxygen to superoxide radical giving rise to appearance in phagocytes of active oxygen forms (superoxide anion radical, hydrogen dioxide, hydroperoxide radicals, etc.), plays an important role. In the present work, we have shown moderate increase in parameters that indicate the respiratory burst intensity induced by CF fucoidan. This suggests the ability of the polysaccharide to somewhat promote destruction of engulfed microorganisms.
In summarizing, we conclude that fucoidan CF substantially activates engulfment of bacteria and yeasts by neutrophils (initial phase of phagocytosis), but not destruction of phagocytized microorganisms mediated by oxygen-dependent mechanisms (completion of phagocytosis). The revealed feature of the polysaccharide action on neutrophils can be considered as balanced stimulating influence on the cell-mediated anti-infection immunity that excludes excessive release of reactive oxygen radicals causing acute tissue damage with developing of organ or even multi-organ insufficiency [24, 25].
Our studies also revealed augmentation of antitumor cytotoxicity by immunocompetent cells under ex vivo conditions after a course of parenteral administration of CF. The purpose of this experiment was determination of the influence of fucoidan on immunoreactivity of tumor-bearing mice to estimate changes in the functional state of innate immunity effectors – natural killer cells participating in antitumor immunity. The chosen research design, on one hand, allowed estimating NK-activity that is an integral parameter of reactivity of innate immunity effectors. On the other hand, it allowed determining antitumor cytotoxicity of lymphocytes towards cells of target (used for subinoculation of mice) tumor – B16 melanoma. Therefore, two cell lines were used in the cytotoxicity test as targets: NK-sensitive K-562 line and B16 melanoma line. As a result, after fucoidan treatment, we not only observed a tendency for NK-activity stimulation during examination of the NK-sensitive K-562 cells (IC of ML was 1.2-1.7-fold increased in comparison with control), but also found statistically reliable augmentation of cytotoxicity towards B16 melanoma cells (IC of ML increased 1.1-1.9-fold as compared to control). It is known that integrins CD11c, being membrane-bound molecules, are associated not only with neutrophils, but also with monocytes and macrophage, NK, and dendritic cells. It is possible that the ability of CF to stimulate antitumor activity of ML, which we discovered, as in the case of neutrophils is mediated by activation of expression of membrane-bound CD11c molecules in NK that provide contact of effectors with target cells.
The data suggest that the molecular mechanism of stimulating effect of fucoidan CF on effectors of anti-infection and antitumor immunity is realized with the participation of integrin CD11c.
This study established that fucoidan CF from the alga C. flagelliformis is able to activate the innate immunity cells by local action in vitro and on systemic level during the course of parenteral administration. It was reliably established that this compound intensifies engulfment of microorganisms by neutrophils increasing both relative number of phagocytes (PI) and their efficiency (PN) along with increase in CD11c concentration in membranes of neutrophils. The systemic action of CF caused an increase in antitumor cytotoxicity (IC) of splenic ML towards melanoma B16 cells (1.7-1.9-fold, p < 0.05) and towards K-562 cells (1.2-1.7-fold, p > 0.05).
This work was supported by the Russian Foundation for Basic Research (KOMFI-grant 13-04-40315-K and its parts 13-04-40313-H, 13-04-314-H, and 13-04-40315-H).
REFERENCES
1.Fitton, J. H. (2011) Therapies from fucoidan;
multifunctional marine polymers, Mar. Drugs, 9,
1731-1760.
2.Pomin, V. H. (2012) Fucanomics and galactanomics:
current status in drug discovery, mechanisms of action and role of the
well-defined structures, Biochim. Biopys. Acta, 1820,
1971-1979.
3.Jiao, G., Yu, G., Zhang, J., and Ewart, S. (2011)
Chemical structures and bioactivities of sulfated polysaccharides from
marine algae, Mar. Drugs, 9, 196-223.
4.Ale, M. T., Mikkelsen, J. D., and Meyer, A. S.
(2011) Important determinants for fucoidan bioactivity: a critical
review of structure-function relations and extraction methods for
fucose-containing sulfated polysaccharides from brown seaweeds, Mar.
Drugs, 9, 2106-2130.
5.Ananikov, V. P., Khokhlova, E. A., Egorov, M. P.,
Sakharov, A. M., Zlotin, S. G., Kucherov, A. V., Kustov, L. M., Gening,
M. L., and Nifantiev, N. E. (2015) Organic and hybrid molecular
systems, Mendeleev Commun., 25, 75-82.
6.Jin, J., and Yu, Q. (2015) Fucoidan delays
apoptosis and induces pro-inflammatory cytokine production in human
neutrophils, Int. J. Biol. Macromol., 73, 65-71.
7.Makarenkova, I. D., Logunov, D. Y., Tukhvatulin, A.
I., Semenova, I. B., Zvyagintseva, T. N., Gorbach, V. I., Ermakova, S.
P., and Besednova, N. N. (2012) Sulfated polysaccharides of brown
seaweeds are ligands of toll-like receptors, Biochem. Mosc. Suppl.
Ser. B. Biomed. Chem., 6, 75-80.
8.Jin, J-O., Zhang, W., Du, J-Y., Wong, K-W., Oda,
T., and Yu, Q. (2014) Fucoidan can function as an adjuvant in
vivo to enhance dendritic cell maturation and function and promote
antigen-specific T-cell immune responses, PLoS One, 9,
e99396.
9.Cumashi, A., Ushakova, N. A., Preobrazhenskaya, M.
E., D’Incecco, A., Piccoli, A., Totani, L., Tinari, N.,
Morozevich, G. E., Berman, A. E., Bilan, M. A., Usov, A. I.,
Ustuzhanina, N. E., Sanderson, C. J., Kelly, M., Rabinovich, G. A.,
Iacobelli, S., and Nifantiev, N. E. (2007) A comparative study of the
antiinflammatory, anticoagulant, antiangiogenic and antiadhesive
activities of nine different fucoidans from brown seaweeds,
Glycobiology, 17, 541-552.
10.Ustyuzhanina, N. E., Ushakova, N. A., Zyuzina, K.
A., Bilan, M. I., Elizarova, A. L., Somonova, O. V., Madzhuga, A. V.,
Krylov, V. B., Preobrazhenskaya, M. E., Usov, A. I., Kiselevskiy, M.
V., and Nifantiev, N. E. (2013) Influence of fucoidans on hemostatic
system, Mar. Drugs, 11, 2444-2458.
11.Ustyuzhanina, N. E., Bilan, M. I., Ushakova, N.
A., Usov, A. I., Kiselevskiy, M. V., and Nifantiev, N. E. (2014)
Fucoidans: pro- or antiangiogenic agents, Glycobiology,
24, 1265-1274.
12.Ustyuzhanina, N. E., Ushakova, N. A.,
Preobrazhenskaya, M. E., Bilan, M. I., Tsvetkova, E. A., Krylov, V. B.,
Anisimova, N. A., Kiselevskiy, M. V., Krukovskaya, N. V., Li, C., Yu,
G., Saran, S., Saxena, R. K., Usov, A. I., and Nifantiev, N. E. (2014)
Fucoidans as a platform for new anticoagulant drugs discovery, Pure
Appl. Chem., 86, 1365-1375.
13.Bilan, M. I., Grachev, A. A., Shashkov, A. S.,
Kelly, M., Sanderson, C. J., Nifantiev, N. E., and Usov, A. I. (2010)
Further studies on the composition and structure of a fucoidan
preparation from the brown alga Saccharina latissima,
Carbohydr. Res., 345, 2038-2047.
14.Bilan, M. I., Vinogradova, E. V., Tsvetkova, E.
A., Grachev, A. A., Shashkov, A. S., Nifantiev, N. E., and Usov, A. I.
(2008) A sulfated glucuronofucan containing both fucofuranose and
fucopyranose residues from the brown alga Chordaria
flagelliformis, Carbohydr. Res., 343, 2605-2612.
15.Bilan, M. I., Shashkov, A. S., and Usov, A. I.
(2014) Structure of a sulfated xylofucan from the brown alga
Punctaria plantaginea, Carbohydr. Res., 393,
1-8.
16.Krylov, V. B., Kaskova, Z. M., Vinnitskiy, D. Z.,
Ustyuzhanina, N. E., Grachev, A. A., Chizhov, A. O., and Nifantiev, N.
E. (2011) Acid-promoted synthesis of per-O-sulfated
fucooligosaccharides related to fucoidan fragments, Carbohydr.
Res., 346, 540-550.
17.Anisimova, N. Yu., Lebedinskaya, O. V., Karpenko,
A. Yu., Kopylov, A. N., and Kiselevskiy, M. V. (2012) Estimation of
activity of blood neutrophils using bacteria and unicellular yeasts as
phagocytosis objects, Vest. Ural. Med. Acad. Sci., 41,
12-13.
18.Karpishchenko, A. I. (ed.) (2002) Medical
Laboratory Technologies. Guidebook [in Russian], Intermedika, St.
Petersburg.
19.Shpakova, A. P., Pavlova, K. S., and Bulycheva,
T. I. (2000) MTT-colorimetric method for detection the cytotoxic
activity of human natural killer cells, Klin. Lab. Diagn., 2,
20-23.
20.Mann, B. S., and Chung, K. F. (2006) Blood
neutrophil activation markers in severe asthma: lack of inhibition by
prednisolone therapy, Resp. Res., 7, 59.
21.Sadhu, C., Ting, H. J., Lipsky, B., Hensley, K.,
Garcia-Martinez, L. F., Simon, S. I., and Staunton, D. E. (2007)
CD11c/CD18: novel ligands and a role in delayed-type hypersensitivity,
J. Leukoc. Biol., 81, 1395-1403.
22.Anisimova, N. Yu., Pluzhnikova, N. A., Gromova,
E. G., Kuznetsova, L. S., Tsvetkov, D. S., and Kiselevskiy, M. V.
(2011) Receptor of apoptosis and adhesion molecules of
leukocytes – promising sepsis markers in cancer patients,
Russ. J. Immunol., 14, 262-265.
23.Anisimova, N. Yu., and Blokhin, N. N. (2014)
Immunological Pathogenesis of Sepsis and Use of Hemosorption for
Treatment of Cancer Patients with Sepsis, Nova Science Publishers
Inc., N. Y.
24.Kumar, V., and Sharma, A. (2010) Neutrophils:
Cinderella of innate immune system, Int. Immunopharmacol.,
10, 1325-1334.
25.Thomas, S., and Balasubramanian, K. A. (2004)
Role of intestine in postsurgical complications: involvement of free
radicals, Free Radic. Biol. Med., 36, 745-756.