Induction of Secondary Carotenogenesis in New Halophile Microalgae from the Genus Dunaliella (Chlorophyceae)
A. E. Solovchenko1,2*, E. A. Selivanova3, K. A. Chekanov1,4, R. A. Sidorov2, N. V. Nemtseva3, and E. S. Lobakova1
1Lomonosov Moscow State University, Faculty of Biology, 119991 Moscow, Russia; E-mail: solovchenko@mail.bio.msu.ru2Timiryazev Institute of Plant Physiology, Russian Academy of Sciences, 127276 Moscow, Russia
3Institute for Cellular and Intracellular Symbiosis, Urals Branch of the Russian Academy of Sciences, 460000 Orenburg, Russia
4National Research Nuclear University “MEPhI”, Center of Humanitarian Studies and Technologies, 115409 Moscow, Russia
* To whom correspondence should be addressed.
Received July 6, 2015; Revision received July 21, 2015
We report on the effects of high light irradiance (480 µmol quanta/(m2·s)) and salinity (160 and 200 g/liter NaCl) on culture growth as well as on cell lipid pigment and fatty acid (FA) composition in three novel strains of halophile microalga from the genus Dunaliella. Based on the ITS1–5.8S rRNA–ITS2 sequence and on the capability of accumulation of secondary (uncoupled from the photosynthetic apparatus) β-carotene, the strains Dunaliella sp. BS1 and BS2 were identified as D. salina and Dunaliella sp. R5 as D. viridis. Under conditions optimal for growth, chlorophylls and primary carotenoids (mainly lutein) dominated the pigment profile of all investigated strains. The main FA were represented by unsaturated C18 FA typical of thylakoid membrane structural lipids. In all studied cells, stressors caused a decline in chlorophylls and an increase in unsaturated C16 and C18 FA associated with reserve lipids. The carotenogenic species D. salina demonstrated 10-fold increase in carotenoids accompanied by a decline in lutein and a drastic increase in β-carotene (up to 75% of total carotenoids). In D. viridis, only 1.5-fold increase in carotenoid content took place, the ratio of major carotenoids remaining essentially unchanged. The role of the carotenogenic response in mechanisms of protection against photooxidative damage is discussed in view of halophile microalgae stress tolerance and application of the new Dunaliella strains for biotechnological production of β-carotene.
KEY WORDS: carotenoids, chlorophylls, fatty acids, molecular identification, photoacclimation, salt tolerance, stressDOI: 10.1134/S0006297915110139
Abbreviations: BNMm, basic nutrition mix modified; Car, carotenoid(s); Chl, chlorophyll(s); FA, fatty acid(s); PAR, photosynthetically active radiation.
Halophile single-celled algae (microalgae) from the genus
Dunaliella (Chlorophyta, Chlorophyceae) are the main
phytoplankton component and primary producer in continental hypersaline
water bodies characterized by volatile and often unfavorable
environmental conditions [1, 2]. Among the halophile microalgae, Dunaliella
salina draws considerable attention for its capability of
significant (up to 10% of cell dry weight) accumulation of secondary
(i.e. structurally and functionally uncoupled from the photosynthetic
apparatus) β-carotene [3-5]. This feature renders D. salina the most
important microalgal source of biotechnologically produced natural
β-carotene [2, 6].
The capability of secondary carotenogenesis, among other factors, determines the high tolerance of certain representatives of the genus Dunaliella to extremely high salinities, irradiances, and temperatures as well as to mineral nutrient deficiency [7-9]. In microalgae including Dunaliella, the stress-induced secondary carotenogenesis is accompanied by a reduction in the photosynthetic apparatus and transition of the lipid metabolism to production of reserve neutral lipids [10-12]. Studies of the coordinated biosynthesis of secondary carotenoids (Car) and reserve lipids are of considerable interest for revealing stress-tolerance mechanisms in single-celled phototrophic organisms. One should also emphasize that a pronounced carotenogenic response to stresses is encountered only in selected representatives of the genus Dunaliella, being a species-specific trait of D. salina.
In consideration of the forgoing, we turned our attention to newly described Dunaliella strains with different carotenogenic capacity isolated from hypersaline water bodies of the Russian Federation and preliminarily identified as D. salina [13]. Here, we report on the updated taxonomic status of these Dunaliella strains, the induction of their carotenogenic response, and the changes in fatty acid (FA) profile of their cell lipids induced by high irradiance and salinity levels. The new findings are discussed in view of halophile microalgae tolerance to photooxidative damage and possible biotechnological application of these Dunaliella strains.
MATERIALS AND METHODS
Microalgae and cultivation conditions. Algological monocultures isolated from shallow ephemeral lagoons near the delta of Bol’shaya Smorogda River flowing into Elton Lake (Volgograd Region, Russia) – strains Dunaliella sp. BS1 and BS2, as well as from hypersaline Razval Lake (Orenburg Region, Russia) – Dunaliella sp. R5, were used in this work. The latter strain was preliminarily identified as D. salina and deposited into the IPPAS collection (Timiryazev Institute of Plant Physiology, Russian Academy of Sciences) under ID IPPAS D-232 [13].
Microalgal cells were grown according to an earlier described method [14] in 600 ml of the BNMm medium containing (g/liter): NaCl, 160; MgSO4·7H2O, 50; KNO3, 2.5; K2HPO4, 0.2; NaHCO3, 1.0; and tap water (to 1 liter) in 1.5-liter glass cylinders (6.6 cm internal diameter). The cells were grown at 27°C under continuous illumination with white light-emitting diode lamps (480 µmol PAR photons/(m2·s) as measured with LiCor 850 (LiCor, USA) quantum sensor) and sparging with atmospheric air (0.3 liter/per liter of culture per min). In certain experiments, NaCl concentration in the medium was elevated to 200 g/liter. The initial culture density was 3·105 (Dunaliella sp. BS1, BS2) or 2.3·106 cells/ml (Dunaliella sp. R5). The microalgal cell preparations were studied under a Nikon Eclipse 90i (Nikon, Japan) photomicroscope. The cells were counted using a hemocytometer.
Genomic DNA isolation, sequencing, and sequence analysis. The cells (2-5 mg dry weight) were pelleted by centrifugation, freeze–thawed three times, and incubated for 1 h in 300 µl of citrate-phosphate buffer (pH 5.0) containing 1 µM EDTA and 2% SDS at 40°C. After addition of 1 M NaCl, the samples were left overnight for protein salting, then DNA was extracted by a standard phenol–chloroform method [15]. The DNA preparation quality was checked by electrophoresis in 1.5% agarose gel stained with 0.2 µg/ml ethidium bromide.
The following oligonucleotide primers were used to amplify the target DNA fragment (~600 bp) containing the internal transcribed spacers ITS1 and ITS2 (fragment) as well as the 5.8S rRNA gene from the nuclear ribosomal gene cluster (ITS1–5.8S rRNA–ITS2): 3′-GCCTGCCTACCCAGTTGCG-5′ and 5′-GAACCTGCGGAAGGATCATTG-3′ [16]. PCR amplification was carried out by a Mastercycler Gradient amplifier (Eppendorf, Germany) using Taq-polymerase (Evrogen, Russia) according to the following program: 95°C, 3 min – initial denaturation; 95°C, 20 s; 55°C, 30 s; and 72°C, 35 s (30 cycles); 72°C, 5 min – final elongation. The PCR product was purified using the Cleanup Standard kit (Evrogen) according to the manufacturer’s manual and sequenced using a 3730 DNA Analyzer automatic sequencer (Applied Biosystems, USA).
Homologous sequences were searched in the NCBI GenBank (nr/nt) database using the online BLAST [17] service. The sequences were analyzed using MEGA 6.06 software [18]; multiple alignment was performed with MUSCLE software [19] using the default parameters (see Supplement to this paper on the site of the journal http://protein.bio.msu.ru/biokhimiya).
Pigment and total cell lipid FA extraction and assay. Photosynthetic pigments were extracted from the microalgal cells with chloroform–methanol mixture (2 : 1 v/v), chlorophylls (Chl) a and b as well as total Car were assayed in the chloroform fraction of the extract spectrophotometrically [20], and FA profile of the total cell lipids was resolved using GC-MS [14].
All experiments were carried out in triplicate. Average values with corresponding standard errors are shown unless stated otherwise.
RESULTS AND DISCUSSION
Studies of secondary carotenogenesis in microalgae are important for deciphering the mechanisms of stress tolerance of phototrophic organisms as well as for the biotechnological production of value-added carotenoid pigments [8, 10]. Therefore, potentially carotenogenic organisms such as the new Dunaliella representatives studied in this work are of considerable interest. Based on cytological and morphological criteria, all the studied strains were preliminarily identified as D. salina. However, significant physiological and biochemical differences between the strains (e.g. in their carotenogenic potential, see below) found in this work poised the need for a revision of their taxonomical status. Towards this end, the genomic DNA fragments including partial sequences of ITS1 and ITS2 as well as complete 5.8S rRNA gene were amplified and sequenced, and the corresponding GenBank IDs are KT013269 (strain BS1), KT013270 (BS2), and KT013271 (strain R5 IPPAS D-232). Comparison of the ITS1–5.8S rRNA–ITS2 sequences with those of known microalgae revealed high (95-99%) homology with representatives of the genus Dunaliella (Volvocales, Dunaliellaceae). The strain Dunaliella sp. R5 clustered (Fig. S1 in Supplement) with strains forming the so-called “viridis clade III”. Accordingly, the strain Dunaliella sp. R5 could be identified as D. viridis [21]. The strains Dunaliella sp. BS1 and BS2 clustered together with the strains belonging to the “salina clade III” [21], thereby allowing to identify them as D. salina.
Since the carotenogenic response was of special interest in the context of the present research, we followed the changes in cell number and morphology at high irradiance (480 µmol PAR quanta/(m2·s)) and NaCl level (160 or 200 g/liter) in the cultivation medium – the most efficient inducers of secondary carotenoid accumulation in Dunaliella [2-5].
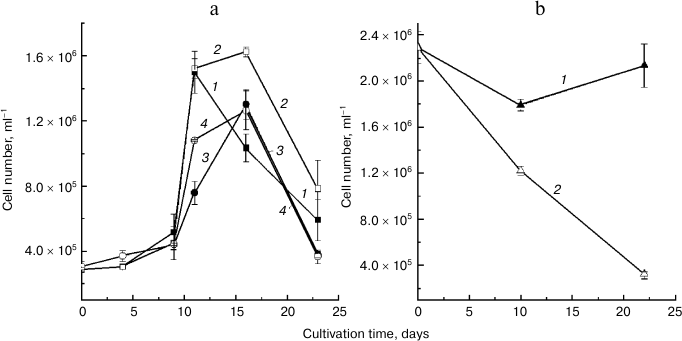
The strains identified as belonging to different species exhibited different trends in cell number changes during cultivation (figure). Thus, the D. salina BS1 and BS2 cultures (figure, panel (a)) had a low growth rate during the first nine days of cultivation. After this period, the growth rate increased sharply, leading to a 3-4-fold increase in cell number. At the 16th (or, in some cases, at the 11th) day of cultivation, the culture growth slowed and a sharp decline in cell number took place. We did not detect a profound effect of NaCl concentration in the medium the growth rate in these strains. Cell death was observed in the D. viridis R5 cultures initiated at the same cell density and grown under similar conditions as the cultures of D. salina (not shown). Although an increase in the initial cell density of the D. viridis R5 cultures prevented the cell death, no detectable culture growth was observed at 160 g/liter NaCl (figure, panel (b), curve 1). Still, there was a sizeable decline in the cell number in the cultures grown at elevated salinity level (200 g/liter NaCl; figure, panel (b), curve 2).
Changes in cell number of microalgae (a) D. salina and (b) D. viridis grown at high irradiance in medium containing 160 (1, 3) or 200 g/liter NaCl (2, 4).
The cultivation of the microalgae under conditions inducing carotenogenesis was accompanied by species-specific changes in cell morphology. In the case of D. viridis R5, the cell coloration changed from green to yellow, and colorless inclusions appeared in the cytoplasm (Fig. S2 in the Supplement). The cells of D. salina BS1 and BS2 increased their size, assumed a roundish form, and accumulated large globules in the cytoplasm. The cells of these strains acquired first yellowish and then uniformly orange-red of brown-red coloration (Fig. S2) typical of the carotenogenic Dunaliella species [1-4].
The pre-cultures of D. viridis R5 grown under the optimal conditions had similar pigment composition, high Chl content and, according to the results of the HPLC analysis, relatively low proportion of β-carotene in total Car (Table 1), typical of primary Car of green microalgae and higher plants grown under optimal conditions [22]. The cells of D. salina BS1 and BS2 pre-cultures were characterized by a 15-20% higher proportion of β-carotene in total Car (Table 1).
Table 1. Pigment composition of studied
halophile microalgae from the genus Dunaliella on the culture
initiation (0 day) and after 23 days of cultivation at 480 µmol
photons/(m2·s) in the presence of different NaCl
concentrations in the medium
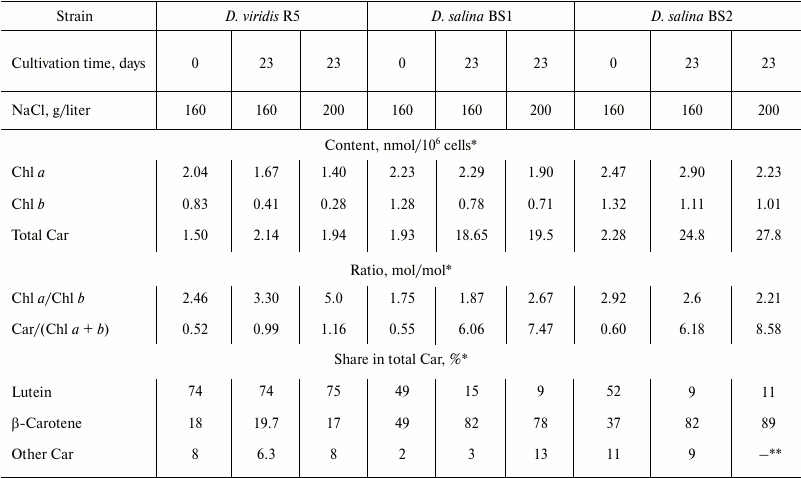
* Standard error (<5% of the average) is not shown.
** Traces.
A decline in total Chl along with increase in Chl a/b ratio was normally observed in the course of cultivation under stressful conditions. An increase in absolute Car content as well as Car/Chl ratio also comprised a common trend, but the studied strains differed dramatically in the magnitude of the changes. Thus, approximately 1.5-fold increase in Car content and Car/Chl ratio was recorded in D. viridis R5, whereas in D. salina BS1 and BS2 these parameters increased more than by one order of magnitude (Table 1). At the same time, the Car profile of D. viridis R5 remained essentially unchanged. By contrast, the Car composition of D. salina BS1 and BS2 was characterized by an increase in the proportion of β-carotene up to 75-88% of total Car, mainly at the expense of a corresponding decline in lutein (Table 1). The decline in absolute content of lutein serving mainly the function of light harvesting is in accord with the observed decline in Chl. These phenomena might manifest a typical for microalgae response to stresses involving the reduction of the light harvesting antenna in order to mitigate the risk of photooxidative damage, which can be high under stress [23].
The processes described above were accompanied by a characteristic dynamics of total cell lipid FA profile (Table 2). In all cases studied, the major FA were myristic (14:0, 0.8-12.8% of total FA), palmitic (16:0, 22.4-38.3%), palmitoleic (Δ9-16:1, 1.8-11.9%), hexadecatetraenoic (Δ4,7,10,13-16:4, 0.8-13.6%), stearic (18:0, 0.7-11.4%), and, in the case of D. viridis, α-linolenic (Δ9,12,15-18:3, 16.8-36.9%) acid as well. The minor FA included pentadecanoic (15:0), margaric (17:0), arachidic (20:0), and cis-vaccenic (Δ7-18:1) acids (<0.3% of total FA). The proportion of myristic acid in total cell lipids of D. salina BS1 grown at 160 g/liter NaCl declined 2.3-fold, whereas in the other strains this FA increased considerably (2.6-12.8 times). At the same time, D. salina BS1 and D. viridis displayed an increase in palmitic and stearic FA, suggesting vigorous biosynthesis of myristate, which is converted to palmitate and further elongated to stearate. The latter two saturated FA are known as the major FA of reserve lipids – triacylglycerols [24]. Similar changes of FA composition were recorded in strain BS2 as well as during cultivation of D. salina in the presence of 200 g/liter NaCl. Noteworthy, trienoic FA (represented mainly by α-linolenic acid associated predominantly with the thylakoid membrane glycolipids [24]) declined considerbly in D. viridis. In cells grown in the presence of 160 g/liter NaCl, this process occurred on the background of an increase in dienoic FA, whereas more intense salinity stress (200 g/liter NaCl) promoted an increase in monoenoic FA. These changes in the FA composition might reflect the operation of two stress acclimation mechanisms: reduction of the thylakoid membrane and induction of reserve lipid biosynthesis providing a sink for the excessive photosynthates appearing as a result of slowing of cell division [25]. This is a plausible explanation of a stronger correlation between accumulation of β-carotene and increase in palmitate or oleate (but not with the increase in total FA) in cells of carotenogenic Dunaliella species [10]. The observed features of FA composition are also consistent with the currently accepted central role of FA biosynthesis in the buildup of carotenogenic response. FA biosynthesis is the source of the building blocks of triacylglycerols, the major constituents of plastoglobuli harboring the secondary β-carotene accumulating under stress [8, 12].
Table 2. Changes in total cell lipid fatty
acid composition of the studied Dunaliella strains grown for
nine days at 480 µmol photons/(m2·s) in medium
containing 160 g/liter NaCl
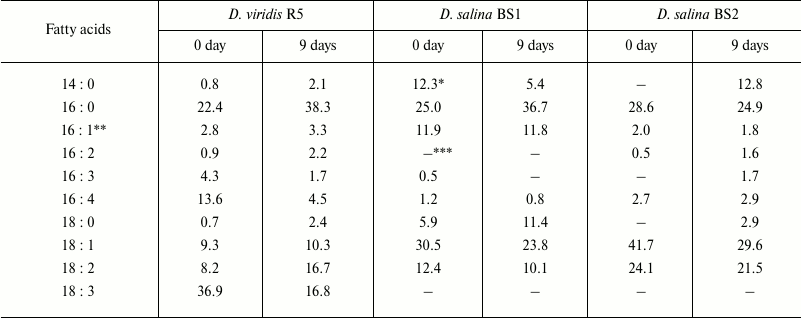
* Percentage of total FA (standard error <5% of average).
** Sums of all isomers of corresponding FA are specified.
*** Not detected.
Summarizing the data available in the literature and obtained in the present work, we conclude that the capability of secondary carotenogenesis is a key determinant of the strategy of acclimation to harsh environmental conditions. Thus, species of Dunaliella lacking the ability to accumulate high amounts of secondary carotenoids (e.g. D. viridis) respond to high light and salinity stresses mostly by decline in Chl, reduction of the thylakoid membrane apparatus [26, 27], and accumulation of osmoprotectants such as glycerol [2]. Carotenogenic Dunaliella species such as D. salina, apart from a decline in Chl, accumulate β-carotene, enhancing cell protection against the stresses by optical screening [28] and a possible local antioxidant effect [29]. Furthermore, the biosynthesis of FA and neutral lipids coordintaed with the accumulation of secondary Car might provide an additional sink for excesive photsynthates, thereby lowering the risk of photooxidative damage, which is especially high under the combination stress (high light intensity and high salinty) [26].
Judging from the growth curves, the carotenogenic species D. salina more efficiently acclimated to the stresses simulated under our experimental conditions that the non-carotenogenic D. viridis; the adverse effects of the elevated salinity were much less expressed in D. salina than in D. viridis (figure). Collectively, a considerably higher stress tolerance together with the capability of β-carotene accumulation suggest that the new D. salina strains BS1 and BS2 have considerable potential for biotechnological production of this value-added pigment.
The authors are grateful to Dr. A. O. Plotnikov for his assistance in obtaining of the microalgal cultures and Dr. T. A. Fedorenko for her help with DNA isolation.
Funding by the Russian Scientific Found is greatly appreciated (project No. 14-50-00029).
REFERENCES
1.Borowitzka, M., and Siva, C. (2007) The taxonomy of
the genus Dunaliella (Chlorophyta, Dunaliellales) with emphasis
on the marine and halophilic species, J. Appl. Phycol.,
19, 567-590.
2.Borowitzka, M. (2013) in Handbook of Microalgal
Culture: Applied Phycology and Biotechnology (Richmond, A., and Hu,
Q., eds.) 2nd Edn., Blackwell, N. Y., pp. 359-368.
3.Ben-Amotz, A., and Shaish, A. (1992) in
Dunaliella: Physiology, Biochemistry, and Biotechnology (Avron,
M., and Ben-Amotz, A., eds.) CRC, Boca Raton, pp. 205-216.
4.Ben-Amotz, A., Katz, A., and Avron, M. (1982)
Accumulation of β-carotene in halotolerant alge: purification and
characterization of β-carotene-rich globules from Dunaliella
bardawil (Chlorophyceae), J. Phycol., 18,
529-537.
5.Oren, A. (2005) in Adaptation to Life at High
Salt Concentrations in Archaea, Bacteria, and Eukarya
(Gunde-Cimerman, N., Oren A., and Plemenitas, A., eds.) Springer, N.
Y., pp. 493-502.
6.Ben-Amotz, A. (2004) in Handbook of Microalgal
Culture: Biotechnology and Applied Phycology (Richmond, A., ed.)
Blackwell, N. Y., pp. 273-280.
7.Semenenko, V., and Abdullaev, A. (1980) Parametric
control of β-carotene biosynthesis in Dunaliella salina
cells under conditions of intensive cultivation, Russ. J. Plant
Physiol., 27, 31-41.
8.Lamers, P., Janssen, M., de Vos, R., Bino, R., and
Wijffels, R. (2012) Carotenoid and fatty acid metabolism in
nitrogen-starved Dunaliella salina, a unicellular green
microalga, J. Biotechnol., 162, 21-37.
9.Ye, Z.-W., Jiang, J.-G., and Wu, G.-H. (2009)
Biosynthesis and regulation of carotenoids in Dunaliella:
progresses and prospects, Biotechnol. Adv., 26,
352-360.
10.Lamers P., Van de Laak, C., Kaasenbrood, P.,
Lorier, J., Janssen M., de Vos R., Bino, R., and Wijffels, R. (2010)
Carotenoid and fatty acid metabolism in light-stressed Dunaliella
salina, Biotechnol. Bioeng., 106, 638-648.
11.Solovchenko, A. (2013) Physiology and adaptive
significance of secondary carotenogenesis in green microalgae, Russ.
J. Plant Physiol., 60, 3-16.
12.Rabbani, S., Beyer, P., Lintig, J., Hugueney, P.,
and Kleinig, H. (1998) Induced β-carotene synthesis driven by
triacylglycerol deposition in the unicellular alga Dunaliella
bardawil, Plant Physiol., 116, 1239-1248.
13.Nemtseva, N., Selivanova, E., Ignatenko, M., and
Sharapova, N. (2013) Characterization of a novel Dunaliella
salina (Chlorophyta) strain and the assessment of its cultivation
parameters, Russ. J. Plant Physiol., 60, 529-535.
14.Chivkunova, O., Fedorenko T., and Lobakova, E.
(2015) Similarity and diversity of the Desmodesmus spp.
microalgae isolated from associations with white sea invertebrates,
Protoplasma, 252, 489-503.
15.Sambrook, J., and Russell, D. W. (2006)
Purification of nucleic acids by extraction with phenol: chloroform,
CSH Protoc., doi: 10.1101/pdb.prot4455.
16.Zedek, F., Smerda, J., Smarda, P., and Bures, P.
(2010) Correlated evolution of Ltr retrotransposons and genome size in
the genus eleocharis, BMC Plant Biol., 10, 265.
17.Altschul, S., Gish, W., Miller, W., Myers, E.,
and Lipman, D. (1990) Basic local alignment search tool, J. Mol.
Biol., 215, 403-410.
18.Tamura, K., Stecher, G., Peterson, D., Filipski,
A., and Kumar, S. (2013) MEGA6: molecular evolutionary genetics
analysis version 6.0, Mol. Biol. Evol., 30,
2725-2729.
19.Edgar, R. C. (2004) Muscle: multiple sequence
alignment with high accuracy and high throughput, Nucleic Acids
Res., 32, 1792-1797.
20.Wellburn, A. (1994) The spectral determination of
chlorophyll a and chlorophyll b, as well as total
carotenoids, using various solvents with spectrophotometers of
different resolution, J. Plant Physiol., 144,
307-313.
21.Assuncao, P., Jaen-Molina, R., Caujape-Castells,
J., Wolf, M., Buchheim, M. A., Jara, A., Freijanes, K., Carmona, L.,
and Mendoza, H. (2013) Phylogenetic analysis of ITS2 sequences suggests
the taxonomic re-structuring of Dunaliella viridis
(Chlorophyceae, Dunaliellales), Phycol. Res., 61,
81-88.
22.Lichtenthaler, H. (1987) Chlorophyll and
carotenoids: pigments of photosynthetic biomembranes, Methods
Enzymol., 148, 331-382.
23.Erickson, E., Wakao, S., and Niyogi, K. K. (2015)
Light stress and photoprotection in Chlamydomonas reinhardtii,
Plant J., 82, 449-465.
24.Guschina, I. A., and Harwood, J. L. (2013) in
Algae for Biofuels and Energy (Borowitzka, M., and Moheimani,
N., eds.) Springer, Dordrecht, pp. 17-36.
25.Solovchenko, A. (2012) Physiological role of
neutral lipid accumulation in eukaryotic microalgae under stresses,
Russ. J. Plant Physiol., 59, 167-176.
26.Dubinsky, Z., and Stambler, N. (2009)
Photoacclimation processes in phytoplankton: mechanisms, consequences,
and applications, Aquatic Microb. Ecol., 56, 163-176.
27.Berner, T., Dubinsky, Z., Wyman, K., and
Falkowski, P. (1989) Photoadaptation and the “package”
effect in Dunaliella tertiolecta (Chlorophyceae), J.
Phycol., 25, 70-78.
28.Ben-Amotz, A., Shaish, A., and Avron, M. (1989)
Mode of action of the massively accumulated β-carotene of
Dunaliella bardawil in protecting the alga against damage by
excess irradiation, Plant Physiol., 86, 1286-1291.
29.Shaish, A., Avron, M., Pick, U., and Ben-Amotz,
A. (1993) Are active oxygen species involved in induction of
β-carotene in Dunaliella bardawil? Planta,
190, 363-368.
Supplementary Methods, Figures S1 and S2 (PDF)