REVIEW: Diseases and Aging: Gender Matters
V. A. Popkov1, E. Yu. Plotnikov1*, D. N. Silachev1, L. D. Zorova2, I. B. Pevzner1, S. S. Jankauskas1, S. D. Zorov3, V. A. Babenko3, and D. B. Zorov1*
1Lomonosov Moscow State University, Belozersky Institute of Physico-Chemical Biology, 119991 Moscow, Russia; fax: +7 (495) 939-0338; E-mail: plotnikov@genebee.msu.ru; zorov@genebee.msu.su2Lomonosov Moscow State University, International Laser Center, 119991 Moscow, Russia
3Lomonosov Moscow State University, Faculty of Bioengineering and Bioinformatics, 119991 Moscow, Russia
* To whom correspondence should be addressed.
Received July 21, 2015; Revision received September 1, 2015
At first glance, biological differences between male and female sex seem obvious, but, in fact, they affect a vast number of deeper levels apart from reproductive function and related physiological features. Such differences affect all organizational levels including features of cell physiology and even functioning of separate organelles, which, among other things, account for such global processes as resistance to diseases and aging. Understanding of mechanisms underlying resistance of one of the sexes to pathological processes and aging will allow taking into consideration gender differences while developing drugs and therapeutic approaches, and it will provide an opportunity to reproduce and enhance such resistance in the more vulnerable gender. Here we review physiological as well as cellular and biological features of disease course including aging that are affected by gender and discuss potential mechanisms behind these processes. Such mechanisms include features of oxidative metabolism and mitochondrial functioning.
KEY WORDS: mortality, aging, females, males, myocardial infarction, stroke, hormones, estrogen, mitochondriaDOI: 10.1134/S0006297915120032
Abbreviations: ROS, reactive oxygen species.
It is commonly accepted that, on average, women live longer than men do:
expected lifespan for women in developed countries is on average five
years longer than for men. Partially, this depends on social roles and
life style men and women follow. Nonetheless, in developed countries,
where men and women have fewer differences in social atmosphere, the
difference in lifespan is bigger, not smaller, than in people of
opposite sexes in developing countries, except those conducting warfare
for a long time. Indeed, lifespan much depends on life style,
inclination to risk, and specific professions: even in Europe,
mortality from injuries in men is 5-8-fold higher than in women.
A 2012 statistical report released by the World Health Organization concerning the European population revealed that among the most common causes of death in all ages, mortality was lower in women than in men (a similar pattern is observed in global population, but the European population was more homogenous in terms of social roles played by men and women). No doubt, social factors still affect these statistical data, but such difference in mortality rate is considered quite ponderable only for social differences. Furthermore, statistics on mortality rate among children under five having vanishingly small sex-related social differences do not come out from the overall picture [1].
INFLUENCE OF GENDER ON SEVERITY OF CLINICAL COURSE OF
DISEASES
In this section, we intend to avoid discussing hormonal and reproduction-related diseases due to extreme sex-dependence, which deserve to be examined separately, and focus on pathologies more common to both genders. Moreover, we review data obtained not only in humans, but in other animals as well, wherein hormonal changes might affect an organism in a completely different way.
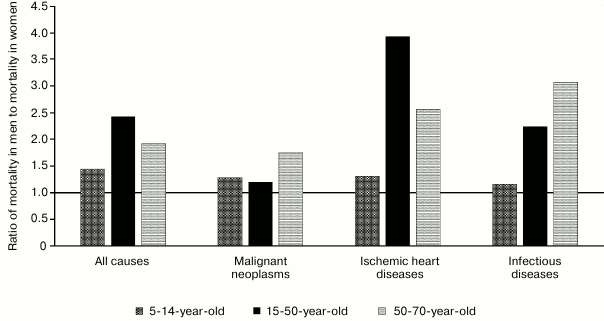
Altogether, mortality from cardiovascular diseases substantially anticipates mortality from all other diseases. The same group of diseases is characterized by one of the most evident differences between men and women, wherein many diseases in women begin to develop with a 10-year delay, including myocardial infarction [2-4], ischemic heart disease [3], heart failure [5], cardiomyopathies [6], and arrhythmias [7]. Many diseases tend to lose “protective effect” related to female gender during the postmenopausal period [8-12]. Mortality of women from myocardial infarction is higher at young age [13], whereas in men – at older age [14]. Death from myocardial rupture is more common in men [15]. Hence, disturbances related to hormone regulation such as diabetes or obesity are more serious risk factors in women with myocardial infarction [2], coronary artery disease [16], and hypertension [17, 18] (Fig. 1).
Fig. 1. Total all-cause, malignant neoplasm, ischemic heart disease, and infectious disease mortality in men and women of age groups 5-14, 15-50, and 50-70 in Europe in 2012 according to the World Health Organization.
On the contrary, autoimmune diseases are much more common in women (occurrence for some of them is higher by up to 10-fold [19]). Hence, men might have poorer prognosis in case a disease has eventually been developed [20, 21].
Renal failure in men develops twice faster [22, 23], and, clinically, male gender is considered as a serious aggravating factor: in particular, in men, the incidence of end-stage renal failure is higher by 10% [24]. However, signs of anemia in men are attenuated during this pathology, whereas in women they are aggravated. In case renal failure is burdened with diabetes, then female gender would be considered as an aggravating factor [25].
In addition, significant differences between males and females are found in manifestation of some neurological diseases. For instance, multiple sclerosis is more common in women [26]; however, men have poorer prognosis of the disease progressing [27]. In women, prognosis of stroke is poorer and its incidence is higher by 2-3-fold [28-30]. In addition, focal epilepsy is more common in men, whereas idiopathic epilepsy – in women [31].
The examples presented above are among those that have very prominent gender differences; however, small differences in pathogenesis and clinical manifestations of diseases as well as mortality rate and survival and reactions to drugs were observed in virtually all disease groups (for more information see [32]).
Nonetheless, sufficient examples were provided allowing understanding that: after occurring, many diseases develop in men and women in a different way, thus partially removing an issue regarding social factors. These include cases when a certain substance acts on representatives of one gender, but not of other one. For example, healthy men respond to injected angiotensin II by elevating glomerular filtration rate, whereas women – do not [33], thus evidencing higher capacity to increase blood pressure in glomeruli and significant influence on response against damaging impacts in men.
MECHANISMS UNDERLYING INFLUENCE OF SEX HORMONES ON DISEASES AND
MORTALITY
Apart from socio-behavioral factors such as risk proneness and dangerous habits, features of disease manifestation and mortality in men and women are apparently determined by biological differences, undoubtedly exhibited at the level of the whole organism. However, they are less evident at the level of separate organs, whereas dissimilarities between single cells, organelles, and molecules are found quite seldom. These differences at all mentioned organizational levels will be discussed below. Unfortunately, many experimental studies were done not with people or human cell cultures, but with animals or animal cells. This imposes routine biological limitations on interpreting such data. Nonetheless, within the scope of this review, we will assume that it is acceptable to use data obtained for other animals for discussing general regularities also being true for humans.
Physiological differences. On average, men’s body weight is greater by 15% compared to women [34]. The former have stronger bones, firmer tendons and ligaments [35], and higher skeletal muscle mass/body weight ratio [36]. In contrast, women have higher percentage of fat tissue. Muscular strength of men exceeds, on average, that of women by 40-60% [37]. In men, lung volume is greater by 56% even after being adjusted for body weight [38].
In men, the heart is larger in terms of mass and size; in particular, the left ventricle is larger than in women by 30% [39]. On the other hand, blood pressure is lower in women, and heart beat rate is higher, even during sleep. In the blood of men, the concentration of red blood cells and hemoglobin is higher and consequently oxygen capacity of the blood, whereas women contain more white blood cells including granulocytes and B- and T-cells. Men are known to have faster wound healing and have higher pain threshold. Due to vasoconstriction, women have cooler outer but warmer inner layers of the skin, giving them lower heat loss.
In addition, women have smaller kidneys [40] containing 15% less glomeruli [41], and such differences become most evident after reaching sexual maturity [42]. This is accounted for by hypertrophy of the cells in proximal and, to a lesser degree, distal tubules in response to male androgens [43]. As a result, blood flow via the kidney is higher in men, whereas women have higher tone of renal vessels, which is also mediated by sex hormones [44].
There is data showing that men’s brain has larger volume when size-adjusted to body weight [45]. The bed nuclei of terminal stria and interstitial nuclei of the anterior hypothalamus are twice bigger in men and contain 2-fold more neurons (interestingly, transsexual subjects have characteristics of the “chosen” sex rather than the one they were born with). It was demonstrated that bodies of myelinated axons are longer in men [46], and the number of synapses per cubic millimeter in cortex was more by 33% [47]. It should be noted that studies on gender differences are numerous yet controversial; therefore, any conclusions on occurrence or lack of differences must be taken with some skepticism.
Differences at cell level. Physiological and anatomical differences between men and women both in normal and pathological settings also imply differences in energy turnover. It is clear that men demand higher oxygen consumption due to differences in structure of muscles, brain, heart functioning, temperature regime, and oxygen capacity of blood. Moreover, the most obvious differences among diseases are observed in a group of cardiovascular pathologies and inflammatory reactions, wherein a significant, if not leading, impact is commonly accepted to be played by mitochondria [48]. Due to this, it is conventional to assume that men and women might have differences in terms of mitochondrial functions, redox homeostasis, and response to oxidative stress.
It has been demonstrated that in women basal metabolism adjusted to body cell mass is lower than in men [49-52]. The system of oxygen transport also depends on gender: partial oxygen pressure at 50% O2-saturation of hemoglobin is significantly higher in women than in men [53], which is imposed upon already mentioned low number of erythrocytes and overall hemoglobin. A series of studies showed that cells derived from men are observed to have redox state shifted towards more oxidized state compared to women [54].
In particular, smooth muscle cells in blood vessels from healthy male rats were found to have higher concentration of hydrogen peroxide, whereas females contained higher concentration of reduced glutathione and higher activity of superoxide dismutase and catalase [54-56]. Moreover, rats were shown to have upregulated amounts of carbonylated proteins in male vs. female rat liver [57] and brain [58].
Smooth muscle cells in blood vessels isolated from male rats were less resistant to UV-induced oxidative stress compared to the cells from females: males were noted to have cells containing higher levels of 4-hydroxynonenal (4-HNE) (a product of membrane lipid oxidation). In addition, more cells from male rats were found to die by anoikis (a type of apoptosis related to losing contact with basal membrane), whereas female cells were resistant to it and were more prone to autophagy rather than to apoptosis. It is suggested that upregulated concentration of reactive oxygen species (ROS) in male cells can activate some metalloproteases, causing degradation of some proteins required for resistance to anoikis. It has been demonstrated that in male cells oxidative stress results in more pronounced drop of such enzymes compared to females [54].
It has been well examined that autophagy, mitochondrial functioning, oxidative stress, and apoptosis are linked to heart and liver diseases, diabetes, and other pathologies [59, 60]. Moreover, it was also observed in terms of gender differences: it was demonstrated that autophagy markers are differently expressed in various tissues depending on gender. On average, males express more such markers that might be related to elevated concentration of ROS [61]. However, oxidative stress or starvation result in upregulated number of autophagy markers in females, but they decline in males, which is accompanied by a greater tendency to apoptosis as shown in cultured neurons, fibroblasts, and vascular smooth muscle cells [54, 62, 63].
It has been found [55] that in males, vascular smooth muscle and endothelial cells had lower expression of RLIP76-transporter required for energy-dependent transport of various substances out of the cells, including GS-HNE, a product of lipid oxidation, which can be accumulated which results in apoptosis. Hence, male cells were more sensitive to the effect of hydrogen peroxide, and males had higher concentration of 4-HNE both in control and under stress. Interestingly, in females, estrogen caused almost 2-fold upregulated expression of RLIP76, but it had no effect in males. Similarly, addition of anti-RLIP76 antibodies or specific siRNA abolished protective effects in females as well as decreased amount of reduced glutathione, but had no effect in males. This quite convincingly demonstrates that differences between females and males are not only of quantitative, but also of qualitative character, and it is obvious that the major defense mechanisms against oxidative stress in males are not based on activity of this protein compared to females.
Even more representative was the case with animals mutated for the histone deacetylase 5 gene (HDAC5) resistant to applied stimuli: such modification also inducing disturbed functions and morphology of heart mitochondria was lethal in 100% of males, but in only 25% of females. Estrogen administered to transgenic males lowered mortality rate to the level observed in females [64].
Differences mediated by hormonal background. Because sex hormones play quite extensive and important roles without “narrowing” the range of their functions, they were not examined above. It is commonly believed that it is estrogen (as a major female hormone) that provides resistance to diseases in women, whereas testosterone (as the major male hormone), in contrast, accounts for severe manifestation of diseases in men. Female mice more easily overcome myocardial infarction, and administration of testosterone worsens outcome, whereas in males administration of estrogen affords protective effect [15]. Androgens elevate blood pressure in males, whereas castration decreases it [65, 66]. Moreover, castration of male rats lowers renal injury during ischemia, whereas excision of ovaries in females aggravates the injury. Testosterone administered to males and females lacking reproductive glands potentiates injuries, whereas estrogen attenuates injury in ovariectomized females [9]. Treatment of postmenopausal women with estrogen ameliorates the manifestation of some types of renal failure [67]. This data cannot be explained by antioxidant properties exhibited by estrogen itself, as its concentration in the blood serum is quite low.
Estradiol displayed remarkable cardioprotective effects in a model of cardiac trauma in rats, which are also mediated by the influence of the hormone on mitochondrial transcription factor A (TFAM), ATP metabolism, and mitochondrial functioning [68].
It is believed that estrogen and testosterone regulate activity of NADPH oxidase, which in females and males results in downregulated and upregulated production of ROS, respectively [69, 70].
Giordano et al. showed that astrocytes from male mice and rats are more sensitive to oxidative stress compared to females. It is considered that this effect is related to expression of paraoxonase 2 (PON2) as an antioxidant protein associated with mitochondria, which was higher in the brain from animals of a control group, but not in males or castrated animals. Cells knocked out by the gene encoding this protein in males and females did not differ in terms of sensitivity to prooxidants such as hydrogen peroxide or 2,3-dimethoxy-1,4-naphthoquinone. Estradiol caused upregulated expression of this protein in both genders and enhanced cell resistance to stress triggered by these agents, but it did not affect PON2 knockout cells [71].
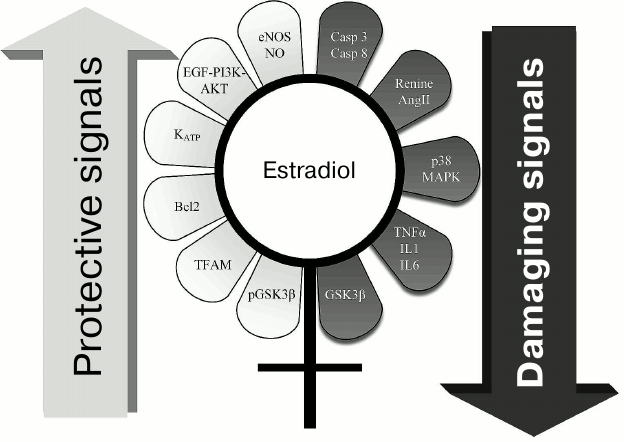
Sex hormones display multifaceted regulatory functions. Gender-dependent differences considered as the most interesting in the light of the discussed pathologies are summarized in Fig. 2. In particular, females contained glycogen synthase kinase 3β (GSK3β) inactivated via phosphorylation, which is a key enzyme in signaling mechanisms in ischemic injury and anti-ischemic defense [72, 73]. Also, the p38 MAPK signaling pathway is more inactivated in females, and they have lower levels of proinflammatory cytokines TNFα, IL-1, IL-6 [74, 75]. On the contrary, endothelial NO synthase is activated in females [76, 77]. Altogether, these changes are related to defense of various tissues against ischemic injuries.
Fig. 2. Range of signaling pathways somehow influenced by estrogen that might be responsible for higher resistance to diseases in females vs. males. Left: mechanisms responsible for antiapoptotic signals and providing higher cell viability are depicted; estrogen stimulates these pathways. Right: potential pathological or even “lethal” estrogen-inhibited signals for cells are depicted.
Study done on expression of the genes associated with mitochondrial functioning demonstrated that gender differences vary over the lifetime and differ with age [78]. In particular, young females and males differed by expression of genes associated with metabolism of fatty acids and apoptosis. At mature age, differences in expression of genes encoding pyruvate dehydrogenase complex added up, and the difference in apoptotic genes becomes more distinct (males are found to have upregulated expression of proapoptotic genes). By old age, differences in expression of genes encoding products of oxidative phosphorylation, particularly complex I and IV, are more upregulated in females. This study agrees both with assumptions regarding differences in mitochondrial metabolism peaking with age and numerous evidences that male cells are more prone to apoptosis.
Expression of the genes responsible for metabolism of fatty acids was more upregulated in females than in males upon hypertrophic response to exercise, which may point at a role of gender differences in terms of availability of energy substrates in the heart [79, 80]. Similar suggestions followed from a study particularly demonstrating that a shift towards using ketone bodies and fatty acids as energy substrates was more pronounced in age-related male vs. female brain, and estradiol antagonized such changes in favor of glucose oxidation [81]. Sterilization of females resulted in profoundly reduced glucose consumption and elevated utilization of ketone bodies and fatty acids [82].
There is data suggesting that estrogen receptors can be activated by various growth factors, not just estrogen alone. Moreover, in various tissues peak activity of these receptors can be observed at different phases of the ovarian cycle in females, regardless of total concentration of estrogens [83].
Clinical data regarding influence of hormone therapy on diseases in humans are not as unambiguous as in animals. Men suffering from prostate cancer and undergoing androgen suppression or estrogen therapy also had higher risk of developing cardiovascular diseases [84-86]. Moreover, men with low testosterone level had higher, but not lower, risk of developing cardiovascular diseases compared to healthy men [87]. Interesting studies were done with transsexual individuals: male-to-female transsexual subjects treated with estrogens and anti-androgens were found to have higher rate of cardiovascular diseases than female-to-male transsexual subjects receiving testosterone or the control population [88].
Indeed, among women mortality from cardiovascular diseases starts to grow after menopause, but such changes are not as pronounced as lowered malignancy of mammary neoplasms during this period of life [89]. This suggests that altered pattern of mortality causes can just reflect age-related processes and has no direct link to a declined level of estrogens. In addition, cardiovascular diseases are characterized by a long-term latent period preceding clinical manifestations. Alternatively, it can be assumed that a greater role is played by accelerated course of latent stage of disease in men at early age rather than proper or attenuated defense of women during the premenopausal or postmenopausal period, respectively.
Genetic differences. All the above-mentioned points to an important role for sex hormones in discussed differences between genders. However, it is important to understand that the cells from men and women also differ genetically at the level of sex chromosomes. Approximately 200 genes are found on the Y-chromosome, among which 72 genes encode proteins. Such a small number of genes is related to the fact that Y-chromosome bears a very high level of mutations caused by high number of deletions occurring upon maturation of spermatozoa as well as due to high levels of oxidative stress due to their active motility. In addition, it is overlaid by an extremely ineffective selection due to inability to recombine with the X-chromosome. Altogether, this results in gradual transfer of vital genes onto the X-chromosome, whereas the genes required exclusively in males and, perhaps, harmful to females stay on the Y-chromosome. In the light of these data, it can be assumed that these genes are harmful for males as well, but required for some reason. It is predicted that quite soon in evolutionary terms the human Y-chromosome would lose all its genes. Among mammals, there are several species that came down to this stage, e.g. the mole vole (Ellobius tancrei), wherein all individual animals demonstrate XX phenotype [90], whereas in transcaucasian mole vole (Ellobius lutescens) all individual animals have X0 phenotype [91]. In men, cells lacking a Y-chromosome can occur with age, which correlates with shortened lifespan as well as with higher probability to develop some types of cancer. Nonetheless, this seems to be rather a consequence than a cause of some pathologies [92]. Moreover, there is no disease unrelated to reproductive function that has been found to have a link with mutations in the genes on Y-chromosome. Lack of vital genes on the Y-chromosome is quite logical, since half of humanity carries good life without them.
For the reason that, experimentally, it is easier to manage the level of hormones than expression of the genes in sex chromosomes, the impact by hormones is much more examined and understood compared to direct effect of genes on sex chromosomes. Direct influence of a genetic component was evidenced by medical statistics on mortality of prepubertal children as well as numerous cases of physiological differences often being already observed postnatally and potentiated after pubertal maturation. However, one should not forget that it is mainly impossible to distinguish between these two effects: impact by sex hormones is exhibited already during embryonic development, so synthesis of testosterone begins in the mid-first trimester of pregnancy, whereas XY-carriers with disturbed testosterone metabolism can phenotypically look like females [93]. Even relatively low level of sex hormones found before pubertal maturation or during embryonic development might significantly influence all organismal systems. Experiments done on isolated cell cultures unexposed to direct influence of blood hormones can be collateral evidence for direct impact of a genetic component. However, again one should not rule out that before isolating cells, hormones might exhibit epigenetic regulatory effects.
GENDER-RELATED INFLUENCE ON MITOCHONDRIAL FUNCTIONS
By considering an impact of mitochondria on gender differences, it should be noted that mitochondria are inherited as much as possible in a gender-related manner: in mammals, mitochondria are stringently inherited along maternal lineage. Evolutionary reasons for this were discussed in detail elsewhere [94] and, presumably, they are mostly accounted for by the need to exclude a “tug-of-war” between paternal and maternal mitochondrial genomes, which would inevitably result in “selfish” behavior of semiautonomous organelles and decreased overall body fitness. Moreover, it is related to a higher levels of oxidative stress applied to mitochondria in male gametes due to their active motility [94].
In humans, the mitochondrial genome encodes only 37 out of around 1000 genes required for their functioning [95]. Disturbed mutual “coordination” between nuclear and mitochondrial genomes [96] is related to a number of pathologies that gave rise to the term “mother’s curse” [97, 98], i.e. disturbed functioning of cells and tissues in males due to discoordinated activity between “paternal” nuclear and “maternal” mitochondrial genomes. Around 500 mutations both in coding and non-coding regions have been found in human mitochondrial DNA that are somehow associated with various diseases [99-103].
The nuclear genome symmetrically inherited from both parental organisms allows incorporation during selection of both paternal and maternal features of physiology and metabolism. However, maternally inherited mitochondria create asymmetry: efficient selection becomes possible only along the maternal lineage. This seems not to be a problem if a trait is common for men and women. However, if there were mutations harmful only to men, but not to women, then they would mainly escape selection unless they are not lethal. Primarily, it would unambiguously be applied to systems related to male reproductive function [100, 104, 105]. As a result, mutations specifically influencing the male organism would be accumulated in the mitochondrial genome [97, 106-110]. In the light of the data presented above, it can be assumed that mitochondria from females and males are not on the same page. Therefore, it would be quite possible to assume that there might exist mutations detrimental to males, but not to females.
Experiments with Drosophila flies having identical nuclear genome, but various mitochondria, showed that the latter profoundly affected lifespan and aging in males, but not females [109]. Moreover, by comparing mitochondrial genomes it was demonstrated that such phenotypic effect was rather linked to the number of small mutations distributed along the mitochondrial genome than mutations in any particular gene.
Technically, investigation of this effect in mammals is a much more complex issue. Nonetheless, male but not female mice with elevated number of mitochondrial mutations (due to modified mitochondrial DNA polymerase) were first shown to have significantly increased arterial blood pressure [111].
In turn, it might be a reason for males of most species with maternally inherited mitochondria to have shorter lifespan and early aging [98, 99, 102, 110, 112-114], as well as being more vulnerable to various diseases due to inability for rapid and proper selection of mitochondrial mutations, which are neutral (or even positive) for females, but detrimental to males. Now, two potential compensatory mechanisms lowering the influence of “mother’s curse” are expected. First, frequent inbreeding might create a sufficient indirect pressure on females for selection of such mutations. Second, another potential mechanism includes compensatory mutations within the nuclear genome to be selected in the male population [110]. This is why study on Drosophila was done by using flies having stringently similar nuclear genome, to avoid its influence.
It should be noted that another existing type of maternal inheritance known as inheritance of microbiota occurs, which is the assortment of microorganisms, primarily bacteria, inhabiting various niches of the multicellular organism. The impact of microbiota on the organism and various diseases is currently being extensively investigated and well appreciated [115]. At the same time, there is a viewpoint considering a parallel between bacteria and mitochondria, i.e. their assemblies known as microbiota and mitobiota [116]. In this case, the logic regarding “mother’s curse” stated above for mitochondria can be applied to microbiota as well, as due to physiological reasons (passage through the birth canal, feeding with mother’s milk) progeny obtains microbiota primarily from females.
GENDER AND AGING
A great body of data suggests that there is a connection between gender and aging. Both medical statistics and experimental data suggest the following: men age faster. In women, the onset for many diseases is shifted forward at least 5-10 years [2-7]. Moreover, earlier we provided evidence that men and women not only age with various speeds, but also differ in terms of age-related changes occurring in some pathways of their metabolism [78].
The mitochondrial theory of aging is in good agreement with the presented data – a large part of the differences between men and women mentioned above is precisely in mitochondrial functions. This raises a question: would it not be accounted for by some evolutionary necessity? On one hand, such differences might be explained by the mentioned “mother’s curse” and inability to efficiently “adjust” female mitochondria (and, perhaps, female microbiota) to the features of the male organism during evolutionary selection. On the other hand, it might be supposed that a reason for this lies in different metabolic rates in females and males. At present, it is known that often the rate of basal metabolism inversely correlates with lifespan [117], which was confirmed in a huge number of studies examining the influence of caloric restriction on lifespan [118]. Given that, it is reasonable to assume that females live longer due to lower energy consumption [50-52], whereas, for the same reason, males “burn down” faster.
Finally, another possible explanation is based on the supposed evolutionary sense of aging. It is believed that aging is necessary for increasing selection pressure on new mutations, positive and negative, which barely affect a healthy and young organism, but may substantially influence a weakened subject. It should be noted that fecundity rate in men and women can differ by an order of magnitude. In particular, the highest fecundity rate in females was around 60 children, whereas in males – approximately 800 children. It is considered that male/female ratio at ~1 : 10 is sufficient to maintain normal population. According to the evolutionary theory of gender [119] and some theories of gender selection [120], an evolutionary pressure on males might be increased via aging and lowered resistance to diseases. By using a hypothetical aging program [121], it is possible to increase selection of males reducing their redundancy, thereby accelerating adaptation and evolution of species as a whole, whereas females are considered as a very precious resource from the point of maintaining population, and their loss might have been more detrimental. Moreover, due to a menopause (evolutionary aspects are also discussed in [122]) in human (and some other species) women over 50, in fact, are no longer involved in reproduction, whereas men retain reproductive capacity over entire lifetime. This results in an idea that aging in postmenopausal women lacks evolutionary “reason”, as they already ceased to be considered as subjects of evolution compared to men. Overall, a clear-cut increased tolerance and death risk, which are programmed in males, fairly fit in the general idea of phenoptosis as a death program [121].
In this review, we did not intend to provide an encyclopedic enumeration of all known gender differences, but attempted to delineate phenotypic manifestations of them at different organizational levels to fuel further analysis of mechanisms of gender influence on the manifestation of diseases and organism aging. In particular, evidence presented here give reason to believe that overall changes in energy turnover and mitochondrial functions in particular are among the main causes underlying gender differences. This is in good agreement with known data regarding mechanisms of many developing diseases. Asymmetry in the manifestation of diseases and the aging process between genders creates a unique setting: it becomes possible to examine features of healthy individuals unaffected by already ongoing pathologies, which will determine either resistance or predisposition to diseases in the future. Thus, studies on differences of disease manifestation occurring in both genders are necessary not only to take them into consideration while designing pharmaceuticals and applying treatment depending on a patient’s gender, but also for proposing novel therapeutic approaches based on a transfer of natural resistance of the more protected gender to the more vulnerable gender.
This study was performed with financial support by the Russian Science Foundation (project No. 14-15-00147).
REFERENCES
1.World Health Organization, Annual Report
(2012).
2.Anand, S. S., Islam, S., Rosengren, A., Franzosi,
M. G., Steyn, K., Yusufali, A. H., Keltai, M., Diaz, R., Rangarajan,
S., and Yusuf, S. (2008) Risk factors for myocardial infarction in
women and men: insights from the INTERHEART study, Eur. Heart
J., 29, 932-940.
3.Hochman, J. S., McCabe, C. H., Stone, P. H.,
Becker, R. C., Cannon, C. P., Defeo-Fraulini, T., Thompson, B.,
Steingart, R., Knatterud, G., and Braunwald, E. (1997) Outcome and
profile of women and men presenting with acute coronary syndromes: a
report from TIMI IIIB, J. Am. Coll. Cardiol., 30,
141-148.
4.Heer, T., Gitt, A. K., Juenger, C., Schiele, R.,
Wienbergen, H., Towae, F., Gottwitz, M., Zahn, R., Zeymer, U., and
Senges, J. (2006) Gender differences in acute non-ST-segment elevation
myocardial infarction, Am. J. Cardiol., 98, 160-166.
5.Deswal, A., and Bozkurt, B. (2006) Comparison of
morbidity in women versus men with heart failure and preserved ejection
fraction, Am. J. Cardiol., 97, 1228-1231.
6.Dimitrow, P., Czarnecka, D., Jaszcz, K., and
Dubiel, J. (1997) Sex differences in age at onset of symptoms in
patients with hypertrophic cardiomyopathy, J. Cardiovasc. Risk,
5, 33-35.
7.Humphries, K. H., Kerr, C. R., Connolly, S. J.,
Klein, G., Boone, J. A., Green, M., Sheldon, R., Talajic, M., Dorian,
P., and Newman, D. (2001) New-onset atrial fibrillation: sex
differences in presentation, treatment, and outcome,
Circulation, 103, 2365-2370.
8.Costenbader, K. H., Feskanich, D., Stampfer, M. J.,
and Karlson, E. W. (2007) Reproductive and menopausal factors and risk
of systemic lupus erythematosus in women, Arthritis Rheum.,
56, 1251-1262.
9.Sandberg, K. (2008) Mechanisms underlying sex
differences in progressive renal disease, Gend. Med., 5,
10-23.
10.Grodstein, F., Stampfer, M. J., Manson, J. E.,
Colditz, G. A., Willett, W. C., Rosner, B., Speizer, F. E., and
Hennekens, C. H. (1996) Postmenopausal estrogen and progestin use and
the risk of cardiovascular disease, N. Engl. J. Med.,
335, 453-461.
11.Harrison-Bernard, L. M., and Raij, L. (2000)
Postmenopausal hypertension, Curr. Hypertens. Rep., 2,
202-207.
12.Rosen, C. J. (2005) Postmenopausal osteoporosis,
Bone, 8, 595-603.
13.Vaccarino, V., Parsons, L., Every, N. R., Barron,
H. V., and Krumholz, H. M. (1999) Sex-based differences in early
mortality after myocardial infarction. National Registry of Myocardial
Infarction 2 Participants, N. Engl. J. Med., 341,
217-225.
14.Lundblad, D., Holmgren, L., Jansson, J.-H.,
Naslund, U., and Eliasson, M. (2008) Gender differences in trends of
acute myocardial infarction events: the Northern Sweden MONICA study
1985-2004, BMC Cardiovasc. Disord., 8, 17.
15.Cavasin, M. A., Tao, Z.-Y., Yu, A.-L., and Yang,
X.-P. (2006) Testosterone enhances early cardiac remodeling after
myocardial infarction, causing rupture and degrading cardiac function,
Am. J. Physiol. Heart Circ. Physiol., 290, 2043-2050.
16.Pajunen, P., Taskinen, M. R., Nieminen, M. S.,
and Syvanne, M. (2000) Angiographic severity and extent of coronary
artery disease in patients with type 1 diabetes mellitus, Am. J.
Cardiol., 86, 1080-1085.
17.Donahue, R. P., Rejman, K., Rafalson, L. B.,
Dmochowski, J., Stranges, S., and Trevisan, M. (2007) Sex differences
in endothelial function markers before conversion to pre-diabetes: does
the clock start ticking earlier among women? The Western New York
study, Diabetes Care, 30, 354-359.
18.Carlsson, A. C., Wandell, P. E., De Faire, U.,
and Hellenius, M. L. (2008) Risk factors associated with newly
diagnosed high blood pressure in men and women, Am. J.
Hypertens., 21, 771-777.
19.Aharon, A., Zandman-Goddard, G., and Shoenfeld,
Y. (1994) Autoimmune multiorgan involvement in elderly men is it SLE?
Clin. Rheumatol., 13, 631-634.
20.Prete, P. E., Majlessi, A., Gilman, S., and
Hamideh, F. (2001) Systemic lupus erythematosus in men: a retrospective
analysis in a Veterans Administration Healthcare System population,
J. Clin. Rheumatol., 7, 142-150.
21.Jimenez-Balderas, F. J., and Mintz, G. (1993)
Ankylosing spondylitis: clinical course in women and men, J.
Rheumatol., 20, 2069-2072.
22.Hannedouche, T., Chauveau, P., Kalou, F.,
Albouze, G., Lacour, B., and Jungers, P. (1993) Factors affecting
progression in advanced chronic renal failure, Clin. Nephrol.,
39, 312-320.
23.Neugarten, J., Acharya, A., and Silbiger, S. R.
(2000) Effect of gender on the progression of nondiabetic renal
disease: a meta-analysis, J. Am. Soc. Nephrol., 11,
319-329.
24.National Institutes of Health, National
Institutes of Diabetes and Digestive and Kidney Disease, D. of K.U. and
H.D. (2011) USRDS 2011 Annual Data Report: Atlas of Chronic Kidney
Disease and End-Stage Renal Disease in the United States, 2011.
25.Manigrasso, M. B., and Maric-Bilkan, C. (2013)
Peptides, sex, diabetes, and the kidney, in Handbook in Biologically
Active Peptides, pp. 1487-1493.
26.Sellner, J., Kraus, J., Awad, A., Milo, R.,
Hemmer, B., and Stuve, O. (2011) The increasing incidence and
prevalence of female multiple sclerosis. A critical analysis of
potential environmental factors, Autoimmun. Rev., 10,
495-502.
27.Beatty, W. W., and Aupperle, R. L. (2002) Sex
differences in cognitive impairment in multiple sclerosis, Clin.
Neuropsychol., 16, 472-480.
28.Howard, V. J., Cushman, M., Pulley, L., Gomez, C.
R., Go, R. C., Prineas, R. J., Graham, A., Moy, C. S., and Howard, G.
(2005) The reasons for geographic and racial differences in stroke
study: objectives and design, Neuroepidemiology, 25,
135-143.
29.Niewada, M., Kobayashi, A., Sandercock, P. A.,
Kaminski, B., and Czlonkowska, A. (2005) Influence of gender on
baseline features and clinical outcomes among 17,370 patients with
confirmed ischaemic stroke in the international stroke trial,
Neuroepidemiology, 24, 123-128.
30.Sheikh, K., and Bullock, C. M. (2007) Effect of
measurement on sex difference in stroke mortality, Stroke,
38, 1085-1087.
31.McHugh, J. C., and Delanty, N. (2008)
Epidemiology and classification of epilepsy: gender comparisons,
Int. Rev. Neurobiol., 83, 11-26.
32.Oertelt-Prigione, V., and Regitz-Zagrosek, V.
(2012) Sex and Gender Aspects in Clinical Medicine,
Springer-Verlag, London.
33.Miller, J. A., Anacta, L. A., and Cattran, D. C.
(1999) Impact of gender on the renal response to angiotensin II,
Kidney Int., 55, 278-285.
34.Ogden, C. L., Fryar, C. D., Carroll, M. D., and
Flegal, K. M. (2004) Mean body weight, height, and body mass index,
United States 1960-2002, Adv. Data, 1-17.
35.Benjamin, M., Toumi, H., Ralphs, J. R., Bydder,
G., Best, T. M., and Milz, S. (2006) Where tendons and ligaments meet
bone: attachment sites (“entheses”) in relation to exercise
and/or mechanical load, J. Anat., 208, 471-490.
36.Janssen, I., Heymsfield, S. B., Wang, Z. M., and
Ross, R. (2000) Skeletal muscle mass and distribution in 468 men and
women aged 18-88 yr, J. Appl. Physiol., 89, 81-88.
37.Miller, A. E. J., MacDougall, J. D., Tarnopolsky,
M. A., and Sale, D. G. (1993) Gender differences in strength and muscle
fiber characteristics, Eur. J. Appl. Physiol. Occup. Physiol.,
66, 254-262.
38.Glucksmann, A. (1981) Sexual Dimorphism in
Human and Mammalian Biology and Pathology, Academic Press,
London-New York.
39.Salton, C. J., Chuang, M. L., O’Donnell, C.
J., Kupka, M. J., Larson, M. G., Kissinger, K. V., Edelman, R. R.,
Levy, D., and Manning, W. J. (2002) Gender differences and normal left
ventricular anatomy in an adult population free of hypertension, J.
Am. Coll. Cardiol., 39, 1055-1060.
40.Neugarten, J., Kasiske, B., Silbiger, S. R., and
Nyengaard, J. R. (2002) Effects of sex on renal structure,
Nephron, 90, 139-144.
41.Nyengaard, J. R., and Bendtsen, T. F. (1992)
Glomerular number and size in relation to age, kidney weight, and body
surface in normal man, Anat. Rec., 232, 194-201.
42.Jean-Faucher, C., Berger, M., Gallon, C., De
Turckheim, M., Veyssiere, G., and Jean, C. (1987) Sex-related
differences in renal size in mice: ontogeny and influence of neonatal
androgens, J. Endocrinol., 115, 241-246.
43.Oudar, O., Elger, M., Bankir, L., Ganten, D.,
Ganten, U., and Kriz, W. (1991) Differences in rat kidney morphology
between males, females, and testosterone-treated females, Ren.
Physiol. Biochem., 14, 92-102.
44.Blantz, R. C., Peterson, O. W., Blantz, E. R.,
and Wilson, C. B. (1988) Sexual differences in glomerular
ultrafiltration: effect of androgen administration in ovariectomized
rats, Endocrinology, 122, 767-773.
45.Ankney, C. D. (1992) Sex differences in relative
brain size: the mismeasure of woman, too? Intelligence,
16, 329-336.
46.Marner, L., Nyengaard, J. R., Tang, Y., and
Pakkenberg, B. (2003) Marked loss of myelinated nerve fibers in the
human brain with age, J. Comp. Neurol., 462, 144-152.
47.Alonso-Nanclares, L., Gonzalez-Soriano, J.,
Rodriguez, J. R., and De Felipe, J. (2008) Gender differences in human
cortical synaptic density, Proc. Natl. Acad. Sci. USA,
105, 14615-14619.
48.Dongworth, R. K., Hall, A. R., Burke, N., and
Hausenloy, D. J. (2014) Targeting mitochondria for cardioprotection:
examining the benefit for patients, Future Cardiol., 10,
255-272.
49.Garaulet, M., Perez-Llamas, F., Fuente, T.,
Zamora, S., and Tebar, F. J. (2000) Anthropometric, computed
tomography, and fat cell data in an obese population: relationship with
insulin, leptin, tumor necrosis factor-alpha, sex hormone-binding
globulin and sex hormones, Eur. J. Endocrinol., 143,
657-666.
50.Ferraro, R., Lillioja, S., Fontvieille, A. M.,
Rising, R., Bogardus, C., and Ravussin, E. (1992) Lower sedentary
metabolic rate in women compared with men, J. Clin. Invest.,
90, 780-784.
51.Buchholz, A. C., Rafii, M., and Pencharz, P. B.
(2001) Is resting metabolic rate different between men and women?
Br. J. Nutr., 86, 641-646.
52.Arciero, P. J., Goran, M. I., and Poehlman, E. T.
(1993) Resting metabolic rate is lower in women than in men, J.
Appl. Physiol., 75, 2514-2520.
53.Humpeler, E., Vogel, S., Schobersberger, W., and
Mairbaurl, H. (1989) Red cell oxygen transport in man in relation to
gender and age, Mech. Ageing Dev., 47, 229-239.
54.Straface, E., Vona, R., Gambardella, L., Ascione,
B., Marino, M., Bulzomi, P., Canu, S., Coinu, R., Rosano, G., Malorni,
W., and Franconi, F. (2009) Cell sex determines anoikis resistance in
vascular smooth muscle cells, FEBS Lett., 583,
3448-3454.
55.Matarrese, P., Colasanti, T., Ascione, B.,
Margutti, P., Franconi, F., Alessandri, C., Conti, F., Riccieri, V.,
Rosano, G., Ortona, E., and Malorni, W. (2011) Gender disparity in
susceptibility to oxidative stress and apoptosis induced by
autoantibodies specific to RLIP76 in vascular cells, Antioxid. Redox
Signal., 15, 2825-2836.
56.Malorni, W., Campesi, I., Straface, E., Vella,
S., and Franconi, F. (2007) Redox features of the cell: a gender
perspective, Antioxid. Redox Signal., 9, 1779-1801.
57.Valle, A., Guevara, R., Garcia-Palmer, F. J.,
Roca, P., and Oliver, J. (2007) Sexual dimorphism in liver
mitochondrial oxidative capacity is conserved under caloric restriction
conditions, Am. J. Physiol. Cell Physiol., 293,
1302-1308.
58.Guevara, R., Santandreu, F. M., Valle, A.,
Gianotti, M., Oliver, J., and Roca, P. (2009) Sex-dependent differences
in aged rat brain mitochondrial function and oxidative stress, Free
Radic. Biol. Med., 46, 169-175.
59.Wang, E. Y., Biala, A. K., Gordon, J. W., and
Kirshenbaum, L. A. (2012) Autophagy in the heart, J. Cardiovasc.
Pharmacol., 60, 110-117.
60.Lee, J., Giordano, S., and Zhang, J. (2011)
Autophagy, mitochondria, and oxidative stress: cross-talk and redox
signalling, Biochem. J., 441, 523-540.
61.Campesi, I., Straface, E., Occhioni, S.,
Montella, A., and Franconi, F. (2013) Protein oxidation seems to be
linked to constitutive autophagy: a sex study, Life Sci.,
93, 145-152.
62.Cosper, P. F., and Leinwand, L. A. (2011) Cancer
causes cardiac atrophy and autophagy in a sexually dimorphic manner,
Cancer Res., 71, 1710-1720.
63.Du, L., Hickey, R. W., Bayir, H., Watkins, S. C.,
Tyurin, V. A., Guo, F., Kochanek, P. M., Jenkins, L. W., Ren, J.,
Gibson, G., Chu, C. T., Kagan, V. E., and Clark, R. S. (2009) Starving
neurons show sex difference in autophagy, J. Biol. Chem.,
284, 2383-2396.
64.Czubryt, M. P., McAnally, J., Fishman, G. I., and
Olson, E. N. (2003) Regulation of peroxisome proliferator-activated
receptor γ coactivator 1α (PGC-1α) and mitochondrial
function by MEF2 and HDAC5, Proc. Natl. Acad. Sci. USA,
100, 1711-1716.
65.Singh, H., Cheng, J., Deng, H., Kemp, R.,
Ishizuka, T., Nasjletti, A., and Schwartzman, M. L. (2007) Vascular
cytochrome P450 4A expression and 20-hydroxyeicosatetraenoic acid
synthesis contribute to endothelial dysfunction in androgen-induced
hypertension, Hypertension, 50, 123-129.
66.Reckelhoff, J. F., Zhang, H., Srivastava, K., and
Granger, J. P. (1999) Gender differences in hypertension in
spontaneously hypertensive rats: role of androgens and androgen
receptor, Hypertension, 34, 920-923.
67.Szekacs, B., Vajo, Z., Varbiro, S., Kakucs, R.,
Vaslaki, L., Acs, N., Mucsi, I., and Brinton, E. A. (2000)
Postmenopausal hormone replacement improves proteinuria and impaired
creatinine clearance in type 2 diabetes mellitus and hypertension,
BJOG, 107, 1017-1021.
68.Hsieh, Y.-C., Choudhry, M. A., Yu, H.-P.,
Shimizu, T., Yang, S., Suzuki, T., Chen, J., Bland, K. I., and Chaudry,
I. H. (2006) Inhibition of cardiac PGC-1α expression abolishes
ERβ agonist-mediated cardioprotection following trauma-hemorrhage,
FASEB J., 20, 1109-1117.
69.Lopez-Ruiz, A., Sartori-Valinotti, J., Yanes, L.
L., Iliescu, R., and Reckelhoff, J. F. (2008) Sex differences in
control of blood pressure: role of oxidative stress in hypertension in
females, Am. J. Physiol. Heart Circ. Physiol., 295,
466-474.
70.Sullivan, J. C., Sasser, J. M., and Pollock, J.
S. (2007) Sexual dimorphism in oxidant status in spontaneously
hypertensive rats, Am. J. Physiol. Regul. Integr. Comp.
Physiol., 292, 764-768.
71.Giordano, G., Tait, L., Furlong, C. E., Cole, T.
B., Kavanagh, T. J., and Costa, L. G. (2013) Gender differences in
brain susceptibility to oxidative stress are mediated by levels of
paraoxonase-2 expression, Free Radic. Biol. Med., 58,
98-108.
72.Haq, S., Choukroun, G., Kang, Z. B., Ranu, H.,
Matsui, T., Rosenzweig, A., Molkentin, J. D., Alessandrini, A.,
Woodgett, J., Hajjar, R., Michael, A., and Force, T. (2000) Glycogen
synthase kinase-3β is a negative regulator of cardiomyocyte
hypertrophy, J. Cell Biol., 151, 117-130.
73.Antos, C. L., McKinsey, T. A., Frey, N.,
Kutschke, W., McAnally, J., Shelton, J. M., Richardson, J. A., Hill, J.
A., and Olson, E. N. (2002) Activated glycogen synthase-3β
suppresses cardiac hypertrophy in vivo, Proc. Natl. Acad.
Sci. USA, 99, 907-912.
74.Kadokami, T., McTiernan, C. F., Kubota, T., Frye,
C. S., and Feldman, A. M. (2000) Sex-related survival differences in
murine cardiomyopathy are associated with differences in TNF-receptor
expression, J. Clin. Invest., 106, 589-597.
75.Janczewski, A. M., Kadokami, T., Lemster, B.,
Frye, C. S., McTiernan, C. F., and Feldman, A. M. (2003) Morphological
and functional changes in cardiac myocytes isolated from mice
overexpressing TNF-α, Am. J. Physiol. Heart Circ.
Physiol., 284, 960-969.
76.Wang, C., Chiari, P. C., Weihrauch, D.,
Krolikowski, J. G., Warltier, D. C., Kersten, J. R., Pratt, P. F., and
Pagel, P. S. (2006) Gender-specificity of delayed preconditioning by
isoflurane in rabbits: potential role of endothelial nitric oxide
synthase, Anesth. Analg., 103, 274-280.
77.Cross, H. R., Kranias, E. G., Murphy, E., and
Steenbergen, C. (2003) Ablation of PLB exacerbates ischemic injury to a
lesser extent in female than male mice: protective role of NO, Am.
J. Physiol. Heart Circ. Physiol., 284, 683-690.
78.Vijay, V., Han, T., Moland, C. L., Kwekel, J. C.,
Fuscoe, J. C., and Desai, V. G. (2015) Sexual dimorphism in the
expression of mitochondria-related genes in rat heart at different
ages, PLoS One, 10, e0117047.
79.Foryst-Ludwig, A., Kreissl, M. C., Sprang, C.,
Thalke, B., Bohm, C., Benz, V., Gurgen, D., Dragun, D., Schubert, C.,
Mai, K., Stawowy, P., Spranger, J., Regitz-Zagrosek, V., Unger, T., and
Kintscher, U. (2011) Sex differences in physiological cardiac
hypertrophy are associated with exercise-mediated changes in energy
substrate availability, Am. J. Physiol. Heart Circ. Physiol.,
301, 115-122.
80.Foryst-Ludwig, A., and Kintscher, U. (2010)
Metabolic impact of estrogen signalling through ERα and ERβ,
J. Steroid Biochem. Mol. Biol., 122, 74-81.
81.Yao, J., Hamilton, R. T., Cadenas, E., and
Brinton, R. D. (2010) Decline in mitochondrial bioenergetics and shift
to ketogenic profile in brain during reproductive senescence,
Biochim. Biophys. Acta, 1800, 1121-1126.
82.Ding, F., Yao, J., Zhao, L., Mao, Z., Chen, S.,
and Brinton, R. D. (2013) Ovariectomy induces a shift in fuel
availability and metabolism in the hippocampus of the female transgenic
model of familial Alzheimer’s, PLoS One, 8,
13-15.
83.Ciana, P., Raviscioni, M., Mussi, P., Vegeto, E.,
Que, I., Parker, M. G., Lowik, C., and Maggi, A. (2003) In vivo
imaging of transcriptionally active estrogen receptors, Nat.
Med., 9, 82-86.
84.Mikkola, A., Aro, J., Rannikko, S., Oksanen, H.,
and Ruutu, M. (2005) Cardiovascular complications in patients with
advanced prostatic cancer treated by means of orchiectomy or
polyestradiol phosphate, Scand. J. Urol. Nephrol., 39,
294-300.
85.Hamilton, E. J., Gianatti, E., Strauss, B. J.,
Wentworth, J., Lim-Joon, D., Bolton, D., Zajac, J. D., and Grossmann,
M. (2011) Increase in visceral and subcutaneous abdominal fat in men
with prostate cancer treated with androgen deprivation therapy,
Clin. Endocrinol. (Oxford), 74, 377-383.
86.Langley, R. E., Cafferty, F. H., Alhasso, A. A.,
Rosen, S. D., Sundaram, S. K., Freeman, S. C., Pollock, P., Jinks, R.
C., Godsland, I. F., Kockelbergh, R., Clarke, N. W., Kynaston, H. G.,
Parmar, M. K., and Abel, P. D. (2013) Cardiovascular outcomes in
patients with locally advanced and metastatic prostate cancer treated
with luteinizing hormone-releasing hormone agonists or transdermal
oestrogen: the randomised, phase 2 MRC PATCH trial (PR09), Lancet
Oncol., 14, 306-316.
87.Haring, R., John, U., Volzke, H., Nauck, M.,
Dorr, M., Felix, S. B., and Wallaschofski, H. (2012) Low testosterone
concentrations in men contribute to the gender gap in cardiovascular
morbidity and mortality, Gend. Med., 9, 557-568.
88.Gooren, L. J., Wierckx, K., and Giltay, E. J.
(2014) Cardiovascular disease in transsexual persons treated with
cross-sex hormones: reversal of the traditional sex difference in
cardiovascular disease pattern, Eur. J. Endocrinol., 170,
809-819.
89.Barrett-Connor, E. (2003) Clinical review 162:
cardiovascular endocrinology 3: an epidemiologist looks at hormones and
heart disease in women, J. Clin. Endocrinol. Metab., 88,
4031-4042.
90.Graves, J. A. (2006) Sex chromosome
specialization and degeneration in mammals, Cell, 124,
901-914.
91.Arakawa, Y., Nishida-Umehara, C., Matsuda, Y.,
Sutou, S., and Suzuki, H. (2002) X-chromosomal localization of
mammalian Y-linked genes in two XO species of the Ryukyu spiny rat,
Cytogenet. Genome Res., 99, 303-309.
92.Forsberg, L. A., Rasi, C., Malmqvist, N., Davies,
H., Pasupulati, S., Pakalapati, G., Sandgren, J., Diaz de Stahl, T.,
Zaghlool, A., Giedraitis, V., Lannfelt, L., Score, J., Cross, N. C.,
Absher, D., Janson, E. T., Lindgren, C. M., Morris, A. P., Ingelsson,
E., Lind, L., and Dumanski, J. P. (2014) Mosaic loss of chromosome Y in
peripheral blood is associated with shorter survival and higher risk of
cancer, Nat. Genet., 46, 624-628.
93.Hughes, I. A., and Deeb, A. (2006) Androgen
resistance, Best Pract. Res. Clin. Endocrinol. Metab.,
20, 577-598.
94.Greiner, S., Sobanski, J., and Bock, R. (2015)
Why are most organelle genomes transmitted maternally?
Bioessays, 37, 80-94.
95.Gregersen, N., Hansen, J., and Palmfeldt, J.
(2012) Mitochondrial proteomics – a tool for the study of
metabolic disorders, J. Inherit. Metab. Dis., 35,
715-726.
96.Woodson, J. D., and Chory, J. (2008) Coordination
of gene expression between organellar and nuclear genomes, Nat. Rev.
Genet., 9, 383-395.
97.Gemmell, N. J., Metcalf, V. J., and Allendorf, F.
W. (2004) Mother’s curse: the effect of mtDNA on individual
fitness and population viability, Trends Ecol. Evol., 19,
238-244.
98.Wolff, J. N., and Gemmell, N. J. (2013)
Mitochondria, maternal inheritance, and asymmetric fitness: why males
die younger, BioEssays, 35, 93-99.
99.Wallace, D. C. (2010) Mitochondrial DNA mutations
in disease and aging, Environ. Mol. Mutagen., 51,
440-450.
100.Ruiz-Pesini, E., Lapena, A. C., Diez-Sanchez,
C., Perez-Martos, A., Montoya, J., Alvarez, E., Diaz, M., Urries, A.,
Montoro, L., Lopez-Perez, M. J., and Enriquez, J. A. (2000) Human mtDNA
haplogroups associated with high or reduced spermatozoa motility,
Am. J. Hum. Genet., 67, 682-696.
101.Wallace, D. C., Singh, G., Lott, M. T., Hodge,
J. A., Schurr, T. G., Lezza, A. M., Elsas, L. J., and Nikoskelainen, E.
K. (1988) Mitochondrial DNA mutation associated with Leber’s
hereditary optic neuropathy, Science, 242, 1427-1430.
102.Greaves, L. C., Reeve, A. K., Taylor, R. W.,
and Turnbull, D. M. (2012) Mitochondrial DNA and disease, J.
Pathol., 226, 274-286.
103.Schapira, A. H. (2012) Mitochondrial diseases,
Lancet, 379, 1825-1834.
104.Ieremiadou, F., and Rodakis, G. C. (2009)
Correlation of the 4977 bp mitochondrial DNA deletion with human sperm
dysfunction, BMC Res. Notes, 2, 18.
105.Holyoake, A. J., McHugh, P., Wu, M.,
O’Carroll, S., Benny, P., Sin, I. L., and Sin, F. Y. (2001) High
incidence of single nucleotide substitutions in the mitochondrial
genome is associated with poor semen parameters in men, Int. J.
Androl., 24, 175-182.
106.White, D. J., Wolff, J. N., Pierson, M., and
Gemmell, N. J. (2008) Revealing the hidden complexities of mtDNA
inheritance, Mol. Ecol., 17, 4925-4942.
107.Zeh, J. A., and Zeh, D. W. (2005) Maternal
inheritance, sexual conflict, and the maladapted male, Trends
Genet., 21, 281-286.
108.Gemmell, N. J., and Sin, F. Y. (2002)
Mitochondrial mutations may drive Y chromosome evolution,
BioEssays, 24, 275-279.
109.Innocenti, P., Morrow, E. H., and Dowling, D.
K. (2011) Experimental evidence supports a sex-specific selective sieve
in mitochondrial genome evolution, Science, 332,
845-848.
110.Clancy, D. J. (2008) Variation in mitochondrial
genotype has substantial lifespan effects which may be modulated by
nuclear background, Aging Cell, 7, 795-804.
111.Golob, M. J., Tian, L., Wang, Z., Zimmerman,
T., Caneba, C., Hacker, T., Song, G., and Chesler, N. C. (2015)
Mitochondria DNA mutations cause sex-dependent development of
hypertension and alterations in cardiovascular function, J.
Biomech., 48, 405-412.
112.Camus, M. F., Clancy, D. J., and Dowling, D. K.
(2012) Mitochondria, maternal inheritance, and male aging, Curr.
Biol., 22, 1717-1721.
113.Kujoth, G. C., Bradshaw, P. C., Haroon, S., and
Prolla, T. A. (2007) The role of mitochondrial DNA mutations in
mammalian aging, PLoS Genet., 3, 161-173.
114.Schapira, A. H. (2012) Mitochondrial diseases,
Lancet, 379, 1825-1834.
115.Sekirov, I., Russell, S. L., Antunes, L. C.,
and Finlay, B. B. (2010) Gut microbiota in health and disease,
Physiol. Rev., 90, 859-904.
116.Zorov, D. B., Plotnikov, E. Y., Silachev, D.
N., Zorova, L. D., Pevzner, I. B., Zorov, S. D., Babenko, V. A.,
Jankauskas, S. S., Popkov, V. A., and Savina, P. S. (2014) Microbiota
and mitobiota. Putting an equal sign between mitochondria and bacteria,
Biochemistry (Moscow), 79, 1017-1031.
117.Speakman, J. R. (2005) Body size, energy
metabolism, and lifespan, J. Exp. Biol., 208,
1717-1730.
118.Fontana, L., Partridge, L., and Longo, V. D.
(2010) Extending healthy life span – from yeast to humans,
Science, 328, 321-326.
119.Geodakian, V. A. (1991) Evolutionary theory of
sex, Priroda, 8, 60-69.
120.Zahavi, A. (1975) Mate selection – a
selection for a handicap, J. Theor. Biol., 53,
205-214.
121.Skulachev, V. P. (2004) Model Systems in
Aging, Springer Berlin Heidelberg, Berlin, Heidelberg.
122.Croft, D. P., Brent, L. J., Franks, D. W., and
Cant, M. A. (2015) The evolution of prolonged life after reproduction,
Trends Ecol. Evol., 30, 407-416.