Effect of Long-Term Treatment with Antioxidant SkQ1 Added to Drinking Water on Cytochromes P450 Level in Rat Liver
K. N. Myasoedova1,2* and D. N. Silachev2,3
1Lomonosov Moscow State University, Faculty of Fundamental Medicine, 119991 Moscow, Russia; E-mail: skulach@genebee.msu.ru; skulach@belozersky.msu.ru2Lomonosov Moscow State University, Institute of Mitoengineering, 119991 Moscow, Russia; fax: +7 (495) 939-5945
3Lomonosov Moscow State University, Belozersky Institute of Physico-Chemical Biology, 119991 Moscow, Russia; fax: +7 (495) 939-0338
* To whom correspondence should be addressed.
Received October 1, 2015
Mitochondria-targeted cationic antioxidant plastoquinonyl decyltriphenylphosphonium (SkQ1) added to drinking water in therapeutic doses (250 nmol/kg per day) for a long time (up to 24 months) does not induce cytochromes P450 in rat liver.
KEY WORDS: cytochrome P450, SkQ1, mitochondria-targeted antioxidantDOI: 10.1134/S0006297915120123
Abbreviations: SkQ1, plastoquinonyl decyltriphenylphosphonium.
The autooxidizable heme proteins cytochromes P450, named for the
specific absorption band at 450 nm in the spectrum of reduced complexes
of these proteins with carbon monoxide, are represented in human and
animal liver by a group of forms having a unique property: they can
catalyze oxidative transformations of exogenous organic compounds of
different chemical nature (xenobiotics). In other tissues, cytochromes
P450 (over 50 genes of these cytochromes have been found in the human
genome) mainly carry out reactions of endogenous substrates, including
the reactions of biosynthesis and metabolism of a number of
physiologically active compounds [1-6].
Xenobiotics (medicines, drugs, toxins, various factors of environmental pollution, etc.), which are transformed by cytochromes P450, can induce a significant reversible increase of the content of these heme proteins in liver due to stimulation of the biosynthesis of one or several of their forms [5].
Forms of cytochrome P450 have unusual kinetic properties and extremely broad substrate specificity, a phenomenon that so far has no clear explanation. For example, one of the best-studied P450 forms in human liver (form 3A4) can metabolize up to 50% of all drugs available on the pharmaceutical market [3, 4, 6].
Until recently, cytochromes P450 were seen as components of the main detoxifying system of an organism, oxidizing and thereby hydrophilizing exogenous compounds, facilitating their excretion from the organism. However, it has been shown that oxidation products are sometimes more harmful (toxic, carcinogenic, etc.) than the initial compounds. For example, activation of potential carcinogens – polycyclic aromatic hydrocarbons, in particular, benzo(a)pyrene, follows this pattern [2, 6]. This circumstance supports the need to study possible induction of cytochromes P450 by new medicines recommended for use in various pathologies.
One such medicine is the synthetic derivative of plastoquinone conjugated with the penetrating cation of decyltriphenylphosphonium (SkQ1). This compound, synthesized in our laboratory [7], passed preclinical and clinical trials [7-12], is recommended for use in the treatment of a number of human diseases, and has been in pharmacies in Russia since 2012.
In our previous work, we showed that therapeutic doses of SkQ1 have no effect on the level of cytochromes P450 in rat liver in short-term experiments (5 days). The ability of rats for inductive response was monitored using the classic inductor phenobarbital, which caused a significant increase in cytochromes P450 content in the liver of such rats [13].
In this study, we measured the total amount of cytochromes P450 in the liver of adult rats that have been daily receiving therapeutic doses of SkQ1 with drinking water for many months (up to 24 months), compared to respective control animals.
Outbred female albino rats were used for the experiments. Starting from the age of 3.5 months, they daily received SkQ1 solution (250 nmol SkQ1/kg body weight per day).
The animals were euthanized at the age of 23 to 28 months. The rats did not receive food for one day before death. Following decapitation, the rat liver was carefully perfused with cold physiological solution, and then it was milled in a blender in the cold in a small volume of 0.15 M KCl with 5 mM EDTA and later homogenized in the same solution at a ratio of 1 : 3 using a Dounce homogenizer with a Teflon pestle. Microsome fraction was obtained by a conventional method of differential centrifugation. The final pellet obtained after centrifugation for 1 h at 105,000g, was carefully washed with buffer containing 100 mM KH2PO4, 100 mM KCl, 1 mM EDTA, 1 mM dithiothreitol, and 20% glycerol, pH 7.4. The pellet was suspended in a small amount of the same buffer. The total amount of P450 cytochromes in the microsomes was determined spectrophotometrically according to the differential scheme of Omura and Sato [14] using light absorption by the reduced P450 complex with carbon dioxide at 450 nm and molar extinction coefficient 91 mM–1·cm–1. Protein content was determined by the Lowry method [15].
The results are provided in the table, which shows the content of cytochromes P450 in rat microsomes for every rat from the control and experimental groups. The data indicate the absence of inducing effect of SkQ1 at therapeutic concentrations on the content of cytochromes P450 in rat liver after many months of antioxidant treatment.
Amount of cytochromes P450 in liver microsomes of control rats and rats
that underwent long-term treatment with 250 nmol/kg body weight of SkQ1
(nmol of cytochrome P450 per mg of microsomal protein)
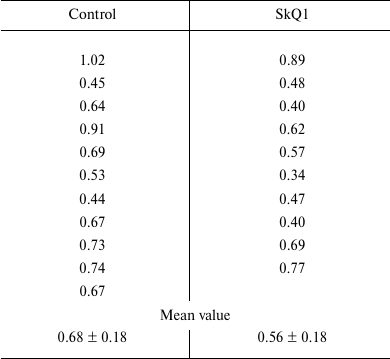
Data obtained with 1.5-2-month-old rats commonly used in studies of cytochromes P450 induction, which were previously reported by us [13], also showed the absence of inductive response to SkQ1, and classic values of cytochromes P450 content in normal rat liver (about 0.8 nmol per mg protein) even under significant induction by phenobarbital. It is known that rats of any age keep their capacity for inductive response to phenobarbital, although certain parameters characterizing the activity of monooxygenases of P450 type decrease with age [5].
The data suggest that the SkQ1 cation either cannot induce cytochromes P450, or its therapeutic doses (250 nmol/kg bogy weight per day) are too low to cause such an undesirable effect, which could be dangerous both in toxicological and carcinogenic aspects.
We are very grateful to O. A. Averina for the opportunity to use the livers of SkQ1-treated animals that were prepared for another study.
This study was supported by the Russian Science Foundation (project No. 14-50-00029).
REFERENCES
1.Furge, L. L., and Guengerich, F. P. (2006)
Cytochrome P450 enzymes in drug metabolism and chemical toxicology: an
introduction, Biochem. Mol. Biol. Educ., 34, 66-74.
2.Coon, M. J. (2002) Enzyme ingenuity in biological
oxidations: a trail leading to cytochrome P450, J. Biol. Chem.,
277, 28351-28363.
3.Ekroos, M., and Sjogren, T. (2006) Structural basis
for ligand promiscuity in cytochrome P450 3A4, Proc. Natl. Acad.
Sci. USA, 103, 13682-13687.
4.Guengerich, F. P. (2006) A malleable catalyst
dominates the metabolism of drugs, Proc. Natl. Acad. Sci. USA,
103, 13565-13566.
5.Lyakhovich, V. V., and Tsyrlov, I. B. (1981)
Induction of the Enzymes of Xenobiotics Metabolism [in Russian],
Nauka, Novosibirsk.
6.Myasoedova, K. N. (2008) New findings in studies on
cytochrome P450, Biochemistry (Moscow), 73, 965-969.
7.Antonenko, Y. N., Avetisyan, A. V., Bakeeva, L. E.,
Chernyak, B. V., Chertkov, V. A., Domnina, L. V., Ivanova, O. Yu.,
Izyumov, D. S., Khailova, L. S., Klishin, S. S., Korshunova, G. A.,
Lyamzaev, K. G., Muntyan, M. S., Nepryakhina, O. K., Pashkovskaya, A.
A., Pletyushkina, O. Yu., Pustovidko, A. V., Roginsky, V. A.,
Rokitskaya, T. I., Ruuge, E. K., Saprunova, V. B., Severina, I. I.,
Simonyan, R. A., Skulachev, I. V., Skulachev, M. V., Sumbatyan, N. V.,
Sviryaeva, I. V., Tashlitsky, V. N., Vassiliev, J. M., Vysokikh, M.
Yu., Yaguzhinsky, L. S., Zamyatnin, A. A., Jr., and Skulachev, V. P.
(2008) Mitochondria-targeted plastoquinone derivatives as tools to
interrupt execution of the aging program. I. Cationic plastoquinone
derivatives: synthesis and in vitro studies, Biochemistry
(Moscow), 73, 1273-1287.
8.Skulachev, V. P., Anisimov, V. N., Antonenko, Y.
N., Bakeeva, L. E., Chernyak, B. V., Erichev, V. P., Filenko, O. F.,
Kalinina, N. I., Kapelko, V. I., Kolosova, N. G., Kopnin, B. P.,
Korshunova, G. A., Lichinitser, M. R., Obukhova, L. A., Pasyukova, E.
G., Pisarenko, O. I., Roginsky, V. A., Ruuge, E. K., Senin, I. I.,
Severina, I. I., Skulachev, M. V., Spivak, I. M., Tashlitsky, V. N.,
Tkachuk, V. A., Vyssokikh, M. Y., Yaguzhinsky, L. S., and Zorov, D. B.
(2009) An attempt to prevent senescence: a mitochondrial approach,
Biochim. Biophys. Acta, 1787, 437-461.
9.Severin, F. F., Severina, I. I., Antonenko, Y. N.,
Rokitskaya, T. I., Cherepanov, D. A., Mokhova, E. N., Vyssokikh, M. Y.,
Pustovidko, A. V., Markova, O. V., Yaguzhinsky, L. S., Korshunova, G.
A., Sumbatyan, N. V., Skulachev, M. V., and Skulachev, V. P. (2010)
Penetrating cation/fatty acid anion pair as a mitochondria-targeted
protonophore, Proc. Natl. Acad. Sci. USA, 107,
663-668.
10.Skulachev, M. V., Antonenko, Y. N., Anisimov, V.
N., Chernyak, B. V., Cherepanov, D. A., Chistyakov, V. A., Egorov, M.
V., Kolosova, N. G., Korshunova, G. A., Lyamzaev, K. G., Plotnikov, E.
Y., Roginsky, V. A., Savchenko, A. Y., Severina, I. I., Severin, F. F.,
Shkurat, T. P., Tashlitsky, V. N., Shidlovsky, K. M., Vyssokikh, M. Y.,
Zamyatnin, A. A., Jr., Zorov, D. B., and Skulachev, V. P. (2011)
Mitochondria-targeted plastoquinone derivatives. Effect on senescence
and acute age-related pathologies, Curr. Drug Targets,
12, 800-826.
11.Neroev, V. V., Archipova, M. M., Bakeeva, L. E.,
Fursova, A. Zh., Grigoryan, E. N., Grishanova, A. Yu., Iomdina, E. N.,
Ivashchenko, Zh. N., Katargina, L. A., Khoroshilova-Maslova, I. P.,
Kilina, O. V., Kolosova, N. G., Kopenkin, E. P., Korshunov, S. S.,
Kovaleva, N. A., Novikova, Yu. P., Filippov, P. P., Pilipenko, D. I.,
Robustova, O. V., Saprunova, V. B., Senin, I. I., Skulachev, M. V.,
Sotnikova, L. F., Stefanova, N. A., Tikhomirova, N. K., Tsapenko, I.
V., Shchipanova, A. I., Zinovkin, R. A., and Skulachev, V. P. (2008)
Mitochondria-targeted plastoquinone derivatives as tools to interrupt
execution of the aging program. 4. Age-related eye disease. SkQ1
returns vision to blind animals, Biochemistry (Moscow),
73, 1317-1328.
12.Yani, Ye. V., Katargina, L. A., Chesnokova, N.
B., Beznos, O. V., Savchenko, A. Yu., Vygodin, Ye. Yu., Gudkova, Ye.
Yu., Zamyatnin, A. A., Jr., and Skulachev, M. V. (2012) The first
experience of using Visomitin in “dry eye” treatment,
Prakt. Med., 1, 134-137.
13.Myasoedova, K. N., and Silachev, D. N. (2014)
Therapeutic doses of SkQ1 do not induce cytochromes P450 in rat liver,
Biochemistry (Moscow), 79, 1130-1132.
14.Omura, T., and Sato, R. (1964) Carbon
monoxide-binding pigment of liver microsomes. I. Evidence for its
hemoprotein nature, J. Biol. Chem., 239, 2370-2378.
15.Dawson, R., Elliott, D., and Jones, K. (1991)
Directory of a Biochemist [Russian translation], Mir,
Moscow.