Activity of Tissue Factor in Microparticles Produced in vitro by Endothelial Cells, Monocytes, Granulocytes, and Platelets
S. G. Khaspekova, O. A. Antonova, O. N. Shustova, V. V. Yakushkin, N. V. Golubeva, E. V. Titaeva, A. B. Dobrovolsky, and A. V. Mazurov*
Russian Cardiology Research and Production Complex, Russian Ministry of Health, 121552 Moscow, Russia; fax: +7 (495) 414-6699; E-mail: avmazurov@list.ru; cclibr@cardio.ru* To whom correspondence should be addressed.
Received July 16, 2015; Revision received September 23, 2015
Activity of tissue factor (TF) in membrane microparticles (MPs) produced in vitro by endothelial cells (ECs), monocytes, THP-1 monocytic cells, granulocytes, and platelets was investigated. ECs were isolated from human umbilical vein, and monocytes, granulocytes, and platelets – from the blood of healthy donors. ECs, monocytes, and THP-1 cells were activated by bacterial lipopolysaccharide, granulocytes – by lipopolysaccharide or phorbol myristate acetate, and platelets – by SFLLRN, thrombin receptor-activating peptide. MPs were sedimented from the culture medium or supernatant of activated cells at 20,000g for 30 min. Coagulation activity of MPs was analyzed in a modified recalcification assay by assessing their effects on coagulation of donor plasma depleted of endogenous MPs (by centrifuging at 20,000g for 90 min). MPs from all cell types accelerated plasma coagulation. Antibodies blocking TF activity prolonged coagulation lag-phase in the presence of MPs from ECs, monocytes, and THP-1 cells (by 2.7-, 2.0-, and 1.8-fold, respectively), but did not influence coagulation in the presence of MPs from granulocytes and platelets. In accordance with these data, TF activity measured by its ability to activate factor X was found in MPs from ECs, monocytes, and THP-1 cells, but not in MPs from granulocytes and platelets. The data obtained indicate that active TF is present in MPs produced in vitro by ECs, monocytes, and THP-1 cells, but not in MPs derived from granulocytes and platelets.
KEY WORDS: membrane microparticles, coagulation, tissue factor, endothelial cells, monocytes, granulocytes, plateletsDOI: 10.1134/S000629791602005X
Membrane microparticles (MPs), or microvesicles, are produced upon activation and/or apoptosis of various cells via shedding of small fragments of plasma membrane. MPs circulating in the blood can be released from platelets, erythrocytes, and leukocytes (monocytes and granulocytes), as well as from endothelial cells (ECs). All MPs exhibit coagulation activity, as they contain on their surface negatively charged phospholipids. These phospholipids (primarily phosphatidylserine) are required as a substrate for the assembly of tenase (Xase) and prothrombinase coagulation complexes responsible for activating factor X and formation of thrombin. Moreover, some MPs may contain tissue factor (TF) – a membrane protein initiating (in a complex with factor VII) reactions of the coagulation cascade [1, 2].
Activated monocytes, like the MPs they produce, are able to express TF. These cells are considered as a major source of the TF circulating in the blood (“blood-borne tissue factor”) [1-5]. TF from the cells of the vascular wall plays the main role in thrombogenesis — its large amount contacts with the blood upon vessel injury. However, TF circulating in the blood may also take part in initiating thrombosis [6]. Apart from monocytes, ECs, granulocytes (polymorphonuclear leukocytes, neutrophils), platelets, and MPs produced by these cells could be also considered as potential sources of blood TF. It was shown that under in vitro conditions activated ECs might express TF [1, 5, 7]. However, an issue regarding the ability of ECs and their MPs to express TF in vivo remains unsolved. ECs containing TF (TF+) were detected in some pathological models in animals (sepsis and sickle-cell anemia) [8, 9]. However, it is proposed that such cells may appear as a result of their interaction with monocyte-derived TF+ MPs [1, 5]. Studies on expression of TF by granulocytes and platelets are highly controversial, which still keeps open the possibility for the release of TF+ MPs by these cells [1, 4, 5, 10-13]. It is assumed that like ECs, granulocytes and platelets and their MPs may not contain TF per se, but obtain it via capturing monocyte-derived MPs. Such interaction was reported for both granulocytes [14] and platelets [15, 16]. Therefore, an issue regarding the presence of TF, and particularly an active form of TF, in MPs derived from various cell types remains unclear.
In our study, we compared activity of TF in MPs obtained in vitro from five cell types – ECs, monocytes, THP-1 monocytic cells, granulocytes, and platelets. Two approaches were applied to all types of MPs: (i) evaluation of the effects of anti-TF antibodies on coagulation activity of MPs, and (ii) direct measurement of TF activity in MPs by assessing its ability to activate factor X.
MATERIALS AND METHODS
Endothelial cells. Endothelial cells were isolated from human umbilical vein as described earlier [17] and cultured under standard conditions (37°C, 5% CO2) in DMEM medium containing 20 mM Hepes, 2 mM L-glutamine, 1 mM sodium pyruvate, penicillin (50 U/ml), streptomycin (100 µg/ml), 10% heat-inactivated fetal bovine serum (FBS), 200 µg/ml vascular endothelial growth factor (all purchased from Gibco Life Technologies, USA), and 5 U/ml heparin (Moscow Endocrine Plant, Russia). Confluent cultures of ECs of the second/third passages were used for their activation and for further isolation of MPs. Just before activation, cells were washed twice and activated with E. coli bacterial lipopolysaccharide (LPS) (Sigma-Aldrich, USA) at concentration 1 µg/ml for 12 h in heparin-free culture medium containing 10% of non-inactivated FBS. Inactivated FBS was replaced for non-inactivated FBS containing intact LPS-binding protein, which enhances LPS cellular effects. After the incubation, culture medium was collected, cells together with large cell fragments were pelleted at 400g for 15 min and further – at 1500g for 15 min at room temperature. A similar centrifugation protocol was used for preparing cell supernatants containing MPs derived from monocytes, granulocytes, and THP-1 cells (see below).
Collection and preparation of blood samples for isolating monocytes and granulocytes. Monocytes and granulocytes were usually isolated from 40-50 ml of peripheral blood from healthy volunteers. Blood was collected using 0.109 M sodium citrate (3.8% solution of 5.5 hydrate) as an anticoagulant at blood/anticoagulant ratio 9 : 1. To reduce the number of platelets, blood was centrifuged at 180g for 10 min at room temperature, platelet-rich plasma was collected, platelets were pelleted (1000g for 15 min at room temperature), and platelet-free plasma was added to the remaining blood. After mixing, the blood sample was supplemented with 6% hydroxyethyl starch solution (Stabizol; Berlin Chemie, Germany) for sedimentation of erythrocytes in a volume of 20% of the blood volume and incubated for 1 h at room temperature. Supernatant obtained after agglutination and sedimentation of erythrocytes (leukocyte-enriched blood fraction) was used for isolating monocytes and granulocytes.
Monocytes. Leukocyte-enriched blood fraction (see above) was placed over Histopaque-1077 solution (density 1.077 g/ml) (Sigma-Aldrich) (1 : 1 v/v) and centrifuged at 400g for 30 min at room temperature. A white blood cell ring (“buffy coat”) was collected, and cells were washed twice with Hanks’ Balanced Salt Solution (HBSS) (Gibco Life Technologies, USA) containing 1% BSA (HBSS/BSA) at 400g for 15 min at room temperature. The cell pellet was resuspended in RPMI 1640 culture medium (Sigma-Aldrich) containing 20 mM Hepes, 2 mM L-glutamine, 1 mM sodium pyruvate, penicillin (50 U/ml), streptomycin (100 µg/ml), and 10% heat-inactivated FBS and seeded into untreated 100-mm Petri dishes at 25·106 cells/dish. After 18 h cultivation (37°C, 5% CO2), adhered monocytes were washed twice and activated with LPS (1 µg/ml) in medium containing 10% non-inactivated FBS (instead of inactivated) for 6 h.
Granulocytes. The cells from the leukocyte-enriched blood fraction (see above) were pelleted at 400g for 10 min at room temperature. Residual erythrocytes were lysed by adding to the pellet 12 ml of cooled (4°C) distilled water. After pipetting for 20 sec, isotonicity of the cell suspension was restored by adding 4 ml 0.6 M KCl solution, and the volume was adjusted up to 50 ml with phosphate-buffered saline (PBS) (150 mM NaCl, 10 mM sodium phosphate, pH 7.4). The cells were pelleted at 400g for 6 min at room temperature and resuspended in 2.5 ml PBS. The cell suspension was placed over 3 ml Histopaque-1077 solution and centrifuged at 400g for 30 min at room temperature. Pelleted granulocytes were washed twice in HBSS/BSA (400g, 10 min, room temperature) and resuspended in the same buffer at the concentration of 3·106 cells/ml. The cells were activated with LPS at concentration 10 µg/ml or with phorbol myristate acetate (PMA) (Sigma-Aldrich) at concentration 20 ng/ml for 30 min at 37°C.
THP-1 cell line. The THP-1 human monocytic cell line was obtained from the American Type Culture Collection (ATCC, Bethesda, MD, USA). The cells were cultured under standard conditions (37°C, 5% CO2) in RPMI 1640 medium containing 20 mM Hepes, 2 mM L-glutamine, 1 mM sodium pyruvate, penicillin (50 U/ml), streptomycin (100 µg/ml), and 10% heat-inactivated FBS. After thawing, the cells were cultured until reaching a stable growth (usually for 1-2 weeks), and such cells were used for activation and further isolations of MPs. After preliminary washings, THP-1 cells were activated with LPS (1 µg/ml) added in the culture medium containing 10% non-inactivated FBS (instead of inactivated) for 6 h.
Platelets. Washed platelets were obtained as described before [18], resuspended in Tyrode/Hepes solution (137 mM NaCl, 2.7 mM KCl, 0.36 mM NaH2PO4, 0.1% dextrose, 1 mM MgCl2, 0.35% BSA, 5 mM Hepes, pH 7.35), and supplemented with 1 mM CaCl2. The concentration of platelets was determined using an Abacus Junior B hematological analyzer (Diatron Ltd., Austria). Washed platelets were used at concentration of 5·108 per ml. Platelets were activated by thrombin receptor-activating peptide (TRAP; sequence – SFLLRN) kindly provided by Dr. M. D. Ovchinnikov (Laboratory of Peptide Synthesis, Russian Cardiology Research and Production Complex, Russian Ministry of Health). Platelets were placed into a cuvette of ALAT-2 platelet aggregation analyzer (BIOLA, Moscow) with magnetic stirrer, supplemented with TRAP to final concentration 10 µM, and incubated for 10 min at 37°C and at stirring rate of 800 rpm. Activation with TRAP resulted in intensive platelet aggregation. After that, platelets were pelleted at 1500g for 15 min, and the supernatant was additionally centrifuged at 2500g 15 min (at room temperature).
Preparation and storage of microparticles. All types of MPs were prepared from the culture media and/or cell supernatants immediately after activating and pelleting the cells and their large fragments (see above). MPs were sedimented at 20,000g for 30 min at 4°C and resuspended in Tris-buffered saline (TBS) (100 mM NaCl, 50 mM Tris-HCl, pH 7.4) containing 1% BSA (TBS/BSA). Usually, 1 ml of suspension contained MPs isolated from 106 ECs, monocytes, THP-1 cells, and granulocytes and from 4.5·108 platelets. Immediately after isolation, MPs were frozen in liquid nitrogen in 0.5-1.0 ml aliquots in 1.5-ml Eppendorf tubes and stored at –70°C for no more than six months. The MPs were thawed immediately before use without undergoing repeated freezing/thawing.
Examination of coagulation activity of MPs (modified plasma recalcification assay). Plasma pools were usually prepared from the blood of 3 or 4 healthy volunteers. Blood was collected using 0.109 M sodium citrate as anticoagulant at blood/anticoagulant ratio 9 : 1. The blood samples were centrifuged at 2500g for 15 min at room temperature, and the supernatant was repeatedly centrifuged under the same conditions. To remove endogenous MPs, the plasma obtained was centrifuged at 20,000g for 90 min at 4°C. After centrifugation, plasma samples from several healthy volunteers were combined (MP– substrate plasma) and stored at –70°C in 1 ml aliquots for no more than three months. Plasma samples were not repeatedly frozen and thawed.
Samples of plasma and MPs were thawed within 5 min at 37°C in a solid-state Thermostat 5320 (Eppendorf, Germany). Thawed MPs were sedimented at 20,000g for 30 min at 4°C and then resuspended in plasma. Plasma samples without or with MPs derived from various cell types were supplemented with an equal volume of Owren-Koller buffer (Diagnostica Stago, France) containing an inhibitor of contact activation (corn trypsin inhibitor (CTI); Institute of Protein Research, Russian Academy of Sciences, Pushchino) at final concentration 150 µg/ml. While examining the effect of anti-TF antibodies (clone HTF-1, BD Pharmingen; BD Biosciences, USA), they were added to the buffer at final concentration 30 µg/ml. Diluted plasma samples in a volume of 100 µl were added into the wells of Costar round-bottom 96-well plates (Corning Inc., USA) pre-siliconized with 5% dichloromethylsilane solution (Acros, Thermo Fisher Scientific, USA) in isopropanol. Plate with the plasma samples was incubated for 5 min in an ST-3M Shaker Thermostat (Elmi, Latvia) at 30°C and 300 rpm, then 50 µl of 25 mM CaCl2 pre-warmed at 30°C (Diagnostica Stago) were added into the plate wells, and after 30 sec, the plate was placed into a pre-warmed (at 30°C) cell of a Thermo Scientific Multiskan Go (Thermo Fisher Scientific, Finland) spectrophotometer. After a 20-sec stirring (mean rate) and 10-sec pause, plasma clotting was measured at 30°C by changes of absorbance at 450 nm (A450) evaluated with 30-sec intervals over 60 min. All measurements were done in triplicates, and mean values were used for analysis. Plasma clotting curves were analyzed using Microsoft Excel 2010 software (Microsoft Corp., USA). The following parameters were assessed: (i) coagulation lag-phase (min) determined as a time required for reaching 5% of the total change of A450 within the assay period (A0 + ΔA(60 min) × 0.05, where A0 is the A450 value at the first time point, and ΔA(60 min) is the total increase of A450 within 60 min; and (ii) the maximum coagulation rate (Vmax, in % A/min) calculated according to the formula ΔA(30 sec)max × 2/ΔA(60 min) × 100%, where ΔA(30 sec)max is the maximum increase of A450 within 30 sec (interval between measurements), and ΔA(60 min) is total increase of A450 within 60 min.
To determine amounts of MPs of various types that result in a marked coagulation acceleration of MP– substrate plasma, in preliminary experiments MPs were titrated in regard of the number of cells producing MPs. The titration strategy for MPs was as follows: ECs – starting from 0.25·106 cells, monocytes, THP-1 cells, and granulocytes – from 0.5·106 cells, and platelets – from 75·106 cells. For further experiments evaluating the effect of anti-TF antibodies on coagulation activity of MPs, the following numbers of producing cells were taken: ECs – 0.125·106, monocytes and THP-1 cells – 0.25·106, granulocytes – 0.5·106, and platelets – 37.5·106 cells. MPs obtained from such numbers of cells caused a significant acceleration of coagulation of MP– plasma (see “Results”).
Detection of TF activity in MPs. Thawed MPs were pelleted at 20,000g for 30 min at 4°C, followed by resuspending in TBS/BSA buffer. The volume of the latter was adjusted according to the number of the cells producing the MPs. Samples (25 µl) contained MPs derived from 0.3·106 ECs, monocytes, or THP-1 cells, from 106 granulocytes, and from 90·106 platelets. Activity of TF in MPs was measured using an Actichrome TF Kit (Sekisui Diagnostics, USA). All measurements evaluating the ability of TF to activate factor X in the presence of activated factor VII were performed in accordance with the manufacturer’s protocol. Formation of activated factor X was registered by the cleavage of chromogenic substrate. Activity of TF in each sample was measured in at least two repeats, and mean values were used for analysis.
Statistical analysis. Statistical analysis was done using Statistica 8 software (StatSoft Inc., USA). The results were presented as mean ± standard error of mean. Significance of the effects of MPs on coagulation parameters (in comparison with MP– plasma) was calculated using Student’s t-test for independent groups, whereas significance of the effects of anti-TF antibodies on coagulation parameters – by paired Student’s t-test.
RESULTS
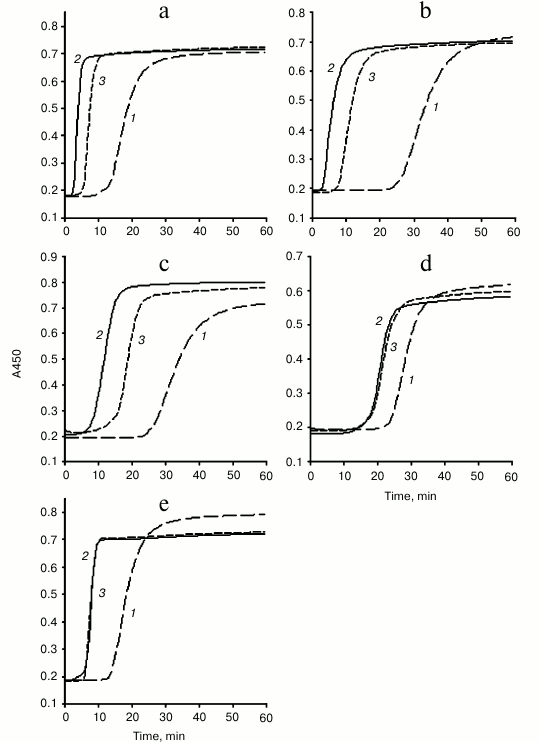
A modified plasma recalcification assay was used to detect coagulation activity of MPs derived from various cell types and to assess the impact of TF in MPs dependent acceleration of plasma clotting (by using anti-TF antibodies). Endogenous MPs were removed from plasma by centrifugation at 20,000g for 90 min to prepare MP– substrate plasma. Tested MPs were obtained by pelleting them at 20,000g for 30 min from culture media and/or supernatants of activated ECs, monocytes, THP-1 monocytic cell line, granulocytes, and platelets. ECs, monocytes, and THP-1 cells were activated with LPS, granulocytes – with LPS or PMA, platelets — with TRAP (for more details, see “Materials and Methods”). Samples of MP– plasma were supplemented with MPs obtained from 0.125·106 ECs, 0.25·106 monocytes and THP-1 cells, 0.5·106 granulocytes, and 37.5·106 platelets. Such amounts of MPs (empirically chosen during preliminary titration experiments, see “Materials and Methods”) caused marked and statistically significant shortening of the lag-phase and increase of the maximum rate (Vmax) of plasma coagulation. These effects were maximal for MPs derived from ECs and minimal for MPs derived from granulocytes (Table 1 and figure). Blocking anti-TF antibodies slowed plasma clotting in the presence of MPs from ECs, monocytes, and THP-1 cells – on average, prolonging the lag-phase by 2.7-, 2.0-, and 1.8-fold, respectively (figure and Table 1). In addition, the maximum rate (Vmax) of plasma coagulation was slightly decreased, though this tendency did not reach the significant level (Table 1). Anti-TF antibodies did not affect plasma clotting in the presence of MPs derived from granulocytes and platelets, as they did not extend the lag-phase or decrease Vmax (figure and Table 1). Also, these antibodies did not affect coagulation parameters of MP– substrate plasma without exogenous MPs (Table 1).
Acceleration of plasma coagulation in the presence of MPs derived from various cell types. Effects of anti-TF antibodies. Coagulation of plasma from healthy volunteers (MP– substrate plasma) was recorded after plasma recalcification by measuring changes of absorbance at 450 nm (A450). Curves: 1) (long dashes), MP– plasma; 2) (solid line), plasma supplemented with MPs in the absence of anti-TF antibodies; 3) (short dashes), plasma supplemented with MPs in the presence of anti-TF antibodies (10 µg/ml). MPs derived from 0.125·106 ECs (a), 0.25·106 monocytes (b), 0.25·106 THP-1 cells (c), 0.5·106 granulocytes (d), and 37.5·106 platelets (e) were added to MP– plasma. The data for granulocyte-derived MPs were obtained upon activation of granulocytes with LPS. Similar results were obtained after their activation with PMA (not shown)
Table 1. Effects of anti-TF antibodies
on parameters of plasma coagulation in the presence of MPs derived from
various cell types
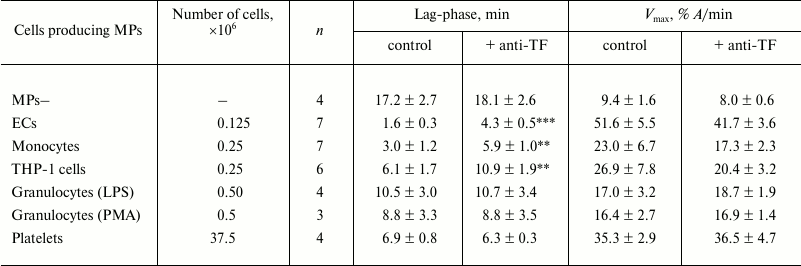
Notes: Lag-phase and maximum rate (Vmax) of plasma
coagulation in the presence of MPs derived from various cell types were
measured. The number of the cells producing MPs that were added to the
plasma samples is presented. Activating agents used for stimulation of
granulocytes are noted (LPS or PMA). MPs–, MP– substrate
plasma (see “Materials and Methods”). Mean values for
coagulation parameters for MP– plasma: lag-phase = 19.9 ±
0.9 min; Vmax = 9.8 ± 0.4% A/min
(n = 31). Significance of differences between coagulation
parameters in the presence of MPs derived from all cell types and in
MP– substrate plasma – p < 0.001. Measurements
were done in the absence (control) and presence of 10 µg/ml
anti-TF antibodies (+ anti-TF); n is the number of
experiments (different preparations of MPs); the data are presented as
mean ± standard error of mean; ** p < 0.01 and ***
p < 0.001 – significances of differences between
“control” and “+ anti-TF” groups. All other
differences between these groups were insignificant (p >
0.05).
TF activity in different types of MPs was detected by its ability to activate factor X in the presence of factor VII (assay with chromogenic substrate). TF activity was registered in MPs derived from ECs, monocytes, and THP-1 cells. However, using this approach, we failed to reveal TF activity in MPs from granulocytes and platelets (Table 2). It should be taken into account that samples were supplemented with MPs obtained from the large numbers of granulocytes (106) and platelets (90·106), which were higher than those that induced substantial acceleration of MP– plasma clotting (Tables 1 and 2).
Table 2. Amount of active TF in MPs
derived from various cell types
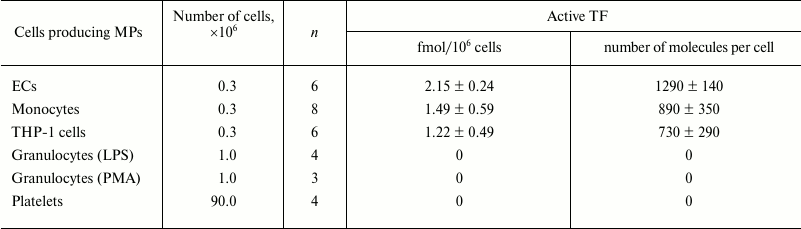
Notes: Amount of active TF in MPs derived from various cell types was
measured. Numbers of cells producing MPs added into the samples are
noted. Granulocytes were stimulated by LPS or PMA; n is the
number of experiments (different preparations of MPs); the data are
presented as mean ± standard error of mean (for non-zero
values); 0 – below sensitivity threshold (<0.02
fmol/sample).
DISCUSSION
We for the first time have compared activity of TF in MPs derived in vitro from five cell types – ECs, monocytes, THP-1 monocytic cells, granulocytes, and platelets. Only erythrocytes as a potential source of MPs in blood were not examined because the possibility to express and/or bind exogenous TF has never been demonstrated for erythrocytes or for erythrocyte-derived MPs [1, 2, 4, 5]. Two approaches were used in the study – inhibition of TF coagulation activity by specific anti-TF antibodies, and direct evaluation of TF activity by its ability to activate factor X. Studies of plasma coagulation were performed in siliconized plates and in the presence of the inhibitor of contact activation (CTI), thereby maximizing the impact of the extrinsic coagulation pathway, i.e. TF pathway. Using the two approaches, we demonstrated the presence of active TF in MPs derived from ECs, monocytes, and THP-1 cells, but not in MPs from granulocytes and platelets. Active TF was detected in all preparations of MPs derived from ECs, monocytes, and THP-1 cells (six to eight for various cell types and for two different assays) despite a marked variability of the examined parameters (coagulation activity of MPs and activity of TF), particularly in monocyte samples. The lag-phase of plasma coagulation was significantly extended by anti-TF antibodies, whereas the slight decrease of the maximal rate of coagulation was insignificant in all cases. These data suggest that TF in MPs primarily affects initiation of clotting, and that after its triggering it has much less influence on the rate of coagulation reactions. For activation of platelets, we used one of the most powerful agonists, TRAP peptide, which acts on thrombin receptor and efficiently stimulates release of MPs (for example, see [19, 20]). Granulocytes were activated either by LPS or PMA, which were previously shown to induce production of MPs in these cells (see review [21]). MPs derived from activated platelets and granulocytes substantially accelerated coagulation of MP– plasma. However, no significant effects from adding anti-TF antibodies on coagulation parameters were found in any of the seven examined samples of granulocyte MPs (four – activated with LPS, three – activated with PMA) and of four samples of platelet MPs. Nor were we able to detect in any sample activity of TF despite collecting MPs from a large number of these cells.
It is known that activated monocytes and MPs produced by them are able to express TF both in vitro and in vivo in various pathological conditions (sepsis, endotoxemia, sickle-cell anemia, etc.) [1, 3-5, 19]. In accordance with these data, we detected active TF in MPs derived from LPS-activated monocytes and THP-1 monocytic cells. We also found active TF in MPs obtained from LPS-activated ECs. In this case, our data are in agreement with the results obtained in similar conditions by Kagawa et al. [22], who also found TF in MPs derived from LPS-treated ECs. Two other studies demonstrated that cultured ECs were able to release TF+ MPs upon activation with tumor necrosis factor and interleukin-1 [23, 24]. Altogether, these data indicate that upon in vitro activation, ECs can produce TF+ MPs. To prove that ECs can in vivo synthesize active TF and produce TF+ MPs is much more difficult, as any possibility to acquire TF due to interaction between ECs and monocyte-derived TF+ MPs must be ruled out. We did not detect TF in MPs derived from granulocytes (activated by LPS and PMA) and platelets (activated by TRAP), which agrees with the data published by others about inability of these cells to synthesize TF [1, 4, 5, 10, 12, 14, 19]. At the same time, there are reports pointing to the presence of TF in granulocytes [11, 25, 26] and platelets [13, 27, 28], and assuming that they might potentially produce TF+ MPs. However, these cells (like ECs), and potentially MPs they produce, might capture TF due to interacting with monocytic TF+ MPs. The ability of granulocytes and platelets to capture such MPs and acquire TF was earlier demonstrated in a model setting [14-16]. In studies performed under in vivo conditions, monocytes are always present in the blood stream, and in in vitro experiments, it is very hard to exclude the possibility of the presence of their contaminations in the preparations of isolated granulocytes and platelets. Recently, Brambilla et al. [28] demonstrated that TF might be synthesized in megakaryocytes and passed on to the platelets developed from them. However, this study was performed in megakaryocyte culture, i.e. in the absence of natural stimuli regulating their biosynthetic activity; therefore, these results do not allow to make a conclusion about in vivo expression of TF in megakaryocytes as well as in circulating platelets and platelet MPs.
This study was financially supported by the Russian Foundation for Basic Research (grant No. 14-04-00179a) and the Ministry of Health of the Russian Federation (grant No. 115061870026).
REFERENCES
1.Owens III, A. P., and Mackman, N. (2011)
Microparticles in hemostasis and thrombosis, Circ. Res.,
108, 1284-1297.
2.Lacroix, R., and Dignat-George, F. (2012)
Microparticles as a circulating source of procoagulant and fibrinolytic
activities in the circulation, Thromb. Res., 129 (Suppl.
2), S27-S29.
3.Angelillo-Scherrer, A. (2012) Leukocytes-derived
microparticles in vascular homeostasis, Circ. Res., 110,
356-369.
4.Osterud, B. (2012) Tissue factor/TFPI and blood
cells, Thromb. Res., 129, Suppl. 2, S27-S29.
5.Osterud, B., and Bjorklid, E. (2012) Tissue factor
in blood cells and endothelial cells, Front. Biosci. (Elite
Ed.), 4, 289-299.
6.Zwicker, J. I., Trenor III, C. C., Furie, B. C.,
and Furie, B. (2011) Tissue factor-bearing microparticles and thrombus
formation, Arterioscler. Thromb. Vasc. Biol., 31,
728-733.
7.Parry, G. C., and Mackman, N. (1995)
Transcriptional regulation of tissue factor expression in human
endothelial cells, Arterioscler. Thromb. Vasc. Biol., 15,
612-621.
8.Lupu, C., Westmuckett, A. D., Peer, G., Ivanciu,
L., Zhu, H., Taylor, F. B., Jr., and Lupu, F. (2005) Tissue
factor-dependent coagulation is preferentially upregulated within
arterial branching areas in a baboon model of Escherichia coli
sepsis, Am. J. Pathol., 167, 1161-1172.
9.Solovey, A., Kollander, R., Shet, A., Milbauer, L.
C., Choong, S., Panoskaltsis-Mortari, A., Blazar, B. R., Kelm, R. J.,
Jr., and Hebbel, R. P. (2004) Endothelial cell expression of tissue
factor in sickle mice is augmented by hypoxia/reoxygenation and
inhibited by lovastatin, Blood, 104, 840-846.
10.Osterud, B. (2004) Tissue factor in neutrophils:
no, J. Thromb. Haemost., 2, 218-220.
11.Nakamura, S., Imamura, T., and Okamoto, K. (2004)
Tissue factor in neutrophils: yes, J. Thromb. Haemost.,
2, 214-217.
12.Bouchard, B. A., Mann, K. G., and Butenas, S.
(2010) No evidence for tissue factor on platelets, Blood,
116, 854-855.
13.Camera, M., Brambilla, M., Toschi, V., and
Tremoli, E. (2010) Tissue factor on platelets is a dynamic event,
Blood, 116, 5076-5077.
14.Egorina, E. M., Sovershaev, M. A., Olsen, J. O.,
and Osterud, B. (2008) Granulocytes do not express but acquire
monocyte-derived tissue factor in whole blood: evidence for a direct
transfer, Blood, 111, 1208-1216.
15.Rauch, U., Bonderman, D., Bohrmann, B., Badimon,
J. J., Himber, J., Riederer, M. A., and Nemerson, Y. (2000) Transfer of
tissue factor from leukocytes to platelets is mediated by CD15 and
tissue factor, Blood, 96, 170-175.
16.Del Conde, I., Shrimpton, C. N., Thiagarajan, P.,
and Lopez, J. A. (2005) Tissue-factor-bearing microvesicles arise from
lipid rafts and fuse with activated platelets to initiate coagulation,
Blood, 106, 1604-1611.
17.Antonov, A. S., Nikolaeva, M. A., Klueva, T. S.,
Romanov, Y. A., Babaev, V. R., Bystrevskaya, V. B., Perov, N. A.,
Repin, V. S., and Smirnov, V. N. (1986) Primary culture of endothelial
cells from atherosclerotic human aorta. Part 1. Identification,
morphological and ultrastructural characteristics of two endothelial
cell subpopulations, Atherosclerosis, 59, 1-19.
18.Mazurov, A. V., Vinogradov, D. V., Kabaeva, N.
V., Antonova, G. N., Romanov, Yu. A., Vlasik, T. N., Antonov, A. S.,
and Smirnov, V. N. (1991) A monoclonal antibody, VM64, reacts with a
130 kDa glycoprotein common to platelets and endothelial cells:
heterogeneity in antibody binding to human aortic endothelial cells,
Thromb. Haemost., 66, 494-499.
19.Aleman, M. M., Gardiner, C., Harrison, P., and
Wolberg, A. S. (2011) Differential contribution of monocyte- and
platelet-derived microparticles towards thrombin generation and fibrin
formation and stability, J. Thromb. Haemost., 9,
2251-2261.
20.Perez-Pujol, S., Marker, P. H., and Key, N. S.
(2007) Platelet microparticles are heterogeneous and highly dependent
on the activation mechanism: studies using a new digital flow
cytometer, Cytometry, Pt. A, 71A, 38-45.
21.Johnson, B. L., III, Kuethe, J. W., and Caldwell,
C. C. (2014) Neutrophil derived microvesicles: emerging role of a key
mediator to the immune response, Endocrin. Metab. Immune Disord.
Drug Targets, 14, 210-217.
22.Kagawa, H., Komiyama, Y., Nakamura, S., Miyake,
T., Miyazaki, Y., Hamamoto, K., Masuda, M., Takahashi, H., Nomura, S.,
and Fukuhara, S. (1998) Expression of functional tissue factor on small
vesicles of lipopolysaccharide-stimulated human vascular endothelial
cells, Thromb. Res., 91, 297-304.
23.Combes, V., Simon, A.-C., Grau, G.-E., Arnoux,
D., Camoin, L., Sabatier, F., Mutin, M., Sanmarco, M., Sampol, J., and
Dignat-George, F. (1999) In vitro generation of endothelial
microparticles and possible prothrombotic activity in patients with
lupus anticoagulant, J. Clin. Invest., 104, 93-102.
24.Abid Hussein, M. N., Boing, A. N., Biro, E.,
Hoek, F. J., Vogel, G. M. T., Meuleman, D. G., Sturk, A., and
Nieuwland, R. (2008) Phospholipid composition of in vitro
endothelial microparticles and their in vivo thrombogenic
properties, Thromb. Res., 121, 865-871.
25.Maugeri, N., Brambilla, M., Camera, M., Carbone,
A., Tremoli, E., Donati, M. B., De Gaetano, G., and Cerletti, C. (2006)
Human polymorphonuclear leukocytes produce and express functional
tissue factor upon stimulation, J. Thromb. Haemost., 4,
1323-1330.
26.Kambas, K., Chrysanthopoulou, A., Vassilopoulos,
D., Apostolidou, E., Skendros, P., Girod, A., Arelaki, S., Froudarakis,
M., Nakopoulou, L., Giatromanolaki, A., Sidiropoulos, P., Koffa, M.,
Boumpas, D. T., Ritis, K., and Mitroulis, I. (2014) Tissue factor
expression in neutrophil extracellular traps and neutrophil derived
microparticles in antineutrophil cytoplasmic antibody associated
vasculitis may promote thromboinflammation and the thrombophilic state
associated with the disease, Ann. Rheum. Dis., 73,
1854-1863.
27.Siddiqui, F. A., Desai, N., Amirkhosravi, A.,
Amaya, M., and Francis, J. L. (2002) The presence and release of tissue
factor from human platelets, Platelets, 13, 247-253.
28.Brambilla, M., Facchinetti, L., Canzano, P.,
Rossetti, L., Ferri, N., Balduini, A., Abbonante, V., Boselli, D., De
Marco, L., Di Minno, M. N., Toschi, V., Corsini, A., Tremoli, E., and
Camera, M. (2015) Human megakaryocytes confer tissue factor to a subset
of shed platelets to stimulate thrombin generation, Thromb.
Haemost., 114, 579-592.