Na+-Translocating Rhodopsin from Dokdonia sp. PRO95 Does Not Contain Carotenoid Antenna
Y. V. Bertsova, A. M. Arutyunyan, and A. V. Bogachev*
Belozersky Institute of Physico-Chemical Biology, Lomonosov Moscow State University, 119991 Moscow, Russia; fax: +7 (495) 939-0338; E-mail: bogachev@belozersky.msu.ru* To whom correspondence should be addressed.
Received November 8, 2015; Revision received December 1, 2015
Carotenoid-binding properties of Na+-translocating rhodopsin (NaR) from Dokdonia sp. PRO95 were studied. Carotenoids were extracted from Dokdonia sp. PRO95 cells. It was found that zeaxanthin is the predominant carotenoid of this bacterium. Incubation of recombinant NaR purified from Escherichia coli cells with carotenoids from Dokdonia sp. PRO95 did not result in any changes in optical absorption or circular dichroism spectra, indicating the absence of binding of the carotenoids by NaR. The same results were obtained using salinixanthin as the carotenoid. These data along with genome analysis of Dokdonia sp. PRO95 and other flavobacteria indicate that NaR from Dokdonia sp. PRO95 and possibly the other flavobacterial Na+-translocating rhodopsins do not contain a carotenoid antenna.
KEY WORDS: Na+-translocating rhodopsin, xanthorhodopsin, carotenoid antenna, zeaxanthin, salinixanthin, flavobacteriaDOI: 10.1134/S000629791604012X
Abbreviations: CD, circular dichroism; DDM, n-dodecyl β-d-maltoside; NaR, Na+-translocating rhodopsin; TLC, thin-layer chromatography; ΔμH+, transmembrane differences in H+ electrochemical potentials.
Transformation of solar energy plays a key role in the functioning of
the Earth’s biosphere. So far, two fundamentally different
mechanisms to conserve energy of light quanta have been discovered in
living organisms: (i) chlorophyll-containing photosynthetic complexes
(photosystems), and (ii) retinal-containing proteins (such as
bacteriorhodopsin, halorhodopsin, and Na+-translocating
rhodopsin). The former mechanism has higher quantum yield and higher
efficiency of energy conservation compared to retinal-containing
proteins. In addition, this mechanism supplies a cell with
low-potential reducing equivalents that along with
ΔμH+ (ATP) are essential for the
autotrophic type of metabolism. These properties probably allowed
chlorophyll-containing photosynthetic complexes to prevail over
retinal-containing proteins in the course of biological evolution [1].
Chlorophyll-containing photosynthetic complexes have an additional advantage over rhodopsins. The capture of light energy by these complexes is significantly enhanced by an array of accessory pigments (light-harvesting complexes or antennae), which assist harvesting light energy over a wider spectral and polarization range as well as greatly increase the cross-section for light absorption [2]. Light-harvesting mechanisms seem to be absent in the case of most rhodopsins. The absence of antennae appears to result in the formation of such exotic bacteriorhodopsin-containing structures as purple membranes of Halobacterium salinarium that in fact represent two-dimensional crystals of bacteriorhodopsin.
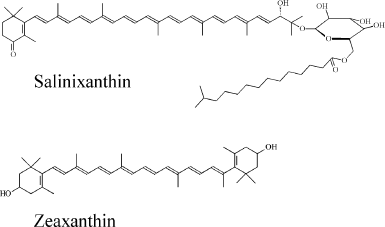
However, it was recently found that a bacteriorhodopsin homolog from the bacterium Salinibacter ruber M31, xanthorhodopsin, contains a tightly bound carotenoid molecule that functions as a simple light-harvesting antenna [3]. Absorption of a light quantum by this antenna results in very fast energy transfer from carotenoid to retinal, and this energy can be further conserved by xanthorhodopsin in the form of ΔμH+ [3, 4]. Xanthorhodopsin uses the main carotenoid of S. ruber, salinixanthin [5], as the antenna chromophore (the chemical structure of salinixanthin is shown in Fig. 1). The three-dimensional structure of xanthorhodopsin was determined by X-ray diffraction [6]. The structure reveals that salinixanthin is bound in xanthorhodopsin in such a way that the angle between the axes of the polyene moiety of the carotenoid and retinal is 46°. The ring moieties of salinixanthin and retinal are within 5 Å of each other, and part of the retinal β-ionone ring is in van der Waals distance of the carotenoid keto-ring [6]. The structural analysis revealed amino acid residues of xanthorhodopsin involved in binding of the carotenoid antenna. Replacement of the bulky Trp151 residue of bacteriorhodopsins (numbering is given in accordance to bacteriorhodopsin from H. salinarium) with the glycine residue in xanthorhodopsins (Fig. 2) plays a critical role in carotenoid binding. This replacement results in the formation of a hydrophobic cavity in xanthorhodopsin located in close proximity to the β-ionone ring of retinal. It is this cavity that is used for binding of the keto-ring of salinixanthin [6].
Fig. 1. Chemical structures of salinixanthin and zeaxanthin.
Fig. 2. Sequence alignment of several rhodopsins. BR, bacteriorhodopsin from H. salinarium (GenBank accession code, AAA72504); XR_Sr and XR_Gv, xanthorhodopsins from S. ruber (ABC44767) and G. violaceus (BAC88139), respectively; NaR, Na+-translocating rhodopsin from Dokdonia sp. PRO95 (AEX55013). The position of the Trp151 residue in BR is marked by an asterisk. Amino acid residues of XR_Sr involved in salinixanthin binding [6] as well as identical to those residues of XR_Gv and NaR are marked by black boxes.
Carotenoid antenna can be removed from xanthorhodopsin and reconstituted in vitro by incubation of this retinal protein with the carotenoid [7, 8]. It was shown that the C=O group of the salinixanthin ring is indispensable for the binding of the carotenoid by xanthorhodopsin. For example, salinixanthin with reduced C=O bond, salinixanthinol, is unable to form a complex with xanthorhodopsin on reconstitution [8, 9]. Apart from xanthorhodopsin of S. ruber, a similar antenna-containing rhodopsin from the cyanobacterium Gloeobacter violaceus has been described. The latter protein apparently uses the keto-carotenoid echinenone as an antenna in vivo, but in vitro it can also form a functional complex with salinixanthin [9, 10].
Previously, various retinal-containing proteins were found to function as light-dependent pumps of H+ or Cl– and as light receptors (for review see [1, 11]). Recently, it was found that one of the rhodopsins of the marine flavobacterium Krokinobacter eikastus NBRC 100814T is capable of light-dependent transport of Na+ from the cytoplasm to the external medium [12]. In the absence of Na+, this protein transfers H+ in the same direction, albeit at a much lower rate [12]. Recently, the three-dimensional structure of Na+-translocating rhodopsin (NaR) from K. eikastus was determined by X-ray diffraction [13, 14]. This approach revealed a high structural similarity of NaR with xanthorhodopsin [13, 14]. Notably, a carotenoid binding site similar to that of xanthorhodopsin is found in NaR [13, 14]. This site was unoccupied in the crystallized NaR. However, as NaR production for crystallography was carried out in Escherichia coli cells unable to synthesize carotenoids, it was assumed that in K. eikastus cells NaR does contain a carotenoid antenna [13, 14].
Recently, we established that one of the rhodopsins of the marine flavobacterium Dokdonia sp. PRO95 (GenBank accession code: AEX55013) having high similarity with NaR from K. eikastus (98% identity) is a primary light-driven Na+-pump [15]. NaR from Dokdonia sp. PRO95 contains the same amino acid residues that are presumably involved in carotenoid antenna binding in NaR from K. eikastus [16], including the key Trp151Gly replacement (Fig. 2). It would be of interest to establish whether NaR from Dokdonia sp. PRO95 contains a carotenoid antenna as was proposed for its analog from K. eikastus. The direct study of this question in Dokdonia sp. PRO95 cells is hampered by a lack of developed genetics techniques for this bacterium. Therefore, we attempted to solve this question by reconstitution of a hypothetical antenna of NaR from Dokdonia sp. PRO95 using carotenoids isolated from this bacterium.
MATERIALS AND METHODS
Bacterial strains and growth conditions. Dokdonia sp. PRO95 and S. ruber M31 were obtained from Leibniz Institute collection of microorganisms and cell cultures (DSMZ). Dokdonia sp. PRO95 cells were grown at 25°C in Difco™ Marine Broth 2216 as described previously [16]. Salinibacter ruber cells were grown at 37°C as described by Anton et al. [17].
Purification of Na+-pumping rhodopsin. Heterologous expression of the gene of NaR from Dokdonia sp. PRO95 in E. coli BL21 and isolation of the recombinant 6×His-tagged protein was performed as described previously [15].
Extraction of carotenoids. Carotenoids of Dokdonia sp. PRO95 and S. ruber were extracted from corresponding bacterial cells with acetone–methanol (7 : 3 v/v) mixture. Phospholipids were removed from the extract by several cycles of precipitation with cold acetone as described previously [5]. Carotenoids of egg yolk were purified as described by Sommerburg et al. [18].
Spectroscopy. Circular dichroism (CD) spectra were recorded using a Chirascan CD spectrometer (Applied Photophysics, Great Britain). Optical absorption spectra were measured on an Agilent-8453 spectrophotometer. The following values of extinction coefficients were used for quantification: salinixanthin in methanol ε480nm ≈ 140 mM–1·cm–1 [19], zeaxanthin in methanol ε450nm = 145 mM–1·cm–1 [20], NaR ε522nm = 33.9 mM–1·cm–1 [12].
Thin-layer chromatography (TLC). Carotenoids were separated by means of TLC on silica gel plates (Sorbfil, 15 × 15 cm) by single development with acetone–hexane (2 : 3 v/v).
Preparation of membrane vesicles from S. ruber cells. Salinibacter ruber cells were disrupted by osmolysis upon overnight dialysis against distilled water. Membranes containing xanthorhodopsin were isolated as described previously [19].
RESULTS AND DISCUSSION
Carotenoid composition of Dokdonia sp. PRO95 cells. Dokdonia sp. PRO95 cells have a bright yellow color indicative of a high carotenoid content in this bacterium. We extracted these carotenoids by treatment of the cells with acetone–methanol mixture, which was done twice. It is noteworthy that after such treatment the cell pellet became uncolored, suggesting completeness of the extraction.
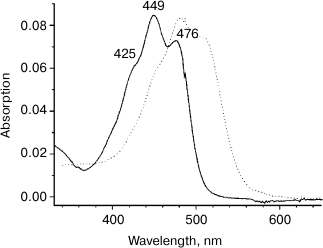
Separation of the extract using TLC resulted in a single colored band (Rf = 0.74, data not shown) that indicates that Dokdonia sp. PRO95 cells contain only one major carotenoid. Figure 3 shows the electronic absorption spectrum of the carotenoid extract in methanol. The shape of the spectrum and the position of its main maximum at 449 nm suggest that the extracted carotenoid is a β-carotene derivative and has 10 conjugated double bonds [21]. Positions of the absorption maxima (Fig. 3) as well as the spectral fine structure III/II = 21% (A476-466/A449-466, %) were identical to those for the earlier determined absorption spectra of zeaxanthin [20, 21]. Furthermore, the carotenoid isolated from Dokdonia sp. PRO95 cells had the same mobility on TLC as the zeaxanthin purified from egg yolk. Thus, we identified the predominant carotenoid of Dokdonia sp. PRO95 as zeaxanthin (the chemical structure of this carotenoid is shown in Fig. 1). This conclusion is in good agreement with results of previous analyses of carotenoid content of different Flavobacteriia, which revealed that zeaxanthin is the most abundant carotenoid among species of this bacterial class [21-24].
Fig. 3. Optical absorption spectra of carotenoids isolated from Dokdonia sp. PRO95 (zeaxanthin, solid curve) and S. ruber (salinixanthin, dotted curve) in methanol. Peak labels refer to maxima of the spectrum of the Dokdonia sp. PRO95 carotenoids.
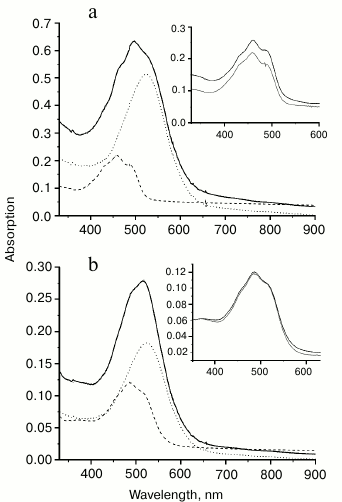
Binding of carotenoids isolated from Dokdonia sp. PRO95 with NaR from this bacterium studied by optical absorption spectroscopy. It was earlier established that xanthorhodopsins reconstitution made by incubation of their carotenoid-free forms with salinixanthin or echinenone is accompanied by a strong sharpening of the vibronic bands in the absorption spectra of the carotenoids [7-10]. We tested this effect on incubation of NaR, produced heterologously and purified from E. coli cells, with carotenoids isolated from Dokdonia sp. PRO95. Figure 4a shows absorption spectra for NaR, carotenoids of Dokdonia sp. PRO95, and their mixture after one hour of incubation. As can be seen from the inset of this figure, incubation of the carotenoids with an excess of NaR did not result in any noticeable changes in the carotenoid spectrum. The same results were obtained after longer incubations with duration up to 8 h. These data indicated that no carotenoid binding to NaR occurred. However, it is important to note that zeaxanthin, the predominant carotenoid of Dokdonia sp. PRO95, in contrast to salinixanthin, does not contain the C=O group in the carotenoid ring (Fig. 1). Thereby, zeaxanthin exhibits a well-structured spectrum even in a solvent (Fig. 3), which can decrease the sensitivity of the method used for the detection of NaR–zeaxanthin interaction. For this reason, it was important to study this interaction by additional technique(s).
Fig. 4. Interaction of NaR with carotenoids measured by optical absorption spectroscopy. Spectra were determined in medium containing 20 mM Tris-HCl (pH 8.0), 100 mM NaCl, and 0.1% DDM. a) Interaction of NaR with carotenoids isolated from Dokdonia sp. PRO95. Dashed curve, spectrum of the carotenoids (2 µM); dotted curve, spectrum of NaR (15 µM); solid curve, spectrum of the carotenoid and NaR mixture (2 and 15 µM, respectively) after 1 h of incubation. b) Interaction of NaR with salinixanthin. Dashed curve, spectrum of salinixanthin (0.86 µM); dotted curve, spectrum of NaR (5.4 µM); solid curve, spectrum of salinixanthin and NaR mixture (0.86 and 5.4 µM, respectively) after 1 h of incubation. Insets on panels (a) and (b) show spectra of corresponding carotenoids before (black curves) and after their incubation with NaR (gray curves). The latter spectra (gray curves) were obtained by subtracting spectra of NaR (dotted curves on the main panels) from spectra of the mixtures (solid curves on the main panels).
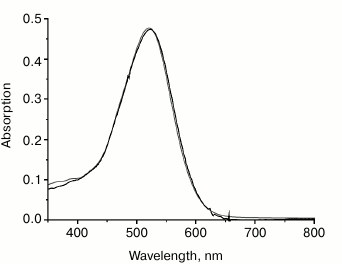
Binding of carotenoids isolated from Dokdonia sp. PRO95 with NaR studied by chromatographic separation. To further test the ability of NaR to bind an antenna carotenoid, membrane vesicles from E. coli cells producing 6×His-NaR [15] were isolated and incubated for 2 h (i) with 1.5% DDM or (ii) with 1.5% DDM and a high excess of the carotenoids from Dokdonia sp. PRO95 (at the carotenoid/NaR molar ratio equal to 5 : 1). We purified NaR from these two mixtures by means of affinity chromatography on a Ni-NTA column and compared spectral properties of the obtained NaR preparations. As Fig. 5 makes clear, the optical absorption spectrum of NaR purified after incubation with the carotenoids was identical to that of NaR not exposed to the carotenoids. Neither spectrum had peaks characteristic for the carotenoids of Dokdonia sp. PRO95 (see Fig. 3). Thus, this technique also indicated the inability of NaR to bind carotenoids isolated from Dokdonia sp. PRO95. It was however possible to assume that NaR has low affinity to carotenoids and they are washed out from the protein during NaR purification on the Ni-NTA column. It was therefore important to study the NaR–carotenoid interaction by a method that does not depend on the stability of this hypothetical complex.
Fig. 5. Optical absorption spectra of different NaR preparations. NaR solubilization from E. coli membrane vesicles had been carried out in the absence (gray curve) or in the presence of Dokdonia sp. PRO95 carotenoids at carotenoid/NaR molar ratio = 5 : 1 (black curve). Purification of NaR was performed as described previously [15].
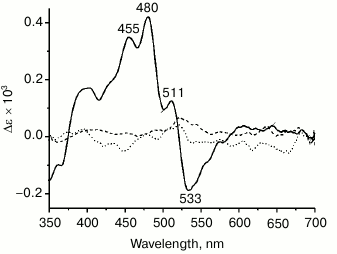
Binding of carotenoids isolated from Dokdonia sp. PRO95 with NaR studied by CD spectroscopy. Due to a very short lifetime of the S2 exited state of carotenoids, a close proximity between carotenoid and retinal is essential for efficient energy transfer from the former to the latter chromophore, i.e. for functioning of the carotenoid as an antenna [3]. Accordingly, the distance between the ring moieties of salinixanthin and retinal is < 5 Å in xanthorhodopsin [6]. Strong excitonic coupling between these chromophores due to such proximity can be easily seen using CD spectroscopy. In line with this, xanthorhodopsin has an intense CD spectrum [7] (see also Fig. 6, solid curve), disappearing if either salinixanthin or retinal is removed from the protein [7, 8]. To further test the ability of NaR to bind an antenna carotenoid, CD spectra of NaR, carotenoids of Dokdonia sp. PRO95, as well as their mixture after 4 h of incubation were determined. As shown in Fig. 6 (dotted curve), incubation of NaR with the carotenoids from Dokdonia sp. PRO95 was not accompanied by the appearance of any additional lines in the CD spectrum. Thus, the results obtained using CD spectroscopy also indicated the absence of a carotenoid antenna in NaR.
Fig. 6. Interaction of NaR with carotenoids measured by CD spectroscopy. Spectra were determined in medium containing 20 mM Tris-HCl (pH 8.0), 100 mM NaCl, and 0.1% DDM. Dotted curve, alteration in CD spectra of NaR and carotenoids of Dokdonia sp. PRO95 after their mixing and joint incubation for 4 h. Dashed curve, alteration in CD spectra of NaR and salinixanthin after their mixing and incubation for 4 h. The curves were obtained by subtracting from the spectrum of NaR and carotenoid mixture (at 11-µM concentration of both components) the individual spectra of NaR and corresponding carotenoid. Solid curve (positive control), CD spectrum of xanthorhodopsin in membrane vesicles of S. ruber, normalized to 11-µM xanthorhodopsin concentration.
Analysis of the Dokdonia sp. PRO95 genome. In summary, all methods used in the current study of the NaR–zeaxanthin interaction did not support the hypothesis of a carotenoid antenna in NaR [13, 14, 16] since we were unable to detect any binding of Dokdonia sp. PRO95 carotenoids by this protein. Nevertheless, there is a possibility that not zeaxanthin, but an additional, minor, and non-extractable carotenoid presumably contained in Dokdonia sp. PRO95 cells operates as the NaR antenna.
Genome analysis of Dokdonia sp. PRO95 reveals that this bacterium contains a gene encoding β-carotene 15,15′-monooxygenase (GenBank accession code, AEX55004) that is essential for retinal synthesis and therefore for production of the active form of rhodopsin. The Dokdonia sp. PRO95 genome also contains a gene for β-carotene hydroxylase CrtZ (AEX55023), an enzyme catalyzing appending of hydroxyl group(s) into β-ionone ring(s) of β-carotene, i.e. the zeaxanthin synthesis [25], which is in good agreement with our data for the carotenoid content of this bacterium.
The C=O group in the carotenoid ring is known to be essential for interaction of antennal carotenoids with xanthorhodopsins [8, 9]. During keto-carotenoid synthesis, the C=O group is appended into the β-ionone ring by β-carotene ketolase CrtO (CrtW) [25]. Genome analysis of Dokdonia sp. PRO95, however, reveals that this bacterium does not contain a gene for this enzyme, which was noted already on the genome sequencing [16]. This means that Dokdonia sp. PRO95 cannot contain an endogenous carotenoid with C=O group in the carotenoid ring, which again argues against the operation of a carotenoid antenna in NaR of this bacterium.
Interestingly, the crtO (crtW) gene is absent not only in Dokdonia sp. PRO95, but also in all other Flavobacteriia with sequenced genomes. This correlates well with previous analyses of carotenoid content of various flavobacteria: only carotenoids with hydroxyl but not keto-group in the carotenoid ring(s) were detected in these microorganisms [21-24]. Thereby, not only NaR from Dokdonia sp. PRO95, but also all other known NaRs (as all of them were identified among different flavobacteria) apparently do not contain a carotenoid antenna.
Due to the high structural similarity between NaR and xanthorhodopsin [13, 14] it is possible to assume that evolutionarily NaR originated from some xanthorhodopsin. The gene of NaR was presumably transferred into an original flavobacterium by horizontal gene transfer, but the crtO gene, essential for keto-carotenoids synthesis, was not acquired during this process. Apparently, this is the reason why NaRs of the present flavobacteria do not contain the carotenoid antenna. It would thus be of interest to determine whether NaR from Dokdonia sp. PRO95 can form antenna with some model keto-carotenoid even if this carotenoid is missing in this flavobacterium.
Binding of salinixanthin by NaR from Dokdonia sp. PRO95. Salinixanthin, a carotenoid with a C=O group in the β-ionone ring [5], was isolated from S. ruber cells and used as the model keto-carotenoid. It is noteworthy that salinixanthin can operate as an antenna in the currently studied xanthorhodopsins from S. ruber and G. violaceus [3, 10]. As can be seen from Fig. 4b, incubation of salinixanthin with an excess of NaR did not result in any changes in the absorption spectrum of this carotenoid, indicating absence of salinixanthin binding by NaR. The same results were obtained using CD spectroscopy. As Fig. 6 (dashed curve) makes clear, incubation of salinixanthin with NaR was not accompanied by the development of the characteristic CD spectrum comparable with that of native xanthorhodopsin in S. ruber membranes (Fig. 6, solid curve) and indicative of strong excitonic coupling between salinixanthin and retinal. These results clearly show that NaR from Dokdonia sp. PRO95 is unable to use carotenoid with the C=O group as the antenna, at least, such as salinixanthin. Apparently, the prolonged evolution of NaR in bacteria unable to synthesize keto-carotenoids resulted in the accumulation of mutations that prevented binding of these chromophores.
Our results indicate that NaR from Dokdonia sp. PRO95 is unable to bind carotenoids in spite of structural similarity of NaR to antenna-containing xanthorhodopsin. Due to very short lifetime of the S2 exited state of carotenoids [3], for efficient energy transfer from carotenoid to retinal close proximity (<5 Å) between these chromophores is required [26]. Since no binding providing such proximity was detected, we conclude that NaR from Dokdonia sp. PRO95 and possibly the other flavobacterial NaRs do not contain a carotenoid antenna. This is an intriguing example when a functionally important and advantageous trait, such as the carotenoid antenna of xanthorhodopsin, was lost in the course of evolution.
We are grateful to Dr. M. D. Mamedov for help in carotenoids extraction.
This work was supported by the Russian Science Foundation (project No. 14-14-00128).
REFERENCES
1.Skulachev, V. P., Bogachev, A. V., and Kasparinsky,
F. O. (2013) Principles of Bioenergetics, Springer.
2.Croce, R., and Van Amerongen, H. (2014) Natural
strategies for photosynthetic light harvesting, Nat. Chem.
Biol., 10, 492-501.
3.Balashov, S. P., Imasheva, E. S., Boichenko, V. A.,
Anton, J., Wang, J. M., and Lanyi, J. K. (2005) Xanthorhodopsin: a
proton pump with a light-harvesting carotenoid antenna, Science,
309, 2061-2064.
4.Boichenko, V. A., Wang, J. M., Anton, J., Lanyi, J.
K., and Balashov, S. P. (2006) Functions of carotenoids in
xanthorhodopsin and archaerhodopsin, from action spectra of
photoinhibition of cell respiration, Biochim. Biophys. Acta,
1757, 1649-1656.
5.Lutnaes, B. F., Oren, A., and Liaaen-Jensen, S.
(2002) New C40-carotenoid acyl glycoside as principal
carotenoid in Salinibacter ruber, an extremely halophilic
eubacterium, J. Nat. Prod., 65, 1340-1343.
6.Luecke, H., Schobert, B., Stagno, J., Imasheva, E.
S., Wang, J. M., Balashov, S. P., and Lanyi, J. K. (2008)
Crystallographic structure of xanthorhodopsin, the light-driven proton
pump with a dual chromophore, Proc. Natl. Acad. Sci. USA,
105, 16561-16565.
7.Balashov, S. P., Imasheva, E. S., and Lanyi, J. K.
(2006) Induced chirality of the light-harvesting carotenoid
salinixanthin and its interaction with the retinal of xanthorhodopsin,
Biochemistry, 45, 10998-11004.
8.Imasheva, E. S., Balashov, S. P., Wang, J. M., and
Lanyi, J. K. (2011) Removal and reconstitution of the carotenoid
antenna of xanthorhodopsin, J. Membr. Biol., 239,
95-104.
9.Balashov, S. P., Imasheva, E. S., Choi, A. R.,
Jung, K. H., Liaaen-Jensen, S., and Lanyi, J. K. (2010) Reconstitution
of gloeobacter rhodopsin with echinenone: role of the 4-keto group,
Biochemistry, 49, 9792-9799.
10.Imasheva, E. S., Balashov, S. P., Choi, A. R.,
Jung, K. H., and Lanyi, J. K. (2009) Reconstitution of Gloeobacter
violaceus rhodopsin with a light-harvesting carotenoid antenna,
Biochemistry, 48, 10948-10955.
11.Grote, M., Engelhard, M., and Hegemann, P. (2014)
Of ion pumps, sensors and channels – perspectives on microbial
rhodopsins between science and history, Biochim. Biophys. Acta,
1837, 533-545.
12.Inoue, K., Ono, H., Abe-Yoshizumi, R., Yoshizawa,
S., Ito, H., Kogure, K., and Kandori, H. (2013) A light-driven sodium
ion pump in marine bacteria, Nat. Commun., 4, 1678.
13.Gushchin, I., Shevchenko, V., Polovinkin, V.,
Kovalev, K., Alekseev, A., Round, E., Borshchevskiy, V., Balandin, T.,
Popov, A., Gensch, T., Fahlke, C., Bamann, C., Willbold, D., Buldt, G.,
Bamberg, E., and Gordeliy, V. (2015) Crystal structure of a
light-driven sodium pump, Nat. Struct. Mol. Biol., 22,
390-395.
14.Kato, H. E., Inoue, K., Abe-Yoshizumi, R., Kato,
Y., Ono, H., Konno, M., Hososhima, S., Ishizuka, T., Hoque, M. R.,
Kunitomo, H., Ito, J., Yoshizawa, S., Yamashita, K., Takemoto, M.,
Nishizawa, T., Taniguchi, R., Kogure, K., Maturana, A. D., Iino, Y.,
Yawo, H., Ishitani, R., Kandori, H., and Nureki, O. (2015) Structural
basis for Na+ transport mechanism by a light-driven
Na+ pump, Nature, 521, 48-53.
15.Bertsova, Y. V., Bogachev A. V., and Skulachev,
V. P. (2015) Proteorhodopsin from Dokdonia sp. PRO95 is a
light-driven Na+-pump, Biochemistry (Moscow),
80, 449-454.
16.Riedel, T., Gomez-Consarnau, L., Tomasch, J.,
Martin, M., Jarek, M., Gonzalez, J. M., Spring, S., Rohlfs, M.,
Brinkhoff, T., Cypionka, H., Goker, M., Fiebig, A., Klein, J.,
Goesmann, A., Fuhrman, J. A., and Wagner-Dobler, I. (2013) Genomics and
physiology of a marine flavobacterium encoding a proteorhodopsin and a
xanthorhodopsin-like protein, PLoS One, 8, e57487.
17.Anton, J., Oren, A., Benlloch, S.,
Rodriguez-Valera, F., Amann, R., and Rossello-Mora, R. (2002)
Salinibacter ruber gen. nov., sp. nov., a novel, extremely
halophilic member of the bacteria from saltern crystallizer ponds,
Int. J. Syst. Evol. Microbiol., 52, 485-491.
18.Sommerburg, O., Keunen, J. E. E., Bird, A. C.,
and Van Kuijk, F. J. G. M. (1998) Fruits and vegetables that are
sources for lutein and zeaxanthin: the macular pigment in human eyes,
Br. J. Ophthalmol., 82, 907-910.
19.Imasheva, E. S., Balashov, S. P., Wang, J. M.,
Smolensky, E., Sheves, M., and Lanyi, J. K. (2008) Chromophore
interaction in xanthorhodopsin: retinal dependence of salinixanthin
binding, Photochem. Photobiol., 84, 977-984.
20.Zang, L. Y., Sommerburg, O., and Van Kuijk, F. J.
(1997) Absorbance changes of carotenoids in different solvents, Free
Radic. Biol. Med., 23, 1086-1089.
21.Shindo, K., Kikuta, K., Suzuki, A., Katsuta, A.,
Kasai, H., Yasumoto-Hirose, M., Matsuo, Y., Misawa, N., and Takaichi,
S. (2007) Rare carotenoids, (3R)-saproxanthin and (3R,2′S)-myxol,
isolated from novel marine bacteria (Flavobacteriaceae) and their
antioxidative activities, Appl. Microbiol. Biotechnol.,
74, 1350-1357.
22.Britton, G., Brown, D. J., Goodwin, T. W.,
Leuenberger, F. J., and Schocher, A. J. (1977) The carotenoids of
Flavobacterium strain R1560, Arch. Microbiol.,
113, 33-37.
23.Takatani, N., Nakanishi, M., Meirelles, P., Mino,
S., Suda, W., Oshima, K., Hattori, M., Ohkuma, M., Hosokawa, M.,
Miyashita, K., Thompson, F. L., Niwa, A., and Sawabe, T. (2014) Draft
genome sequences of marine flavobacterium Algibacter lectus
strains SS8 and NR4, Genome Announc., 2, e01168-14, doi:
10.1128/genomeA.01168-14.
24.Prabhu, S., Rekha, P. D., and Arun, A. B. (2014)
Zeaxanthin biosynthesis by members of the genus Muricauda,
Pol. J. Microbiol., 63, 115-119.
25.Sieiro, C., Poza, M., De Miguel, T., and Villa,
T. G. (2003) Genetic basis of microbial carotenogenesis, Int.
Microbiol., 6, 11-16.
26.Gruszecki, W. I., Zelent, B., and Leblanc, R. M.
(1990) Fluorescence of zeaxanthin and violaxanthin in aggregated forms,
Chem. Phys. Lett., 171, 563-568.