Effect of Fibrinogen on Platelet Reactivity Measured by the VerifyNow P2Y12 Assay
A. B. Dobrovolsky1*, P. S. Laguta1, E. V. Guskova1, E. B. Yarovaya2, E. V. Titaeva1, A. N. Storozhilova1, and E. P. Panchenko1
1Russian Cardiology Research and Production Complex, Ministry of Healthcare, 121552 Moscow, Russia; fax: +7 (495) 414-6699; E-mail: abdobrovolsky@inbox.ru2Lomonosov Moscow State University, Faculty of Mechanics and Mathematics, 119899 Moscow, Russia
* To whom correspondence should be addressed.
Received January 15, 2016; Revision received February 12, 2016
The VerifyNow assay is based upon the ability of activated platelets to cross-link beads coated with fibrinogen. However, fibrinogen is an abundant protein of blood, and therefore it may affect test results by competing with fibrinogen of beads for binding to platelets. To test this assumption, we assessed the influence of artificial alteration of fibrinogen level in blood samples obtained from donors (n = 9) and patients on clopidogrel therapy (n = 8) on the results of the VerifyNow P2Y12 assay. Fibrinogen level was altered by adding to blood samples 1/10 volume of fibrinogen solution (10.56 g/liter) or corresponding buffer. Relative to baseline, addition of buffer significantly increased platelet reactivity, whereas addition of fibrinogen decreased it. Analysis of the relationship between change in platelet reactivity values (dBase and dPRU) and change in fibrinogen concentration (dFg) revealed strong negative correlations: dBase = −63.3 × dFg − 27.1 (r = −0.924, p < 0.0005) and dPRU = −54.4 × dFg − 21.8 (r = −0.764, p < 0.0005). Thus, the results of our experiments suggest that: (i) blood fibrinogen strongly influences results of the VerifyNow P2Y12 assay, and (ii) correcting for fibrinogen effect may be needed to improve the accuracy of the test in the measuring of antiplatelet effect of clopidogrel therapy.
KEY WORDS: platelets, fibrinogen, P2Y12 inhibitors, VerifyNow testDOI: 10.1134/S0006297916050011
Abbreviations: AU, arbitrary units of platelet aggregation; Base, platelet reactivity mediated by PAR-1 and -4; CVD, cardiovascular diseases; Fg, fibrinogen; GP IIb-IIIa, glycoprotein complex binding Fg; PAR-1 and -4, receptors activated by proteases; PRU, platelet reactivity units mediated by P2Y12; P2Y12, platelet ADP receptor.
Platelet activation and formation of thrombi play a pivotal role in the
pathogenesis of atherosclerosis and related cardiovascular disorders
(CVD), which are the major cause of death and disability in the
populations of developed countries [1].
Antithrombotic therapy is a mainstay in the management of patients with
CVD. However, treatment with antithrombotic drugs is associated with
increased risk of bleeding. Plots of relationship between the rate of
adverse events (both thrombotic and bleeding complications) and the
intensity of anticoagulation are U-shaped. This indicates the existence
of a therapeutic window for the intensity of anticoagulation within
which the lowest risk for adverse events is observed. Implementation of
laboratory control improved the safety of therapy with vitamin K
antagonists and heparins, but so far all attempts to improve outcomes
in patients receiving antiplatelet treatment by dose adaptation
according to results of platelet reactivity testing were not successful
[2, 3].
The individual response to clopidogrel is highly heterogeneous because of limited intestinal absorption and complex mechanism of biotransformation of this thienopyridine prodrug to the active metabolite, which irreversibly inhibits P2Y12 receptors of ADP in platelets. Therefore, many efforts have been undertaken for the development of laboratory tests for the evaluation of pharmacodynamic effect of clopidogrel [2, 3].
One of latest developments in this field is the VerifyNow P2Y12 assay. This test is based upon the ability of activated platelets to bind and aggregate beads coated with fibrinogen (Fg) [4]. Although this test did overcome some technical and methodological limitations of the previous assays, one should take into consideration that Fg of blood might affect test results by competing with Fg of beads for binding to activated platelets. If so, then the result of this competition will be apparent decrease in platelet reactivity with the increase in concentration of Fg in blood. However, it should be mentioned that according to data of epidemiological studies, an increase of 1 g/liter in plasma Fg level is associated with about two-fold increase in risk of CVD [5]. Therefore, one may speculate that: (i) fibrinogen might be a factor interfering with correct identification of high risk patients according to results of the VerifyNow P2Y12 assay; and (ii) incorrect identification of high risk patients might explain why dose adaptation according to results of platelet reactivity testing by the VerifyNow P2Y12 assay have not been successful so far.
This experimental study was undertaken to define the relationship between plasma fibrinogen and platelet reactivity measured by the VerifyNow P2Y12 assay.
MATERIALS AND METHODS
The VerifyNow system. Platelet reactivity was measured using VerifyNow P2Y12 cartridges (Accumetrics, USA) designed to monitor the therapy with drugs inhibiting the P2Y12 receptors of ADP on platelets. Each cartridge comprises two channels with measuring cells containing fibrinogen-coated beads and two types of platelet activators. Cells of the test channel contain 20 µM of ADP and 22 nM of prostaglandin E1 that increases the test specificity for the P2Y12 receptor pathway. Cells of the control channel contain two peptide agonists of protease-activated receptors (PAR-1 and PAR-4). Activated platelets form aggregates with fibrinogen-coated beads. This results in an increase in light transmittance of the sample in proportion to the extent of platelet activation. The change in light transmittance in control cells is converted by the instrument into Base value, which represents maximal platelet aggregation, and that of test cells is converted into platelet reactivity units (PRU), which represents platelet aggregation mediated by the P2Y12 receptor pathway. The instrument also calculates the percent of inhibition of platelet aggregation according to formula: PI (%) = (1 − PRU/Base) × 100%.
Fibrinogen. Human fibrinogen with clotability >95% was purified from outdated fresh frozen plasma according to the method of Vila et al. (1985) with slight modifications described by us earlier [6]. At the final purification step, Fg was dialyzed against 25 mM Hepes-NaOH, pH 7.35, containing 140 mM NaCl. The dialyzed sample was centrifuged at 20,000g for 20 min at 4°C to remove any precipitate, dispensed in Eppendorf microtubes, and stored at −70°C. The concentration of Fg in the batch used in this study was 10.56 g/liter. Immediately before experiment, an aliquot of Fg was quickly thawed at 37°C for 10 min.
Patients and study design. Blood samples were obtained from nine apparently healthy volunteers (mainly laboratory employees, further referred to as donors) and eight patients on clopidogrel therapy, who provided informed consent to participate in the study. Blood was drawn from a peripheral vein into a 10-ml Monovette containing 1 ml of 0.109 M trisodium citrate (Sarstedt, Germany). Blood obtained from each study participants was divided into 3-ml aliquots, which were transferred to tubes labeled “Baseline”, “+Fg”, and “+Buffer”. To alter fibrinogen level we added to tubes “+Fg” and “+Buffer” 0.3 ml of Fg solution (10.56 g/liter) or corresponding buffer, respectively. After gentle mixing, 2 ml of blood from these tubes was transferred into Vacuette tubes (Greiner, Austria), from which citrate was previously removed. These tubes were used for platelet reactivity testing. The remaining blood was centrifuged at 2000g for 10 min at room temperature to obtain platelet poor plasma, which was used for measurements of Fg level in the plasma. Fibrinogen was measured by the clotting assay of Clauss using the STA-fibrinogen kit and STA-compact analyzer (Diagnostica Stago, France).
Statistical analysis. Calculations were performed using SPSS (version 11.5) and STATISTICA (version 7.0) software. Continuous variables are expressed as mean ± standard deviation (M ± SD), median (Me), and interquartile range (IQR), and max and min values. Comparisons between donors and patients and analysis of platelet reactivity dynamics after change in Fg concentration were performed using the Mann–Whitney U-test. Linear regression analysis was performed to quantify the relationship between changes in Fg concentrations and platelet reactivity variables. Evaluation of correlations was performed by calculating Pearson correlation coefficients (r).
RESULTS
Donors and patients on clopidogrel therapy did not differ in the initial level of Base values (p = 0.65), which characterize platelet activation independent of P2Y12 receptor activity. For the donors, initial PRU values revealed high variability; nevertheless, all measured values were within the reference range (194-418) reported by the manufacturer for individuals not treated with P2Y12 inhibitors. As should be expected, patients receiving clopidogrel had lower PRU, but higher Fg values than donors (table).
Influence of change in fibrinogen level on platelet reactivity in the
VerifyNow P2Y12 assay

Notes: Data are presented as mean ± standard deviation (M
± SD), median (Me), and interquartile range (IQR, 25-75th
percentile), and maximal and minimal values (max, min).
* p < 0.02, significance of differences between donors and
patients groups in baseline levels of Fg and PRU. Significances of
changes in platelet reactivity after addition of buffer or Fg solution
within groups are shown in corresponding cells of table.
Addition of buffer to blood significantly increased Base and PRU values measured in samples obtained from both donors and patients. Addition of Fg solution significantly decreased Base and PRU values in donors and Base values in patients. The decrease in PRU values in blood of patients was not significant. This might be explained by two reasons. First, because of initially higher level of Fg in patients, addition of the same amount of exogenous Fg caused lesser increase in its concentration in patients than in donors’ blood. Second, owing to initially low PRU values in patients, the amplitude of their change was lower compared with donors. This could increase the impact of measurement imprecision in evaluation of the relationship between change of Fg and PRU in patients. Indirect conformation of latter assumption might be that decrease in Base, initial values of which were similar in patients and donors, was significant in both groups.
Inverse directions of changes in platelet reactivity after the addition of buffer or Fg solution indicates that the observed effects are caused by the change in Fg concentration, since the content of all other blood components, which may influence test results, changed equally after adding buffer or Fg solution. However, analysis of relationships between Fg and platelet reactivity by the VerifyNow P2Y12 assay revealed only a tendency towards inverse correlations of initial Fg levels with PRU (r = −0.57, p = 0.11) and Base (r = −0.61, p = 0.08) values measured in donors. In patients all p were >0.3.
The small size of the study population along with high variability of the individual data may explain why observed in experiments influence of Fg on platelet reactivity by the VerifyNow P2Y12 assay did not appear in this correlation analysis. Therefore, to better define the impact of Fg level on results of the VerifyNow P2Y12 assay, we decided to analyze the relationship between magnitudes of change in platelet reactivity values (dBase and dPRU) and Fg concentration (dFg) caused by addition of buffer or Fg solution to blood. Magnitudes of changes were calculated as the difference between values measured in tube “Baseline” and values measured in tubes “+Fg” and “+Buffer”. It should be noted that, although we added standard volume of buffer or Fg to all samples, the magnitude of change in Fg concentration depended on its baseline level. The increase in Fg level after addition of exogenous Fg was more pronounced in samples with initially lower Fg levels, whereas the decrease after addition of buffer was more pronounced in samples with initially higher Fg levels.
Analysis of the relationship between magnitudes of change in platelet reactivity values (dBase and dPRU) and Fg concentration (dFg) revealed strong negative correlations. The strongest correlations were observed between dFg and dBase in donors (r = −0.948) and in patients (r = −0.892), and dPRU in donors (r = −0.821), p < 0.0005 for each. In patients on clopidogrel therapy, the correlation between dFg and dPRU was slightly weaker than in donors, but nevertheless remained highly significant (r = −0.711, p = 0.002). Given that, all correlation coefficients were similar in both groups and that PRU values in donors represent those, which could be measured in patients resistant to clopidogrel, we decided to combine data for further analysis.
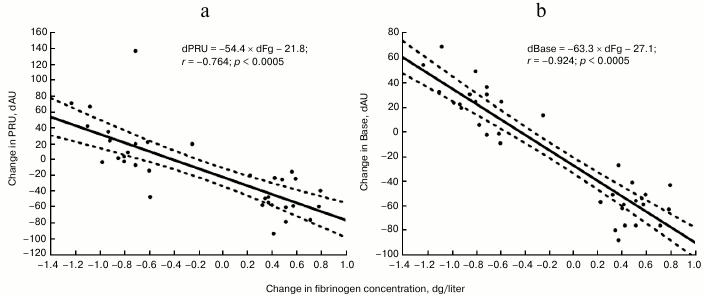
Regression analysis of pooled data showed inverse linear relationships between change in Fg concentration and platelet reactivity values, which are described by equations: dPRU = −54.4 × dFg − 21.8, r = −0.764, p < 0.0005; and dBase = −63.3 × dFg − 27.1, r = −0.924, p < 0.0005 (figure).
Relationships between magnitudes of change in PRU (a) and Base (b) values (Y-axis) and Fg concentration (X-axis) in blood. Magnitudes of changes were calculated as the difference between values measured in tube “Baseline” and values measured in tubes “+Fg” and “+Buffer”. Thus, we obtained 34 pairs of values, which on graphs formed two data arrays showing the change in platelet reactivity with the increase or decrease in Fg level in blood of each studied individual. For better visibility on the graphs, only the regression lines (solid lines) and corresponding 95% confidence intervals (broken lines) derived for pooled data are presented
According to the regression coefficients, the change in Fg concentration by 1 g/liter is associated with change in PRU and Base by 54 and 63 AU, respectively. It should be mentioned that Fg levels are highly variable between patients. Even among those who were included in our study, Fg levels ranged from 2.78 to 5.87 g/liter. This indicates that Fg may exert significant effect on platelet reactivity by the VerifyNow P2Y12 assay and therefore correction for Fg effect may be needed to improve the test specificity in the determination of pharmacodynamics effect of clopidogrel.
The simplest way to correct for Fg effect may consist in the calculation of PRU and Base values corresponding to mean Fg concentration, which according to data of epidemiologic studies is 3.2 g/liter [5, 7]. Using this value and the obtained regression coefficients, corrected for Fg effect Base and PRU values can be calculated by the formulae:
BASE(Fg-corrected) = BASEmeasured + 63 × (Fgpatient − 3.2);
PRU(Fg-corrected) = PRUmeasured + 54 × (Fgpatient − 3.2).
Because with increasing Fg level platelet reactivity by the VerifyNow P2Y12 assay decreases, corrected values compared with measured will be higher in patients with Fg > 3.2 g/liter and lower in those with Fg < 3.2 g/liter. It should be mentioned that since Fg exerts about the same influence on both PRU and Base, Fg has little influence on the percent of inhibition of platelet reactivity calculated on the basis of PRU/Base ratio.
DISCUSSION
Platelet activation causes conformational changes in GP IIb-IIIa receptors, which expose sites with high affinity (Kd ~ 0.1 µM) for Fg. Fibrinogen is a symmetrical molecule comprising of three pairs of nonidentical polypeptide chains, and therefore it contains at least two sites binding with GP IIb-IIIa. This allows Fg to bridge activated platelets and thus promote formation of platelet aggregates. The concentration of Fg in plasma is much higher than the number of GP IIb-IIIa receptors on the surface of platelets in circulation [8]. Therefore, formation of platelet aggregates depends mainly on the extent of GP IIb-IIIa activation [9].
The VerifyNow method is based on the measurement of change in light transmittance when activated platelets bind and aggregate beads coated with Fg. The manufacturer does not specify what amount of Fg is immobilized on beads. However, given mean concentration of Fg in plasma is ~9 µM (~3.2 g/liter), it seems unlikely that the amount of Fg immobilized on beads surface could be much higher than that in the analyzed sample. If these values are comparable, then binding of platelets with beads will depend not only on the extent of platelet activation, but also on blood Fg level. The increase in PRU and Base values after the addition of buffer and their decrease after the addition of Fg shown in our experiments indicates competition between two pools of fibrinogen for binding with activated platelets. Thus, these data demonstrate that the VerifyNow P2Y12 assay results are significantly influenced by Fg level in the analyzed samples.
It should be emphasized that this effect of Fg is an in vitro phenomenon due to the influence of Fg on the method of measurement rather than on intrinsic platelet reactivity. In fact, Fg is the main factor supporting platelet aggregation in vivo, and elevated Fg level is associated with increased risk of ischemic events whose prevention is a goal of antiplatelet therapy in patients with CVD [5].
Large prospective studies GRAVITAS and ARCTIC failed to demonstrate any advantage of antiplatelet therapy tailored according to results of the VerifyNow P2Y12 assay over standard dosing. Several reasons have been postulated to explain why results of these studies were disappointing. One is that the used PRU ≥ 230 as a cutoff value, which was established in previous studies assessing the relationship between platelet reactivity and outcomes in patients treated with clopidogrel, was too high [2, 10, 11]. Indeed, subsequent analysis of the GRAVITAS study revealed that the achievement of PRU < 208 was associated with significant reduction in ischemic events, and the Working Group on Thrombosis of the European Society of Cardiology suggested PRU ≥ 208 as a better threshold to define high platelet reactivity in patients treated with P2Y12 inhibitors [3]. However, the data of the large-scale ADAPT-DES registry showed that even after lowering the cutoff value to PRU ≥ 208, the sensitivity of the test is only 65.2% at specificity 57.5% [12]. The low predictive values of the test might be explained by the fact that the risk of ischemic events depends on multiple factors including coexisting patient comorbidities, which can also interfere with platelet function testing [2, 3, 13, 14].
One of the factors influencing both risk for adverse outcomes and the VerifyNow P2Y12 assay results is hematocrit level. To adjust for the impact of hematocrit on PRU, correction algorithms consisting in the calculation of PRU corresponding to mean hematocrit level in studied patients were proposed [15, 16]. Pendyala et al. recently showed that the addition of PRU, hematocrit, and interaction between the hematocrit and PRU significantly improved the discriminatory power of a logistic model for prediction of adverse events in patients on clopidogrel therapy [17].
The first data indicating that Fg may affect platelet reactivity by the VerifyNow test were obtained by Mahmud et al. in the study of platelet inhibition by eptifibatide – a reversible GP IIb-IIIa antagonist, reported in 2007 [18]. The observed inverse relationship between Fg levels and the extent of platelet inhibition (PI) was explained by a competition of Fg and eptifibatide for binding with GP IIb-IIIa. However, soon it was shown that in diabetic patients elevated Fg level was associated with impaired response to clopidogrel, which is an irreversible inhibitor of P2Y12 [19]. Furthermore, later the same authors showed that Fg level is an even more significant predictor of ischemic events than platelet reactivity by the VerifyNow P2Y12 assay [20].
The finding that elevated Fg level was associated with ischemic outcomes is not surprising. There are several potential mechanisms by which Fg may promote atherothrombosis, and numerous epidemiological studies have shown that Fg is strong risk factor for CVD [5, 7]. As an acute phase protein, Fg shows high variability and positive correlation with most of the conventional cardiovascular risk factors. At the same time, as our experiments showed, in the VerifyNow P2Y12 assay an increase in Fg level is associated with apparent decrease in platelet reactivity, which indeed is an artefact interfering with correct evaluation of pharmacodynamics effect of clopidogrel. This indicates that accounting for the effects of Fg may improve the accuracy of the VerifyNow P2Y12 assay in identification of patients with high risk for adverse outcomes. Unfortunately, this assumption may be verified only in large prospective studies. However, measurement of Fg is a routine coagulation test. This allows us to hope that Fg was measured in many patients included in previous studies. If so, a retrospective analysis of available data might be performed as a first step to determine whether correction for Fg could improve the diagnostic utility the VerifyNow P2Y12 assay.
REFERENCES
1.Sanz, J., Moreno, P. R., and Fuster, V. (2013) The
year in atherothrombosis, J. Am. Coll. Cardiol., 62,
1131-1143.
2.Cattaneo, M. (2012) Response variability to
clopidogrel: is tailored treatment, based on laboratory testing, the
right solution? J. Thromb. Haemost., 10, 327-336.
3.Aradi, D., Kirtane, A., Bonello, L., Gurbel, P. A.,
Tantry, U. S., Huber, K., Freynhofer, M. K., Ten Berg, J., Janssen, P.,
Angiolillo, D. J., Siller-Matula, J. M., Marcucci, R., Patti, G.,
Mangiacapra, F., Valgimigli, M., Morel, O., Palmerini, T., Price, M.
J., Cuisset, T., Kastrati, A., Stone, G. W., and Sibbing, D. (2015)
Bleeding and stent thrombosis on P2Y12-inhibitors:
collaborative analysis on the role of platelet reactivity for risk
stratification after percutaneous coronary intervention, Eur. Heart
J., 36, 1762-1771.
4.Malinin, A., Pokov, A., Spergling, M., Defranco,
A., Schwartz, K., Schwartz, D., Mahmud, E., Atar, D., and Serebruany,
V. (2007) Monitoring platelet inhibition after clopidogrel with the
VerifyNow-P2Y12 rapid analyzer: the VERIfy Thrombosis risk
ASsessment (VERITAS) study, Thromb. Res., 119,
277-284.
5.Fibrinogen Studies Collaboration (2005) Plasma
fibrinogen level and the risk of major cardiovascular diseases and
nonvascular mortality: an individual participant meta-analysis,
JAMA, 294, 1799-1809.
6.Titaeva, E. V., Dobrovolsky, A. B., and Titov, V.
N. (1988) A simple method for isolation of highly purified preparations
of fibrinogen from human plasma, Lab. Delo, 1, 29-32.
7.Dobrovolsky, A. B., Titaeva, E. V., Yarovaya, E.
B., Storozhilova, A. N., Zhernakova, Yu. V., Oschepkova, E. V.,
Panchenko, E. P., Trubacheva, I. A., Serebryakova, V. N., Kaveshnikov,
V. S., Chazova, I. E., and Karpov, R. S. (2013) Coagulation risk
factors of heart disease in the adult population of Tomsk, System
Hypertension, 10, 50-54.
8.Bennett, J. S. (2015) Regulation of integrins in
platelets, Biopolymers, 104, 323-333.
9.Yakushkin, V. V., Zyuryaev, I. T., Khaspekova, S.
G., Sirotkina, O. V., Ruda, M. Y., and Mazurov, A. V. (2011)
Glycoprotein IIb-IIIa content and platelet aggregation in healthy
volunteers and patients with acute coronary syndrome, Platelets,
22, 243-251.
10.Price, M. J., Angiolillo, D. J., Teirstein, P.
S., Lillie, E., Manoukian, S. V., Berger, P. B., Tanguay, J. F.,
Cannon, C. P., and Topol, E. J. (2011) Platelet reactivity and
cardiovascular outcomes after percutaneous coronary intervention: a
time-dependent analysis of the Gauging Responsiveness with a VerifyNow
P2Y12 assay: impact on thrombosis and safety (GRAVITAS)
trial, Circulation, 124, 1132-1137.
11.Montalescot, G., Range, G., Silvain, J., Bonnet,
J. L., Boueri, Z., Barthelemy, O., Cayla, G., Belle, L., Van Belle, E.,
Cuisset, T., Elhadad, S., Pouillot, C., Henry, P., Motreff, P., Carrie,
D., Rousseau, H., Aubry, P., Monsegu, J., Sabouret, P., O’Connor,
S. A., Abtan, J., Kerneis, M., Saint-Etienne, C., Beygui, F., Vicaut,
E., and Collet, J. P.; ARCTIC Investigators (2014) High on-treatment
platelet reactivity as a risk factor for secondary prevention after
coronary stent revascularization: a landmark analysis of the ARCTIC
study, Circulation, 129, 2136-2243.
12.Stone, G. W., Witzenbichler, B., Weisz, G.,
Rinaldi, M. J., Neumann, F. J., Metzger, D. C., Henry, T. D., Cox, D.
A., Duffy, P. L., Mazzaferri, E., Gurbel, P. A., Xu, K., Parise, H.,
Kirtane, A. J., Brodie, B. R., Mehran, R., and Stuckey, T. D.;
ADAPT-DES Investigators (2013) Platelet reactivity and clinical
outcomes after coronary artery implantation of drug-eluting stents
(ADAPT-DES): a prospective multicentre registry study, Lancet,
382, 614-623.
13.Siller-Matula, J. M., Trenk, D., Schror, K.,
Gawaz, M., Kristensen, S. D., Storey, R. F., and Huber, K. (2015) How
to improve the concept of individualized antiplatelet therapy with
P2Y12 receptor inhibitors – is an algorithm the answer?
Thromb. Haemost., 113, 37-52.
14.Legrand, D., Barbato, E., Chenu, P., Magne, J.,
Vrolix, M., Wijns, W., and Legrand, V. (2015) The STIB score: a simple
clinical test to predict clopidogrel resistance, Acta Cardiol.,
70, 516-521.
15.Kakouros, N., Kickler, T. S., Laws, K. M., and
Rade, J. J. (2013) Hematocrit alters VerifyNow P2Y12 assay
results independently of intrinsic platelet reactivity and clopidogrel
responsiveness, J. Thromb. Haemost., 11, 1814-1822.
16.Kim, Y. G., Suh, J.-W., Park, J. J., Oh, I.-Y.,
Yoon, C.-H., Cho, Y. S., Youn, T. J., Chae, I. H., and Choi, D. J.
(2014) Different influences of hematocrit on the results of two
point-of-care platelet function tests, the VerifyNow assay and multiple
electrode platelet aggregometry, PLoS One, 9,
e114053.
17.Pendyala, L. K., Loh, J. P., Lhermusier, T.,
Minha, S., Magalhaes, M., Torguson, R., Chen, F., Satler, L. F.,
Pichard, A. D., and Waksman, R. (2014) Does baseline hematocrit
influence the assays of on-treatment platelet reactivity to
clopidogrel? Am. Heart J., 168, 545-551.
18.Mahmud, E., Cavendish, J. J., Tsimikas, S., Ang,
L., Nguyen, C., Bromberg-Marin, G., Schnyder, G., Keramati, S.,
Palakodeti, V., Penny, W. F., and DeMaria, A. N. (2007) Elevated plasma
fibrinogen level predicts suboptimal response to therapy with both
single- and double-bolus eptifibatide during percutaneous coronary
intervention, J. Am. Coll. Cardiol., 49, 2164-2171.
19.Ang, L., Palakodeti, V., Khalid, A., Tsimikas,
S., Idrees, Z., Tran, P., Clopton, P., Zafar, N., Bromberg-Marin, G.,
Keramati, S., and Mahmud, E. (2008) Elevated plasma fibrinogen and
diabetes mellitus are associated with lower inhibition of platelet
reactivity with clopidogrel, J. Am. Coll. Cardiol., 52,
1052-1059.
20.Ang, L., Bin Thani, K., Ilapakurti, M., Lee, M.
S., Palakodeti, V., and Mahmud, E. (2013) Elevated plasma fibrinogen
rather than residual platelet reactivity after clopidogrel
pre-treatment is associated with an increased ischemic risk during
elective percutaneous coronary intervention, J. Am. Coll.
Cardiol., 61, 23-34.