Molecular and Cellular Mechanisms of Inflammation
D. V. Kuprash1,2* and S. A. Nedospasov1,2
1Engelhardt Institute of Molecular Biology, Russian Academy of Sciences, 119991 Moscow, Russia; E-mail: kuprash@gmail.com2Lomonosov Moscow State University, 119991 Moscow, Russia
* To whom correspondence should be addressed.
Received August 15, 2016
Inflammation is one of the most fundamental and pronounced protective reactions of the organism. From ancient times to the present day, complex and diverse patterns of inflammation development and their role in various diseases have attracted attention of investigators. This issue of Biokhimiya/Biochemistry (Moscow) includes experimental studies and reviews dedicated to various aspects of this important and interesting problem.
KEY WORDS: signs of inflammation, infection, tissue damage, phagocytosis, innate immunity, inflammatory mediators, polymorphismDOI: 10.1134/S0006297916110018
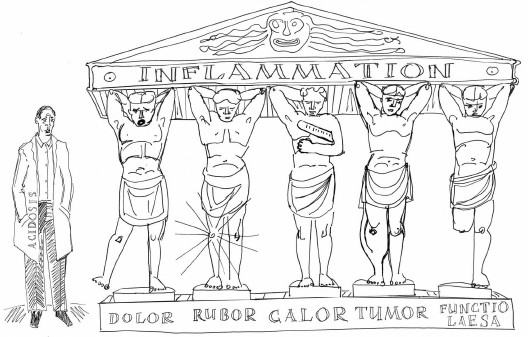
Inflammatory reactions were phenomenologically described in ancient times. Already in the IV century B.C., Hippocrates depicted signs of inflammation that in a familiar classic view were articulated by Celsius, a contemporary of Jesus of Nazareth: redness (rubor), pain (dolor), heat (calor), and swelling (tumor). The famous Roman physician Galen, who lived and worked in the II century A.D. proposed a fifth sign – disturbance of function (functio laesa). Since then, over the last two thousand years only one more sign has been added to a general clinical picture of inflammation (figure) [1]: a shift of base-acid balance to acidic pH (acidosis) due to hypoxia [2].
Known since ancient times, clinical signs of inflammation are often jocularly depicted as the Atlanteans each suffering from a certain symptom. This cartoon is usually considered to have been inspired by the British scientist Derek Willoughby known for developing models of inflammatory pathologies in laboratory animals and studies of physiological inflammatory mediators including histamine [1]. Inflammatory mediators recruit leukocytes to the site of inflammation, simultaneously activating blood vessel endothelium and increasing its permeability. As a result, blood inflow is manifested by redness, locally elevated temperature, and swelling. Compression of the nerve endings elicits pain sensation. The latter combined with altered mechanical properties of organs may disturb their function. Impaired oxygen tissue supply and glycolytic shift with subsequently lowered pH result from swelling as well [2]. In addition, local tissue acidification is also related to pain sensation
Even Galen rightly pointed out that inflammation is a natural body defense reaction [3], and the outstanding Scottish military surgeon John Hunter assigned an important role to inflammation during healing of gunshot wounds [4]. Now we understand that inflammation is tightly bound to innate immunity and its molecular mechanisms, and many inflammatory mediators are a part of innate immunity inherent to all multicellular organisms [5]. However, great physicians of the foretime were mainly guided by intuition and inferences based on the works of their predecessors as well as on personal experience, whereas inflammation theory became scientifically justified only in the second half of the XIXth century owing to studies by Rudolf Virchow [6], Ilya Metchnikoff [7], and their followers. Virchow first pointed to damaged cells at the site of inflammation as a plausible triggering mechanism, whereas Metchnikoff discovered the phenomenon of phagocytosis that mechanistically explained the need for recruiting leukocytes to inflamed tissues. It took a hundred more years until the phenomenon of inflammation was gradually described in molecular terms, including both cells and inflammatory mediators, to understand what cell types elicit or sense certain molecular signals.
They distinguish acute inflammation, which, strictly speaking, is manifested by cardinal signs of inflammation, and chronic inflammation that can occur in the absence of some of them. Chronic inflammation can contribute to emergence of serious pathological conditions such as autoimmunity, diabetes, and cancer [8, 9]. Although usually inflammation is not a primary cause, it plays an important role in development of these diseases, and treatment aimed at suppressing inflammation in many cases can improve the clinical picture [10].
This special issue of Biokhimiya/Biochemistry (Moscow) contains 15 reviews and experimental papers discussing inflammation that give an idea about some developing trends, both in basic biological and in clinical areas.
Innate immunity, apart from the abovementioned role in protecting the body against infections and tissue damage, is also involved in maintaining stability of the body’s internal environment, or homeostasis. It is believed that myeloid cells are a core of innate immunity [11], although recently a significant role was assigned to innate lymphoid cells [12]. The most important inflammatory mediators include cytokines and chemokines mounting local inflammatory reaction [13], as well as low molecular weight mediators acting on other body systems such as the nervous, cardiovascular, and endocrine systems [14]. These mediators are synthesized by various cell types resulting from triggered signaling cascades, some of which are initiated by innate immunity receptors. The latter may be located both on the cell surface and inside cells or cell organelles, and become activated in response to microbiota, tissue damage, or stress signals [15]. Inflammasomes as intracellular signaling platforms involved in reactions of innate immunity and inflammation are able to respond not only to pathogen-derived components but also to various endogenous signals that may occur under sterile conditions [16, 17], to inorganic environmental components [18] or to vaccine adjuvants [19].
This special issue of Biokhimiya/Biochemistry (Moscow) contains studies with laboratory animals and cell-based models as well as projects aimed at examining biological samples obtained from patients and volunteers. Genetic heterogeneity in the human population is a fundamental obstacle inherent to investigating human diseases. It creates specific difficulties upon cell or tissue grafting, whereas in other cases it results in substantial variability of inflammatory and other reactions. At the gene expression level, the majority of statistically significant variations are associated with single nucleotide polymorphisms (SNP), whereas the remainder – with copy number variations [20]. In most cases, SNP association may only point to a certain chromosomal locus, although there are many cases when direct causal relation was found between an SNP and a functional variation. In particular, while examining various inflammation-related disorders, SNPs were found in coding regions and promoters of cytokine genes that profoundly affected either their functional activity [21] or expression level [22].
The articles collected in this issue cover many, yet not all, research areas in the biology of inflammation. The limited space of one journal issue did not allow proper consideration of a number of essential topics such as the role of acute phase proteins, glucocorticoids, and nonsteroidal antiinflammatory drugs in diagnostics and treatment of cardiovascular diseases [23-25], inflammation in developing type 2 diabetes [26], and inflammatory pathologies in neurodegenerative diseases [27].
Acknowledgements
We thank D. Bogolyubova-Kuznetsova for the creative cartoon.
This work was performed with financial support from the Russian Science Foundation (project No. 14-14-01140).
REFERENCES
1.Spector, W. G., and Willoughby, D. A. (1964)
Vasoactive amines in acute inflammation, Ann. N. Y. Acad. Sci.,
116, 839-846.
2.Menkin, V., and Warner, C. R. (1937) Studies on
inflammation. XIII. Carbohydrate metabolism, local acidosis, and the
cytological picture in inflammation, Am. J. Pathol., 13,
25-44.1.
3.Rocha e Silva, M. (1994) A brief survey of the
history of inflammation. 1978, Agents Actions, 43,
86-90.
4.Turk, J. L. (1994) Inflammation: John
Hunter’s “A treatise on the blood, inflammation and
gun-shot wounds”, Int. J. Exp. Pathol., 75,
385-395.
5.Medzhitov, R. (2010) Inflammation 2010: new
adventures of an old flame, Cell, 140, 771-776.
6.Molenaar, J. C. (2003) From the library of the
Netherlands Journal of Medicine. Rudolf Virchow: die Cellularpathologie
in ihrer Begrundung auf physiologische und pathologische Gewebelehre;
1858, Ned. Tijdschr. Geneeskd., 147, 2236-2244.
7.Gordon, S. (2008) Elie Metchnikoff: father of
natural immunity, Eur. J. Immunol., 38, 3257-3264.
8.Shacter, E., and Weitzman, S. A. (2002) Chronic
inflammation and cancer, Oncology, 16, 217-226, 229;
discussion 230-212.
9.Hotamisligil, G. S. (2006) Inflammation and
metabolic disorders, Nature, 444, 860-867.
10.Laveti, D., Kumar, M., Hemalatha, R., Sistla, R.,
Naidu, V. G., Talla, V., Verma, V., Kaur, N., and Nagpal, R. (2013)
Anti-inflammatory treatments for chronic diseases: a review,
Inflamm. Allergy Drug Targets, 12, 349-361.
11.Gabrilovich, D. I., and Nagaraj, S. (2009)
Myeloid-derived suppressor cells as regulators of the immune system,
Nat. Rev. Immunol., 9, 162-174.
12.Spits, H., and Cupedo, T. (2012) Innate lymphoid
cells: emerging insights in development, lineage relationships, and
function, Annu. Rev. Immunol., 30, 647-675.
13.Luster, A. D. (1998) Chemokines –
chemotactic cytokines that mediate inflammation, N. Engl. J.
Med., 338, 436-445.
14.Libby, P., Ridker, P. M., and Maseri, A. (2002)
Inflammation and atherosclerosis, Circulation, 105,
1135-1143.
15.Kawai, T., and Akira, S. (2010) The role of
pattern-recognition receptors in innate immunity: update on Toll-like
receptors, Nat. Immunol., 11, 373-384.
16.Wallin, R. P., Lundqvist, A., More, S. H., Von
Bonin, A., Kiessling, R., and Ljunggren, H. G. (2002) Heat-shock
proteins as activators of the innate immune system, Trends
Immunol., 23, 130-135.
17.Martinon, F., Petrilli, V., Mayor, A., Tardivel,
A., and Tschopp, J. (2006) Gout-associated uric acid crystals activate
the NALP3 inflammasome, Nature, 440, 237-241.
18.Dostert, C., Petrilli, V., Van Bruggen, R.,
Steele, C., Mossman, B. T., and Tschopp, J. (2008) Innate immune
activation through Nalp3 inflammasome sensing of asbestos and silica,
Science, 320, 674-677.
19.Eisenbarth, S. C., Colegio, O. R.,
O’Connor, W., Sutterwala, F. S., and Flavell, R. A. (2008)
Crucial role for the Nalp3 inflammasome in the immunostimulatory
properties of aluminium adjuvants, Nature, 453,
1122-1126.
20.Stranger, B. E., Forrest, M. S., Dunning, M.,
Ingle, C. E., Beazley, C., Thorne, N., Redon, R., Bird, C. P., De
Grassi, A., Lee, C., Tyler-Smith, C., Carter, N., Scherer, S. W.,
Tavare, S., Deloukas, P., Hurles, M. E., and Dermitzakis, E. T. (2007)
Relative impact of nucleotide and copy number variation on gene
expression phenotypes, Science, 315, 848-853.
21.Ozaki, K., Ohnishi, Y., Iida, A., Sekine, A.,
Yamada, R., Tsunoda, T., Sato, H., Sato, H., Hori, M., Nakamura, Y.,
and Tanaka, T. (2002) Functional SNPs in the lymphotoxin-alpha gene
that are associated with susceptibility to myocardial infarction,
Nat. Genet., 32, 650-654.
22.Kubaszek, A., Pihlajamaki, J., Komarovski, V.,
Lindi, V., Lindstrom, J., Eriksson, J., Valle, T. T., Hamalainen, H.,
Ilanne-Parikka, P., Keinanen-Kiukaanniemi, S., Tuomilehto, J.,
Uusitupa, M., Laakso, M., and Finnish Diabetes Prevention Study (2003)
Promoter polymorphisms of the TNF-α (G-308A) and IL-6 (C-174G)
genes predict the conversion from impaired glucose tolerance to type 2
diabetes: the finnish diabetes prevention study, Diabetes,
52, 1872-1876.
23.Ridker, P. M., Hennekens, C. H., Buring, J. E.,
and Rifai, N. (2000) C-reactive protein and other markers of
inflammation in the prediction of cardiovascular disease in women,
N. Engl. J. Med., 342, 836-843.
24.Barnes, P. J., and Adcock, I. M. (2009)
Glucocorticoid resistance in inflammatory diseases, Lancet,
373, 1905-1917.
25.Ridker, P. M., Cushman, M., Stampfer, M. J.,
Tracy, R. P., and Hennekens, C. H. (1997) Inflammation, aspirin, and
the risk of cardiovascular disease in apparently healthy men, N.
Engl. J. Med., 336, 973-979.
26.Shoelson, S. E., Lee, J., and Goldfine, A. B.
(2006) Inflammation and insulin resistance, J. Clin. Invest.,
116, 1793-1801.
27.Glass, C. K., Saijo, K., Winner, B., Marchetto,
M. C., and Gage, F. H. (2010) Mechanisms underlying inflammation in
neurodegeneration, Cell, 140, 918-934.