Effects of Fibroin Microcarriers on Inflammation and Regeneration of Deep Skin Wounds in Mice
A. Y. Arkhipova1#, M. A. Nosenko1,2#, N. V. Malyuchenko1, R. V. Zvartsev2, A. M. Moisenovich2, A. S. Zhdanova1,2, T. V. Vasil’eva1, E. A. Gorshkova1,2, I. I. Agapov3, M. S. Drutskaya1,2*, S. A. Nedospasov1,2,4, and M. M. Moisenovich1
1Lomonosov Moscow State University, Biological Faculty, 119991 Moscow, Russia; E-mail: marinadru@gmail.com2Engelhardt Institute of Molecular Biology, 119991 Moscow, Russia
3Shumakov Research Institute of Transplantation and Artificial Organs, Russian Ministry of Health, 113182 Moscow, Russia
4Lomonosov Moscow State University, Belozersky Institute of Physico-Chemical Biology, 119991 Moscow, Russia
# These authors contributed equally to this work.
* To whom correspondence should be addressed.
Received June 21, 2016; Revision received July 20, 2016
The process of tissue regeneration following damage takes place with direct participation of the immune system. The use of biomaterials as scaffolds to facilitate healing of skin wounds is a new and interesting area of regenerative medicine and biomedical research. In many ways, the regenerative potential of biological material is related to its ability to modulate the inflammatory response. At the same time, all foreign materials, once implanted into a living tissue, to varying degree cause an immune reaction. The modern approach to the development of bioengineered structures for applications in regenerative medicine should be directed toward using the properties of the inflammatory response that improve healing, but do not lead to negative chronic manifestations. In this work, we studied the effect of microcarriers comprised of either fibroin or fibroin supplemented with gelatin on the dynamics of the healing, as well as inflammation, during regeneration of deep skin wounds in mice. We found that subcutaneous administration of microcarriers to the wound area resulted in uniform contraction of the wounds in mice in our experimental model, and microcarrier particles induced the infiltration of immune cells. This was associated with increased expression of proinflammatory cytokines TNF, IL-6, IL-1β, and chemokines CXCL1 and CXCL2, which contributed to full functional recovery of the injured area and the absence of fibrosis as compared to the control group.
KEY WORDS: proinflammatory cytokines, microparticles, fibroin, IL-6, TNFDOI: 10.1134/S0006297916110031
Abbreviations: CXCL1(2), CXC-chemokine ligand 1(2); F, fibroin; FG, fibroin-gelatin; FG-MC, fibroin microcarriers supplemented with gelatin; F-MC, fibroin microcarriers; IL-1β, interleukin 1β; IL-6, interleukin 6; PBS, phosphate buffered saline; TNF, tumor necrosis factor.
Tissue damage is followed by the launch of a cascade of local and
systemic immune reactions providing effective protection and repair.
Inflammation plays an integral role in the regeneration of skin after
injury. Once the integrity of the epidermal barrier is violated,
proinflammatory cytokines, the key mediators of inflammation IL-1, TNF,
and IL-6 are released [1]. These cytokines mediate
the basic protective inflammatory response against pathogens, increase
permeability of blood vessels, and attract and activate effector cells.
The role of the immune system in tissue repair involves elimination of
damaged or dead cells and pathogens, formation of inflammatory
microenvironment, providing signals for migration, proliferation, and
differentiation of tissue progenitor cells, and induction of
angiogenesis [2]. Various effector molecules such
as cytokines and chemokines, VEGF, and TGF-β1, as well as other
growth factors, regulate these processes [3]. A key
role in the proliferative and repair phases of wound healing is carried
out by wound-healing macrophages. Depletion of these cells slows wound
healing, delays fibroblast proliferation, disrupts angiogenesis, and
enhances fibrosis [4]. Fibroblasts that are
associated with wound healing actively synthesize different types of
collagen and generate granulation tissue, which serves as a temporary
framework and provides mechanical and regulatory functions [5]. Granulation tissue is then extensively remodeled
into mature dermis, although not always resulting in full functional
recovery of the injured tissue. In some cases, the damaged area is
filled with fibrous tissue that is unable to fully perform the required
functions. There is now progress in the treatment of skin wounds,
including complicated ones. One of the innovative areas is related to
bioengineered materials used not only to accelerate, but also to
improve the quality of the healing of the injured skin [6, 7].
Fibroin – the main component of silk of the silkworm Bombyx mori – has a number of unique properties and is widely used in biomedicine [8-12]. Fibroin is a nontoxic biocompatible natural polymer; its degradation in vivo is accompanied by the formation of nontoxic derivatives, which in some cases are even beneficial for the regeneration [13, 14]. Many biomaterials based on fibroin were shown to be effective in the regeneration of lesions of skin [15], bone [9], cartilage [16], heart [17], vessels [18], and liver [19] and also can serve as carriers for medical compounds with controlled release [20]. For the treatment of skin lesions, fibroin is used mainly in the form of films or three-dimensional sponges, and it promotes the healing process [15, 21]. At the same time, the results of another study show that fibroin increases not only the speed of healing, but also the quality of regeneration resulting in more effective functional recovery of the damaged tissue [22]. A promising new approach in bioengineering of fibroin-based materials is generation of microcarriers, small biopolymer particles of sizes up to several hundred micrometers, which can be used for delivery of substrate-dependent cells involved in regeneration and biologically active substances. Such delivery can be achieved by the injection of microparticles without the need for a surgical procedure.
Application of the fibroin polymer as a biomedical material requires a better understanding of its immunomodulating activity, as this will allow further improvement of the fibroin derivatives, which will use helpful potential of the immune response for enhancing the regenerative properties. Fibroin has long been regarded as a bioinert material [23]. However, this was based on the studies that used fibroin macroscaffolds in the form of a dense crystalline structure formed by antiparallel β-sheets of repetitive amino acid sequence of fibroin molecules. Such fibroin-based structures are characterized by high density and durability and are associated with slow degradation when implanted in vivo. Small fibroin products, on the other hand, can induce a mild inflammatory response because due to their size they are subject to more intense attack by macrophages, which then subsequently become activated and produce proinflammatory cytokines. Uff et al. [24] first demonstrated that soluble factors, released after the sonication of surgical fibroin suture, can cause activation of macrophages, enhance their phagocytosis ability and production of proinflammatory cytokines. The study of Panilaitis et al. [23] showed that porous fibroin particles of irregular shape with size of 10-200 µm induce significant production of TNF by macrophages in vitro. Cui et al. [25] demonstrated that fibroin particles with size 10-45 and 45-125 µm induce strong TNF production and weaker production of IL-1β and IL-6 in RAW 264.7 macrophage cell line after 24 h of culture. Experiments have shown that the production of proinflammatory cytokines TNF, IL-1β, and IL-6 by peripheral blood monocytes only occurred when cultured on fibroin 3D-matrix, but not on 2D-films, and is associated with the induction of p38 MAP kinase pathway followed by activation of the transcription factor NF-κB [26]. Thus, some evidence of the immunomodulatory activity of the fibroin particles redefines the common opinion of the inertness of this material. It is possible that some effects that contribute to the regeneration of damaged skin are associated with the development of mild inflammation caused by introduction of fibroin microcarriers.
The aim of this study was to evaluate the effect of inflammation induced by fibroin microcarriers on the process of tissue regeneration in the experimental model of deep skin wound in mice.
MATERIALS AND METHODS
Microparticles. Fibroin matrices (F) and composite fibroin matrices supplemented with 30% gelatin (FG) were prepared as previously described [27]. Microcarriers (MC) were obtained by cryodestruction of the matrices to generate fibroin (F-MC) and fibroin–gelatin (FG-MC) microparticles, respectively. The microcarriers were fractionated by passing through a laboratory strainer with pores ranging from 100 to 250 µm (LLC Kraft, Russia).
Mice. Female C57Bl/6N mice at the age of 8-10 weeks were used in the experiments. The animals were bred and maintained under specific pathogen-free conditions at the Pushchino Animal Facility, Branch of Shemyakin and Ovchinnikov Institute of Bioorganic Chemistry, Russian Academy of Sciences.
Generation of deep skin wounds. The mice were subjected to anesthesia by i.p. injection of 100 µl of Zoletil 100 (Virbac, France) and Rometar (Bioveta, Czech Republic) in sterile phosphate-buffered saline (PBS) at concentrations of 10 and 20% (v/v), respectively. After the injection of anesthetic and muscle relaxant, surgery was initiated after observation of complete absence of reflexes in the limbs. Shaved back skin was subjected to brief treatment with depilatory cream to remove residual hair; the skin surface was then cleaned with water and tissue following by decontamination with 70% ethanol. Deep skin wounds were generated in aseptic conditions using a 4 mm sterile disposable biopsy stylet (EPITHEASY; Medax, Italy). The microcarriers were administered by three subcutaneous injections of 60 µl suspensions to the area surrounding the wound, and the control group was injected with PBS solution. The experimental and control groups contained four mice each with two deep skin wounds per mouse.
Gene expression analysis. For RNA isolation, skin samples were subjected to instant freezing in liquid nitrogen, followed by mechanical homogenization with a pre-cooled pestle in a mortar in order to generate a homogeneous substance. Total RNA was isolated using TRI Reagent® (Sigma Aldrich, USA) according to the manufacturer’s recommendations. Total RNA was quantitated with a NanoDrop® ND-1000 spectrophotometer (NanoDrop Technologies, Inc., USA). Next, 1 µg of RNA was treated with DNase I (Thermo Scientific, USA) and used as a template for reverse transcription. First strand cDNA synthesis was performed using a kit for reverse transcription (Thermo Scientific) with oligo(dT)18 as a primer (Thermo Scientific). The resulting cDNA was diluted 10-fold with DNase- and RNase-free water (Invitrogen, USA). Gene expression was assessed by the quantitative real time PCR (qPCR) using a 7500 Real-Time PCR System (Applied Biosystems, USA) with the following amplification protocol: 95°C – 5 min; 45 cycles of 95°C – 15 s, 61°C – 20 s, 72°C – 30 s. After 45 cycles of DNA synthesis, a DNA melting curve was generated in the range of 61-95°C in order to confirm the purity of amplification products. For qPCR 2 µl of cDNA, reaction mixture qPCRmix-HS SYBR + LowROX (Evrogen, Russia) and primers at a concentration of 0.4 µM each in the final volume of 20 µl were used per each reaction according to the manufacturer’s recommendations. The primer sequences are provided in the table.
Primers sequences used for RT-PCR

Gene expression was assessed by relative quantitative analysis (method 2–ΔΔCt, Pfaffl [28]). Actb served as the reference gene. Data were normalized to the level of the expression of genes in naive mouse skin set to be equal to 1. Statistical processing of gene expression data was performed using Student’s t-test in MS Excel.
Morphometric analysis. Photographs of the wounds were taken in macro mode on days 0, 2, 5, and 14 to assess the healing rate. The change of the wound area was evaluated using ImageJ (National Institutes of Health, USA). To this end, wound area was selected on the image and the pixel area was calculated. The area of the wound was assessed as relative to the initial point for the four animals in each group by the formula: (wound area on day n/initial wound area) × 100.
Histology. Samples of skin measuring 1 × 1 cm were spread out and fixed in Buen solution (saturated aqueous picric acid, formalin, and glacial acetic acid in ratio of 15 : 5 : 1) for 24 h. Each sample was cut into two pieces along the middle, dehydrated in alcohols of increasing concentration, and embedded in Histomix® (BioVitrum, Russia). Sections of 7 µm were prepared, which were then deparaffinized, rehydrated, and stained with Mallory stain. Stained samples were embedded in balsam and examined using an Axiovert 200M inverted microscope (Carl Zeiss, Germany) and AxioCam MRC 5 camera (Carl Zeiss). The area of muscle and adipose tissue and the thickness of loose connective tissue in the wound were also evaluated using the ImageJ application. Tissue area, connective tissue thickness, and the number of hair follicles were assessed on five histological sections for each group.
RESULTS
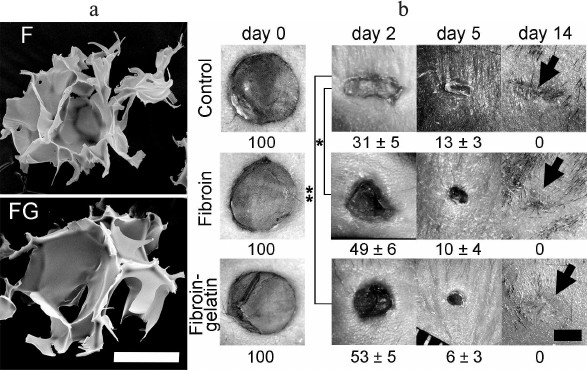
Microcarriers change the dynamics of skin wound contraction. Two types of fibroin microcarriers were used in the experiments, F-MC and FG-MC. Composite FG-MC were supplemented with 30% gelatin, which is optimal for supporting adhesion, proliferation, and migration of cells involved in the regeneration of skin wounds [29]. The microcarriers were three-dimensional porous sponges allowing cell access and nutrient penetration (Fig. 1). The size of the microparticles (100-250 µm) was suitable for subcutaneous injection.
Fig. 1. Effect of microcarriers on the speed of deep skin wound contraction and scar formation within 14 days after wounding. a) Scanning electron microscopy of microcarriers. Scale bar is 100 µm. b) Healing of deep skin wounds after subcutaneous injection of microcarriers based on fibroin (F) or fibroin modified by addition of gelatin (FG). The control group was injected with PBS. The numbers correspond to the percentage of wound area relative to the initial point in the three experimental groups, each consisting of four animals with two deep skin wounds. Statistically significant differences between the experimental and control groups are marked with asterisk (* p < 0.05; ** p < 0.01; n = 8). Wound healing areas and scar formation on day 14 in control and two experimental groups are marked with black arrows. The scale bar is 2 mm.
After the generation of deep skin wounds, fibroin microcarriers F-MC and FG-MC were injected subcutaneously on the perimeter of the wound area. The dynamics of wound contraction was assessed by measuring the wound area. Macrophotographs of the wounds were obtained on days 0, 2, 5, and 14 following the surgery (Fig. 1). Administration of microcarriers decreased the contraction rate of the wounds on day 2 as compared to the control group, which was characterized by rapid skin contraction (Fig. 1; day 2). Thus, on day 2 following the injury the areas of the wound in two experimental groups were significantly greater than in the control group. Further regenerative process was similar (Fig. 1; day 5), and complete healing of the wounds in all three groups was observed on day 10 after the initiation of the experiment. Visual analysis of the wounds on day 14 showed that more effective healing occurred in the groups receiving microcarriers compared to the control group with evident scar formation, whereas animals in the two experimental groups did not show significant scar formation (Fig. 1; day 14).
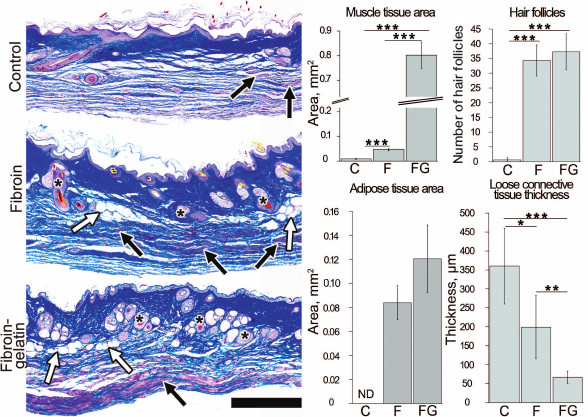
Microcarriers facilitate regeneration of deep skin wounds. Comparative histological analysis of the tissue formed at the site of the injury was carried out to assess the effect of the microcarriers on healing and regeneration of the damaged skin (Fig. 2). In the control group of mice, subcutaneous adipose tissue and muscle layer were completely missing at the site of the wounding one month after the injury (Fig. 2). The connective tissue in the dermis was highly dense, while recovery of the sebaceous glands and hair follicles was not observed. At the sites where hypodermis and subcutaneous muscle should be formed, fibrosis was observed, characterized by outgrowth of loose connective tissue. The thickness of this tissue in the control group was 360 ± 100 µm. Injection of F-MC and FG-MC contributed to the decrease in the thickness of the loose connective tissue to 199 ± 84 and 67 ± 16 µm, respectively. In the newly formed hypodermis in the two experimental groups, there was a restoration of adipose tissue, the total area of which on histological sections in the area of injury was 0.084 ± 0.014 mm2 for the F-MC and 0.12 ± 0.03 mm2 for FG-MC. Administration of F-MC to the injury site resulted in muscle tissue formation with a total area of newly formed tissue of 0.050 ± 0.006 mm2. In the group with FG-MC, this area was 0.80 ± 0.05 mm2 (Fig. 2). Moreover, blood vessels and nerves outgrowth was observed in the regenerating muscle tissue and subcutaneous hypodermis. In the control group, isolated hair follicles were detected in the recovered dermis, while in the two experimental groups, F-MC and FG-MC, their number was significantly increased to 34 ± 5 and 37 ± 6, respectively, over the entire surface area of the wound section. These data indicate that administration of fibroin microcarriers facilitated more effective restoration of normal tissue at the site of the deep skin wounds, whereas supplementing of fibroin with gelatin enhanced the regenerative potential.
Fig. 2. Histological analysis of tissue formed at the site of injury one month after wounding and subcutaneous injection of fibroin and fibroin-gelatin microcarriers. PBS was injected as a control. Black arrows indicate muscles, white arrows – adipose tissue, and hair follicles are marked with asterisks. Scale bar is 500 µm. Bars represent the area of newly formed adipose tissue and muscle, the thickness of loose connective tissue, and the number of hair follicles in the site of injury. Measurements were carried out in five histological sections using ImageJ software (* p < 0.05; ** p < 0.01; *** p < 0.001).
Microcarriers induce local inflammation. It has previously been established that fibroin microparticles induce local inflammation in several experimental models [23-26]. The morphometric analysis of the damage two days after the introduction of the microcarriers showed the presence of edema in the tissues surrounding the wound, as well as the characteristic inflammatory swelling on the periphery of the wound, which was retained until day 5. Edema in the control group was significantly less pronounced on day 2 (Fig. 1) and was completely absent on day 5.
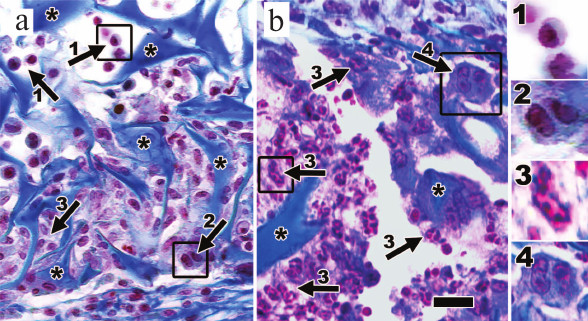
Histological examination revealed the presence of cellular infiltrate consisting of neutrophils, macrophages, and mononuclear cells on day 3 after the injury and FG-MC microparticle injection (Fig. 3a). Infiltrate containing immune cells was also detected at the site of F-MC administration (data not shown). Ten days after the injury, there were signs of degradation of FG-MC characterized by significant reduction in the number of these particles and the area of the fragments detected on histological sections (Fig. 3b). Some remnants of microparticle clusters were surrounded by a thin layer of connective tissue. The infiltrate consisted mainly of neutrophils; in addition, giant multinucleated cells were also observed (Fig. 3b). In the group that received injections of F-MC, there were no residual microcarriers or sign of inflammation detected in the wound area, indicating their accelerated biodegradation as compared to FG-MC. Signs of inflammation were also absent in the control group on day 10 (data not shown). Thus, in the group receiving injections of FG-MC, immune cell infiltration foci were preserved longer than in the other groups.
Fig. 3. Microcarrier clusters and their infiltration by immune cells at days 3 and 10 after injury. On day 3, (a) various immune cells including mononuclear cells, macrophages, and neutrophils can be identified around microcarrier fragments. On day 10, (b) changes in the cellular composition of the infiltrate can be observed, including increase in number of neutrophils and appearance of giant multinucleated cells. 1) Mononuclear cells; 2) macrophages; 3) neutrophils; 4) giant cells. Fragments of microparticles are marked with asterisk. Scale bar is 50 µm.
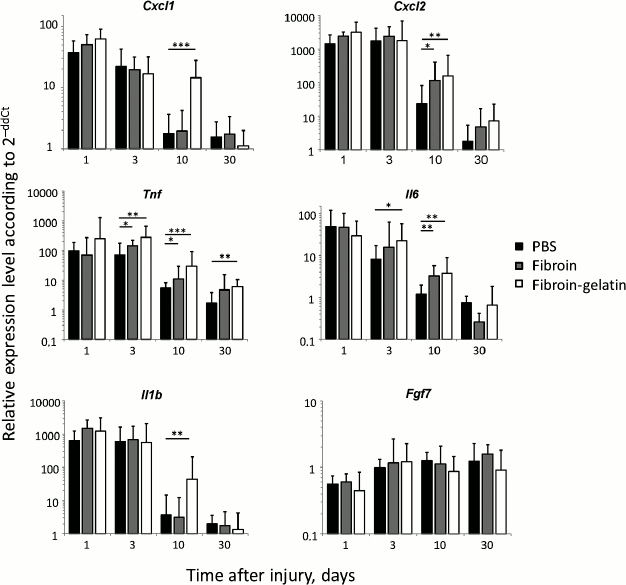
Microcarriers affect expression of inflammatory and regenerative factors in skin. To further study the effect of microcarriers on inflammation in the healing skin, expression of genes of proinflammatory cytokines TNF, IL-6, and IL-1β, chemokines CXCL1 and CXCL2, and regeneration factor FGF7 was investigated (Fig. 4). As anticipated, the dynamics of gene expression of proinflammatory cytokines and chemokines was at maximum during the first days following the injury, with a gradual decline in expression as healing progressed in all three groups. Remarkably, the introduction of fibroin microcarriers, especially those supplemented with gelatin, led to a prolongation of high-level expression of inflammatory factors in the skin at the later regeneration stage (Fig. 4; day 10). The most significant effect of microcarriers was detected for Tnf and Il6 genes. Expression of Il6 was significantly increased at days 3 and 10 after F-MC and FG-MC administration in comparison to the control group. After one month, Il6 expression in the wound area of mice injected with F-MC and FG-MC dropped to the level of controls. Expression of Tnf was increased significantly in the presence of both types of microcarriers on days 3 and 10 after the injury, while in the case of FG-MC even on day 30 of the experiment the level of expression was significantly higher compared to the control group. Introduction of FG-MC resulted in the increase in gene expression of Cxcl1, Cxcl2, and Il1b on day 10 after the wounding, which was not observed when fibroin microparticles without gelatin were introduced. A month later, the expression of Cxcl1, Cxcl2, and Il1b at the site of the injury in mice injected with FG-MC dropped to the level of the control group. Thus, fibroin particles, especially those supplemented with gelatin, contributed to the induction of proinflammatory cytokines and chemokines during skin healing. In addition, in the skin samples expression of the Fgf7 gene encoding one of the fibroblast growth factors involved in regeneration of skin wounds was analyzed. As expected, gene expression was minimal at day 1 and was gradually increased during the healing process, but no significant differences were detected between the experimental and control groups.
Fig. 4. Gene expression of cytokines in the skin during regeneration. Analysis of gene expression of Cxcl1, Cxcl2, Tnf, Il6, Il1b, and Fgf7 was carried out at days 1, 3, 10, and 30 after the administration of the deep skin wounds (n = 6). Gene expression levels are presented as mean with standard deviation. All values were normalized to the expression levels of the genes in naive skin, which were set to be equal to 1; * p < 0.05; ** p < 0.01; *** p < 0.001.
DISCUSSION
We have demonstrated that the introduction of fibroin microcarriers contributes to a better recovery of damaged skin following injury in vivo. Morphometric analysis of the dynamics of wound contraction revealed a significant decrease in scar formation in groups injected with microcarriers 14 days after the induction of deep skin wounds (Fig. 1). In the control group of mice receiving subcutaneous injection of phosphate buffered saline, we observed formation of fibrous tissue at the injury site one month after wounding, while skin appendages, such as hypodermis and muscular layer, were absent, indicating inefficient regenerative processes (Fig. 2). In contrast, in the two experimental groups of mice one month after the damage and the administration of fibroin microcarriers we observed full restoration of the dermis, including skin appendages and the epidermis (Fig. 2). In the dermis, we observed formation of thick, tightly packed, ordered collagen fibers. It should be noted that the introduction of FG-MC promoted regeneration of damaged areas to a greater extent than that of F-MC, since it was associated with a more intense recovery of subhypodermic muscle layer and adipose tissue (Fig. 2), sprouting of blood vessels and nerves in the regenerating hypodermis and better developed muscular layer (data not shown).
It is known that inflammation is an integral step in wound healing, including skin wounds. Inflammatory processes have roles in protection against pathogens that can enter the body through the damaged tissue barrier, removing disrupted and dead cells, tissue remodeling, and production of regenerative factors [30]. Thus, the controlled induction of inflammatory processes should have a beneficial effect on wound healing. It has been shown that the introduction of fibroin particles to deep skin wounds promotes prolongation of the inflammatory process. Morphometric analysis of the wounds showed that inflammatory edema was significantly less pronounced in the control group than in the groups with the introduction of microparticles (Fig. 1). Since deep skin wounds were applied in aseptic conditions, the swelling around the wound in the control group was mainly due to the release of “danger signals” (danger associated molecular patterns, DAMPs) from the cells destroyed during the administration of the wounds and “pathogenic signals” (pathogen associated molecular patterns, PAMPs) from the skin microbiota. Reduction in the swelling two days after wounding, and its absence after five days in the control group of mice, apparently happen due to the successful removal of the components of the disrupted cells and microorganisms mainly by neutrophils infiltrating the damaged area in the first hours after the injury. Absence of proinflammatory PAMPs and DAMPs leads to a reduction of the inflammatory response and consequently to a decreased edema at the wound site. In contrast, skin inflammation in the animals from the experimental groups is apparently further induced by the fibroin microcarriers that remained in the wound area for a long time after administration, thereby prolonging the inflammatory phase of the regeneration.
Presumably, the evident proinflammatory effect of fibroin microcarriers can be associated with increased adhesion and interaction between immune cells and the microcarriers [25, 31, 32]. Histological analysis of the specimens on day 3 after wounding revealed that the microcarriers are surrounded by numerous neutrophils and to a less degree – by macrophages (Fig. 3a). On the day 10 of the experiment, engulfment of the microcarriers by macrophages with consequent generation of giant cells was detected, as well as the presence of neutrophils (Fig. 3b). Gelatin modified fibroin microcarriers were surrounded by macrophages and giant cells in even larger degree, which probably happens due to the involvement of integrin-binding motifs from gelatin, resulting in increased cells adhesion to the microcarrier [29]. Indeed, it was previously established that modification of fibroin scaffolds with gelatin results in their better adhesive properties to cultured cells in vitro [27]. Thus, injection of fibroin microcarriers, particularly FG-MC, caused prolonged local inflammatory response. In support of this, we found that the introduction of fibroin microcarriers resulted in increased expression of inflammatory chemokines and cytokines in the wounded skin. Il6 and Tnf expression were significantly increased at days 3 and 10 post-wounding in the case of F-MC and FG-MC compared to the control group (Fig. 4). We observed increased expression of genes Cxcl1, Cxcl2, and Il1b in the skin on day 10 after wounding in the FG-MC but not in the F-MC group. CXCL1 and CXCL2 are potent chemoattractants that attract neutrophils and other blood cells to the site of inflammation. One month after wounding and injection of fibroin particles, expression of Il6, Cxcl1, Cxcl2, and Il1b in the damaged skin was decreased to the level of uninjured skin, indicating the completion of the inflammatory process and the absence of chronic manifestations. It seems that such a brief but significant prolongation of inflammatory reactions has a positive effect on the healing of the skin wounds – regeneration is more successful, and the functional properties of the damaged area are more efficiently recovered. One month after the injury, we observed a complete restoration of the dermis skin appendages (hair follicles and sebaceous glands) while in the control group formation of fibrous tissue was detected. Apparently, the elevated levels of proinflammatory cytokines and chemokines contribute to attraction, differentiation, and activation of immune cells, which then promote the regenerative process.
Among the proinflammatory cytokines, IL-6 plays a special role. It was shown that in IL-6 knockout mice skin wound healing is attenuated, while expression of other proinflammatory factors, particularly IL-1β and CXCL1, is decreased, and the migration of immune cells to the wound site is disrupted [33, 34]. In addition, IL-6 plays an important role in de novo formation of hair follicles during skin wound healing [35], although recent studies indicate the involvement of other members of the IL-6 family of cytokines in this process [36]. IL-1β also has an important immunoregulatory function in skin regeneration: during the formation of granulation tissue, IL-1β stimulates growth and metabolism of connective tissue and influences the re-epithelialization of wounds. It was shown in vitro that IL-1α and IL-1β enhance proliferation of fibroblasts and their ability to produce glycosaminoglycans [37]. IL-1β promotes the differentiation of epidermal tissue by induction of keratinocyte growth factor (KGF) expression in fibroblasts. Furthermore, keratinocytes themselves produce IL-1β, and thus IL-1β is a major autocrine growth factor for keratinocytes [38, 39]. The role of TNF in the skin wound healing is considered controversial. Interestingly, TNFR1 knockout mice have accelerated wound healing compared to wild type mice, and this was associated with reduced infiltration of immune cells into the inflammatory site [40]. Remarkably, fibrosis formation in TNFR1–/– mice during wound healing was not studied in this work. However, pharmacological agents that decrease the expression of several proinflammatory chemokines and cytokines during the inflammatory phase were shown to impair regeneration of skin wounds [41, 42]. Recent study also considered the role of TNF in attraction of a class III of newly described innate lymphoid cells (ILC3), which play an important role in maintaining homeostasis of the barrier tissues [43]. However, there is evidence concerning negative effects of TNF on skin wound healing [44, 45]. Apparently, the contradictory role of this cytokine in wound healing is associated with its production by various cell types in different forms (soluble and membrane-bound) as well as the presence of two functional receptors of TNF. Possibly tissue-specific inactivation of TNF in various cell types can help clarify better its role in the regeneration process.
Thus, temporarily prolonged moderate inflammation caused by the introduction of fibroin microcarriers alters the immune response balance towards strengthening of the regenerative processes, while avoiding the negative effects of chronic inflammation and fibrosis. The role of inflammation associated with microcarriers in the induction of direct regenerative factors in the deep skin wound model in mice remains an open question. So far, we did not find significant differences in the expression of Fgf7, one of the regenerative factors (Fig. 4); however, perhaps microcarriers induce other regenerative factors. For example, TGF-β1 plays a key role in regeneration of skin after damage [46, 47], participating in the regulation of fibroblast proliferation and their further differentiation into myofibroblasts, collagen synthesis, and the formation of granulation tissue [48, 49]. The details of exactly how injected fibroin microparticles induce the shift in the balance of inflammatory and regenerative factors toward the effective recovery of damaged tissue should be further investigated.
In conclusion, we suggest that moderate controlled inflammation can be useful for regeneration of deep skin wounds, and this can be used in the future to develop new bioresorbable microcarriers designed for delivery to the damaged area. Control of the induced inflammatory responses may be enhanced by modifications of the microcarriers with immunomodulators and other factors that affect regeneration. Furthermore, the vitalization of microcarriers with cell compositions consisting of various populations of immune cells and cells involved in regeneration may be a prominent approach to achieve more efficient skin regeneration.
Acknowledgements
This work was supported by the Russian Science Foundation (project No. 14-25-00160; Figs. 1 and 4) and by the Russian Foundation for Basic Research (project No. 15-29-04903).
REFERENCES
1.Eming, S. A., Krieg, T., and Davidson, J. M. (2007)
Inflammation in wound repair: molecular and cellular mechanisms, J.
Invest. Dermatol., 127, 514-525.
2.Martin, P., and Leibovich, S. J. (2005)
Inflammatory cells during wound repair: the good, the bad and the ugly,
Trends Cell Biol., 15, 599-607.
3.Barrientos, S., Stojadinovic, O., Golinko, M. S.,
Brem, H., and Tomic-Canic, M. (2008) Growth factors and cytokines in
wound healing, Wound Repair Regen., 16, 585-601.
4.Wynn, T. A., and Vannella, K. M. (2016) Macrophages
in tissue repair, regeneration, and fibrosis, Immunity,
44, 450-462.
5.Gurtner, G. C., Werner, S., Barrandon, Y., and
Longaker, M. T. (2008) Wound repair and regeneration, Nature,
453, 314-321.
6.Boateng, J., and Catanzano, O. (2015) Advanced
therapeutic dressings for effective wound healing – a
review, J. Pharm. Sci., 104, 3653-3680.
7.Broussard, K. C., and Powers, J. G. (2013) Wound
dressings: selecting the most appropriate type, Am. J. Clin.
Dermatol., 14, 449-459.
8.Kapoor, S., and Kundu, S. C. (2016) Silk
protein-based hydrogels: promising advanced materials for biomedical
applications, Acta Biomater., 31, 17-32.
9.Melke, J., Midha, S., Ghosh, S., Ito, K., and
Hofmann, S. (2016) Silk fibroin as biomaterial for bone tissue
engineering, Acta Biomater., 31, 1-16.
10.Kanokpanont, S., Damrongsakkul, S.,
Ratanavaraporn, J., and Aramwit, P. (2013) Physico-chemical properties
and efficacy of silk fibroin fabric coated with different waxes as
wound dressing, Int. J. Biol. Macromol., 55, 88-97.
11.Arkhipova, A. Y., Kotlyarova, M. S., Novichkova,
S. G., Agapova, O. I., Kulikov, D. A., Kulikov, A. V., Drutskaya, M.
S., Agapov, I. I., and Moisenovich, M. M. (2016) New silk fibroin-based
bioresorbable microcarriers, Bull. Exp. Biol. Med., 160,
491-494.
12.Moisenovich, M. M., Arkhipova, A. Yu., Orlova, A.
A., Drutskaya, M. S., Volkova, S. V., Zacharov, S. E., Agapov, I. I.,
and Kirpichnikov, M. P. (2014) Composite scaffolds containing silk
fibroin, gelatin, and hydroxyapatite for bone tissue regeneration and
3D cell culturing, Acta Naturae, 6, 96-101.
13.Wang, Y., Rudym, D. D., Walsh, A., Abrahamsen,
L., Kim, H. J., Kim, H. S., Kirker-Head, C., and Kaplan, D. L. (2008)
In vivo degradation of three-dimensional silk fibroin scaffolds,
Biomaterials, 29, 3415-3428.
14.Park, S. H., Gil, E. S., Kim, H. J., Lee, K., and
Kaplan, D. L. (2010) Relationships between degradability of silk
scaffolds and osteogenesis, Biomaterials, 31,
6162-6172.
15.Thurber, A. E., Omenetto, F. G., and Kaplan, D.
L. (2015) In vivo bioresponses to silk proteins,
Biomaterials, 71, 145-157.
16.Foss, C., Merzari, E., Migliaresi, C., and Motta,
A. (2013) Silk fibroin/hyaluronic acid 3D matrices for cartilage tissue
engineering, Biomacromolecules, 14, 38-47.
17.Chi, N. H., Yang, M. C., Chung, T. W., Chou, N.
K., and Wang, S. S. (2013) Cardiac repair using
chitosan-hyaluronan/silk fibroin patches in a rat heart model with
myocardial infarction, Carbohydr. Polym., 92,
591-597.
18.Lovett, M., Cannizzaro, C., Daheron, L., Messmer,
B., Vunjak-Novakovic, G., and Kaplan, D. L. (2007) Silk fibroin
microtubes for blood vessel engineering, Biomaterials,
28, 5271-5279.
19.Yang, Z., Xu, L. S., Yin, F., Shi, Y. Q., Han,
Y., Zhang, L., Jin, H. F., Nie, Y. Z., Wang, J. B., Hao, X., Fan, D.
M., and Zhou, X. M. (2012) In vitro and in vivo
characterization of silk fibroin/gelatin composite scaffolds for liver
tissue engineering, J. Dig. Dis., 13, 168-178.
20.Liu, Q., Liu, H., and Fan, Y. (2015) Preparation
of silk fibroin carriers for controlled release, Microsc. Res.
Tech., doi: 10.1002/jemt.22606.
21.Roh, D. H., Kang, S. Y., Kim, J. Y., Kwon, Y. B.,
Young Kweon, H., Lee, K. G., Park, Y. H., Baek, R. M., Heo, C. Y.,
Choe, J., and Lee, J. H. (2006) Wound healing effect of silk
fibroin/alginate-blended sponge in full thickness skin defect of rat,
J. Mater. Sci. Mater. Med., 17, 547-552.
22.Moisenovich, M. M., Pustovalova, O., Shackelford,
J., Vasiljeva, T. V., Druzhinina, T. V., Kamenchuk, Y. A., Guzeev, V.
V., Sokolova, O. S., Bogush, V. G., Debabov, V. G., Kirpichnikov, M.
P., and Agapov, I. I. (2012) Tissue regeneration in vivo within
recombinant spidroin 1 scaffolds, Biomaterials, 33,
3887-3898.
23.Panilaitis, B., Altman, G. H., Chen, J., Jin, H.
J., Karageorgiou, V., and Kaplan, D. L. (2003) Macrophage responses to
silk, Biomaterials, 24, 3079-3085.
24.Uff, C. R., Scott, A. D., Pockley, A. G., and
Phillips, R. K. (1995) Influence of soluble suture factors on in
vitro macrophage function, Biomaterials, 16,
355-360.
25.Cui, X., Wen, J., Zhao, X., Chen, X., Shao, Z.,
and Jiang, J. J. (2013) A pilot study of macrophage responses to silk
fibroin particles, J. Biomed. Mater. Res. A, 101,
1511-1517.
26.Bhattacharjee, M., Schultz-Thater, E., Trella,
E., Miot, S., Das, S., Loparic, M., Ray, A. R., Martin, I., Spagnoli,
G. C., and Ghosh, S. (2013) The role of 3D structure and protein
conformation on the innate and adaptive immune responses to silk-based
biomaterials, Biomaterials, 34, 8161-8171.
27.Orlova, A. A., Kotlyarova, M. S., Lavrenov, V.
S., Volkova, S. V., and Arkhipova, A. Y. (2014) Relationship between
gelatin concentrations in silk fibroin-based composite scaffolds and
adhesion and proliferation of mouse embryo fibroblasts, Bull. Exp.
Biol. Med., 158, 88-91.
28.Pfaffl, M. W. (2001) A new mathematical model for
relative quantification in real-time RT-PCR, Nucleic Acids Res.,
29, e45.
29.Moisenovich, M. M., Kulikov, D. A., Arkhipova, A.
Y., Malyuchenko, N. V., Kotlyarova, M. S., Goncharenko, A. V., Kulikov,
A. V., Mashkov, A. E., Agapov, I. I., Paleev, F. N., Svistunov, A. A.,
and Kirpichnikov, M. P. (2015) Fundamental bases for the use of silk
fibroin-based bioresorbable microvehicles as an example of skin
regeneration in therapeutic practice, Ter. Arkh., 87,
66-72.
30.Landen, N. X., Li, D., and Stahle, M. (2016)
Transition from inflammation to proliferation: a critical step during
wound healing, Cell. Mol. Life Sci., doi:
10.1007/s00018-016-2268-0.
31.Wilson, C. J., Clegg, R. E., Leavesley, D. I.,
and Pearcy, M. J. (2005) Mediation of biomaterial-cell interactions by
adsorbed proteins: a review, Tissue Eng., 11, 1-18.
32.Franz, S., Rammelt, S., Scharnweber, D., and
Simon, J. C. (2011) Immune responses to implants – a review
of the implications for the design of immunomodulatory biomaterials,
Biomaterials, 32, 6692-6709.
33.Lin, Z. Q., Kondo, T., Ishida, Y., Takayasu, T.,
and Mukaida, N. (2003) Essential involvement of IL-6 in the skin
wound-healing process as evidenced by delayed wound healing in
IL-6-deficient mice, J. Leukoc. Biol., 73, 713-721.
34.Gallucci, R. M., Simeonova, P. P., Matheson, J.
M., Kommineni, C., Guriel, J. L., Sugawara, T., and Luster, M. I.
(2000) Impaired cutaneous wound healing in interleukin-6-deficient and
immunosuppressed mice, FASEB J., 14, 2525-2531.
35.Nelson, A. M., Reddy, S. K., Ratliff, T. S.,
Hossain, M. Z., Katseff, A. S., Zhu, A. S., Chang, E., Resnik, S. R.,
Page, C., Kim, D., Whittam, A. J., Miller, L. S., and Garza, L. A.
(2015) dsRNA released by tissue damage activates TLR3 to drive skin
regeneration, Cell Stem Cell, 17, 139-151.
36.Nelson, A. M., Katseff, A. S., Resnik, S. R.,
Ratliff, T. S., Zhu, A. S., and Garza, L. A. (2016) Interleukin-6 null
mice paradoxically display increased STAT3 activity and wound-induced
hair neogenesis, J. Invest. Dermatol., 136,
1051-1053.
37.Thornton, S. C., Por, S. B., Walsh, B. J., Penny,
R., and Breit, S. N. (1990) Interaction of immune and connective tissue
cells: I. The effect of lymphokines and monokines on fibroblast growth,
J. Leukoc. Biol., 47, 312-320.
38.Mizutani, H., Black, R., and Kupper, T. S. (1991)
Human keratinocytes produce but do not process pro-interleukin-1 (IL-1)
beta. Different strategies of IL-1 production and processing in
monocytes and keratinocytes, J. Clin. Invest., 87,
1066-1071.
39.Chen, J. D., Lapiere, J. C., Sauder, D. N.,
Peavey, C., and Woodley, D. T. (1995) Interleukin-1α stimulates
keratinocyte migration through an epidermal growth factor/transforming
growth factor-α-independent pathway, J. Invest. Dermatol.,
104, 729-733.
40.Mori, R., Kondo, T., Ohshima, T., Ishida, Y., and
Mukaida, N. (2002) Accelerated wound healing in tumor necrosis factor
receptor p55-deficient mice with reduced leukocyte infiltration,
FASEB J., 16, 963-974.
41.Hasegawa, M., Higashi, K., Matsushita, T.,
Hamaguchi, Y., Saito, K., Fujimoto, M., and Takehara, K. (2013)
Dermokine inhibits ELR+CXC chemokine expression and delays
early skin wound healing, J. Dermatol. Sci., 70,
34-41.
42.Heise, R., Skazik, C., Marquardt, Y., Czaja, K.,
Sebastian, K., Kurschat, P., Gan, L., Denecke, B., Ekanayake-Bohlig,
S., Wilhelm, K. P., Merk, H. F., and Baron, J. M. (2012) Dexpanthenol
modulates gene expression in skin wound healing in vivo, Skin
Pharmacol. Physiol., 25, 241-248.
43.Li, Z., Hodgkinson, T., Gothard, E. J.,
Boroumand, S., Lamb, R., Cummins, I., Narang, P., Sawtell, A., Coles,
J., Leonov, G., Reboldi, A., Buckley, C. D., Cupedo, T., Siebel, C.,
Bayat, A., Coles, M. C., and Ambler, C. A. (2016) Epidermal Notch1
recruits RORγ+ group 3 innate lymphoid cells to
orchestrate normal skin repair, Nat. Commun., 7,
11394.
44.Buck, M., Houglum, K., and Chojkier, M. (1996)
Tumor necrosis factor-α inhibits collagen α1(I) gene
expression and wound healing in a murine model of cachexia, Am. J.
Pathol., 149, 195-204.
45.Lai, J. J., Lai, K. P., Chuang, K. H., Chang, P.,
Yu, I. C., Lin, W. J., and Chang, C. (2009) Monocyte/macrophage
androgen receptor suppresses cutaneous wound healing in mice by
enhancing local TNF-α expression, J. Clin. Invest.,
119, 3739-3751.
46.Quaglino, D., Jr., Nanney, L. B., Ditesheim, J.
A., and Davidson, J. M. (1991) Transforming growth factor-β
stimulates wound healing and modulates extracellular matrix gene
expression in pig skin: incisional wound model, J. Invest.
Dermatol., 97, 34-42.
47.Mustoe, T. A., Pierce, G. F., Thomason, A.,
Gramates, P., Sporn, M. B., and Deuel, T. F. (1987) Accelerated healing
of incisional wounds in rats induced by transforming growth
factor-β, Science, 237, 1333-1336.
48.Clark, R. A., Nielsen, L. D., Welch, M. P., and
McPherson, J. M. (1995) Collagen matrices attenuate the
collagen-synthetic response of cultured fibroblasts to TGF-β,
J. Cell Sci., 108, 1251-1261.
49.Desmouliere, A., Geinoz, A., Gabbiani, F., and
Gabbiani, G. (1993) Transforming growth factor-β1 induces
α-smooth muscle actin expression in granulation tissue
myofibroblasts and in quiescent and growing cultured fibroblasts, J.
Cell Biol., 122, 103-111.