Mediators and Biomarkers of Inflammation in Meningitis: Cytokine and Peptidome Profiling of Cerebrospinal Fluid
A. A. Belogurov, Jr.1,2*, O. M. Ivanova1, Y. A. Lomakin1,2, R. H. Ziganshin1,3, M. I. Vaskina1, V. D. Knorre1, E. A. Klimova4, A. G. Gabibov1,2,5, V. T. Ivanov1, and V. M. Govorun1,3
1Shemyakin–Ovchinnikov Institute of Bioorganic Chemistry, 117997 Moscow, Russia; E-mail: belogurov@mx.ibch.ru2Institute of Fundamental Medicine and Biology, Kazan Federal University, 420012 Kazan, Russia
3Research Institute of Physical-Chemical Medicine, Federal Medical and Biological Agency, 119435 Moscow, Russia
4Evdokimov Moscow State University of Medicine and Dentistry, 127473 Moscow, Russia
5Lomonosov Moscow State University, 119991 Moscow, Russia
* To whom correspondence should be addressed.
Received June 2, 2016; Revision received August 15, 2016
Differential diagnosis of bacterial and viral meningitis is an urgent problem of the modern clinical medicine. Early and accurate detection of meningitis etiology largely determines the strategy of its treatment and significantly increases the likelihood of a favorable outcome for the patient. In the present work, we analyzed the peptidome and cytokine profiles of cerebrospinal fluid (CSF) of 17 patients with meningitis of bacterial and viral etiology and of 20 neurologically healthy controls. In addition to the identified peptides (potential biomarkers), we found significant differences in the cytokine status of the CSF of the patients. We found that cut-off of 100 pg/ml of IL-1β, TNF, and GM-CSF levels discriminates bacterial and viral meningitis with 100% specificity and selectivity. We demonstrated for the first time the reduction in the level of two cytokines, IL-13 and GM-CSF, in the CSF of patients with viral meningitis in comparison with the controls. The decrease in GM-CSF level in the CSF of patients with viral meningitis can be explained by a disproportionate increase in the levels of cytokines IL-10, IFN-γ, and IL-4, which inhibit the GM-CSF expression, whereas IL-1, IL-6, and TNF activate it. These observations suggest an additional approach for differential diagnosis of bacterial and viral meningitis based on the normalized ratio IL-10/IL-1β and IL-10/TNF > 1, as well as on the ratio IFN-γ/IL-1β and IFN-γ/TNF < 0.1. Our findings extend the panel of promising clinical and diagnostic biomarkers of viral and bacterial meningitis and reveal opposite changes in the cytokine expression in meningitis due to compensatory action of pro- and antiinflammatory factors.
KEY WORDS: inflammation, meningitis, cytokines, biomarkers, peptidome, cerebrospinal fluidDOI: 10.1134/S0006297916110079
Abbreviations: BM, bacterial meningitis; CSF, cerebrospinal fluid; GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN-γ, interferon-gamma; IL-1β, interleukin-1β; TNF, tumor necrosis factor; VM, viral meningitis.
Meningitis is an acute inflammatory process affecting pia mater
of the brain and spinal cord. The etiology of meningitis varies. Two
types of meningitis are generally described: bacterial [1] and viral (or serous) [2].
Meningitis is usually a result of penetration of various pathogens into
the brain tunic, starting from viruses (Coxsackie-ECHO enteroviruses,
Flavivirus, epidemic parotitis virus, herpesviruses, etc.) [3] and bacteria (Neisseria meningitidis,
Streptococcus pneumoniae, mycobacteria, Haemophilus
influenzae, spirochetes, etc.) [4] to protozoa
[5]. In the majority of cases, viral meningitis has
a favorable prognosis, whereas the course of bacterial meningitis is
much more severe and in some cases may have lethal outcome. Success in
the treatment of meningitis greatly depends on correct diagnosis during
the first hours of the disease. Strategies of terminating bacterial and
viral meningitis are very different; therefore, the problem of their
differential diagnosis is evidently urgent. Bacterial meningitis can be
detected and confirmed by reliable, rapid, and inexpensive tests [3-7], but diagnosis of viral
meningitis is not trivial: it is difficult to determine the viral agent
because of its significant diversity [3]. Sampling
the cerebrospinal fluid (CSF) by lumbar puncture and its analysis helps
to solve this problem.
Among classic biochemical and immunological markers of meningitis are the contents in the CSF of total protein, lactate, glucose, monocytes, and neutrophils [8]. Recently, increasing significance is ascribed to the cytokine status of CSF of patients with meningitis. Cytokines appear in the CSF due to the secretion by perivascular macrophages and by resident cells of the CNS: astrocytes and glial and neural cells. Endogenous IL-1 and TNF are generally thought to be necessary for active protection in pneumococcal meningitis [9]. A dramatic increase in the TNF level in the CSF of rats with meningitis induced by St. pneumoniae was detected as soon as after 6 h [10], and the authors consider this observation promising for early diagnosis of bacterial meningitis. Increase in the expression levels of TNF and IL-1β in the brain during experimental pneumococcal meningitis has been confirmed on the level of mRNAs encoding them [11]. Among five parameters of CSF in bacterial meningitis, which include quantities of neutrophils and monocytes and levels of protein and glucose, the IL-6 content was the most demonstrative. Moreover, patients with the IL-6 concentration in CSF distributed in the fourth quartile had the highest risk of neurological complications on the background of meningitis [12]. In the CSF of children with bacterial meningitis [13], the levels of IL-6 and IL-8 were increased 10- and 20-fold. Average levels of IL-6, interferon-gamma (IFN-γ), TNF, and IL-8 cytokines were significantly higher in the cohort of survived AIDS-infected patients with meningitis, whereas the levels of IL-10, MCP-1, MIP-1β, G-CSF, and GM-CSF similar [14]. Recent wide-scale profiling of CSF of patients with meningitis caused by Listeria monocytogenes revealed a significant increase in the levels of 51 proteins from the representative panel of cytokines, chemokines, and complement system factors [15].
Obviously, the spectra of protein mediators responsible for coordination of activities of the immune system components in response to penetration of a viral or bacterial pathogen are different. During the last decade, this difference stimulated the active development of approaches to differential diagnosis of meningitis based on analysis of the cytokine status of a patient’s CSF. Using the CSF levels of three cytokines, TNF, IL-6, and IL-8, the authors [13] discriminated bacterial and viral meningitis in children with 100% specificity and sensitivity. The threshold levels established for IL-6 and IL-8 as 100 and 75 pg/ml, respectively, differentiated bacterial meningitis with the 100% sensitivity and specificity. The levels of IL-6 and IL-8 in the CSF of patients with meningitis were also studied [16], and the IL-8 concentration was acknowledged by the authors as a biomarker for discriminating bacterial and viral meningitis. Surprisingly, there are a very few studies using proteomic analysis of CSF of patients with meningitis. Thus, in work [17] the authors identified 208 proteins and measured their contents in the CSF of patients with tuberculous meningitis. Among these proteins there were nine proteins with the twofold difference from the control (the levels of six proteins were increased and the levels of three proteins – decreased). Several studies describe identification of biomarkers or suggest models for discriminating forms of meningitis [18-22]. However, we have not found in the literature careful studies on the CSF peptidome in meningitis. At the same time, it is evident that information about the intensity and specificity of protein degradation in the CSF could be useful for both, understanding the pathogenetic mechanisms of meningitis and development of new approaches of differential diagnosis.
In the present work, we performed multiplex analysis of levels of 17 cytokines and chemokines in the CSF of patients with bacterial and viral meningitis in comparison with controls as an attempt to fundamentally describe the inflammatory process and to reveal cytokines benefitial for differential clinical diagnosis. We also found characteristic differences in the peptide profiles, which together with definite cytokine markers allowed us to create a multiparametric system for differential diagnosis of bacterial and viral meningitis.
MATERIALS AND METHODS
Patients with meningitis and controls. We studied six patients with bacterial meningitis (four men, two women, median 28 years old, the interquartile interval was 22-39 years) (the diagnosis was verified according to standards adopted by the Health Care Ministry of Russia and confirmed by microbiological examination) and 11 patients with viral meningitis (six men, five women, median 26.5 years old, the interquartile interval was 23-38 years) that was confirmed by PCR. All analyses were performed in the Evdokimov Moscow State University of Medicine and Dentistry. The group of controls included 20 patients without neurological disorders or oncologic diseases (10 men, 10 women, median 59 years old, interquartile interval was 41-85 years) from the Therapeutic Rehabilitation Center of the Health Care Ministry of Russia. Sterile samples of CSF were obtained using the standard technique of lumbar puncture. The CSF samples (1 ml) were placed into sterile tubes without conservants before spinal anesthesia. The investigation protocol was approved by ethics committees of both Evdokimov Moscow State University of Medicine and Dentistry and the Therapeutic Rehabilitation Center. All patients gave their informed agreement for participation in the study.
Multiplex analysis of CSF cytokines. The CSF samples were analyzed using multiplex system (Bio-Rad, USA) according to the manufacturer’s recommendations. The CSF samples were analyzed for the simultaneous presence of 17 cytokines (in parentheses the corresponding sensitivity level is indicated in pg/ml for each cytokine according to the manufacturer’s specifications): IL-1β (0.6), IL-2 (1.6), IL-4 (0.7), IL-5 (0.6), IL-6 (2.6), IL-7 (1.1), IL-8 (1.0), IL-10 (0.3), IL-12(p70) (3.5), IL-13 (0.7), IL-17 (3.3), interferon-gamma (IFN-γ) (6.4), tumor necrosis factor (TNF) (6.0), granulocytic colony-stimulating factor (G-CSF) (1.7), granulocyte-macrophage colony-stimulating factor (GM-CSF) (2.2), MCP-1 (1.1) and MIP-1β (2.4). The measurements were performed using a Bio-Plex Pro Human Cytokine 17-plex Assay kit (Bio-Rad). Fifty microliters of threefold-diluted CSF sample and of the cytokine standard were mixed with magnetic microspheres carrying surface-immobilized antibodies (50 µl) in a 96-well nonsorbing plate (Nunc, Denmark) and incubated for 30 min at room temperature. The plate was washed, and afterwards 25 µl of solution of biotinylated antibodies against the studied cytokines (Bio-Rad) were added into the well and incubated for 30 min at room temperature. Streptavidin–phycoerythrin conjugate (Bio-Rad) was introduced into every well and incubated for 10 min at room temperature. After the final washing, the magnetic microspheres were resuspended in 125 µl of a reaction buffer (Bio-Rad) and analyzed using a Suspension Array System (Bio-Plex 200 System; Bio-Rad). The cytokine concentrations were calculated based on calibration standard curves for each cytokine.
Peptidome analysis. Peptides were isolated from CSF using solid-phase extraction on reversed-phase Discovery DSC-18 cartridges (50 mg sorbent; Supelco, USA). CSF samples (100 µl) of the patients with meningitis and 500 µl of CSF of neurologically healthy controls were heated on a boiling water bath for 10 min, cooled to room temperature, diluted twofold with 0.1% trifluoroacetic acid (TFA) aqueous solution, and placed onto a preconditioned cartridge for the reverse-phase extraction. After the sample was placed, the cartridge was washed with 1 ml of 0.1% aqueous TFA solution and eluted with 1 ml of 80% acetonitrile solution in 0.1% TFA. The eluate was dried and re-diluted in 20 µl of 5% acetonitrile in 0.1% TFA. Before mass-spectrometry analysis, the samples were ultrasonicated on an ultrasonic bath for 2 min.
Liquid chromatography combined with mass-spectrometry (LC-MS/MS analysis). Analysis was performed using a TripleTOF 5600+ mass-spectrometer equipped with a NanoSpray III ionization source (ABSciex, Canada) combined with a nano-flow NanoLC Ultra 2D+ (Eksigent, USA) chromatographic system as reported previously [23]. The HPLC system was used in the configuration of a precolumn–separating column. The solution for sample loading and buffer A was the following: 98.9% water, 1% methanol, 0.1% formic acid (v/v). Buffer B consisted of 99.9% acetonitrile and 0.1% formic acid (v/v). The samples were placed onto the Chrom XP C18 precolumn (3 µm, 120 Å, 350 µm × 0.5 mm; Eksigent) at the flow rate of 3 µl/min for 10 min. The peptides were identified using the data-dependent regimen of the mass-spectrometer (IDA). Each cycle included an averaged MS1 spectrum with subsequent 50 dependent MS2 spectra. For analyzing MS1, the parameters were as follows: masses for analysis and subsequent selection of ions for fragmentation analysis were within 300-1250 m/z, signal accumulation time was 250 ms. For MS2 analysis, ions were taken based on the ionic current intensity with the threshold value of 200 pulses/s and the charge from +2 to +5. For analyzing MS2, the following parameters were used: quadrupole resolution UNIT (0.7 Da), masses within 200-1800 m/z, optimization of the ion beam for obtaining the maximum sensitivity (resolution ~20,000), signal accumulation time was 50 ms for each ion. Nitrogen was used for collision-induced dissociation. The collision energy was the same for all ions and changed linearly from 25 to 55 V during 50 ms of the signal accumulation time. Ions for which the MS2 spectrum was obtained were introduced into the dynamic exclusion list for 15 s to increase the probability of obtaining the MS2 spectrum in the highest point of the peptide chromatographic peak (the peak average width for the components was 30 s). Solution of trypsin hydrolysate of β-galactosidase (20 fmol) was used with the 15-min gradient (from 5 to 25% buffer B) for calibration of the mass-spectrometer and control of productivity, stability, and reproducibility of the system.
Analysis of LC-MS/MS data. Based on MS/MS data obtained with the ProteinPilot program (version 4.5; ABSciex), lists of peaks were generated and recalibrated and then analyzed in parallel using MASCOT (version 2.2.07) and X!Tandem (version CYCLONE, 2013.2.01) programs against the UniProt Human Protein Knowledgebase database (version 2015_03). Admissible deviations from the ion precursor and fragment masses were 20 ppm and 0.04 Da, respectively. Searching parameters in the database were unspecific enzyme and dynamic/flexible methionine oxidation. The identification results and their meta-analysis were validated using the Scaffold 4 program (version 4.2.1, Proteome Software Inc., USA). Peptides suitable by the reliability level for the identification massive with Global FDR 1% were considered as reliably identified. The list of precursor proteins was composed based on the reliably identified peptides (FDR 1%). The subcellular localization and functions of the identified precursor proteins were determined using the UniProtKB/Swiss-Prot, Gene Ontology (GO), and KEGG databases.
Statistical processing of data. The data were analyzed using Sigma-Plot 12.5 and GraphPad Prism 5.0 software. Statistically significant differences were evaluated with the paired t-test (at significant difference of p < 0.05). When the cytokine concentration was below the detectable level, the values were substituted by the corresponding threshold concentrations. The levels of cytokines and chemokines were analyzed in diagrams using ROC (Receiver Operator Characteristic) of the curves, which presented graphic characteristics of the binary classifier quality. The characteristics determined the dependence of the fraction of true positive classification on the fraction of false positive classifications.
RESULTS
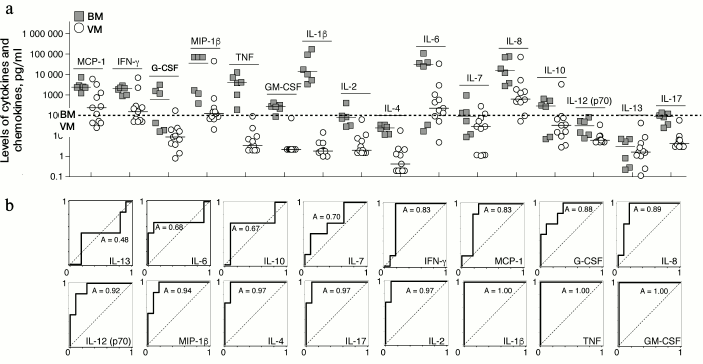
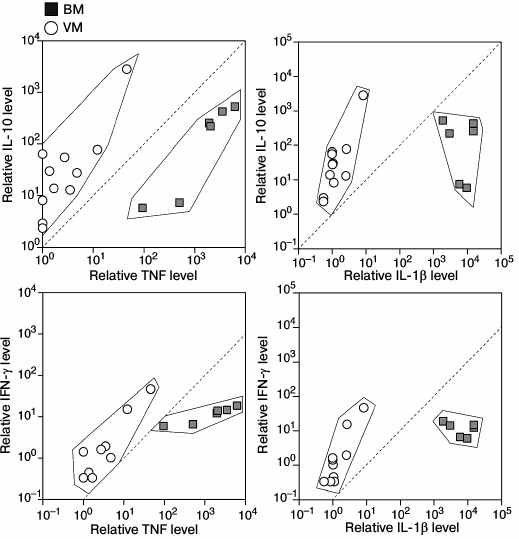
Determination of cytokine status of CSF of patients with bacterial and viral meningitis. Results of the multiplex analysis of the CSF of patients with meningitis and the controls are presented in Table 1 and Fig. 1a. The levels of all cytokines and chemokines in the CSF of patients with bacterial meningitis were increased. The increase in the concentrations of MCP-1, IFN-γ, IL-10, IL-12(p70), IL-13, and IL-17 in the CSF of patients with bacterial meningitis was within one order of magnitude, the concentrations of G-CSF, MIP-1β, GM-CSF, IL-2, IL-4, IL-6, and IL-7 were increased up to 100 times, and the concentrations of TNF and IL-1β were increased by three and four orders of magnitude, respectively. For all the studied cytokines and chemokines, ROC curves were plotted. These curves reveal biological markers with the highest diagnostic potential, whereas the area under the curve, which is described by the coefficient A, tended to 1 for the ideal diagnostic parameter. Figure 1b demonstrates that the increased levels of cytokines IL-1β, TNF, and GM-CSF with 100 pg/ml cutoff differentiated bacterial and viral meningitis with 100% specificity and selectivity. Similar values with the area under the curve with coefficient A = 0.97 were found for the ROC curves for cytokines IL-2, IL-4, and IL-17. A significant dispersion of the MCP-1, IL-6, IL-7, and IL-8 concentrations in the patients with different meningitis forms, which in the case of IL-6 could reach four orders of magnitude, significantly decreases the potential of these factors as parameters for differential diagnosis of meningitis. It should be noted that the increase in the levels of cytokines IL-10 and IFN-γ significantly correlated with the increase in the levels of cytokines TNF and IL-1β in the CSF of patients with viral meningitis, whereas in bacterial meningitis no such dependence was observed, or it was much less pronounced (Fig. 2).
Table 1. Mean values of cytokine and
chemokine concentrations (pg/ml) in CSF of patients with meningitis and
of neurologically healthy controls
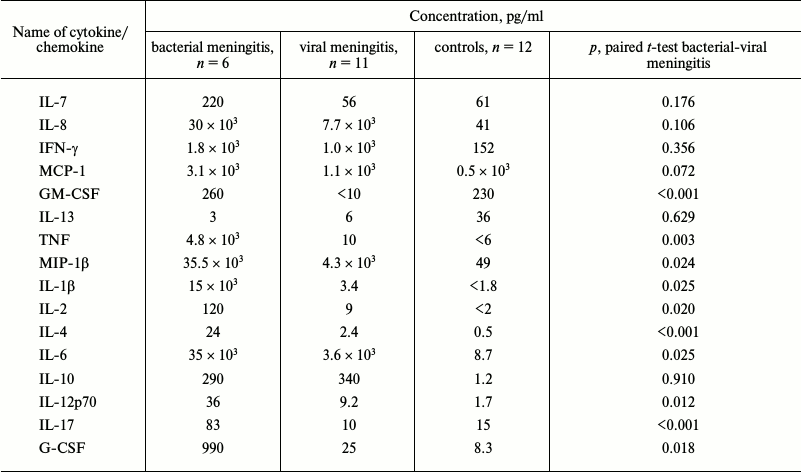
Fig. 1. Levels of cytokines and chemokines in CSF of patients with bacterial meningitis (BM) in comparison with data for viral meningitis (VM) (a) and the corresponding ROC-curves (b). In panel (a), individual values and the median are shown.
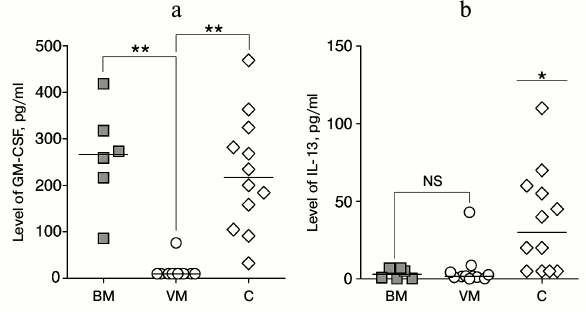
Fig. 2. Levels of GM-CSF (a) and IL-13 cytokines (b) in CSF of patients with bacterial (BM) and viral (VM) meningitis as compared to the controls (C). Individual values and median are shown. NS, not significant; * p < 0.05, ** p < 0.01 (paired t-test).
Comparison of cytokine and chemokine levels in patients with meningitis and in healthy donors revealed a general tendency of their increase in meningitis without dependence on its etiology (Table 1). Nevertheless, the GM-CSF level was significantly decreased in the CSF of patients with viral meningitis as compared to healthy donors, and the level of IL-13 was pronouncedly decreased in the CSF of both groups of patients with meningitis in comparison with the controls (Fig. 3). However, it should be noted that this observation could be caused by specifics of the control group, which consisted of old patients subjected to the epidural anesthesia before prostatectomy or replacement of knee endoprostheses, because sampling CSF of volunteers is prohibited by ethical norms.
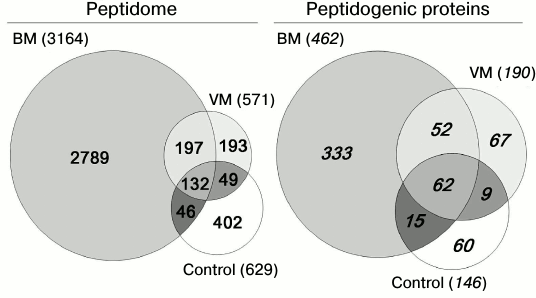
Fig. 3. Venn diagrams representing the distribution of peptides and their protein precursors identified in CSF in three groups of samples: bacterial meningitis (BM), viral meningitis (VM), and control.
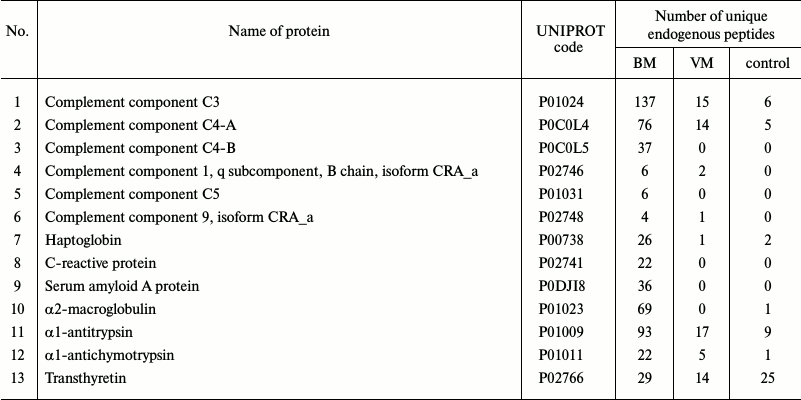
Peptidome profiling of CSF of patients with bacterial and viral meningitis. The LC-MS/MS analysis of peptide fractions of CSF of 20 controls, four patients with bacterial meningitis and four patients with viral meningitis revealed 3808 unique peptides that were fragments of 598 proteins (Tables S1-S3 are given in the Supplement to this paper at the journal site http://protein.bio.msu.ru/biokhimiya and Springer site Link.springer.com). In the sample groups, these peptides were distributed as follows: 3164 peptides (fragments of 462 proteins) were identified in the CSF samples from patients with bacterial meningitis, 571 peptides (fragments of 190 proteins) were identified in the samples of CSF of patients with viral meningitis, and 629 peptides (fragments of 146 proteins) were identified in samples of CSF of the control group. Venn diagrams representing distributions of the identified peptides and their protein precursors are shown in Fig. 4. The peptides identified in the CSF of all subjects were fragments of blood plasma major proteins and of neurospecific proteins including neuropeptides. Thus, in CSF we identified peptide fragments corresponding to 21 of 283 known human neuropeptides. Amino acid sequences of these neuropeptides are listed in the NeuroPep database (Table 2). Significantly more peptide fragments of some neuropeptides, e.g. chromogranin A, secretogranins-1/2/3, somatostatin, and neurosecretory protein VGF, were detected in the CSF of patients with bacterial meningitis than in the CSF of the control subjects and patients with viral meningitis. This could be due to both, an increase in the exchange level of these neuropeptides and an increase in their total content in the CSF during bacterial meningitis. In opposite, for neuropeptide Y and the inhibitor of proprotein convertase 1 (proSAAS), the number of identified peptide fragments was lower in the CSF of patients with viral and bacterial meningitis than in the CSF of the controls. It should be noted that in the peptidome of the CSF of patients with bacterial meningitis we did not find fragments of procalcitonin, which is considered very promising for diagnosis of bacterial meningitis [24, 25]. Peptide fragments of some other proteins associated with acute inflammation (Table 3) were present in significantly larger amounts in bacterial meningitis than in the control. In particular, fragmentation of the complement system proteins serpin clade A, cytoplasmic actin, serum amyloid A, and zyxin in bacterial meningitis was significantly higher than in viral meningitis.
Fig. 4. Correlation analysis of relative increase in levels of cytokines IL-10 and IFN-γ versus IL-1β and TNF in CSF of individual patients with bacterial and viral meningitis (BM and VM, respectively) in comparison with controls. The data were normalized to the mean value of each cytokine in the CSF of the controls.
Table 2. Neuropeptides identified as peptide
fragments in CSF samples
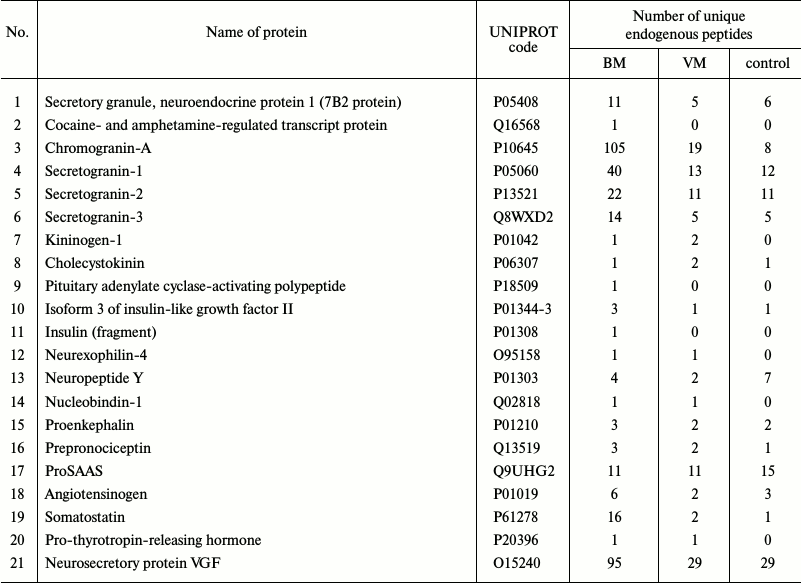
Table 3. Proteins functionally connected
with inflammation that were identified as peptide fragments in CSF
samples
DISCUSSION
Study on the cytokine status based on biochemical and immunological analysis of CSF in meningitis is now an urgent problem of clinical immunology, and such studies are actively performed in many laboratories throughout the world. The cytokines IL-1β and TNF observed herein are considered as the “gold standard” for differential diagnosis of meningitis with bacterial and viral etiology. These two cytokines initially discovered in the 1980s [26, 27] are one of the most important mediators of inflammation. The concentration of IL-1β above 20 pg/ml was found in the CSF of 78% of patients with bacterial meningitis, in 4% of patients with viral meningitis, and in 8% of controls [28]. These data completely confirm our observations of the four-order-of-magnitude difference in the average IL-1β concentrations in patients with bacterial and viral meningitis. In study [29], levels of TNF, IL-1β, and IL-8 were determined in CSF of patients with bacterial and viral meningitis, and TNF level above 75.8 pg/ml was acknowledged as 100% discriminating bacterial and viral meningitis. These data are consistent with the [TNF] cut off value of 100 pg/ml established in the present study.
The levels of cytokines IL-6 and IL-8 were also considered as a diagnostic test of meningitis. Increased levels of TNF, IL-1β, and IL-6 in CSF were shown to be biomarkers of neonatal meningitis, and IL-6 was the most demonstrative [30]. In study [31], the authors discriminated bacterial and viral meningitis in children based on the levels of cytokines IL-6 and IL-12 (the increase was threefold on average), whereas the difference between the levels of these cytokines in CSF in the neurologically healthy control group and in the patients with viral meningitis was insignificant. In bacterial meningitis, the level of IL-12 grew more rapidly than the level of IL-6. It seems that IL-6 is a cytokine of “second line” with delayed increase in concentration and subsequent long-term clearance from the CNS. In the case of enterococcus-caused neonatal meningitis reported in [32], significantly increased levels of IL-6, IL-10, and TNF were observed, and 20-fold increase in the IL-6 level remained on the 28th day of the disease. It should be noted that we have observed significantly overlapping values of the IL-6 and IL-8 concentrations in CSF of patients with bacterial and viral meningitis, although the average values of IL-6 and IL-8 concentrations were increased 10- and 4-fold, respectively. We think that such overlapping lowers the clinical significance of these cytokines for differential diagnosis. In study [33], the authors showed that the TNF level in children with viral and bacterial meningitis differed, respectively, 10- and 100-fold and more from the control, at the same time the difference in the level of IL-6 was insignificant. They suggested that these results could be explained by heterogeneity of the studied cohort and by different moment of the CSF sampling in terms of the disease peak.
In the present study, we report for the first time a decrease in the level of two cytokines, namely IL-13 and GM-CSF, in the CSF of patients with viral meningitis. IL-13 was also significantly decreased in the CSF of patients with bacterial meningitis. The function of GM-CSF is to stimulate the growth and differentiation of hemopoietic cells. Expression of GM-CSF is increased under the influence of IL-1, IL-6, and TNF and is decreased under the influence of IL-10, IFN-γ, and IL-4. The observed decrease in the GM-CSF level in CSF of patients with viral meningitis can be explained by a disproportionate growth of the second group cytokines in comparison with those of the first group. Thus, the concentrations of IL-10 and IFN-γ in the CSF of patients with viral meningitis were comparable with their concentrations in patients with bacterial meningitis, whereas the levels of IL-1β and TNF in the CSF of patients with bacterial meningitis were more than 1000 times higher than in the CSF of patients with viral meningitis. Therefore, if in the case of bacterial meningitis the increase in the IL-10 and IFN-γ concentrations was compensated by an explosive growth of the amounts of IL-1β and TNF, in viral meningitis the inhibitory action of IL-10 and IFN-γ dominated over the activation of GM-CSF expression under the influence of IL-1β and TNF. Correlation analysis of individual values of cytokines confirms these conclusions, because the concentrations of IL-10 and IFN-γ in the CSF of patients with viral meningitis grow more rapidly than the concentrations of IL-1β and TNF, whereas in patients with bacterial meningitis, there is no such dependence or it is much less pronounced. Notwithstanding the absence of a significant difference in the IL-10 and IFN-γ levels in the CSF of patients with different forms of meningitis, utilization of a multiparametric test allows us to establish diagnosis of viral meningitis more accurately at TNF and IL-1β concentrations close to the threshold values, which are specific for bacterial meningitis. These observations suggest an additional approach for differential diagnosis of meningitis based on the normalized ratios IL-10/IL-1β and IL-10/TNF with cut-off >1 and also IFN-γ/IL-1β and IFN-γ/TNF with cut-off >0.1, which discriminates bacterial and viral meningitis.
In addition to determination of cytokine levels in the CSF of patients with meningitis of different etiologies, we identified the spectrum of peptides produced either during the proteolytic degradation of CSF proteins or as a result of activation of neuropeptide protein precursors processing being a component of the human body defense in response to infection localized in the central nervous system. Data presented in Tables 2 and 3 show that number of peptide fragments present in CSF varies differently in various forms of meningitis in comparison with neurologically healthy controls. Our findings open a unique possibility for designing peptide panels for rapid and effective determination of meningitis form at early stages and for performing large-scale clinical studies based on these data. According to the peptidome analysis proteins of complement system, serpin clade A, cytoplasmic actin, serum amyloid A and zyxin are the best candidates for monitoring their presentation in the CSF of patients with different forms of meningitis. Multiple reaction monitoring (MRM) methodology of mass spectrometry seems to be the most beneficial because of its high specificity and large dynamic range of ~107, which is comparable to modern immunochemical analysis. This approach allows researchers to quantitatively determine amount of neuropeptides and to identify cytokines that are present in CSF in picomolar concentrations. These measurements can be performed rather quickly and economically to rapidly discriminate bacterial and viral meningitis at early stages of the disease. We note in conclusion that our data have extended the panel of biomarkers of viral and bacterial meningitis with clinical and diagnostic potential and revealed different trends in cytokine changes in meningitis as a result of compensatory effects of pro- and antiinflammatory cytokines.
Acknowledgements
This work was supported by the Russian Science Foundation (project No. 14-24-00106).
Alexey Belogurov is a laureate of the Stipendium of President of Russian Federation SP-4262.2016.4.
REFERENCES
1.Reimann, H. A. (1935) Micrococcus tetragenus
infection: I. Review of the literature, report of a non-fatal case with
septicemia, meningitis and arthritis, and bacteriologic studies, J.
Clin. Invest., 14, 311-319.
2.Lepow, M. L., Coyne, N., Thompson, L. B., Carver,
D. H., and Robbins, F. C. (1962) A clinical, epidemiologic and
laboratory investigation of aseptic meningitis during the four-year
period, 1955-1958. II. The clinical disease and its sequelae, N.
Engl. J. Med., 266, 1188-1193.
3.Logan, S. A., and MacMahon, E. (2008) Viral
meningitis, BMJ, 336, 36-40.
4.Mace, S. E. (2008) Acute bacterial meningitis,
Emerg. Med. Clin. North Am., 26, 281-317.
5.Finsterer, J., and Auer, H. (2013) Parasitoses of
the human central nervous system, J. Helminthol., 87,
257-270.
6.Bahr, N. C., and Boulware, D. R. (2014) Methods of
rapid diagnosis for the etiology of meningitis in adults, Biomark.
Med., 8, 1085-1103.
7.Cunha, B. A. (2013) The clinical and laboratory
diagnosis of acute meningitis and acute encephalitis, Expert. Opin.
Med. Diagn., 7, 343-364.
8.Knight, J. A., Dudek, S. M., and Haymond, R. E.
(1981) Early (chemical) diagnosis of bacterial meningitis –
cerebrospinal fluid glucose, lactate, and lactate dehydrogenase
compared, Clin. Chem., 27, 1431-1434.
9.Zwijnenburg, P. J., Van der Poll, T., Florquin, S.,
Roord, J. J., and Van Furth, A. M. (2003) IL-1 receptor type 1
gene-deficient mice demonstrate an impaired host defense against
pneumococcal meningitis, J. Immunol., 170, 4724-4730.
10.Barichello, T., Dos Santos, I., Savi, G. D.,
Florentino, A. F., Silvestre, C., Comim, C. M., Feier, G., Sachs, D.,
Teixeira, M. M., Teixeira, A. L., and Quevedo, J. (2009) Tumor necrosis
factor alpha (TNF-alpha) levels in the brain and cerebrospinal fluid
after meningitis induced by Streptococcus pneumoniae,
Neurosci. Lett., 467, 217-219.
11.Izadpanah, K., Freyer, D., Weber, J. R., and
Braun, J. S. (2014) Brain parenchymal TNF-alpha and IL-1-beta induction
in experimental pneumococcal meningitis, J. Neuroimmunol.,
276, 104-111.
12.Takahashi, W., Nakada, T. A., Abe, R., Tanaka,
K., Matsumura, Y., and Oda, S. (2014) Usefulness of interleukin-6
levels in the cerebrospinal fluid for the diagnosis of bacterial
meningitis, J. Crit. Care, 29, 693 e691-696.
13.Prasad, R., Kapoor, R., Srivastava, R., Mishra,
O. P., and Singh, T. B. (2014) Cerebrospinal fluid TNF-alpha, IL-6, and
IL-8 in children with bacterial meningitis, Pediatr. Neurol.,
50, 60-65.
14.Siddiqui, A. A., Brouwer, A. E., Wuthiekanun, V.,
Jaffar, S., Shattock, R., Irving, D., Sheldon, J., Chierakul, W.,
Peacock, S., Day, N., White, N. J., and Harrison, T. S. (2005)
IFN-gamma at the site of infection determines rate of clearance of
infection in cryptococcal meningitis, J. Immunol., 174,
1746-1750.
15.Koopmans, M. M., Brouwer, M. C., Geldhoff, M.,
Seron, M. V., Houben, J., Van der Ende, A., and Van de Beek, D. (2014)
Cerebrospinal fluid inflammatory markers in patients with meningitis,
BBA Clin., 1, 44-51.
16.Pinto Junior, V. L., Rebelo, M. C., Gomes, R. N.,
Assis, E. F., Castro-Faria-Neto, H. C., and Boia, M. N. (2011) IL-6 and
IL-8 in cerebrospinal fluid from patients with aseptic meningitis and
bacterial meningitis: their potential role as a marker for differential
diagnosis, Braz. J. Infect. Dis., 15, 156-158.
17.Ou, Q., Liu, X., and Cheng, X. (2013) An iTRAQ
approach to quantitative proteome analysis of cerebrospinal fluid from
patients with tuberculous meningitis, Biosci. Trends, 7,
186-192.
18.Cordeiro, A. P., Silva Pereira, R. A.,
Chapeaurouge, A., Coimbra, C. S., Perales, J., Oliveira, G., Sanchez
Candiani, T. M., and Coimbra, R. S. (2015) Comparative proteomics of
cerebrospinal fluid reveals a predictive model for differential
diagnosis of pneumococcal, meningococcal, and enteroviral meningitis,
and novel putative therapeutic targets, BMC Genomics, 16
(Suppl. 5), S11.
19.Goonetilleke, U. R., Scarborough, M., Ward, S.
A., and Gordon, S. B. (2010) Proteomic analysis of cerebrospinal fluid
in pneumococcal meningitis reveals potential biomarkers associated with
survival, J. Infect. Dis., 202, 542-550.
20.Jesse, S., Steinacker, P., Lehnert, S., Sdzuj,
M., Cepek, L., Tumani, H., Jahn, O., Schmidt, H., and Otto, M. (2010) A
proteomic approach for the diagnosis of bacterial meningitis, PLoS
One, 5, e10079.
21.Kataria, J., Rukmangadachar, L. A., Hariprasad,
G., Jithesh, O., Tripathi, M., and Srinivasan, A. (2011)
Two-dimensional difference gel electrophoresis analysis of
cerebrospinal fluid in tuberculous meningitis patients, J.
Proteom., 74, 2194-2203.
22.Segawa, S., Sawai, S., Murata, S., Nishimura, M.,
Beppu, M., Sogawa, K., Watanabe, M., Satoh, M., Matsutani, T.,
Kobayashi, M., Iwadate, Y., Kuwabara, S., Saeki, N., and Nomura, F.
(2014) Direct application of MALDI-TOF mass spectrometry to
cerebrospinal fluid for rapid pathogen identification in a patient with
bacterial meningitis, Clin. Chim. Acta, 435, 59-61.
23.Kovalchuk, S. I., Anikanov, N. A., Ivanova, O.
M., Ziganshin, R. H., and Govorun, V. M. (2015) Bovine serum albumin as
a universal suppressor of non-specific peptide binding in vials prior
to nano-chromatography coupled mass-spectrometry analysis, Anal.
Chim. Acta, 893, 57-64.
24.Konstantinidis, T., Cassimos, D., Gioka, T.,
Tsigalou, C., Parasidis, T., Alexandropoulou, I., Nikolaidis, C.,
Kampouromiti, G., Constantinidis, T., Chatzimichael, A., and
Panopoulou, M. (2015) Can procalcitonin in cerebrospinal fluid be a
diagnostic tool for meningitis? J. Clin. Lab. Anal., 29,
169-174.
25.Li, Y., Zhang, G., Ma, R., Du, Y., Zhang, L., Li,
F., Fang, F., Lv, H., Wang, Q., Zhang, Y., and Kang, X. (2015) The
diagnostic value of cerebrospinal fluids procalcitonin and lactate for
the differential diagnosis of post-neurosurgical bacterial meningitis
and aseptic meningitis, Clin. Biochem., 48, 50-54.
26.Nedospasov, S. A., Hirt, B., Shakhov, A. N.,
Dobrynin, V. N., Kawashima, E., Accolla, R. S., and Jongeneel, C. V.
(1986) The genes for tumor necrosis factor (TNF-alpha) and lymphotoxin
(TNF-beta) are tandemly arranged on chromosome 17 of the mouse,
Nucleic Acids Res., 14, 7713-7725.
27.March, C. J., Mosley, B., Larsen, A., Cerretti,
D. P., Braedt, G., Price, V., Gillis, S., Henney, C. S., Kronheim, S.
R., Grabstein, K., Conlon, P. J., Hopp, T. P., and Cosman, D. (1985)
Cloning, sequence and expression of two distinct human interleukin-1
complementary DNAs, Nature, 315, 641-647.
28.Tang, R. B., Lee, B. H., Chung, R. L., Chen, S.
J., and Wong, T. T. (2001) Interleukin-1-beta and tumor necrosis
factor-alpha in cerebrospinal fluid of children with bacterial
meningitis, Childs Nerv. Syst., 17, 453-456.
29.Bociaga-Jasik, M., Garlicki, A., Ciesla, A.,
Kalinowska-Nowak, A., Sobczyk-Krupiarz, I., and Mach, T. (2012) The
diagnostic value of cytokine and nitric oxide concentrations in
cerebrospinal fluid for the differential diagnosis of meningitis,
Adv. Med. Sci., 57, 142-147.
30.Krebs, V. L., Okay, T. S., Okay, Y., and Vaz, F.
A. (2005) Tumor necrosis factor-alpha, interleukin-1-beta and
interleukin-6 in the cerebrospinal fluid of newborn with meningitis,
Arq. Neuropsiquiatr., 63, 7-13.
31.Hsieh, C. C., Lu, J. H., Chen, S. J., Lan, C. C.,
Chow, W. C., and Tang, R. B. (2009) Cerebrospinal fluid levels of
interleukin-6 and interleukin-12 in children with meningitis, Childs
Nerv. Syst., 25, 461-465.
32.Ikeda, N., Suganuma, H., Ohkawa, N., Nagata, S.,
Shoji, H., and Shimizu, T. (2014) Measurement of cytokine levels in
cerebrospinal fluid over time in neonatal Enterococcal
meningitis, Pediatr. Int., 56, e45-47.
33.Mukai, A. O., Krebs, V. L., Bertoli, C. J., and
Okay, T. S. (2006) TNF-alpha and IL-6 in the diagnosis of bacterial and
aseptic meningitis in children, Pediatr. Neurol., 34,
25-29.
Supplementary Tables S1, S2 and S3 (PDF)