Recombinant MHC Tetramers for Isolation of Virus-Specific CD8+ Cells from Healthy Donors: Potential Approach for Cell Therapy of Posttransplant Cytomegalovirus Infection
A. S. Vdovin1, S. Y. Filkin1, P. R. Yefimova1,2, S. A. Sheetikov1,2, N. M. Kapranov1, Y. O. Davydova1, E. S. Egorov3, E. G. Khamaganova1, M. Y. Drokov1, L. A. Kuzmina1, E. N. Parovichnikova1, G. A. Efimov1*, and V. G. Savchenko1
1National Research Center for Hematology, Ministry of Healthcare of the Russian Federation, 125167 Moscow, Russia; E-mail: efimov.g@blood.ru, grigory@efimov.info2Lomonosov Moscow State University, Faculty of Biology, 119991 Moscow, Russia
3Shemyakin–Ovchinnikov Institute of Bioorganic Chemistry, Russian Academy of Sciences, 117997 Moscow, Russia
* To whom correspondence should be addressed.
Received June 28, 2016; Revision received July 28, 2016
Patients undergoing allogeneic hematopoietic stem cell transplantation have a high risk of cytomegalovirus reactivation, which in the absence of T-cell immunity can result in the development of an acute inflammatory reaction and damage of internal organs. Transfusion of the virus-specific donor T-lymphocytes represents an alternative to a highly toxic and often ineffective antiviral therapy. Potentially promising cell therapy approach comprises transfusion of cytotoxic T-lymphocytes, specific to the viral antigens, immediately after their isolation from the donor’s blood circulation without any in vitro expansion. Specific T-cells could be separated from potentially alloreactive lymphocytes using recombinant major histocompatibility complex (MHC) multimers, carrying synthetic viral peptides. Rapid transfusion of virus-specific T-cells to patients has several crucial advantages in comparison with methods based on the in vitro expansion of the cells. About 30% of hematopoietic stem cell donors and 46% of transplant recipients at the National Research Center for Hematology were carriers of the HLA-A*02 allele. Moreover, 94% of Russian donors have an immune response against the cytomegalovirus (CMV). Using recombinant HLA-A*02 multimers carrying an immunodominant cytomegalovirus peptide (NLV), we have shown that the majority of healthy donors have pronounced T-cell immunity against this antigen, whereas shortly after the transplantation the patients do not have specific T-lymphocytes. The donor cells have the immune phenotype of memory cells and can be activated and proliferate after stimulation with the specific antigen. Donor lymphocytes can be substantially enriched to significant purity by magnetic separation with recombinant MHC multimers and are not activated upon cocultivation with the antigen-presenting cells from HLA-incompatible donors without addition of the specific antigen. This study demonstrated that strong immune response to CMV of healthy donors and prevalence of HLA-A*02 allele in the Russian population make it possible to isolate a significant number of virus-specific cells using HLA-A*02–NLV multimers. After the transfusion, these cells should protect patients from CMV without development of allogeneic immune response.
KEY WORDS: cytomegalovirus, cell therapy, adoptive transfer, allogeneic hematopoietic stem cell transplantation, MHC multimersDOI: 10.1134/S0006297916110146
Abbreviations: allo-HSCT, allogeneic hematopoietic stem cell transplantation; APC, allophycocyanin; CMV, cytomegalovirus; CSFE, carboxyfluorescein succinimidyl ester; GVHD, “graft-versus-host” disease; HSC, hematopoietic stem cells; IFN-γ, interferon-gamma; MHC, major histocompatibility complex; NLV, immunodominant peptide of cytomegalovirus (NLVPMVATV); PE, phycoerythrin; SSC, side-scattered light; TNF, tumor necrosis factor.
Cytomegalovirus (CMV) is widely distributed in the human population. In
different countries, antibodies specific to CMV antigens are present in
40-100% of the population [1]. In Russia, such
antibodies are found in ~80% of cases [2]. The
presence of circulating IgG in the bloodstream indicates previous
infection, which is usually asymptomatic or the symptoms are not
specific. After primary infection subsides, the virus becomes latent
and remains in the human organism indefinitely; its occasional
reactivation is effectively controlled by the T-lymphocytes. However,
for patients in the immune deficiency state CMV and some other viruses
(Epstein–Barr virus, human herpesvirus 6) are extremely
dangerous. Secondary or in rare cases primary infections developing on
the background of suppressed T-cell immunity provoke a strong
inflammatory reaction and lead to the development of pneumonia, and
lesions of the gastrointestinal tract, kidneys, and other internal
organs. Uncontrolled inflammation can often lead to the lethal outcome
(up to 50% of cases) [3].
CMV can infect various types of cells, which determines the variety of pathologies caused by this virus. CMV-infected cells undergo different physiological changes – triggering intracellular signaling cascades begins from activation of the Toll-like receptor 2 that leads to secretion of the proinflammatory cytokines IL-6 and IL-8 [3]. CMV infection is also associated with an increased level of tumor necrosis factor (TNF) in the blood plasma. It seems that this cytokine plays the key role in the CMV pathogenesis [4, 5]. Pre-transplantation conditioning damages healthy tissues, including the barrier epithelium, and the microflora penetrates across them, resulting in activation of the innate immunity and development of inflammation [6]. The inflammatory reaction, on one hand, promotes CMV reactivation from the latent state, and, on the other hand, it is one of the key components of “graft-versus-host” disease (GVHD). The correlation between GVHD and CMV infection has been repeatedly mentioned in the literature [7].
Patients after allogeneic hematopoietic stem cell transplantation (allo-HSCT) are in the state of immune deficiency and, consequently, have a risk of reactivation of CMV infection [8]. Prior to allo-HSCT, the patient’s own hematopoietic system is eliminated by radiation and/or chemotherapy, and as a result, in the blood and tissues of such patients during several months after the transplantation and until the immune reconstitution by donor cells there are no lymphocytes capable of controlling CMV infection. Moreover, during the post-transplantation period, many recipients receive immunosuppressive treatment for preventing cytotoxicity of the graft on their normal tissues, and this additionally increases their susceptibility to viruses.
Before the development of effective antiviral therapy, ~35% of all allo-HSCT procedures were accompanied by CMV reactivation with lethal outcome in 85% of cases. At present, notwithstanding the current prophylaxis and antiviral therapy, the problem of secondary herpesvirus infections is still urgent [9]. Moreover, currently used antiviral drugs are highly toxic. Therefore, the search for alternative approaches to therapy of opportunistic infections in immunodeficient patients is required. The goal of the present work was to assess the applicability of one such approach, namely, the adoptive transfer of virus-specific T-cells.
CMV infection is controlled by cytotoxic CD8+ T-lymphocytes specific to viral antigens (peptides derived from the viral proteins and presented in the context of the patient’s own MHC I alleles). Although the virus-specific lymphocytes usually occur in blood with high frequency, transfusion of the total fraction of CD8+ T-cells for therapy of CMV is associated with a significant risk of development of alloreactivity because in the donor’s blood there are also cells capable of recognizing patient-specific peptides encoded by nucleotide sequences containing nonsynonymous single nucleotide polymorphisms [10]. Therefore, it is desirable to obtain an enriched population of the virus-specific cells, which can be performed either using in vitro expansion of T-lymphocytes specific for the antigen or their ex vivo selection from the leukapheresis product by the use of recombinant MHC–antigen complexes. Because the T-cell receptor affinity for the peptide–MHC complex is relatively low, MHC multimers that provide polyvalent interaction are used for isolation of the antigen-specific T-lymphocytes [11, 12].
The first transfusions (adoptive transfer) of the virus-specific cells of a HSC donor for treatment of CMV infection were attempted in the 1990s [13, 14]. The transfer of cytotoxic lymphocytes recognizing viral epitopes was accompanied by a rapid decrease in the virus titer. Up to date several different experimental protocols for cell therapy of CMV and other herpesvirus infections have been described. The majority of these protocols include a stage of in vitro expansion of donor’s cytotoxic lymphocytes with the viral antigens [15-17]. However, in vitro cultivation of T-lymphocytes is expensive and time-consuming (the procedure requires up to 60 days including the time for isolation and maturation of antigen-presenting cells), that limits its applicability for patients with acute CMV infection [18]. Moreover, a significant percentage of the cells lose their proliferative potential because of the long-term in vitro cultivation [19].
Immunodominant epitopes of herpesviruses are well studied for the widely distributed alleles of the MHC I class [20, 21]. Thus, allele HLA-A*02:01 (A02) carried by more than 20% of people in various European populations [22] presents the peptide NLVPMVATV (NLV peptide) derived from the CMV matrix protein pp65. CD8+ lymphocytes specific to the A02–NLV complex are found in 75% of seropositive healthy donors with the frequency of 0.1-4.7% of the total amount of CD8+ T-lymphocytes [23]. Due to such high frequencies of cytotoxic T-lymphocytes specific to the CMV antigens in the bloodstream, it is possible to perform the transfusion of virus-specific cells without preliminary expansion. It was shown that injection of even a small dose of the epitope-specific cells (103 cells per kg of the patient’s weight) could stop development of CMV infection [24]. Considering the high frequency of the A02 allele in the European population, we chose the NLV peptide of CMV as a model antigen for study of applicability of virus-specific cell adoptive transfer for Russian patients after allo-HSCT.
Antigen-specific cells can be isolated from donor’s blood with the help of recombinant MHC I multimers in complex with synthetic viral peptides [25-27]. When using MHC multimers, there is no need for cell expansion in vitro. Using this strategy, one can obtain material in the volume necessary for adoptive transfer in only a few hours after the exfusion. Transfusion of the virus-specific cells immediately after their isolation from the donor’s blood is advantageous because these cells express the native immune phenotype, whereas after long-term in vitro cultivation the cells usually transform to an effector subtype and lose the ability for proliferation. Note that transfusion of even individual central memory cells (TCM) is capable of providing antiviral immunity due to their high proliferative potential [28]. The purpose of the present work was to investigate the possibility of obtaining an enriched population of virus-specific cells by using recombinant MHC tetramers and to characterize the effector functions of the resulting cells and their safety in terms of absence of the allogeneic immune response.
MATERIALS AND METHODS
Preparation of MHC multimers. Peptides NLVPMVATV (a.a. 495-503 of CMV protein pp65) and GLCTLVAML (a.a. 259-267 of BMLF-1 protein of the Epstein–Barr virus) were synthesized by solid-phase peptide synthesis (Peptide 2.0).
Plasmids. The following plasmids were used in this work: pMBio encoding the extracellular domain of HLA-A*02:01 (a.a. 1-276), the peptide sequence GSGGSGGSAGGGLNDIFEAQKIEW that serves as a substrate for BirA biotin ligase; pET3a encoding a.a. 1-100 of β2-microglobulin; pET-21b encoding BirA biotin ligase. These plasmids were kindly provided by Ton Schumacher (The Netherlands Cancer Institute, Amsterdam, The Netherlands).
Preparation of recombinant biotin ligase. Escherichia coli cells (strain BL21(DE3) pLysS (Novagen, Germany)) were transformed with a plasmid containing the BirA gene of biotin ligase and the sequence encoding the polyhistidine label. The bacterial culture was obtained in 1 liter of LB medium at 37°C. When the culture reached OD600 = 0.5, isopropylthiogalactoside (IPTG) was added to the final concentration of 1 mM to induce the protein expression, which was performed at 30°C. After 10 h, the bacterial culture was harvested, lysed by ultrasonication in 20 mM Tris, 100 mM NaCl (pH 8.0), and centrifuged for 10 min at 10,000g at 4°C. The precipitate was discarded, and the supernatant was filtered through a 0.22 µm filter (Millipore, USA). The protein of interest was purified by nickel affinity chromatography (application buffer 20 mM Tris, 100 mM NaCl, 5 mM imidazole (pH 8.0); washing buffer 20 mM Tris, 100 mM NaCl, 10 mM imidazole (pH 8.0); elution buffer 20 mM Tris, 100 mM NaCl, 50 mM imidazole (pH 8.0)). To the purified protein, β-mercaptoethanol and glycerol were added to the final concentrations of 5 mM and 5%, respectively.
Assembly of peptide–MHC complexes. Complexes of recombinant MHC I for tetramerization were prepared as described earlier [29, 30]. Escherichia coli cells of strain BL21(DE3) pLysS (Novagen) were transformed by plasmids containing genes of HLA-A*02:01 or of β2-microglobulin. The two recombinant proteins were expressed independently according to the same protocol. The bacterial culture was obtained in 2 liters of LB medium at 37°C. When the culture reached OD600 = 0.6, expression was induced by addition of IPTG to the final concentration of 1 mM. The bacterial culture was harvested 4 h later, lysed by ultrasonication, and precipitated. The precipitate was washed with a solution containing 1 mM EDTA and 0.5% Triton X-100. The inclusion bodies were dissolved in 100 mM Tris, 8 M urea (pH 8.0). Refolding was performed by dilution: 0.15 µmol HLA-A*02:01 and 0.3 µmol β2-microglobulin were successively diluted in three steps in 50 ml of buffer (100 mM Tris, 400 mM L-arginine, 2 mM EDTA, 5 mM glutathione, 0.5 mM glutathione disulfide (pH 8.0)). The buffer also contained 3 µmol of synthetic peptide that represents an immune dominant epitope of the CMV matrix pp65 (a.a. 495-503) NLVPMVATV. After incubation at 10°C for 72 h, the solution was concentrated to 3 ml by filtration during centrifugation for 15 min (3000g, 4°C) on an Amicon Ultra-15 Unit semipermeable membrane (Millipore). The prepared complex was purified by gel-filtration chromatography using a NGC chromatographic system (Bio-Rad, USA) equipped with a SEC650 column (Bio-Rad). The fractions were analyzed by electrophoresis under denaturing conditions, and the desired fraction was biotinylated using recombinant biotin ligase in accordance with the earlier published protocol [30].
Preparation of MHC multimers. Biotinylated complex of MHC I with the NLV peptide was tetramerized by incubation with streptavidin covalently bound to fluorescent protein phycoerythrin (PE) or allophycocyanin (APC) (S866, S868; Life Technologies, USA). The tetramerization was monitored by titration of biotinylated streptavidin—PE complex followed by chromatographic separation on a SEC650 column.
HLA genotyping. Genotyping of five HLA loci (HLA-A*, -B*, -C*, -DRB1*, and -DQB1*) was performed using the PCR-SSP method (PCR with allele-specific primers; Protrans, Germany) or by PCR-SSO (PCR followed by hybridization with LifeCodes oligonucleotide probes; Immucor Transplant Diagnostics, USA) on a multiplex analyzer platform (Luminex, USA).
The frequency of allelic groups corresponding to HLA antigens and of their haplotypes was determined using the ARLEQUIN maximum likelihood algorithm [31].
Flow cytometry. Peripheral blood mononuclear cells were isolated from healthy donors, patients after allo-HSCT, and HSC donors by differential centrifugation using Histopaque-1077 solution (Sigma, USA) according to the standard protocol. All procedures were approved by the local ethics committee.
For staining with tetramers and antibodies, 106 cells were taken in a volume of 50 µl; 0.2 µg of tetramer and antibodies in the concentration recommended by the manufacturer were used per 106 cells. The incubation was carried out for 30 min in the dark at room temperature in phosphate buffered saline (PBS) supplemented with 0.5% BSA. Before analysis by flow cytofluorimetry, the cells were washed twice in PBS. The commercial multimer NLV-A*02:01 PeliMer (M2427NLVPM; Sanquin, The Netherlands) was used as a reference.
For immune phenotyping, the following antibodies were used: CD45RO FITC, CD28 PE, CD95 PerCP-Cy5.5, CD197 PE-Cy-7, and CD8 APC-Cy7 (BD Biosciences, USA) and CD3-FITC/CD8-PE (Beckman Coulter, USA). The T-cell proliferation was analyzed by staining with carboxyfluorescein succinimidyl ester (CFSE) (Molecular Probes, USA) at the working concentration of 100 µM. The isolated population of NLV-specific T-cells was labeled. Stimulation by NLV peptide (10 µg/ml) was performed with 50 U/ml IL-2 for 3 days in the presence of autologous mononuclear cells depleted of the CD8 marker. To evaluate the cross-reactivity, the CFSE-labeled population of NLV-specific cells was cultivated with allogeneic mononuclear cells without addition of the peptide. Prior to analysis of proliferation, the cells were stained with the tetramer, and the CFSE fluorescence was recorded in the NLV-specific population. Synthesis of IFN-γ was detected using antibodies IFN-γ PE-Cy7 with the parallel immune phenotyping CD3 APC, CD8 FITC (BD Biosciences). TNF production was assessed using TNF PE antibodies (BD Biosciences). The cells were activated with the NLV peptide at the concentration of 10 µg/ml, and 3 h later brefeldin A (GolgiPlug; BD Biosciences) was added to the final concentration of 3 µg/ml. After 3 h of cultivation in the presence of brefeldin A, the cells were fixed and stained with antibodies.
Cytofluorimetric analysis was performed using a BD FACSCanto™ II (BD Biosciences). The data were processed using the FlowJo 7.6.2 software (FlowJo, LLC).
Magnetic sorting. For magnetic separation, we used Anti-PE MicroBeads and CD8 MicroBeads and magnetic LS Columns and a QuadroMACS™ Separator (Miltenyi Biotec, Germany). The separation procedure was performed according to the protocol recommended by the manufacturer. For labeling with the tetramer, before the separation 0.5 µg tetramer conjugated with PE was applied per 107 cells, the incubation was performed at room temperature for 30 min, and after the incubation, the cells were washed twice in PBS supplemented with 0.5% BSA and 2 mM EDTA.
Cultivation of lymphocytes. To analyze lymphocyte proliferation by staining with CFSE, NLV-specific cells selected by magnetic separation with Anti-PE Microbeads particles (Miltenyi Biotec) were added to an irradiated (30-40 Gy) autologous feeder culture depleted of CD8+, with the ratio of 103 epitope-specific lymphocytes per 2·106 feeder cells. The cultivation was carried out in RPMI1640 medium (Gibco, USA) supplemented with 5% human serum, 100 U/ml penicillin, 100 µg/ml streptomycin, and 50 U/ml IL-2. The concentration of the feeder cells was 106 cells/ml.
For antigen-specific proliferation, peripheral blood mononuclear cells were isolated by centrifugation in a density gradient. In 15-ml tubes, 3·106 cells were cultivated at the concentration of 106 cells/ml in RPMI1640 medium (Gibco) in the presence of 5% human serum, 100 U/ml penicillin, 100 µg/ml streptomycin, and 10 µg/ml specific or control peptide. After 48 h, an equivalent volume of medium was added to the cell suspension, which contained 100 U/ml IL-2, and the cells were cultured for 8 days, every 2 days half the medium being replaced by fresh medium containing IL-2 at the concentration of 50 U/ml. On the 10th day, the antigen-specific population was detected by flow cytofluorimetry.
Allogeneic immune response in a mixed lymphocyte culture was analyzed according the following protocol: NLV-specific cells from three donors with known HLA-genotype (donor 1 — A*02/A*24, B*13/B*39, C*06/C*07; donor 2 — A*02/A*02, B*27/B*35, C*01/C*04; donor 3 — A*02/A*24, B*15/B*35, C*04/C*04) were selected by magnetic separation and added to autologous or heterologous cell culture from the same three donors in the ratio of 103 epitope-specific lymphocytes per 2·106 feeder cells.
RESULTS
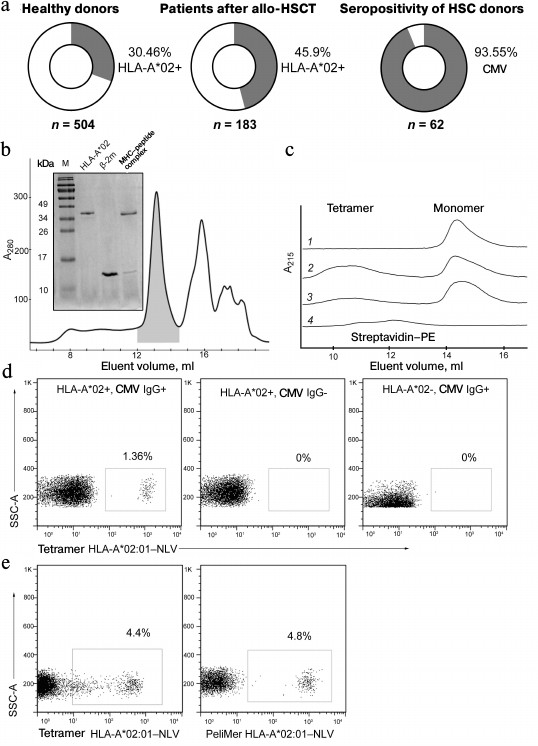
Analysis of HLA-A*02 allele and CMV-seropositivity distribution among patients and healthy donors. Since virus-specific T-lymphocytes can recognize the viral peptide only in the context of a certain (so-called restricting) HLA-allele, for adoptive transfer it occurs most effective to use cells specific to the most frequent HLA alleles. In the Russian population, the allele HLA-A*02:01 is the most common. Among 504 healthy blood donors analyzed by us 30.46% were HLA-A*02-positive (Fig. 1a). Among 183 patients who had undergone allo-HSCT in the Hematological Research Center of Russian Ministry of Healthcare during the period from 1991 to 2016, 46% carried the HLA-A*02 allele; 94% of 62 donors were CMV-seropositive.
Fig. 1. Production of tetramer of the most common in the Russian population allele HLA-A*02:01 in the complex with the CMV peptide NLVPMVATV. a) HLA-A*02 allele carriers and CMV-seropositive individuals in healthy donors and patients. The percentage of HLA-A*02 or anti-CMV IgG carriers and the total number of specimens analyzed in the sample are indicated. b) Assembly of the recombinant complex HLA-A*02:01—NLV. The elution profile of the complex HLA-A*02:01—NLV refolding fractions obtained by gel filtration chromatography is presented. The first elution fraction contains aggregations of the MHC I heavy chain. The fraction shown by gray color contains the HLA-A*02:01—NLV complex, the following fraction contains the unbound β2-microglobulin (β2-m). The inset shows SDS-electrophoresis in 15% polyacrylamide gel of the indicated fractions. M, molecular weight marker. c) Formation of HLA-A*02:01—NLV tetramers by binding to fluorescently labeled streptavidin. Elution profiles of biotinylated HLA-A*02:01—NLV complexes and streptavidin—PE combined in different proportions are shown. Lanes: 1) HLA-A*02:01–NLV without streptavidin–PE; 2) HLA-A*02:01–NLV and 2 µg streptavidin–PE; 3) HLA-A*02:01–NLV and 1 µg streptavidin–PE; 4) 2 µg streptavidin–PE without HLA-A*02:01–NLV. In the absence of streptavidin–PE, the HLA-A*02:01–NLV complex is present only as the monomer, whereas an increase in the streptavidin–PE concentration enhances production of HLA-A*02:01–NLV–streptavidin–PE tetramers. d) Staining of cytotoxic T-cells with NLV-tetramers is specific and depends on presence of the HLA-A*02 allele and on the donor’s serum status. Flow cytometry data are presented for three healthy donors. The presence or absence of the HLA-A*02 allele and IgG to CMV are shown at the top. Percentage of CD8+ cells stained with HLA-A*02:01–NLV tetramers are presented. e) Staining of CD8+ cells with the resulting NLV-A*02 tetramer and the commercial NLV-A*02 multimers (PeliMer; Sanquin, The Netherlands).
Preparation of MHC multimers. Recombinant β2-microglobulin and the heavy chain of HLA-A*02:01 were expressed in a bacterial system with the yield of 3 and 2.5 mg/liter bacterial culture, respectively. By refolding in the presence of the synthetic viral peptide NLVPMVATV (NLV), a complex of peptide–MHC was formed (the refolding efficiency was ~20%). The resulting complex, which had on the C-end of the MHC heavy chain the recognition sequence for biotin ligase BirA, was purified by gel filtration (Fig. 1b) and specifically biotinylated by recombinant biotin ligase. It should be noted that during gel filtration the complex is eluted between its components, because the heavy chain of MHC is unstable in solution in the absence of peptide and β2-microglobulin and forms aggregates (Fig. 1b). The biotinylation efficiency was 93%. The resulting biotinylated monomers were tetramerized by incubation with streptavidin conjugated with a fluorochrome (PE or APC) (Fig. 1c). Tetramers A02–NLV were shown to specifically bind with the CD3+CD8+ subpopulation of T-cells of CMV-seropositive donors carrying the HLA-A*02 allele and did not stain the cells of CMV-seronegative HLA-A*02-positive and of seropositive donors lacking the HLA-A*02 allele (Fig. 1d). The resulting tetramer was compared with the commercial analog PeliMer (Sanquin, The Netherlands) (Fig. 1e). The size of the stained population of the CD8+ T-cells estimated in the same sample was virtually equal.
Thus, we concluded that the produced HLA-A*02–NLV tetramer selectively recognizes CD8+ T-lymphocytes that have a receptor specific to the NLV peptide presented in the HLA-A*02 context.
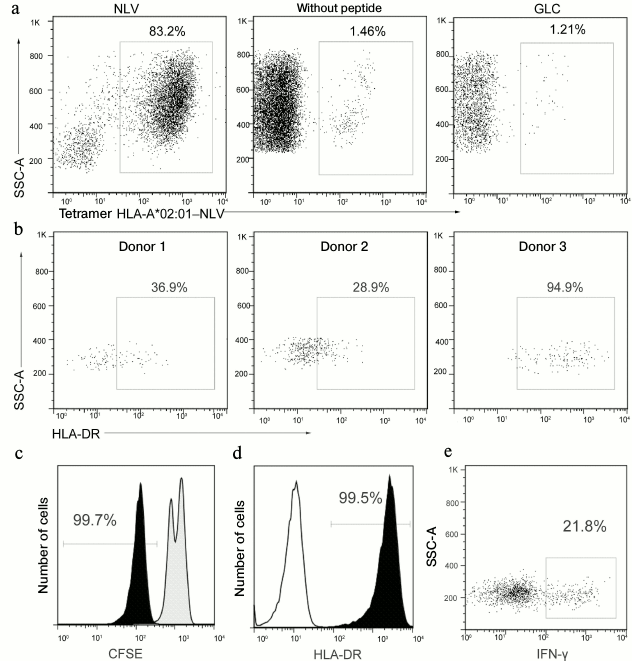
Analysis of epitope-specific T-cell immune response to CMV in healthy donors. We analyzed the functional activity of tetramer-stained cells. These cells were isolated by magnetic separation and cultured with autologous feeder culture supplemented with or without NLV peptide. In the presence of the specific antigen, the virus-specific cell fraction significantly increased (from 1 to >80%), whereas in the absence of the antigen or upon addition of the control antigen (GLC peptide of the Epstein–Barr virus) there was no growth of the antigen-specific cells (Fig. 2a). The expression level of the surface activation marker HLA-DR significantly varied in the population of NLV-specific T-cells isolated from blood of different donors (Fig. 2b).
Fig. 2. Determination of functional activity of cells binding with the A02-NLV tetramer. a) Assessment of the A02-NLV-positive cell fraction after incubation of peripheral blood mononuclear cells from a CMV-seropositive donor during 10 days with the synthetic NLV peptide, with the control peptide from the Epstein–Barr virus or without the peptide (indicated above). b) Assessment of HLA-DR expression level in the population of the NLV-specific T-cells by flow cytofluorimetry in three CMV-seropositive donors. c) A02-NLV-positive cells were isolated from peripheral mononuclear cells by magnetic separation, labeled with CFSE, and cultured for three days with the NLV peptide (black histogram) or without it (gray histogram). The staining with CFSE is presented, as well as the percentage of cells with lowered staining intensity caused by proliferation. d) All cells of the NLV-specific population of T-cells after 10 days of antigen-dependent expansion express the surface activation marker HLA-DR. Data are presented for a representative donor. The open histogram presents the unstained control sample. e) Separated A02-NLV-positive cells were cultured for 6 h with the NLV peptide, and then the production of IFN-γ by the cells was determined.
Proliferation of the NLV-specific cells during antigenic stimulation was confirmed by staining with CFSE (Fig. 2c). After the expansion for two weeks with the NLV peptide, all specific T-cells expressed on the surface the activation marker HLA-DR (Fig. 2d). Synthesis of IFN-γ was also observed in the population of the NLV-specific cells in response to addition of the peptide (Fig. 2e).
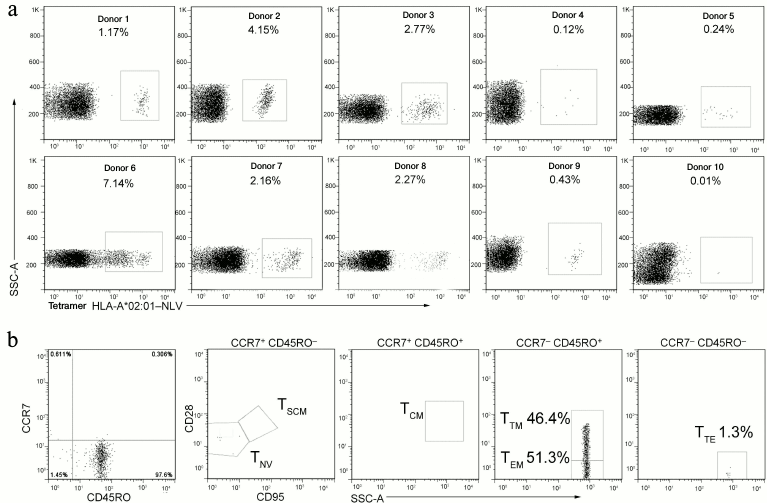
Analysis of T-cell response to CMV in healthy donors. The T-cell response to the NLV epitope was observed in nine of ten analyzed healthy CMV-seropositive carriers of HLA-A*02. Despite of the limited conclusions based on such a small sample size, these findings were in agreement with the published data. In these nine donors the fraction of NLV-specific T-lymphocytes detected in the blood flow was, on average, 2.7% (0.12-7.14%) of the CD8+ cells (Fig. 3a). Because in clinical experiments on adoptive transfer of virus-specific T-lymphocytes, the effect was observed on transfusion of 103-104 cells per 1 kg of the patient’s weight [24, 32, 33], such a high concentration of epitope-specific cells in blood would allow to prepare without difficulty a therapeutic dose of cells from the standard volume of leukapheresis and to escape the expensive and long-term procedure of in vitro expansion. Moreover, for three donors we also analyzed the immune phenotype of the NLV-specific T-cells. The population of NLV-specific cells, as expected, did not contain naïve cells and consisted mainly of terminal memory cells (TTM) and effector memory cells (TEM) (Fig. 3b). These cells were characterized by pronounced effector functions, but unlike the terminal effectors (TTE or TEMRA), they retained a certain proliferative potential [34].
Fig. 3. Fraction and immune phenotype of cells specific to the immune dominant antigen NLV in healthy seropositive carriers of HLA-A*02. a) Analysis of cell number reacting with the NLV peptide of CMV in healthy seropositive carriers of HLA-A*02. Cytofluorimetric analysis of HLA-A*02:01–NLV tetramer staining for 10 donors is presented. The percentage of NLV-specific cells is indicated. b) Analysis of the immune phenotype of the NLV-specific cells for a representative donor. The NLV-specific cells were isolated by magnetic separation and stained for markers characterizing the T-lymphocyte subclasses. Subpopulations of naïve T-cells (TNV) and of stem memory cells (TSCM) were detected in the CCR7+CD45RO– population and divided by the expression of CD28 and CD95. TCM were determined by the CCR7+CD45RO+CD28+ phenotype. TEM and TTM were detected in the CCR7–CD45RO+ population and divided by the expression of CD28. TTE were determined by the CCR7–CD45RO–CD28– phenotype. The analyzed population is indicated above the point diagrams. Subpopulations of T-lymphocytes and the corresponding percentage are shown near the gates.
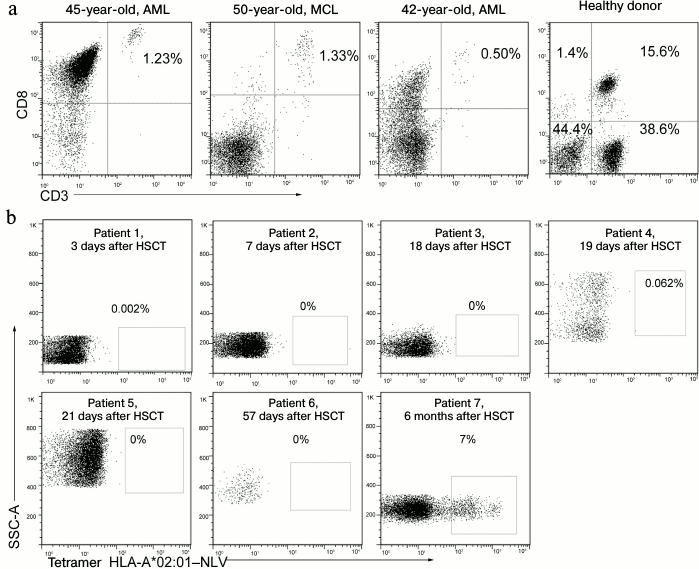
During a short time after allo-HSCT, patients do not have T-cell immunity against CMV. After allo-HSCT, the patients had lymphopenia and their content of cytotoxic CD3+CD8+ T-lymphocytes in peripheral blood was sharply decreased compared to healthy donors (Fig. 4a). During the first months after the transplantation, hematopoiesis gradually recovered, but the population of NLV-specific cells was not detected, these cells appeared only some months later (Fig. 4b). The absence of T-cells reacting to the immunodominant antigen was associated with a high risk of CMV reactivation on the background of immunodeficiency.
Fig. 4.{{anchor|MHC}} Blood concentration of cytotoxic T-lymphocytes and NLV-specific cells in patients during a short time after transplantation. a) Concentration of cytotoxic T-lymphocytes (CD3+/CD8+) in patients and healthy donors, the patient’s diagnosis and age shown above. AML, acute myeloid leukemia; MCL, mantle cell lymphoma. b) Analysis of NLV-specific T-cell population in HLA-A*02-positive patients at different times after allo-HSCT. The post-transplantation time is shown above. Percentage of the NLV-specific cells among all CD8+ lymphocytes are presented.
Antigen-specific T-lymphocytes can be isolated ex vivo to pronounced purity using MHC multimers. MHC multimers carrying immunodominant viral epitopes can be used for screening and choosing potential donors for adoptive transfer of virus-specific T-lymphocytes. Moreover, these recombinant molecules can be used for magnetic separation of antigen-specific cells using antibodies to fluorescent proteins (PE, APC) bound with magnetic particles.
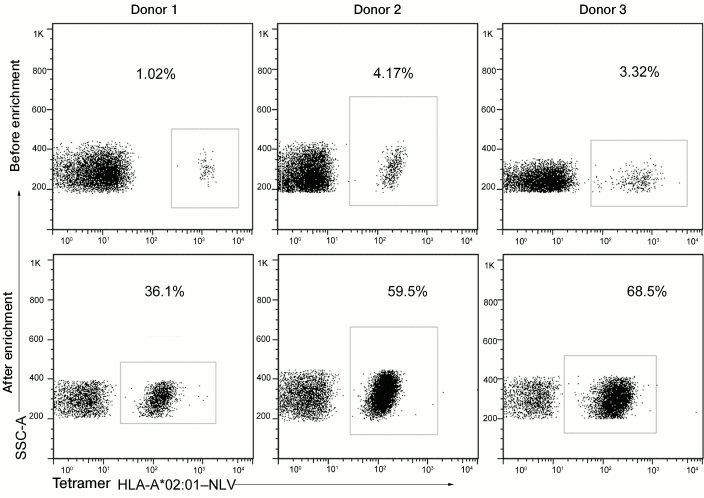
We enriched virus-specific T-lymphocytes from three CMV-seropositive donors carrying HLA-A*02 and possessing NLV-specific CD8+-T-lymphocytes. Before the enrichment, the concentration of these cells was 1-4%, and it was increased to 36-69% by magnetic separation (Fig. 5). Injection of antigen-specific cells enriched only to 10-20% in the dose of 104-105 cells per kg body weight of the patient, according to the literature, did not induce an allogeneic immune response; therefore, we can state that the enrichment level obtained by us was sufficient for cell transfusion to patients.
Fig. 5. Magnetic separation of NLV-specific T-cells using the HLA-A*02–NLV tetramers. The NLV-specific cells from three donors were enriched by magnetic separation using the HLA-A*02–NLV tetramers labeled by the fluorescent protein PE and antibodies to phycoerythrin bound with magnetic nanoparticles. Data on staining with the HLA-A*02–NLV tetramers before and after the enrichment are presented. Percentages of the NLV-specific cells are indicated.
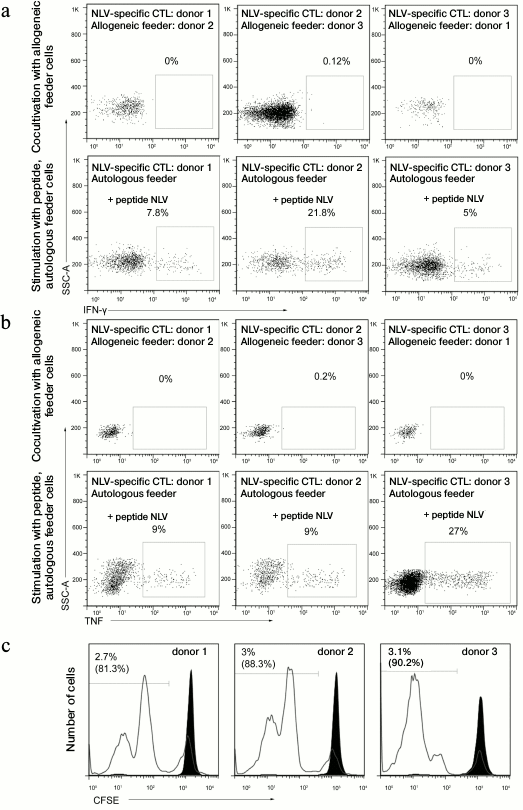
Analysis of in vitro cross-reactivity of T-cells specific to viral antigens. We analyzed the ability of NLV-specific T-lymphocytes to induce allogeneic immune response via cross-reactivity mechanism using a mixed culture of lymphocytes. The NLV-specific cells from three donors were isolated by magnetic separation and cultivated with a heterologous feeder culture (CD8-depleted peripheral mononuclear cells) in the presence of the NLV antigen or without it. The donors of the NLV lymphocytes and of the feeder cells were matched only in the HLA-A*02 allele (Fig. 6). The cross-reactivity was assessed by the release of IFN-γ (Fig. 6a), TNF (Fig. 6b), and by proliferation of the specific population of T-cells (Fig. 6c). No cross-reactivity was observed in all of the three mixed cultures.
Fig. 6. Determination of the ability of virus-specific T-lymphocytes for alloreactive response. The NLV-specific cells were isolated from three donors by magnetic separation, added to the allogeneic or autologous feeder culture (CD8+-depleted peripheral mononuclear cells), and then incubated with or without the NLV peptide. Then production by the cells of IFN-γ (a) and TNF (b) was analyzed. The origin of the feeder culture is indicated above; the stimulatory antigen is shown on the left. The percentage of the cells producing the corresponding cytokine is given. CTL, cytotoxic T-lymphocytes. c) NLV-specific cells chosen by magnetic separation were stained with CFSE and then cultured together with allogeneic or autologous peripheral CD8+-depleted mononuclear cells. The cocultures with the autologous cells were stimulated by addition of the NLV peptide. On the third day, the cells were stained with the NLV tetramer and proliferation of the specific population was analyzed by staining with CFSE. The filled histogram illustrates the cultivation with the allogeneic feeder cells; the open histogram shows the cultivation with the autologous cells. The percentage of the NLV-specific lymphocytes that have divided in response to the allogeneic cells or to the NLV peptide is shown (indicated in parentheses).
DISCUSSION
At present, the problem of CMV reactivation after the allo-HSCT procedure is still extremely relevant. In this work, we have shown that on the background of general leucopenia, in the patient’s blood after the transplantation population of virus-specific cells is absent, and this results in a high risk of secondary infections. Currently in Russia, no alternatives to antiviral drugs are used for therapy and prevention of post-transplantation herpesvirus infections. Adoptive transfer of virus-specific cytotoxic cells is a promising and intensively studied approach. Among donors of hematopoietic stem cells at the Research Center of Hematology, 94% were CMV-seropositive, and therefore it is quite easy to choose a donor with high concentration of the virus-specific lymphocytes. Hence, using MHC multimers for the ex vivo isolation of antiviral T-cells and their immediate transfusion seems to be more effective economically than their in vitro expansion. The absence of the cultivation stage allows to perform the procedure quickly, which sometimes can be crucial; it also decreases the contamination risk, excludes the possibility of changes in the cellular phenotype and exhaustion of the effector potential caused by the long-term antigenic stimulation, and promotes the retention of the proliferative potential of the cells. The viral peptide NLV is considered to be a promising target because it is an immunodominant epitope and restricted to the most distributed in Russia allele HLA-A*02; it occurs in 46% of patients of the Research Center of Hematology. In the literature, there are reports about cross-reactivity of the virus-specific T-lymphocytes with another HLA allele, in particular with HLA-B*27:05 [35, 36], but in the present work we have not observed such alloreactivity, although one of the donors had the HLA-B*27:05 allele. The data have shown that on choosing a donor for adoptive transfer, it is not necessary to take into account the compatibility by the HLA alleles except the restricting allele; nevertheless, this question has to be studied additionally.
Produced MHC multimers specifically bound T-cells that were activated in response to the viral antigen, and the tetramer-labeled cells manifested functional activity. The staining efficiency of the virus-specific population with the resulting tetramer was comparable to staining with the commercial analogs. Thus, the resulting tetramers can be used, first, for choosing the optimal donor with a high content of the specific cells, second, for extracting the donor’s T-lymphocytes specific to the viral antigens from the leukapheresis product, and, third, for monitoring the transplanted cells in the patient’s blood.
In addition to the size of the virus-specific cell population, the donor specimens under study were different in staining intensity with the tetramer, which could correlate with the affinity and/or the T-cell receptor expression. This parameter might also have to be taken into account on choosing the donor for adoptive transfer.
The cell product can be additionally enriched before the transfusion by depletion of natural killers (NK-cells) and monocytes, which carry receptors to the MHC I class and bind with MHC multimers independently of the presented peptide. However, most likely, for these cells there is no risk of development of allogeneic immune response.
Combining adoptive transfer of virus-specific T-lymphocytes with sequencing T-cell receptor repertoire is also interesting. This can help to analyze the proliferation abilities of antiviral cytotoxic cell clonotypes in the patient’s organism, whether they can proliferate or disappear. It would also be interesting to combine analysis of the repertoires with the assessment of the T-cell receptor affinity for the antigen with study on differences in immune phenotypes of the clonotypes present in the subpopulations.
Acknowledgements
We are grateful to Ton Schumacher for the kindly providing the plasmids and to D. N. Penkov for the help in their transporting, and to the nurses of the procedure room of the chemotherapy department: L. A. Baranova, T. I. Davydova, I. A. Kalinina, and V. I. Mironova for their regular help in taking the biological material.
REFERENCES
1.Vilibic-Cavlek, T., Kolaric, B., Ljubin-Sternak,
S., Kos, M., Kaic, B., and Mlinaric-Galinovic, G. (2015) Prevalence and
dynamics of cytomegalovirus infection among patients undergoing chronic
hemodialysis, Indian J. Nephrol., 25, 95-98.
2.Zebrun, A. B., Kuliasheva, L. B., Ermolenko, K. D.,
and Zakrevskaia, A. V. (2013) Spread of herpesvirus infections in
children and adults in St. Petersburg according to seroepidemiologic
study data, Zh. Mikrobiol. Epidemiol. Immunobiol., 6,
30-36.
3.Compton, T., Kurt-Jones, E. A., Boehme, K. W.,
Belko, J., Latz, E., Golenbock, D. T., and Finberg, R. W. (2003) Human
cytomegalovirus activates inflammatory cytokine responses via CD14 and
Toll-like receptor 2, J. Virol., 77, 4588-4596.
4.Humar, A., St Louis, P., Mazzulli, T., McGeer, A.,
Lipton, J., Messner, H., and MacDonald, K. S. (1999) Elevated serum
cytokines are associated with cytomegalovirus infection and disease in
bone marrow transplant recipients, J. Infect. Dis., 179,
484-488.
5.Barry, S. M., Johnson, M. A., and Janossy, G.
(2000) Cytopathology or immunopathology? The puzzle of cytomegalovirus
pneumonitis revisited, Bone Marrow Transplant., 26,
591-597.
6.Efimov, G. A., Vdovin, A. S., Grigor’ev, A.
A., Filkin, S. Y., Bykova, N. A., and Savchenko, V. G. (2015)
Immunobiology of acute reaction “graft-versus-host”,
Med. Immunol., 17, 499-516.
7.Varani, S., and Landini, M. P. (2011)
Cytomegalovirus-induced immunopathology and its clinical consequences,
Herpesviridae, 2, 6.
8.Ariza-Heredia, E. J., Nesher, L., and Chemaly, R.
F. (2014) Cytomegalovirus diseases after hematopoietic stem cell
transplantation: a mini-review, Cancer Lett., 342,
1-8.
9.Meijer, E., Boland, G. J., and Verdonck, L. F.
(2003) Prevention of cytomegalovirus disease in recipients of
allogeneic stem cell transplants, Clin. Microbiol. Rev.,
16, 647-657.
10.Bykova, N. A., Malko, D. B., Vdovin, A. S., and
Efimov, G. A. (2016) In silico analysis of immunogenic potential
of one-nucleotide polimorphism at the fully HLA-compatible
transplantation, Ros. Immunol. Zh., 10, 38-48.
11.Kryuchkov, N. A., and Khaitov, M. R. (2008)
Detection of antigen-specific populations of T-cells using MHC-peptide
tetramers, Immunologiya, 29, 187-190.
12.Karpenko, L. I., Mechetina, L. V., and Reguzova,
A. Iu. (2011) MHC-multimers and their application in studies of
antiviral immune response, Zh. Mikrobiol. Epidemiol.
Immunobiol., 2, 112-119.
13.Riddell, S. R., Watanabe, K. S., Goodrich, J. M.,
Li, C. R., Agha, M. E., and Greenberg, P. D. (1992) Restoration of
viral immunity in immunodeficient humans by the adoptive transfer of
T-cell clones, Science, 257, 238-241.
14.Rooney, C. M., Smith, C. A., Ng, C. Y., Loftin,
S., Li, C., Krance, R. A., Brenner, M. K., and Heslop, H. E. (1995) Use
of gene-modified virus-specific T lymphocytes to control
Epstein–Barr-virus-related lymphoproliferation, Lancet,
345, 9-13.
15.Leen, A. M., Bollard, C. M., Mendizabal, A. M.,
Shpall, E. J., Szabolcs, P., Antin, J. H., Kapoor, N., Pai, S. Y.,
Rowley, S. D., Kebriaei, P., Dey, B. R., Grilley, B. J., Gee, A. P.,
Brenner, M. K., Rooney, C. M., and Heslop, H. E. (2013) Multicenter
study of banked third-party virus-specific T-cells to treat severe
viral infections after hematopoietic stem cell transplantation,
Blood, 121, 5113-5123.
16.Peggs, K. S., Thomson, K., Samuel, E., Dyer, G.,
Armoogum, J., Chakraverty, R., Pang, K., Mackinnon, S., and Lowdell, M.
W. (2011) Directly selected cytomegalovirus-reactive donor T cells
confer rapid and safe systemic reconstitution of virus-specific
immunity following stem cell transplantation, Clin. Infect.
Dis., 52, 49-57.
17.Bao, L., Cowan, M. J., Dunham, K., Horn, B.,
McGuirk, J., Gilman, A., and Lucas, K. G. (2012) Adoptive immunotherapy
with CMV-specific cytotoxic T-lymphocytes for stem cell transplant
patients with refractory CMV infections, J. Immunother.,
35, 293-298.
18.O’Reilly, R. J., Prockop, S., Hasan, A. N.,
Koehne, G., and Doubrovina, E. (2016) Virus-specific T-cell banks for
“off the shelf” adoptive therapy of refractory infections,
Bone Marrow Transplant., doi: 10.1038/bmt.2016.17.
19.McGoldrick, S. M., Bleakley, M. E., Guerrero, A.,
Turtle, C. J., Yamamoto, T. N., Pereira, S. E., Delaney, C. S., and
Riddell, S. R. (2013) Cytomegalovirus-specific T cells are primed early
after cord blood transplant but fail to control virus in vivo,
Blood, 121, 2796-2803.
20.Ljungman, P., Hakki, M., and Boeckh, M. (2011)
Cytomegalovirus in hematopoietic stem cell transplant recipients,
Hematol. Oncol. Clin. North Am., 25, 151-169.
21.Gratama, J. W., Van Esser, J. W., Lamers, C. H.,
Tournay, C., Lowenberg, B., Bolhuis, R. L., and Cornelissen, J. J.
(2001) Tetramer-based quantification of cytomegalovirus (CMV)-specific
CD8+ T-lymphocytes in T-cell-depleted stem cell grafts and
after transplantation may identify patients at risk for progressive CMV
infection, Blood, 98, 1358-1364.
22.Nunes, J. M., Buhler, S., Roessli, D.,
Sanchez-Mazas, A., and HLA-net 2013 collaboration (2014) The HLA-net
Gene[rate] pipeline for effective HLA data analysis and its application
to 145 population samples from Europe and neighbouring areas, Tissue
Antigens, 83, 307-323.
23.Hebart, H., Rauser, G., Stevanovic, S., Haenle,
C., Nussbaum, A. K., Meisner, C., Bissinger, A. L., Tenzer, S., Jahn,
G., Loeffler, J., Rammensee, H. G., Schild, H., and Einsele, H. (2003)
A CTL epitope from human cytomegalovirus IE1 defined by combining
prediction of HLA binding and proteasomal processing is the target of
dominant immune responses in patients after allogeneic stem cell
transplantation, Exp. Hematol., 31, 966-973.
24.Cobbold, M., Khan, N., Pourgheysari, B., Tauro,
S., McDonald, D., Osman, H., Assenmacher, M., Billingham, L., Steward,
C., Crawley, C., Olavarria, E., Goldman, J., Chakraverty, R., Mahendra,
P., Craddock, C., and Moss, P. A. (2005) Adoptive transfer of
cytomegalovirus-specific CTL to stem cell transplant patients after
selection by HLA-peptide tetramers, J. Exp. Med., 202,
379-386.
25.Ramirez, N., and Olavarria, E. (2013)
Viral-specific adoptive immunotherapy after allo-SCT: the role of
multimer-based selection strategies, Bone Marrow Transplant.,
48, 1265-1270.
26.Casalegno-Garduno, R., Schmitt, A., Yao, J.,
Wang, X., Xu, X., Freund, M., and Schmitt, M. (2010) Multimer
technologies for detection and adoptive transfer of antigen-specific
T-cells, Cancer Immunol. Immunother., 59, 195-202.
27.Yee, C. (2003) Adoptive T-cell
therapy – immune monitoring and MHC multimers, Clin.
Immunol., 106, 5-9.
28.Stemberger, C., Graef, P., Odendahl, M.,
Albrecht, J., Dossinger, G., Anderl, F., Buchholz, V. R., Gasteiger,
G., Schiemann, M., Grigoleit, G. U., Schuster, F. R., Borkhardt, A.,
Versluys, B., Tonn, T., Seifried, E., Einsele, H., Germeroth, L.,
Busch, D. H., and Neuenhahn, M. (2014) Lowest numbers of primary
CD8+ T-cells can reconstitute protective immunity upon
adoptive immunotherapy, Blood, 124, 628-637.
29.Garboczi, D. N., Hung, D. T., and Wiley, D. C.
(1992) HLA-A2-peptide complexes: refolding and crystallization of
molecules expressed in Escherichia coli and complexed
with single antigenic peptides, Proc. Natl. Acad. Sci. USA,
89, 3429-3433.
30.Rodenko, B., Toebes, M., Hadrup, S. R., Van Esch,
W. J., Molenaar, A. M., Schumacher, T. N., and Ovaa, H. (2006)
Generation of peptide-MHC class I complexes through UV-mediated ligand
exchange, Nat. Protoc., 1, 1120-1132.
31.Excoffier, L., and Lischer, H. E. (2010) Arlequin
suite ver 3.5: a new series of programs to perform population genetics
analyses under Linux and Windows, Mol. Ecol. Res., 10,
564-567.
32.Uhlin, M., Gertow, J., Uzunel, M., Okas, M.,
Berglund, S., Watz, E., Brune, M., Ljungman, P., Maeurer, M., and
Mattsson, J. (2012) Rapid salvage treatment with virus-specific T-cells
for therapy-resistant disease, Clin. Infect. Dis., 55,
1064-1073.
33.Uhlin, M., Okas, M., Gertow, J., Uzunel, M.,
Brismar, T. B., and Mattsson, J. (2010) A novel haplo-identical
adoptive CTL therapy as a treatment for EBV-associated lymphoma after
stem cell transplantation, Cancer Immunol. Immunother.,
59, 473-477.
34.Mahnke, Y. D., Brodie, T. M., Sallusto, F.,
Roederer, M., and Lugli, E. (2013) The who’s who of T-cell
differentiation: human memory T-cell subsets, Eur. J. Immunol.,
43, 2797-2809.
35.Amir, A. L., D'Orsogna, L. J., Roelen, D. L., Van
Loenen, M. M., Hagedoorn, R. S., De Boer, R., Van der Hoorn, M. A.,
Kester, M. G., Doxiadis, I. I., Falkenburg, J. H., Claas, F. H., and
Heemskerk, M. H. (2010) Allo-HLA reactivity of virus-specific memory
T-cells is common, Blood, 115, 3146-3157.
36.Nguyen, T. H., Rowntree, L. C., Pellicci, D. G.,
Bird, N. L., Handel, A., Kjer-Nielsen, L., Kedzierska, K., Kotsimbos,
T. C., and Mifsud, N. A. (2014) Recognition of distinct cross-reactive
virus-specific CD8+ T-cells reveals a unique TCR signature
in a clinical setting, J. Immunol., 192, 5039-5049.