Evolution of Aging Theories: Why Modern Programmed Aging Concepts Are Transforming Medical Research
Theodore C. Goldsmith
Azinet LLC; E-mail: tgoldsmith@azinet.com
Received June 29, 2016
Programmed aging refers to the idea that senescence in humans and other organisms is purposely caused by evolved biological mechanisms to obtain an evolutionary advantage. Until recently, programmed aging was considered theoretically impossible because of the mechanics of the evolution process, and medical research was based on the idea that aging was not programmed. Theorists struggled for more than a century in efforts to develop non-programmed theories that fit observations, without obtaining a consensus supporting any non-programmed theory. Empirical evidence of programmed lifespan limitations continued to accumulate. More recently, developments, especially in our understanding of biological inheritance, have exposed major issues and complexities regarding the process of evolution, some of which explicitly enable programmed aging of mammals. Consequently, science-based opposition to programmed aging has dramatically declined. This progression has major implications for medical research, because the theories suggest that very different biological mechanisms are ultimately responsible for highly age-related diseases that now represent most research efforts and health costs. Most particularly, programmed theories suggest that aging per se is a treatable condition and suggest a second path toward treating and preventing age-related diseases that can be exploited in addition to the traditional disease-specific approaches. The theories also make predictions regarding the nature of biological aging mechanisms and therefore suggest research directions. This article discusses developments of evolutionary mechanics, the consequent programmed aging theories, and logical inferences concerning biological aging mechanisms. It concludes that major medical research organizations cannot afford to ignore programmed aging concepts in assigning research resources and directions.
KEY WORDS: medical research policy, aging theories, senescence, programmed aging, evolutionDOI: 10.1134/S0006297916120026
Programmed (or adaptive) aging refers to the idea that humans and most other complex organisms possess biological mechanisms that purposely limit their internally determined lifespans beyond a certain species-specific age and that these mechanisms are adaptations in that they evolved because aging, per se, creates an evolutionary advantage. According to this concept, these senescence programs are ultimately responsible for most occurrences of highly age-related diseases and conditions such as cancer, heart disease, and Alzheimer’s disease.
Biological aging theories are essentially determined by underlying evolutionary mechanics theories that describe the operation of the evolution process. Darwin’s survival-of-the-fittest evolutionary mechanics concept, as described by Darwin and widely taught, is obviously incompatible with evolved aging programs. High school students can easily determine that deterioration and death due to senescence do not aid organisms in living longer or reproducing more. As late as 2002, many senior bioscientists (e.g. [1]) dismissed programmed aging as obviously theoretically impossible and therefore scientifically ridiculous. Some compared programmed aging (originally proposed in 1882 [2]) to Intelligent Design and other popular but scientifically absurd concepts regarding evolution. However, developments summarized here provide strong theoretical support for programmed aging, which now represents the best science on senescence.
INDIVIDUAL VERSUS POPULATION BENEFIT
Modern programmed aging theories are based on any of several post-1962 evolutionary mechanics theories to the effect that population benefit can influence the evolution process.
We can say that an evolved inheritable organism design characteristic or trait produces an individual benefit if individuals possessing that trait have an increased probability of producing adult descendants under wild conditions. This is essentially the definition of Darwinian fitness, which we can now describe as individual fitness.
A trait produces a population benefit if a wild population of organisms possessing that trait has an increased probability of avoiding extinction or producing descendant species.
We can use three questions to examine this issue:
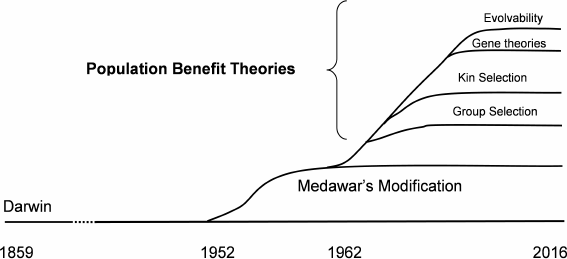
1) Could a trait produce a population benefit but at an individual cost? Human societies are full of behavioral restrictions (laws, rules, even religious commandments) that limit individual behavior in favor of a population. Theorists noticed that various presumably evolved and inherited animal behaviors such as animal altruism also favored populations at the expense of individuals. This led to the first population benefit theory, known as group selection [3], in 1962.
There is no current scientific disagreement with the idea that a hypothetical trait could result in a population benefit and simultaneous individual cost. In addition to altruism, sexual reproduction, some genomic design features, some mating rituals, and apparently unnecessary delay in reproductive maturity (especially in males) have been suggested as instances of such a population versus individual relationship. Since then other population benefit theories including kin selection [4], small-group selection [5], isolated-population selection, gene-oriented selection [6], and evolvability theories [7] have appeared.
2) Can limiting individual lifespan produce a population benefit? Theorists (e.g. [2, 5, 8-11]) have suggested many ways in which limiting individual lifespan beyond a species-specific age produces a population benefit. Scientific arguments against any specific rationale have not appeared.
3) Can a population benefit trade-off against or offset an individual disadvantage creating a net benefit and allowing the evolution and retention of a trait that produces population benefit but individual cost? Historically, this is the big issue. In addition to the gross incompatibility between survival-of-the-fittest and what amount to suicide mechanisms mentioned above, analysis performed in the 1960s (e.g. [12]) suggested that Darwin’s mechanics concept (random mutations, natural selection) is incompatible with a population versus individual trade-off (more below). However, very extensive discoveries (some rather recent) concerning the nature of biological inheritance (central to any evolutionary mechanics theory) have exposed many issues with Darwinian mechanics and suggested multiple ways [8] in which a population benefit could indeed trade-off against individual cost, thus allowing evolution and retention of an individually adverse trait. Scientific arguments against these specific proposals have not appeared.
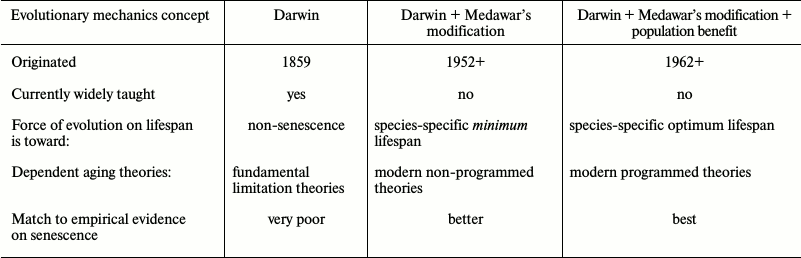
Legacy (pre-1952) aging theories based on unmodified Darwinian mechanics, while still popular with the public, failed to explain many observations, such as the huge inter-species variation in internally determined lifespans and are now deprecated by most gerontologists and medical researchers. These theories include “wear and tear” theories, random damage or stochastic theories, and other theories to the effect that aging is the result of fundamental limitations (i.e. laws of physics or chemistry) that cannot be overcome by the evolution process.
In 1952, P. Medawar introduced a now widely accepted modification to Darwin’s mechanics [13] suggesting that the evolutionary force toward living and reproducing longer declines with age following the age at which a species can complete an initial reproduction. We can summarize this idea by saying that there is clearly no evolutionary benefit from an organism possessing the internal capability for living and reproducing beyond the species-specific age at which essentially all the members of a wild age-cohort, even if lacking any internal limitations on lifespan, would be dead from external causes such as predators, starvation, lack of habitat, environmental conditions, or infectious diseases. This idea is central to modern programmed and non-programmed theories and explains why biochemically very similar species (e.g. mammals) have such huge differences in their internally determined lifespans (more than 200 to 1). How many wild mice would survive beyond 3 years of age even if they had no internal limitations on their lifespans or fitness, i.e. did not exhibit senescence? Modern non-programmed theories of mammal aging based on Medawar’s modification include the mutation accumulation theory [13], antagonistic pleiotropy theory [14], and disposable soma theory [15]. There is no agreement, even within the non-programmed faction, as to which of these theories is valid (table).
In effect, Medawar’s modification means that for each species there is a specific age at which the individual disadvantage of aging is small and that therefore the compensating population benefit of aging could be small. Rejecting programmed aging on evolutionary grounds (while accepting Medawar’s modification) therefore requires the assumption that all the multiple population benefit theories are so utterly invalid that they could not have an even tiny effect on the evolution process. As described below, senior proponents of modern non-programmed theories have largely abandoned efforts to validate such an assumption and have therefore essentially conceded the evolutionary basis of programmed aging.
Evolutionary mechanics concepts and dependent biological aging theories
CONTINUING DIFFICULTIES WITH NON-PROGRAMMED AGING THEORIES
Despite more than a century of effort, bioscientists have been unable to agree on a non-programmed aging theory. The recent reemergence of programmed aging theories exposed many other issues with non-programmed theories [16].
There was and is little disagreement (after 1952) with the idea that there is some species-specific age at which the individual benefit of further survival and reproduction is negligible. However, in 1957 G. Williams pointed out [14] that at least in humans and other larger mammals, deterioration in individual fitness (e.g. speed, strength, etc.) occurred at too young an age to have negligible evolutionary effect. Studies of large mammals in the wild [17] showed that adult death rates increased with age. If aging was having negligible effect on wild organisms, we would expect adult death rates to be constant or even decline with age because older animals have more experience in dealing with their external world. Williams consequently concluded that aging must produce a compensating individual fitness benefit. He further suggested that this benefit resulted from some unspecified individually beneficial property or properties that were somehow permanently linked to aging (more below) in a way that prevented the evolution process from producing an organism design that possessed the beneficial properties without the linked adverse property (in this case, aging). Since then, many theories to the effect that aging is permanently linked to some beneficial property have appeared with no agreement in the non-adaptive faction.
Recall that in 1957 population benefit theories did not exist. Modern programmed aging theories contend that the population benefit of aging is the compensating benefit.
Non-programmed aging theories tend to be written with a limited scope and target audience. For example, fundamental limitation theories based on Darwin’s mechanics provide a plausible fit with empirical evidence if one considers only human aging and therefore avoids having to explain the huge inter-species lifespan variations. Such theories could well appeal to those only interested in human aging, especially if they have been taught to believe that Darwin’s mechanics concept is sacrosanct. Non-programmed “mammal” aging theories such as previously described typically ignore non-mammals and therefore ignore or deprecate non-mammal evidence like explicit suicide mechanisms (e.g. octopus [18]), worm experiments [19], and apparently non-senescing fish [20].
Modern programmed aging theories consider that purposely limited lifespan generally provides a population benefit and that therefore non-mammals may be relevant because humans may have inherited elements of their aging mechanisms from very distant ancestors. The difficulty here is that evolution applies to all organisms. If some or most organisms are excluded from an evolution-based aging theory, a convincing rationale for such exclusion is needed but not provided.
Programmed theories are based on the idea that there is evolutionary force toward achieving and not exceeding some species-specific optimum lifespan and therefore explain the lifespan variations. Modern non-programmed theories are based on the idea that there is evolutionary force toward achieving a species-specific minimum lifespan and that there is no evolutionary disadvantage from living too long. Any lifespan greater than the minimum (including non-senescence) would satisfy the theory. Attempts to explain the observed variety of lifespans typically involve implausible assumptions [16] involving random deteriorative processes.
DEVELOPMENTS ENABLING POPULATION BENEFIT AND PROGRAMMED
AGING
As described above, the overriding objection to population benefit theories (and dependent aging theories) has been the incompatibility with Darwin’s mechanics rather than empirical evidence or any other consideration.
Darwin’s evolutionary mechanics concept as widely understood involves two steps:
Mutations. Random changes in the inheritable design of an organism occur.
Natural selection. If a change causes the possessing individuals to live longer and breed more, it propagates in a population.
If this description is both valid and comprehensive, clearly only individual benefit or cost can influence the evolution process. No one denies that mutations and natural selection are important to the evolution process. However, discoveries in genetics and other observations showed that the evolution process involves many other steps or sub-processes the existence of which enables population benefit and thereby programmed aging as follows:
Inter-trait linkage. Discoveries concerning the very complex mechanisms of biological inheritance in complex organisms (genes, chromosomes, meiosis, genetic crossover, introns, transposons, complex genomic designs, mitochondrial DNA, sex-linking, digital nature of genomic information, and inter-species genomic comparisons) revealed many ways [8] that a trait could be linked to another trait in such a way as to make it more difficult and therefore more time-consuming for the evolution process to produce an organism that possessed the one trait without also possessing the other.
It has been established [21] that traits controlled by genes that are physically near each other on the same chromosome tend to be inherited as a unit, creating a linkage between traits controlled by those genes. G. Williams in 1957 described another linkage mechanism ([14] pleiotropy) that is caused by the fact that a single gene often controls more than one trait and that therefore a mutational change to such a gene would affect more than one trait, causing a linkage. He suggested that if an adverse trait was linked to a beneficial trait (antagonistic pleiotropy) in a way that produced a net benefit, the adverse trait could resist being “selected-out” by natural selection.
Many other genetic linkage mechanisms [8] have been identified that operate on vastly different timescales. That is, the time required by the evolution process to overcome such a linkage and produce the beneficial trait without the linked adverse trait varies enormously depending on the linkage mechanism. For example, a single letter mutation (single nucleotide polymorphism) can alter the functioning of a gene and traits controlled by that gene. However, the creation of a functionally new gene is a vastly more complex and time-consuming process, and consequently genes tend to be conserved between mammal species. In other words, “genes live longer than species”, which is the basis of gene-oriented population benefit theories. Separating a trait from some other trait might require a new gene or genes. The various types of linkage would act to protect an individually-adverse trait from being deleted for long enough that long-term population benefits could be obtained, thus causing retention of the individually adverse trait.
Williams’ 1957 proposal was that pleiotropy would create a permanent inter-trait linkage that would last for all evolutionary time (~3.8 billion years) or at least the period since emergence of complex senescing organisms, and used this idea in support of his non-adaptive aging theory [14] that considered aging, per se, to be adverse. However, he could not explain why, if the evolution process was unable to separate an adverse trait from some beneficial trait in the case of aging, mammals could acquire all their other myriad inter-species differences, somehow without being impeded by antagonistic pleiotropy. More recent inter-species genomic comparisons [8] also suggest that pleiotropic linkage can be overcome on a species timescale.
Excepting evolvability, the various population benefit theories differ mainly regarding the size of the population and therefore the time-scale separating individual disadvantage and population benefit. For example, some theorists accept “small-group” or “kin” selection but reject longer-term population benefit concepts. However, some identified linkage mechanisms such as described above have timescales that are longer than or comparable to the time a typical mammal species has existed. This suggests that all of the population benefit theories including “species-level” group selection are valid! Recall that no one denies that population extinction alters the course of evolution. The question is whether a long-tern population benefit can offset a short-term individual disadvantage during the evolution process.
Evolvability. Darwin’s mechanics concept assumes that the ability to evolve (mutations, natural selection) is an inherent invariant property of all living organisms. However, it is now obvious that organisms can possess evolved traits that alter their ability to evolve (evolvability) and that most such traits produce an individual disadvantage [8]. Illustrative example: The Bighorn sheep have a mating ritual [22] that involves strength (head-butting) contests to determine mating rights. Such a trait aids the evolution and retention of strength traits. However, this behavioral trait is individually-adverse in that it reduces the probability that an individual will reproduce. There has been no scientific objection to the idea that increasing an organism’s ability to adapt more rapidly or more comprehensively to changes in its environment represents an evolutionary advantage. Modern evolvability theories (e.g. [23] 1996) are relatively new.
Theorists have suggested many ways [2, 8, 9], in which limiting individual lifespan increases evolvability. In addition to mating behaviors and aging, evolvability provides explanations for other apparent conflicts with Darwinian mechanics, such as sexual reproduction and delayed reproductive maturity. Modern scientific arguments against these specific proposals have not appeared.
Evolvability is substantively different from the other population benefit theories because a change in evolvability would have an instant effect on the evolution process going forward and would not involve the long-term vs. short-term issue. Evolvability operates on the same timescale as the evolution process [8].
The multiple enabling discoveries suggest that all the population benefit theories mentioned above are valid!
RECENT OBJECTIONS TO PROGRAMMED AGING
Some senior theorists still object to programmed aging. For example, in 2011, T. Kirkwood, defending his 1979 non-adaptive disposable soma theory, published an article [24] (with S. Melov) criticizing programmed aging as well as competing non-programmed theories. However, the article concedes that population benefit can influence the evolution process, concedes that programmed aging could be valid under some circumstances, and does not argue against specific circumstances proposed by various programmed aging theories. Even founding members of the non-programmed faction no longer consider programmed aging to be “impossible”! Today, evolutionary mechanics does not provide a scientific rationale for considering programmed aging to be less likely than non-programmed aging!
Modern arguments against programmed aging such as [24] typically suggest that non-programmed theories are more popular and have been more popular for many decades, and that therefore we should not switch without overwhelming proof and lengthy reflection. They typically also demonstrate that it is possible to devise non-programmed, non-adaptive explanations for various observations that support programmed aging such as aging genes and the existence of non-aging “negligibly-senescent” species. Comparing the plausibility of the non-programmed explanations to the corresponding programmed explanations is left to the reader.
Conversely, there are now extensive critiques describing circular logic, other logical issues, implausible assumptions, and empirical conflicts associated with non-programmed theories (e.g. [16, 25]). The non-programmed theories were developed during a period when programmed aging was considered impossible and therefore competed only with each other.
The proliferation of evolutionary mechanics theories (figure) demonstrates that our collective confidence that we understand the details of the evolution process has declined since about 1950. Of course, those details are crucial to the programmed/non-programmed issue. Few bioscientists consider that we are anywhere near to completely understanding biological inheritance, and so developments such as described above can be expected to continue.
Proliferation of evolutionary mechanics theories between 1859 and the present
NATURE OF PROGRAMMED AGING MECHANISMS
If we accept that there is an evolutionary need to limit lifespan, we can envision many different biological senescence mechanisms that could produce that result. Perhaps each cell possesses its own clock and independently decides when to senesce. Perhaps we possess a very explicit suicide mechanism such as observed in some non-mammals [18]. However, there is now a substantial theoretical and empirical basis [19] for believing that the senescence function is like other biological functions like reproduction that involve a single organism-wide “clock” and logic activity that then controls many biological activities, signaling to coordinate these activities in different tissues and systems, and the capability for sensing internal or external conditions that affect the optimum operation of the function.
Recall that all the modern aging theories consider that potentially temporary or local external conditions like predation and food supply are critical to determining an organism’s need for lifespan and that therefore sensing those conditions and regulating an individual’s lifespan in response would provide additional evolutionary benefit with respect to a scheme that was entirely genetically determined. Evidence [8] of just such regulation exists: lifespan extension resulting from caloric restriction [27] suggests detection of famines and lifespan extension from exercise suggests detection of predation.
Further, it is widely agreed that organisms possess many different maintenance and repair mechanisms that act to delay the many different manifestations of senescence. A regulated programmed aging mechanism that operates by downregulating these maintenance and repair mechanisms provides the best fit with empirical evidence [26].
MEDICAL RESEARCH IMPLICATIONS OF THE THEORIES
It is obvious that the immediate causes of different age-related diseases and conditions are generally very different, and consequently medical research has historically been based on and successful in developing disease-specific methods for treatment and prevention. Non-programmed theories strongly support this paradigm and suggest or state that the many manifestations of aging are functionally independent of each other and that therefore there is generally no treatable common factor involved in causing the different manifestations. We can continue to find new treatments for individual diseases or conditions, but senescence, per se, is an untreatable condition.
Programmed theories strongly suggest the existence of a common biological mechanism that is ultimately substantially responsible for senescence manifestations. In addition to disease-specific interventions, we can find ways to interfere with this common mechanism to generally delay or ameliorate senescence and age-related diseases, i.e. anti-aging medicine.
Researchers following non-programmed theories typically are looking at disease-specific damage mechanisms, disease-specific biological repair mechanisms that act to offset the damage, and ways to reduce or correct damage or enhance repair. Researchers following programmed theories are looking at biological clocks, signaling, and even biological detection of external or internal conditions, all of which are typical of evolved biological programs.
NON-SCIENCE FACTORS
Many non-science factors [8] tend to bias public and medical opinion in favor of earlier aging theories. For example, Darwin’s theory is much more widely taught than the later evolutionary mechanics concepts and dependent aging theories. Modern programmed aging theories are even more recent and date from about 1988.
Researchers publicly declaring a belief in programmed aging could suffer adverse career consequences if their managers or institutions still consider programmed aging to be theoretically impossible. Surveys therefore tend to understate the popularity of programmed aging concepts.
RATIONAL MEDICAL RESEARCH MANAGEMENT
During the long period when programmed aging was considered “impossible”, it was reasonable and proper for medical research managers to assign all their budgets and other resources based on the assumption that aging was non-programmed. However, today a rational and conscientious research manager, having reviewed the current situation summarized above, would need to consider the following in making such decisions:
– programmed aging, if valid, represents a second parallel path toward developing ways to treat or prevent highly age-related diseases that can be exploited in addition to traditional disease-specific approaches;
– programmed aging, if valid, represents an opportunity for “low-hanging fruit”. Since it is a fundamentally new approach, it could be expected to yield rapid initial progress;
– all the modern aging theories (that provide even minimal fit to observations) require modifications to Darwinian mechanics. Evolutionary mechanics concepts have diverged since 1950;
– physicians and patients tend to be more concerned with a treatment’s effectiveness than its theoretical basis. Certification procedures largely concentrate on efficacy and safety;
– there are now at least 26,000 physicians (and presumably corresponding patients) who believe in anti-aging medicine [28]. Public and physician acceptance of anti-aging medicine and supporting theory is increasing;
– a drug developed using programmed aging principles could nevertheless be designated, tested, and certified for treatment of a disease or condition.
Issues concerning the evolutionary nature of senescence surfaced soon after publication of Origin in 1859 [29] and have never been resolved. Market forces [8] act to discourage dissemination of newer evolutionary mechanics ideas and dependent aging theories. We can expect academic disagreements to continue, possibly for another 150 years.
However, as summarized here, there now exists substantial theoretical and empirical support for programmed aging. There is little certainty in medical research. It seems unlikely that managers in a competitive venue (e.g. pharmaceutical company), having performed a due-diligence review of the current situation, would conclude that they could afford to ignore programmed aging theories in allocating resources and determining research directions. We can therefore expect substantial investment in research based on programmed aging concepts.
The public perception that aging is an untreatable condition adversely affects their attitudes regarding medical research on age-related diseases. How can we cure cancer if most cancers are caused by aging and aging is unavoidable? Increasing acceptance of modern theories can be expected to increase funding for aging-related research.
REFERENCES
1.Olshansky, S., Hayflick, L., and Carnes, B. (2002)
No truth to the fountain of youth, Sci. Am. (reprinted July
2004, 14).
2.Weismann, A. (1882) Uber die Dauer des
Lebens, Fischer, Jena.
3.Wynne-Edwards, V. (1962) Animal Dispersion in
Relation to Social Behaviour, Oliver & Boyd, Edinburgh.
4.Hamilton, W. (1963) The evolution of altruistic
behavior, Am. Naturalist, 97, 354-356.
5.Travis, J. (2004) The evolution of programmed death
in a spatially structured population, J. Gerontol., 59,
301-305.
6.Dawkins, R. (1986) The Selfish Gene, Oxford
University Press.
7.Goldsmith, T. (2008) Aging, evolvability, and the
individual benefit requirement; medical implications of aging theory
controversies, J. Theor. Biol., 252, 764-768.
8.Goldsmith, T. (2014) The Evolution of Aging,
3rd Edn., Azinet Press, Annapolis.
9.Skulachev, V. (1997) Aging is a specific biological
function rather than the result of a disorder in complex living
systems: biochemical evidence in support of Weismann’s
hypothesis, Biochemistry (Moscow), 62, 1191-1195.
10.Mittledorf, J. (2006) Chaotic population dynamics
and the evolution of ageing, Evol. Ecol. Res., 8,
561-574.
11.Libertini, G. (1988) An adaptive theory of
increasing mortality with increasing chronological age in populations
in the wild, J. Theor. Biol., 132, 145-162.
12.Williams, G. (1966) Adaptation and Natural
Selection: A Critique of Some Current Evolutionary Thought,
Princeton.
13.Medawar, P. (1952) An Unsolved Problem of
Biology, H. K. Lewis & Co., London.
14.Williams, G. (1957) Pleiotropy, natural selection
and the evolution of senescence, Evolution, 11,
398-411.
15.Kirkwood, T., and Holliday, F. (1979) The
evolution of ageing and longevity, Proc. R. Soc. Lond. B,
205, 531-546.
16.Goldsmith, T. (2013) Arguments against
non-programmed aging theories, Biochemistry (Moscow), 78,
971-978.
17.Loison, A., Fiesta-Bianchet, M., Gaillard, J.,
Jorgenson, J., and Jullien, J. (1999) Age-specific survival in five
populations of ungulates: evidence of senescence, Ecology,
80, 2539-2554.
18.Wodinsky, J. (1977) Hormonal inhibition of
feeding and death in octopus: control by optic gland secretion,
Science, 198, 948-951.
19.Apfeld, J., and Kenyon, C. (1999) Regulation of
lifespan by sensory perception in Caenorhabditis elegans,
Nature, 402, 804-809.
20.Bennett, J. T., Boehlert, G. W., and Turekian, K.
K. (1982) Confirmation on longevity in Sebastes diploproa
(Pisces: Scorpaenidae) from 210Pb/226Ra measurements in otoliths,
Marit. Biol., 71, 209-215.
21.Bateson, W., Saunders, E., and Punnett, R. (1904)
Report II. Experimental studies in the physiology of heredity, Rep.
Evol. Com. R Soc., 2, 1-154.
22.Valdez, R., and Krausman, P. (1999) Mountain
Sheep of North America, The University of Arizona Press,
Tucson.
23.Wagner, C., and Altenberg, L. (1996) Perspective:
complex adaptations and the evolution of evolvability,
Evolution, 50, 3.
24.Kirkwood, T., and Melov, S. (2011) On the
programmed/non-programmed nature of ageing within the life history,
Curr. Biol., 21, R701-R707.
25.Skulachev, V. (2011) Aging as a particular case
of phenoptosis, the programmed death of an organism (A response to
Kirkwood–Melov “On the programmed/non-programmed nature of
aging within the life history”), Aging (Albany N. Y.),
3, 1120-1123.
26.Goldsmith, T. (2016) Emerging programmed aging
mechanisms and their medical implications, Med. Hypotheses,
86, 92-96.
27.Weindruch, R., Walford, R., Fligiel, S., and
Guthrie, D. (1986) The retardation of aging in mice by dietary
restriction: longevity, cancer, immunity and lifetime energy intake,
J. Nutr., 116, 641-654.
28.American Academy of Anti-Aging Medicine (A4M),
website, 6/2016.
29.Darwin, C. (1872) The Origin of Species,
6th Edn., Murray, London.