Adaptation of Drosophila melanogaster to Unfavorable Growth Medium Affects Lifespan and Age-Related Fecundity
E. U. Yakovleva1*, E. B. Naimark2, and A. V. Markov2,3
1Lomonosov Moscow State University, Faculty of Economics, 119991 Moscow, Russia; E-mail: e.u.yakovleva@gmail.com2Paleontological Institute of Russian Academy of Sciences, 117997 Moscow, Russia
3Lomonosov Moscow State University, Biological Faculty, Department of Biological Evolution, 119991 Moscow, Russia; E-mail: markov_a@inbox.ru
* To whom correspondence should be addressed.
Received July 11, 2016
Experimental adaptation of Drosophila melanogaster to nutrient-deficient starch-based (S) medium resulted in lifespan shortening, increased early-life fecundity, accelerated reproductive aging, and sexually dimorphic survival curves. The direction of all these evolutionary changes coincide with the direction of phenotypic plasticity observed in non-adapted flies cultured on S medium. High adult mortality rate caused by unfavorable growth medium apparently was the main factor of selection during the evolutionary experiment. The results are partially compatible with Williams’ hypothesis, which states that increased mortality rate should result in relaxed selection against mutations that decrease fitness late in life, and thus promote the evolution of shorter lifespan and earlier reproduction. However, our results do not confirm Williams’ prediction that the sex with higher mortality rate should undergo more rapid aging: lifespan shortening by S medium is more pronounced in naïve males than females, but it was female lifespan that decreased more in the course of adaptation. These data, as well as the results of testing of F1 hybrids between adapted and control lineages, are compatible with the idea that the genetic basis of longevity is different in the two sexes, and that evolutionary response to increased mortality rate depends on the degree to which the mortality is selective. Selective mortality can result in the development of longer (rather than shorter) lifespan in the course of evolution. The results also imply that antagonistic pleiotropy of alleles, which increase early-life fecundity at the cost of accelerated aging, played an important role in the evolutionary changes of females in the experimental lineage, while accumulation of deleterious mutations with late-life effects due to drift was more important in the evolution of male traits.
KEY WORDS: experimental evolution, adaptation, lifespan, fecundity, aging, life cycle evolution, evolutionary trade-offDOI: 10.1134/S0006297916120063
Experimental study of evolution is a promising and rapidly developing field which helps to understand patterns of evolutionary development of various properties including life cycle parameters such as lifespan, age-related dynamics of fecundity, and mortality [1]. The ability of laboratory populations of Drosophila melanogaster for selection-induced rapid changes of these parameters makes this species a convenient object for studying plasticity and evolution of the life cycle. The nature of selection factors underlying lifespan evolution remains controversial. In some cases, lifespan shortening may be interpreted as a useful adaptation increasing evolutionary potential of a population [2-4], which is supported by natural selection [5], group selection, or “second-order selection for evolvability” [6]. A set of ideas called the classical evolutionary theory of aging [7, 8] is better known and is probably more widely applicable. It is based on Williams’ hypothesis [9]. According to the original idea by Medawar [10] on the age-related reduction of the efficiency of selection (starting from the age of puberty), the less is the number of individuals surviving by a given age, the weaker is the effect of selection (and the stronger is the drift effect) on mutations that manifest their harmful effect after that age. It contributes to the accumulation of deleterious mutations with late effect, especially when they are manifested after the end of the period of active reproduction, when the power of selection is close to zero. Williams, however, drew attention to the fact that in many species viability starts to decline long before the loss of reproductive ability, at the age reached by many individuals. This observation can hardly be explained only by passive (due to drift) accumulation of mutations, because the power of selection against deleterious mutations manifested in the reproductive age is nonzero. Therefore Williams suggested that antagonistic pleiotropy of alleles that have different effects on fitness at different ages is an important factor in the evolution of aging. Alleles increasing fecundity or other fitness components at a young age at the expense of fitness decrease later in life will be supported by selection despite their harmful effects, because the power of selection is higher for properties manifested at the beginning of adult life [9]. If evolution of aging is really related to the age-dependent weakening of selection, then we can expect the fulfillment of some obvious consequences. These include the effect of high exogenous mortality caused, for example, by predation or infections. By reducing individual chances for a long life, high mortality should support evolution of accelerated reduction of fitness with age (aging). This leads to inherited reduction of average lifespan even under favorable conditions [7, 9-11]. Williams’ hypothesis has another verifiable consequence: evolutionary changes of lifespan are coupled to age-related dynamics of fecundity. Williams pointed out that successful selection for longevity should lead to the reduction of vitality early in life [9]. The shorter is life, the higher should be fecundity at the early stages of the life cycle compared to the later ones, and, vice versa, the longer is life, the more pronounced is the shift of reproduction to later life stages. Results consistent with this hypothesis were obtained in experiments on artificial selection [12, 13]. For example, Rose [14] compared lifespan and age-related dynamics of fecundity in laboratory lines of D. melanogaster that existed in the mode of short life cycle (reproduction at the age of 2 weeks after the egg stage) for 180 generations (~7 years), and in lines derived from them that were for 2 years subjected to selection for late reproduction (the age of imago admitted for reproduction was gradually increased from 28 to 70 days). Rose has shown that selection for late reproduction resulted in a significant increase in Drosophila lifespan, and maximal fecundity moved to a later term. If, on the contrary, selection is aimed at early reproduction (or increased mating frequency at an early age), it leads to lifespan reduction and shifts reproductive effort towards younger age (“live fast, die young” strategy) [13, 15-19]. A carefully performed evolutionary experiment has shown that high mortality rate that is caused by external reasons and does not depend on age (regular randomized elimination of some individuals in the experimental lines) leads to the same result: inheritable reduction of lifespan and reproduction shift towards earlier age [20]. This confirmed the assumption that accelerated aging (increase in “internal” mortality with age) could be an evolutionary response to high “external” mortality. Similarity between evolutionary consequences of high mortality due to external causes and selection for early reproduction can apparently be explained by the fact that high mortality that does not depend on age automatically leads to selection for early reproduction: when chances for long life are minimal, selective advantage is granted to individuals that effectively reproduce at an early age.
The inverse correlation between lifespan and early fecundity is consistent with the idea of “evolutionary trade-off”, i.e. redistribution of limited physiological resources between functions of reproduction and maintenance of viability in response to critical environmental requirements. This trade-off should result in antagonistic pleiotropy postulated by Williams: alleles increasing early fecundity are more likely to reduce viability later in life, and vice versa [9, 21-23].
Simple and logical hypotheses on the reduction of selection pressure at later stages of the life cycle and on the inevitable redistribution of physiological resources were later refined and expanded based on experimental data, in particular, with D. melanogaster. It was shown that it is important to take into account the conditions and duration of the maintenance of laboratory lines when interpreting data on lifespan changes. The correlations between lifespan and reproductive parameters are different in lines that exist in different selection modes. However, it may result not only from selection, but also from the loss of genetic diversity in the course of long laboratory maintenance and accumulation of deleterious mutations due to shift especially pronounced in small populations. Linnen et al. [24] compared lifespan in several old laboratory lines of drosophila. They have shown that coupled changes in lifespan and fecundity may partially result from the accumulation of deleterious mutations in old laboratory lines. Evolutionary patterns characteristic of natural populations may be masked by the manifestations of these mutations, which are unlikely to have been entrenched in large natural populations.
Results of these evolutionary experiments considerably strengthened the positions of the supporters of Williams’ hypothesis as well as the “classical” evolutionary aging models including the ideas on antagonistic pleiotropy and accumulation of deleterious mutations with late effect (the latter are based on Williams’ hypothesis). However, other studies have shown that the effects of exogenous mortality on lifespan evolution are not always consistent with Williams’ hypothesis [25-28]. It was shown that increase in exogenous mortality is sometimes accompanied not by reduction, but by increase in lifespan. For example, in guppy populations with high predation pressure, reproduction shifts to earlier age, which is consistent with Williams’ hypothesis, but at the same time genetically determined lifespan instead of being reduced, is even increased [26, 29]. An evolutionary experiment on the nematode Caenorhabditis remanei showed that random nonselective elimination leads (in accordance with Williams’ hypothesis) to the reduction of lifespan. However, equally intense elimination caused by a directed effect of a specific environmental factor (e.g. temperature increase) leads to the opposite result – increase in lifespan that is not accompanied by reduction in fecundity [28]. Apparently, this means that selection for resistance to the factor causing increased mortality can increase general resistance of an organism, thus slowing aging [30]. If mortality caused by an external factor is selective (depends on the state of the organism) and differences between individual organisms in the ability to resist this factor are at least partially hereditary, then the evolutionary response to high exogenous mortality will take the form of increased resistance to this factor, which may eventually lead to the increase and not reduction in lifespan. From this perspective, an opposite situation when increase in exogenous mortality leads to reduction of lifespan in accordance with Williams’ hypothesis can be interpreted as “evolutionary surrender” caused by inability of the population to resist the factor causing mortality increase.
There are not so many good examples of the effects of directed selection on lifespan and fecundity that was stated in the cited study of C. remanei [28]. To understand this phenomenon, we need evolutionary experiments on various model objects under controlled conditions. In particular, especially relevant are experiments on D. melanogaster – the object that has been exceptionally well-studied in terms of genetics and life cycle characteristics.
In this work, we analyze the results of the evolutionary experiment on the adaptation of D. melanogaster laboratory lines recently derived from a natural population, to unfavorable food substrate [31, 32]. Apparently, such an experiment better addresses the question of the relationship between exogenous mortality and lifespan evolution in nature, since adaptation of the population to unfavorable food is a realistic laboratory model imitating natural evolutionary processes caused by changes in environmental conditions. We present the changes in lifespan and age-dependent dynamics of fecundity that developed in flies during a year of adaptation to adverse food and discuss the possible reasons for these changes in the context of ideas about the mechanisms of life cycle evolution.
MATERIALS AND METHODS
Design of evolutionary experiments and experimental populations. The original D. melanogaster population was obtained in September 2014 from 30 wild individuals caught in south-west Moscow. Until January 2015, the population was cultured on favorable food medium N (see below). Then experimental lines cultured on various food substrates were isolated from this population. Flies were kept at temperature 22-25°C and natural lighting in plexiglass boxes. The food in each box was put into 12 open cylindrical glass tubes (diameter 22 mm, height 100 mm) containing 10 ml of food. Every week, four tubes with fresh food were placed in the boxes, and four tubes with old food which had been in the boxes for three weeks were removed. In addition to food tubes, each box had a drinking bowl – a small container with moistened cotton wool, which was replaced weekly. We used two lines of Drosophila, which by the beginning of the preliminary test had lived in their boxes for eight months, by the beginning of the main test – 10 months, and by the beginning of the test that involved hybrids (see below) – 12 months (the minimal time of generational turnover in the experiment was about two weeks); abbreviations: Dn – control line cultured on standard (normal) food medium (inactivated yeast 50 g, semolina 35 g, sugar 50 g, crushed raisins 45 g, agar 8 g, propionic acid 2 g per liter of food). This growth medium will be referred to as N; Ds – line cultured on a nutrient-deficient starch-based medium (inactivated yeast 50 g, starch 30 g, agar 8 g, propionic acid 2 g per liter of food). This growth medium will be referred to as S.
Testing of lifespan and age-dependent dynamics of fecundity. All the flies from the Dn and Ds populations as well as their hybrids used in tests were grown for one generation prior to testing in standard tubes with N medium covered with cotton stoppers. This was done to remove possible maternal effects and direct effects of nutrient-deficient growth medium S on the adaptive parameters [31, 33]. This procedure is commonly recommended for such experiments [1]. In a preliminary test, Dn and Ds flies were tested only on N medium, eggs were not counted, and no division into male and female was performed when counting dead flies. In the main test, Dn and Ds flies were tested on N and S media, males and females were counted separately, and once every three days we counted the number of eggs laid on fresh food during 1 h. In the hybrid test, flies were tested only on N medium, everything else being performed as in the main test. Testing was carried out as follows. Flies that emerged from pupae during three days (a number of flies sufficient for testing were accumulated during this time) were released into a box similar to those where the evolutionary experiment was carried out. The second day of the three-day interval was conventionally considered the “birth date” of the tested flies. About 100-200 flies were released into each box depending on the available number of individuals grown under standard conditions. All the tested cohorts were prepared and released into boxes simultaneously so that random fluctuations of uncontrolled environmental factors (such as atmospheric pressure, lighting, humidity) affected synchronously all the tested lines. N or S medium was placed into boxes in Petri dishes (diameter 35 mm, height 7 mm); they were replaced every three days. Food replacement was performed after dark (between 9.00 pm and midnight). One hour after fresh food had been placed into the boxes, food containers were removed to count the number of laid eggs, and after that they were put back into the boxes. Dead flies were counted daily until the death of the last fly.
To obtain F1 hybrids, we selected virgin males and females from Dn and Ds lines that had emerged from pupae in the temporarily cotton-stopper-covered tubes from the main experimental boxes. To obtain Dsn hybrids, we used 20 virgin Ds females and 15 virgin Dn males, and for Dns hybrids – 20 virgin Dn females and 15 virgin Ds males. The age of all parents was five days after emerging from the pupae. The parents were placed for five days into a box with four open tubes with N medium. Hybrid offspring that had emerged in these tubes was used for testing according to the above-described scheme. Flies obtained simultaneously under the same conditions from 20 Dn females and 15 Dn males were used as control.
Evaluation of mortality of Dn and Ds flies on N and S media in the evolutionary experiment. To assess real imago mortality in the course of the evolutionary experiment, we conducted an additional test. Dn and Ds flies prepared as for the main test were released into a box where N or S fodder was placed in tubes (not in Petri dishes as in lifespan tests). The food was replaced once a week (and not every three days). Then we counted the daily decline in the number of males and females in each box.
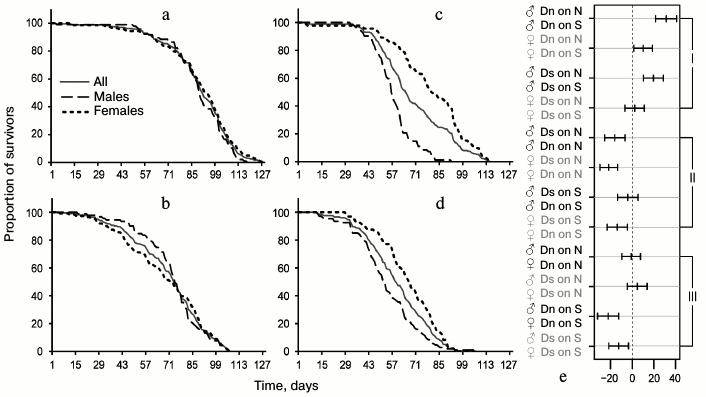
Data analysis. Data on age-related dynamics of mortality are presented as survival curves – graphs with imago age on the horizontal axis (starting from the moment of release from the pupa) and the percentage of individuals surviving by the given age on the vertical axis (Figs. 1 and 3). Lifespan data were used to calculate the average and median lifespan as well as the standard deviation, coefficient of variation (CV), and quartile coefficient of dispersion (QCD = ((Q3 – Q1)/2·Me)·100%) [34] in each experimental line for all the flies without division into sexes and separately for males and females (Tables 1 and 2).
The effects of environment, line, and gender of the flies on the lifespan were assessed using multifactorial dispersion analysis. The search for significant differences between average lifespans of flies from different lines was performed using Tukey’s test, which unlike Student’s t-test suitable only for paired comparisons, takes into account and solves the multiple comparisons problem [35].
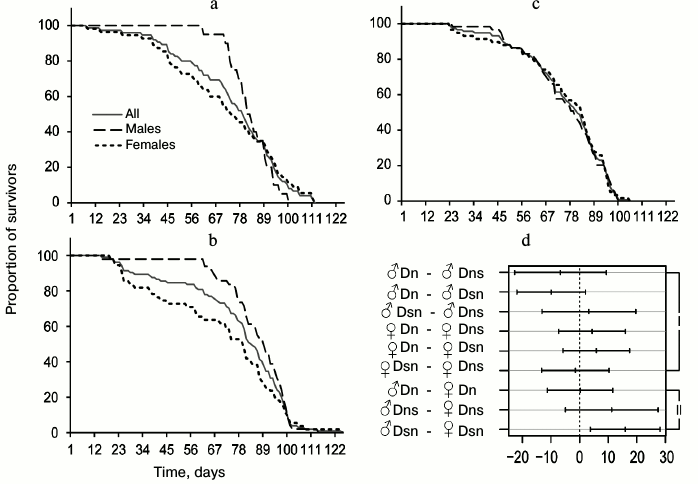
In the main test, there were 12 pairs of groups of flies suitable for meaningful pairwise comparison of average lifespan: males and females of the same line tested on the same medium (4 pairwise comparisons); males from different lines or different media (i.e. we compared Dn males tested on S and N media, Dn and Ds males tested on N medium, etc., all together 4 pairs); females with only one differing characteristic (4 pairs). The results of pairwise comparisons are presented in Fig. 1e [36]. The middle of each segment corresponds to the difference between the average lifespan values for the compared pair (lifespan of the population indicated as the second, is subtracted from the lifespan of the group indicated as the first), the length of the segment – 95% – constitutes the confidence interval. If it crosses the vertical line corresponding to zero difference, lifespan difference is not significant, and vice versa, if the entire segment fits to the right or left of the zero mark, then lifespan difference is significant. In the case of the hybrid test, 9 pairs of groups were chosen for pairwise comparison of average lifespan based on the same principle (Fig. 3d).
We used the assessment of fecundity based on the number of eggs laid during 1 h. It reflects not so much the fecundity per se, but rather the ability of flies for the rapid use of a fresh substrate to lay eggs. We assume that this ability is an important component of the adaptability of D. melanogaster in natural conditions, as this species of fruit flies is specialized in using ephemeral food substrates. In view of these explanations, in the future we will call the number of eggs laid on a fresh food medium during 1 h, “fecundity”.
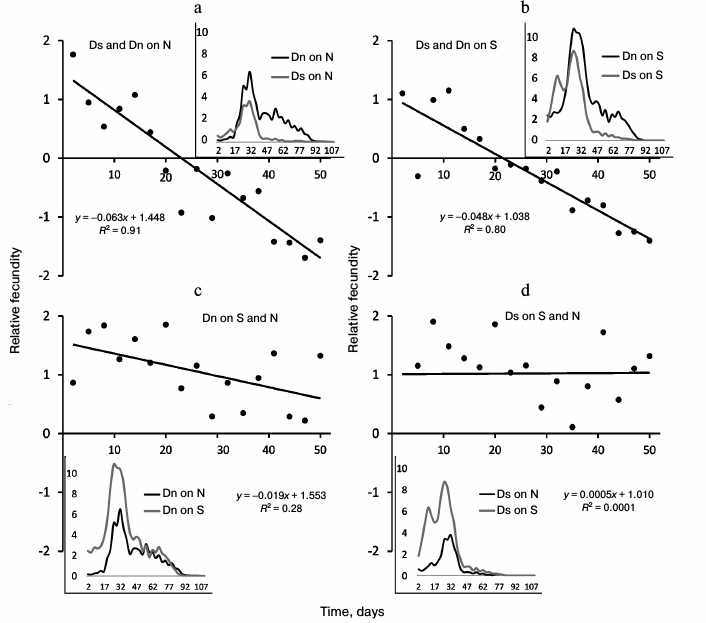
Based on the data on the number of eggs laid in the first hour after food change, we calculated the average number of eggs per female. Fecundity between different lines was compared for the first 50 days of the life of flies. For this purpose, we used the indicator of “relative fecundity” – normalized difference in the average number of eggs, which was calculated as follows: the mean number of eggs per female from the first and second lines was calculated, and then the difference between these two values was divided by their half-sum.
Values of the relative fecundity index are shown by dots in Figs. 2 and 4. The linear trend is applied for the convenience of graph interpretation. If the slope of the linear trend is significantly less than zero, it means that fecundity of the first of the two compared lines decreases with age (when compared to the second line fecundity). If the trend intersects the horizontal axis, it means that at the age corresponding to the intersection point, fecundity of two lines coincides. The slope of the trend reflects the rate of decrease of the first line fecundity compared to the second line. Insets in Figs. 2 and 4 show the dynamics of the average number of eggs per female (“absolute fecundity”) smoothed by the method of moving average. The presented data correspond to the entire period when the flies laid non-zero number of eggs during the first hour after the food change. To compare fecundity of lines, we chose the first 50 days of life because a significant number of flies remain alive during this time (including females), and the majority of eggs are laid in the first 7 weeks of life (see insets in Figs. 2 and 4). In addition, data on late fecundity may be distorted by the original cohort heterogeneity (if the most viable females, an unrepresentative sample of the general population, survive to old age).
RESULTS
A preliminary lifespan test was conducted to check whether there were no lifespan differences between the tested lines. A total of 92 Dn and 108 Ds flies were used in the test. In the course of testing, the food was first provided in tubes, which were changed every three days. However, as the flies were aging, it was becoming more and more difficult for them to fly into high tubes, which led to simultaneous death of many individuals on the days of food change Therefore, starting from the 57th experimental day, the food was provided in a Petri dish, which immediately reduced mortality. Because of these changes in the course of testing, the results are not suitable for detailed analysis. However, they show that average lifespan in Ds line flies is significantly lower than in Dn line (39.3 and 54.8 days; the differences are significant: p < 0.0001, Mann–Whitney test). This gave grounds for a more rigorous test, the results of which are presented below.
The main test. Phenotypic plasticity: direct effects of food medium on lifespan. To identify phenotypic plasticity, we need to compare the chosen indicators for flies from the same line on different food media. This comparison will show the effect of food on lifespan excluding evolutionary changes. Multifactorial dispersion analysis showed that food medium (p < 0.0001), belonging to a line (Dn or Ds) (p < 0.0001), and gender (p < 0.0001) – all affect the average lifespan. Males of both lines had shorter lifespan on S medium than on N medium (Fig. 1e and Table 1). The negative effect of S medium was somewhat more pronounced in Dn (the highest difference of all pairs) than in Ds males, although this difference did not reach the level of statistical significance. Dn females (as well as males) lived longer on N medium than on S medium. However, Ds females showed approximately the same lifespan on both media. Thus, S medium shortens lifespan of flies from both lines, but in case of Ds line adapted to S medium this negative effect is less pronounced and extends only to males, while in unadapted Dn line it is more pronounced and affects both genders.
Fig. 1. Survival curves of D. melanogaster and comparison of average lifespan in different lines: for Dn line on standard food medium N (a) and on depleted starch-based medium S (c); for Ds line of food medium N (b) and S (d). Showing curves for all individuals without division into sexes and separate curves for males and females. In (a-d) the cohort age in days is marked on the horizontal axis, and the proportion of survivors in % – on the vertical axis; e) pairwise comparisons of average lifespan based on Tukey’s test; the differences between average lifespan for compared pairs (marked by one color) are shown on the horizontal axis with 95% confidence intervals; I – comparisons reflecting phenotypic plasticity, II – evolutionary changes, III – sexual dimorphism.
Table 1. Characteristics of lifespan of Dn
and Ds flies on N and S media
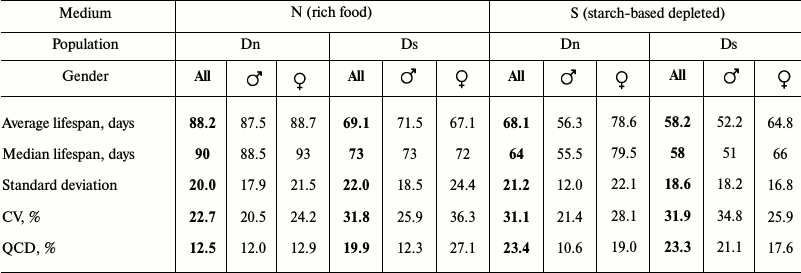
When analyzing lifespan, it is important to consider the differences between males and females as sexual dimorphism concerning this property can be quickly formed in the course of evolution; it serves as an indicator of inhomogeneous genetic basis of lifespan in males and females [28, 37]. In the case of the Dn line on N medium, we observed no sexual dimorphism on any characteristic of survival curves (they are practically identical for males and females; Fig. 1a). Culturing flies from the same line on the depleted S medium led to drastic sexual dimorphism: the lifespan of males was reduced significantly more than that of females (Fig. 1e). For the Ds line, sexual dimorphism can be observed in survival curves of flies cultured on both foods, but the character of this dimorphism differs. In the case of Ds males on N medium, early mortality is reduced and late mortality is increased compared to females; therefore, their survival curve is more convex, although average lifespan values of the two genders are very similar (Fig. 1, b and e). Difference in the degree of convexity of the survival curves is adequately reflected in the values of coefficient of variation of lifespan (CV; Table 1): 25.9% for males, 36.3% for females (the lower the CV, the more convex is the survival curve [32]). When cultured on S medium, Ds males have reduced average lifespan compared to females, and the curve becomes less convex (Fig. 1d and Table 1). In general, sexual dimorphism of the survival curves of Ds flies on S medium is of the same character as that of Dn flies on the same medium, but its absolute magnitude is smaller (Fig. 1, c and d); this difference does not reach the level of statistical significance (Fig. 1e).
Thus, lifespan reduction and enhanced sexual dimorphism of the survival curves are the direct effect of the depleted S medium. This effect is more pronounced in flies from Dn than Ds line.
Main test. Evolutionary changes in lifespan. To see the evolutionary effect of adaptation for depleted food medium, we need to compare lifespans and survival curves of Dn and Ds lines cultured on the same media (to compare Fig. 1a with Fig. 1b, and Fig. 1c – with Fig. 1d). In each of the two media, Dn flies live longer than Ds flies (the difference is 19.1 days on N medium and 9.9 days on S medium). For females this difference is statistically significant in both media, and for males it reaches the level of statistical significance only on N medium (Fig. 1e). In the case of medium S, the average lifespan of Ds males is only slightly reduced compared to Dn males (Fig. 1e and Table 1).
Thus, adaptation to the depleted medium S resulted in inheritable lifespan reduction in females and to a lesser extent in Ds males compared to the control Dn line. The direction of evolutionary changes coincided with the direction of phenotypic plasticity: lifespan reduction caused by unfavorable medium underwent genetic assimilation [38] and started to be manifested even when cultured on the favorable medium.
Main test. Differences in age-related dynamics of number of laid eggs. The age-related dynamics of fecundity is presented in Fig. 2. In all the cases, maximal individual fecundity is observed at the age of 4-5 weeks (Fig. 2, graphs on the insets), but other properties of age-related dynamics of fecundity are different in different lines and with different food media.
Fig. 2. Age-related dynamics of absolute (graphs in the boxes) and relative fecundity. a) Comparison of fecundity of Ds and Dn lines on N medium; b) comparison of the same lines on S medium; c) comparison of fecundity of Dn line on S and N media; d) comparison of fecundity of Ds line on S and N media. Age of flies (in days) starting from their release from the pupa is marked on the horizontal axis; the vertical axis shows relative fecundity, i.e. normalized difference of fecundity of two compared lines (main graphs) and absolute fecundity – average number of eggs per female (graphs in the boxes). Data on absolute fecundity are smoothed using the moving average method.
Differences in the age-related dynamics of fecundity between Dn and Ds lines are illustrated by graphs of relative fecundity (trend lines on Fig. 2, a and b). Positive relative fecundity values mean that the first of the two compared lines (Ds) is characterized by a higher fecundity than the second one (Dn); negative values indicate the reduced fecundity of the first line compared to the second one. Thus, the inclined trend lines on the Fig. 2 (a and b) indicate that Ds flies have higher fecundity at a young age than Dn flies, but their relative fecundity decreases with age. Fecundity of the two lines becomes the same around the age of 3 weeks, and later this characteristic of Dn flies more and more outpaces that of Ds flies. This pattern is clearly observed in tests both on N medium (Fig. 2a) and S medium (Fig. 2b). Thus, adaptation of Ds flies to life on depleted S medium resulted in the shift of reproductive effort to an earlier age and to accelerated reduction of fecundity with age (reproductive aging) compared to control Dn flies.
The direct effect of the food medium (N or S) is revealed when comparing fecundity of one line cultured on different media (Fig. 2, c and d). Both lines, Dn and Ds, demonstrate increased fecundity on S medium compared to N medium during the entire reproductive period. In other words, S medium stimulates accelerated egg laying on fresh food in both lines of flies compared to N medium. The difference between the lines is that age-related decrease in relative fecundity is observed in Dn line on S medium (inclined trend in Fig. 2c), while in Ds line relative fecundity on S medium does not change with age (dependence of absolute fecundity on age is the same in this line on both media, as indicated by the horizontal trend line on Fig. 2d).
Thus, in the case of non-adapted Dn flies, life on S medium stimulates accelerated laying of eggs on fresh food, especially at a young age, and facilitates reproductive aging (Fig. 2c). This is the direction of phenotypic plasticity when transiting to a depleted medium. In Ds flies, adaptation to S medium resulted in evolutionary changes of a similar direction. Their fecundity is increased at a young age not only on S, but also on N medium. Also, judging by a similar dynamics of the relative fecundity of Ds compared to Dn (similar inclinations of trend lines in Fig. 2, a and b), reproductive aging manifests itself in Ds line similarly on both media, and it is faster than in Dn line on S medium. As in the case of lifespan, the direction of evolutionary changes of age-related dynamics coincides with the direction of phenotypic plasticity: the increase in early fecundity and acceleration of reproductive aging stimulated by S medium in control Dn flies were subjected to genetic assimilation and became inheritable in Ds flies adapted to S medium. Interestingly, in the course of adaptation to S medium, Ds flies lost phenotypic plasticity of the rate of reproductive aging in response to the change in food medium: culturing of control Dn flies on S medium accelerates reproductive aging compared to N medium (inclined trend line in Fig. 2c), and in case of Ds flies, the character of age-related fecundity changes is the same for both media.
Hybrid testing. Differences in lifespan. Results of the tests on F1 hybrids (Dns and Dsn) are presented in Figs. 3 and 4 and Table 2. This test was performed only on the favorable food medium N, so it does not address the question of the effects of medium S on the studied parameters and adaptation to this medium. However, it gives an idea about the nature of inheritance of evolutionary changes identified in the main test.
Fig. 3. Survival curves of experimental lines of D. melanogaster on N medium and comparison of average lifespans. a) Survival curves of Dns hybrids (F1 hybrids obtained by crossing Dn females and Ds males); b) Dsn hybrids (F1 hybrids obtained by crossing Ds females and Dn males); c) control Dn flies; d) pairwise comparisons of average lifespans based on Tukey’s test. Symbols are as in Fig. 1; I – comparisons reflecting differences between lines, II – sexual dimorphism.
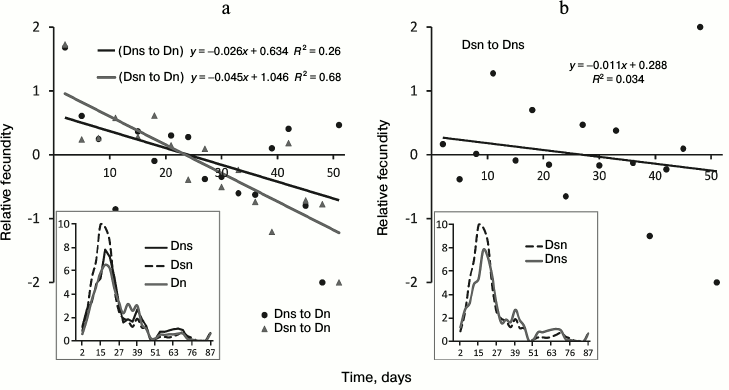
Fig. 4. Age-related dynamics of fecundity in the test involving hybrids on N medium. a) Comparison of fecundity of hybrids Dns and Dsn with fecundity of control Dn line; b) comparison of Dsn and Dns hybrids. Symbols as in Fig. 2.
Table 2. Lifespan characteristics of Dn,
Dns, and Dsn flies tested on N medium
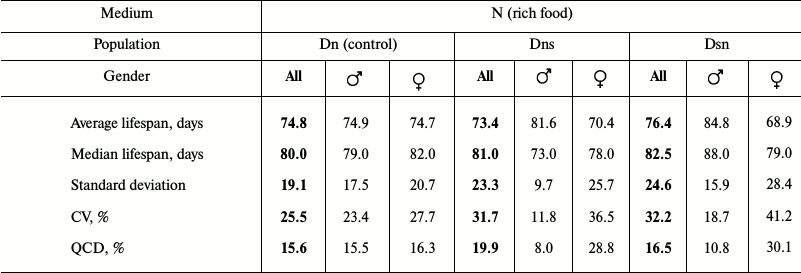
Average lifespan in all three compared cohorts was found to be approximately the same. In contrast to Ds flies that showed reduced lifespan in the main test compared to Dn, the lifespan of hybrids Dns and Dsn is not shorter than that of control Dn flies. As in the main test, Dn flies tested on N medium had no sexual dimorphism in the survival curves: these curves were practically identical in Dn males and females (Fig. 3c). In contrast, hybrids were shown to have pronounced sexual dimorphism similar to that observed in Ds flies in the main test on the N medium (Fig. 1b): early male mortality was significantly lower than female mortality, thus their survival curves were more convex (Fig. 3, a and b). CV and QCD values for hybrids are very illustrative in this respect. These values differ significantly for hybrid males and females, whereas the difference between corresponding parameters for Dn males and females is minimal (Table 2). It should be noted that only Dsn males and females significantly differ in their lifespan (Fig. 3d), although the overall shape of Dsn and Dns curves is very similar (Fig. 3, a and b). Thus, in terms of their lifespan, hybrids are similar to Dn, and in terms of sexual dimorphism in the survival curves – to Ds.
Hybrid testing. Differences in age-related dynamics of fecundity. Age-related fecundity dynamics of Dn, Dns, and Dsn flies is reflected in Fig. 4. Maximal fecundity was observed in all three lines at the age of about 2-3 weeks, i.e. earlier than in the main test. The similarity with the main test is that in both cases maximal fecundity was practically synchronous in simultaneously tested lines. Differences in the age-related fecundity dynamics between hybrids and control Dn line are illustrated by trend lines in Fig. 4a (black trend for Dns to Dn (p < 0.05) and gray trend for Dsn to Dn (p < 0.0001)). Sloping trend lines indicate that at a young age, hybrids (similar to Ms in the main test) have higher fecundity than Dn flies, but their relative fecundity decreases with age. Around three weeks of age, the fecundity of all three lines becomes the same, and later Dn flies increasingly outperform hybrids. Thus, in hybrids as well as in Ds flies, we observe a shift of reproductive effort to an earlier age and accelerated fecundity decrease with age (reproductive aging) compared to control Dn flies. The difference between the age-related fecundity dynamics of Dsn hybrids compared to Dns hybrids is not significant (p > 0.05 for the coefficient of the trend slope in Fig. 4b).
Thus, hybrids demonstrate a mixture of their parents’ properties: long lifespan as in Dn, and pronounced sexual dimorphism of survival curves, increased early fecundity, and accelerated reproductive aging as in Ds.
Analysis of mortality under conditions of the evolutionary experiment. Under the conditions of the evolutionary experiment on S medium, by the end of a weekly cycle we observe mass death of imagoes, although larvae and pupae survive. In contrast, when on N medium, most imagoes survive until the next food change. As a result, the density of flies is high in the box with Dn line, while in the box with Ds it is lower by orders of magnitude. In an additional experiment, we verified an assumption on high imago mortality on S medium, when the food is changed once a week and is served in high tubes. In the main test, life on S medium (compared to N medium) reduced the average lifespan of the control Dn flies by 23% (by 20 days; Table 1). In the additional test, when food was served as in the evolutionary experiment (in tubes, once every 7 days), life on S medium (compared to N medium) reduced the average lifespan of the control Dn flies by 52 days (by 87% or 7.7 times).
DISCUSSION
Effect of uncontrolled factors on parameters of drosophila life cycle during testing. Characteristics of the life cycle of Dn flies were tested twice – in the main test and in hybrid tests. This allowed us to assess the effect of uncontrolled conditions. In both tests, survival curves of Dn flies have a similar shape. This is confirmed by similar CV values (22.7 in the main test and 25.5 in the hybrid tests). In addition, there is no sexual dimorphism in both cases (male and female survival curves are practically the same), which is a specific feature of Dn flies on N medium. All other cohorts (Dn flies on S medium, Ds flies on both media, and hybrids on N medium) demonstrate sexual dimorphism in the shape of survival curves. The difference is that in the main test Dn flies survived on N medium, on average, almost 2 weeks longer (average lifespan in the main test was 88.2 days, and in the hybrid test – 74.8 days). This difference is probably due to uncontrolled factors affecting lifespan. These may include barometric pressure, humidity, temperature fluctuations (we did not allow it to go beyond 22-25°C, but within these limits temperature was freely fluctuating), illumination mode, time of year (the main test was carried out in winter, and the hybrid one – during spring). In addition, flies involved in the main test were adapting to their food for 10 months, whereas in the hybrid test – for 12 months. Two months might have been enough for changes to develop in Dn line. Similarities in other parameters (the shape of survival curves, absence of sexual dimorphism) apparently indicate that these parameters are relatively stable, and the above-mentioned factors hardly affect them. This is probably the case of “temporal scaling” of the survival curves [32, 39].
Thus, when comparing the results of tests performed at different times, we should apparently not pay too much attention to the differences in the average lifespan, whereas comparison of results on the shape of survival curves and sexual dimorphism may be meaningful. Results on different lines of flies tested synchronously are comparable, because they were tested under the same conditions and were subjected to the same fluctuations of uncontrolled factors.
Adaptation of D. melanogaster to depleted food medium. Ds flies apparently rather successfully adapted to the depleted food medium S in the course of the evolutionary experiment. Earlier, we used the number of adult offspring produced during a fixed time by a pair of young (0-4 days after leaving the pupa) parents on the given medium as an adaptability criterion. After the removal of the maternal effect (after living for one generation on N medium), Ds flies showed higher adaptability to S medium than Dn flies and higher adaptability to N medium even without the removal of the maternal effect [31].
New data show that fecundity of young Ds flies is increased compared to Dn flies of the same age on both foods. Obviously, high fecundity of young Ds imagoes provides a satisfactory explanation to the previously obtained results. The shift of reproductive effort to early stages of the life cycle may serve as a “broad profile adaptation” increasing reproductive success of individuals on different food substrates. In the wild, high exogenous mortality is an important condition of the efficiency of such “universal adaptation”: it reduces the chances of flies for a long life, and as a result – for reproduction at an old age.
The results show also another aspect of drosophila adaptation to depleted food medium – lifespan change. In case of Ds line, lifespan on S medium is moderately reduced compared to N medium: it is 10.9 days shorter (15.8%). In Dn flies, the negative reaction to S medium is more pronounced (20.1 days, 22.8%). Therefore, not only the efficiency of reproduction at young age is increased in Ds flies, but also resistance to unfavorable effect of S medium, which generally became less unfavorable for Ds flies than for Dn flies. Probably, the reduction of phenotypic plasticity of the rate of reproductive aging in Ds flies also supports this assumption. In this line, in contrast to Dn, S medium does not accelerate reproductive aging compared to N medium (Fig. 2, c and d). This may be the manifestation of Ds flies adaptation to unfavorable food.
Effect of high exogenous mortality on evolution of life cycle parameters. Ds line is subjected to a drastically increased exogenous mortality of imagoes in the course of the evolutionary experiment. This is evidenced by the results of the main test, when fresh food was supplied every three days, and even more so by the results of the additional test, when food was supplied once a week as in the evolutionary experiment. Lifespan of Dn flies in the main test was reduced on S medium compared to N medium by 20 days (23%), and in the additional test by 52 days (87%). Thus, transition from N to S medium results in a sharp increase in mortality of wild-type flies. Non-adapted males were less enduring than females to substrate change (lifespan reduction on S medium compared to N medium was more pronounced in males than in females).
According to Williams’ hypothesis, high exogenous mortality should lead to inheritable lifespan reduction and reproduction shift to an early age [9, 20]. Based on the assumption about the increase in the resistance of organisms affected by selective exogenous mortality [28, 30], adaptation to unfavorable conditions may lead, on the contrary, to lifespan increase. High mortality of imagoes on S medium under experimental conditions may be somewhat selective (be dependent on the organisms’ condition) and lead to directed selection, similar to the experiments on C. remanei, where high mortality was induced by heat shock [28]. Additional studies are required to assess the level of mortality selectivity.
The results are in general agreement with Williams’ hypothesis. Indeed, we observed the reduction in lifespan, increase in relative fecundity at a young age, and accelerated reproductive aging in Ds flies compared to control Dn flies in favorable conditions on N medium (Figs. 1 and 2). The results indicate that evolutionary changes in the life cycle predicted by Williams’ hypothesis may occur not only in response to artificially induced mortality or artificial selection for early (or late) reproduction, but also in the course of regular evolutionary process – adaptation of populations to adverse environment.
However, our results do not match Williams’ hypothesis completely. Williams argued that if his theory were correct, then accelerated aging and reduced lifespan under favorable conditions should be characteristic of the gender with higher exogenous mortality [9]. Our data indicate that food medium S increases mortality of males more than that of females (Fig. 1 and Table 1): average lifespan of Dn males on S medium is 31.2 days shorter than on N medium, while in case of females this difference is only 10.1 days. Therefore, according to Williams’ hypothesis, lifespan of Ds males on N medium (i.e. under favorable conditions) should be reduced compared to females. But in reality average lifespan of Ds males on N medium was 71.5 days, and that of females – 67.1 days (Table 1). Thus, lifespan of males adapted to S medium is not shorter than lifespan of females (the difference between genders is not statistically significant). This discrepancy might be explained by the fact that high exogenous mortality of Ds flies in the course of the evolutionary experiment is selective (depends on the state of the organism), and in addition to that it differently affects males and females. Such a phenomenon has been described in the literature: high mortality of C. remanei males caused the development of sexual dimorphism in lifespan [37]. In this experiment, both random and selective mortality were simulated (in the latter case only males with high reproductive activity were left). Selective elimination of males rapidly led to the increase in their lifespan, so that by the end of the experiment it became dramatically different from female lifespan, which remained unchanged. No sexual dimorphism in lifespan was found in the case of non-selective elimination. Development of sexual dimorphism is interpreted as an example of a mismatch in genetic regulation of lifespan in males and females [40, 41]. Our results support the incomplete coupling of adaptive lifespan routes in males and females, which opens the possibility for the development of sexual dimorphism in life cycles in case of directed selection.
It is believed that selection is often directed primarily on females in the course of adaptation to adverse conditions. This will be the case if under critical conditions reproductive success of females is more dependent on inherited characteristics of their physiology, behavior, and life cycle parameters, than reproductive success of males on similar male characteristics. In this case, males become “secondary material” and have to settle for alleles suboptimal for their reproductive and other vital tasks, if only those alleles significantly increase reproductive efficiency of females (conflict of genetic regulations) [38].
Our experiment confirms the formation of sexual dimorphism in the course of adaptation to adverse conditions, expanding the circle of animals demonstrating this phenomenon. For example, despite the higher male mortality, lifespan of Ds females was more reduced (compared to Dn females) than male lifespan. This result is more consistent with the assumption on a higher selectivity of male mortality (compared to females) in the course of our experiment, which resulted in the growth of male stability, than with the idea on regulatory conflict.
Thus, high and possibly partially selective exogenous mortality resulted in inheritable reduction of lifespan in both genders, reproduction shift towards an earlier age, and the development of sexual dimorphism of lifespan. The latter is presumably explained by an increased “evolutionary resistance” of males compared to females, possibly due to the more efficient selection affecting males or due to the fact that males pay a smaller physiological “price” for the increased reproductive effort at a young age [23]. This explanation is not part of the mainstream of the discussions on evolution of aging, and therefore it requires a broader empirical confirmation.
Causes of inheritable lifespan reduction in flies adapted to depleted food medium may be different. On one hand, as stated above, this result is consistent with Williams’ hypothesis and may be due to the weakening of selection at the late stages of the life cycle due to high exogenous mortality, which promotes accumulation of deleterious mutations with late effect and pleiotropic alleles increasing early fecundity at the cost of decreased viability at older age. Another possible explanation embraces accumulation of deleterious mutations due to a drift in a small population and inbreeding depression based on the transition of harmful recessive alleles into homozygous state. Indeed, the effective size of Ds population in the course of the evolutionary experiment was lower than that of Dn population (weekly Ds imago hatching was 2-4 times lower than Dn, and the number of live imagoes due to high mortality was 1-2 times lower). In the middle of the previous century, it was shown that lifespan was reduced in highly inbred drosophila lines [42]. However, the idea of the dependence of laboratory drosophila lifespan on the degree of inbreeding found no further evidence. For example, Linnen et al. [24] assessed lifespan in several old laboratory lines, including those subjected to selection for early or late reproduction. They concluded that the effect of inbreeding on lifespan reduction was insignificant compared to the effect of selection for early reproduction. If lifespan reduction is due to inbreeding depression or accumulation of recessive alleles with late adverse effect, it should be expected that hybrids obtained by crossing of different lines will demonstrate increased lifespan due to increased heterozygosity (“hybrid vigor” effect). However, it was shown that this effect neither slows aging nor increases the lifespan of flies that have developed a shorter life cycle due to selection for early reproduction. According to the interpretation proposed in the article, this result does not confirm the role of passive accumulation of deleterious mutations with late effect in evolution of aging, but it is consistent with the idea of antagonistic pleiotropy [43].
In our experiment, Dsn and Dns hybrids demonstrated as high lifespan as simultaneously tested control Dn flies. At the same time, in the main experiment Ds flies were characterized by a shorter lifespan compared to Dn flies. This result is consistent with the assumption of “hybrid vigor” and accumulation of recessive alleles that reduce lifespan. On the other hand, it also does not contradict antagonistic pleiotropy assuming that the effects of pleiotropic alleles that negatively affect survival at an old age are recessive.
It should be noted that hybrids’ fecundity parameters, in contrast to lifespan, were closer to those of Ds than Dn. Hybrids, similarly to Ds, are characterized by increased early fecundity compared to Dn and accelerated reproductive aging (sloping trend lines in Figs. 2a, 2b, and 4a). This result can hardly be explained by the removal of inbreeding depression and “hybrid vigor”. Apparently, it indicates the dominance of alleles responsible for the changes in age-related dynamics of fecundity in the Ds line (or dominance of those effects of pleiotropic alleles that affect fecundity combined with recessiveness of their effects on lifespan). In addition, this result confirms that the connection between high early fecundity and short lifespan, postulated by Williams’ hypothesis, is not unbreakable (see below).
Sexual dimorphism of survival curves is another property in relation to which hybrids were closer to Ds than to Dn. Control Dn flies on N medium have no dimorphism, while in Dsn and Dns hybrids it is even more pronounced than in Ds (Fig. 3). This feature of hybrids is also difficult to explain by the removal of inbreeding depression. In this context, it seems interesting to recall that lifespan differences in male and female Drosophila have an impressive genetic base: lifespan genetic determinants are very different in the two genders [40, 41]. Apparently, genetic changes with different effects on male and female lifespan were selected in Ds flies in the course of their adaptation to depleted food medium. As a result, sexual dimorphism became inheritable, and even hybrid offspring kept it. Another feature observed in hybrids is also consistent with this: enhancement of sexual dimorphism of survival curves (compared to Ds) due to the fact that lifespan is increased and early mortality is reduced in hybrid males compared to females. One possible explanation is that lifespan reduction in Ds males is more related to the passive accumulation of recessive deleterious mutations with late effect (and therefore lifespan of hybrid males increases dramatically due to “hybrid vigor”), while lifespan reduction in Ds females is mainly explained by antagonistic allele pleiotropy increasing early fecundity at the cost of viability decrease in old age. Such an assumption seems logical, given that selection for the increase in reproductive effort at an early age under the conditions of high exogenous mortality should have a more pronounced effect on females than on males, and its price may be higher for them [23].
Is lifespan reduction an unavoidable price paid for high fecundity at early age? Adaptation of flies to depleted food medium led to increase in the intensity of reproduction at early age coupled with growth of “internal” mortality and reduced reproduction later in life. This is consistent with the results of previous experiments [12-14, 20] traditionally explained by antagonistic pleiotropy [9] based on evolutionary trade-off between survival and reproduction functions. It is assumed that increased mortality in old age is the price paid for the increased intensity of early reproduction. Experiments on female drosophila from long- and short-lived lines artificially deprived of the possibility to breed confirm this assumption [23].
However, our results demonstrate that such a price is not required, and that these two effects may be separated. In case of hybrids, life as long as that of Dn flies is combined with increased early fecundity similar to that of Ds flies. A similar situation is observed in Trinidad guppies living in conditions of severe predation: their reproduction is shifted to early age, but genetically determined lifespan instead of being reduced, even increases [26, 29]. Discordance between long lifespan and reduced fecundity was registered in experiments on nematodes [44] and mice [45]. This possibly means that lifespan and age-related dynamics of fecundity are determined by different (although partially overlapping) genetic complexes [46], which contradicts the model of antagonistic pleiotropy. An alternative explanation of the results of our experiment is that antagonistic pleiotropy does exist, but those effects of pleiotropic alleles that contribute to the increase in early fecundity are dominant (and therefore are manifested in hybrids), whereas the effects of the same alleles contributing to the reduced viability at older age are recessive (and therefore are not manifested in hybrids). Our results, especially those obtained on males, are also consistent with the model of aging evolution by passive (due to drift) accumulation of deleterious mutations with late effect. Since the effects of such mutations are often recessive, lifespan of hybrids is expected to be increased compared to the ancestral line (Ds line in our case). At the same time, as noted above, available data suggest a more important role of antagonistic pleiotropy in lifespan evolution in females, and passive accumulation of mutations with late effect – in males.
Connection between phenotypic plasticity and direction of evolutionary changes. All the indicators of adaptation of Ds flies (increased efficiency of reproduction at early age, increased stability of life cycle parameters on food change) as well as lifespan reduction and sexual dimorphism of the survival curves are apparently interrelated and reflect the complex changes of the life cycle when changing to unfavorable food substrate. These changes may be both inheritable and non-inheritable (modificational). To differentiate these two options and so to compare modificational changes with evolutionary ones, we tested the flies from both lines on favorable N medium and on stressful S medium in the main experiment. The character of inheritance of evolutionary changes that developed in Ds line in the course of adaptation is partially clarified by the results of the test with F1 hybrids.
Our results (as well as the results of other experiments consistent with Williams’ hypothesis) confirm that lifespan reduction caused by adverse environmental conditions may eventually become inheritable: the property which was originally caused by the environmental conditions becomes genetically determined, i.e. “genetic assimilation” takes place [38, 47]. The evolutionary role of phenotypic plasticity, both adaptive (useful) and non-adaptive, is discussed in the literature [48, 49]. It was shown that non-adaptive phenotypic plasticity may contribute to evolutionary changes, the direction of which is opposite to that of plastic changes [50]. For example, the development of many poikilotherms is slowed in response to temperature decrease. This feature does not promote successful adaptation to high latitudes, where, on the contrary, rapid development is advantageous due to a short summer. In such a situation, non-adaptive phenotypic plasticity will contribute to enhanced selection for accelerated development. This leads to the so-called “genetic compensation”, when non-adaptive plastic (non-inherited) changes are compensated by adaptive inherited ones [51]. It is also assumed that non-adaptive phenotypic plasticity and maternal effects may inhibit divergence, contributing to the replacement of locally adapted individuals by migrants from favorable habitats [49]. Empirical confirmation of this idea was obtained in our evolutionary experiment [31].
Life on S medium stimulates flies to more intensive oviposition. We can assume that a diet high in starch affects fecundity via increased activity of a signaling cascade involving insulin-like peptides (ILP). This cascade is also known to affect lifespan [52, 53]. This effect is especially pronounced in young Dn flies that are not adapted to S medium (Fig. 2c). In other words, starch-based food increases early fecundity, which is clearly an adaptive property increasing reproductive success on unfavorable food substrate S. In case of Ds flies, this change became hereditary in the course of adaptation to S medium; directions of evolutionary and plastic changes coincided (Fig. 2a). This is a vivid example of genetic assimilation of an adaptive phenotypic change caused by the environment. Lifespan reduction directly caused by adverse food substrate can be interpreted as non-adaptive phenotypic plasticity. In this case, inherited lifespan reduction in Ds flies is an example of genetic assimilation of the non-adaptive plastic change. However, the statement on non-adaptive character of lifespan reduction on a depleted food medium requires additional discussion. On one hand, D. melanogaster flies are known to reproduce until death, producing viable offspring even at a terminal age [54]. Therefore, early death, all other parameters being equal, reduces individual reproductive success. On the other hand, we cannot rule out the possibility that early death of imagoes on a depleted food substance is beneficial for the general population, for example, if the scarce food resources unused by dead imagoes are more effectively utilized by larvae. However, early death as an “adaptation beneficial for the group” cannot be supported by a regular selection at an individual level. Theoretically, it might be supported by a group selection (but it does not work within a single experimental line), kin selection [5], or “second-order selection for evolutionary perspective” [6], but these forms of selection too can hardly be active under the experimental conditions since the resources released due to imago death are not in exclusive possession of its offspring, but become equally available for the offspring of all the flies in the population. It appears that under experimental conditions only conventional individual selection is effective, i.e. only individual adaptive benefit is relevant, and from this perspective lifespan reduction is a non-adaptive phenotype change. Nevertheless, after a number of generations there develops genetic assimilation of this change, which is originally caused by the direct environmental effects, but later becomes hereditary. Thus, in this case the direction of evolution coincides with the direction of non-adaptive change caused by the environment. This situation is not trivial, because it is believed that genetic assimilation of modificational changes is due to the selection for more stable realization of the adaptive phenotype, and it means that only adaptive plastic changes can be subjected to genetic assimilation [38, 47]. This situation is most easily explained in terms of Williams’ hypothesis, since this non-adaptive plastic change (lifespan reduction due to deterioration of living conditions) takes the late stages of the life cycle out of the pressure of selection; disappearance of these late stages is the essence of the plastic change that later becomes hereditary. Mutations cause atrophy of the late stages, similar to the fate of unused organs.
Thus, consistent with Williams’ hypothesis, cases of evolutionary lifespan reduction due to increase in exogenous mortality broaden our understanding of the evolutionary role of phenotypic plasticity and possible correlations between the direction of plastic and evolutionary changes. Evolutionary changes of age-related dynamics of male mortality in the course of adaptation to food medium S apparently should be interpreted as the result of a combination of two processes. On one hand, average lifespan reduction of Ds males on N medium compared to Dn males indicates genetic assimilation of the non-adaptive plastic change. On the other hand, change in the shape of the survival curve (reduced early mortality of Ds males on N medium compared to females), as well as a lesser negative effect of S medium on Ds male lifespan compared to Dn males, indicate that partial genetic compensation was also involved in this case.
In the course of adaptation to a depleted food medium, flies from Ds line underwent complex changes in life cycle parameters such as average lifespan and age-related dynamics of fecundity and mortality. Increased imago mortality caused by life on unfavorable food was apparently the main factor that determined the character of selection. According to Williams’ hypothesis which became the basis for the currently dominant ideas on the mechanisms of evolution of the above-mentioned parameters, mortality increase leads to weakening of selection against mutations reducing fitness in old age, and as a result – to reproduction shift to earlier age. Indeed, inheritable lifespan reduction and increase in relative fecundity at young age combined with accelerated reproductive aging were registered in Ds line compared to control Dn line. An alternative explanation based on the assumption that under the experimental conditions, imago lifespan reduction was beneficial for the general population, seems unlikely, since under the conditions of this experiment the forms of selection capable of supporting such an “adaptation beneficial for the group” apparently could not function.
Unlike previous experiments with similar results, in our experiment we used neither artificial selection for reproduction at a given age, nor artificially induced mortality. Our results complement the available data on lifespan evolution indicating that predicted by Williams’ evolutionary response to increased mortality can be observed within the frame of a normal natural process – adaptation of a population to a hostile environment.
It is true though that not all our results are consistent with Williams’ hypothesis. The prediction that it is the gender with higher mortality in the natural conditions that will have shorter lifespan under favorable conditions, was not confirmed. Life on S medium reduced the lifespan of non-adapted males more than that of females, i.e. it causes higher male mortality. However, in the course of adaptation, lifespan of Ds males was reduced even a little less (and not more) than that of females. In addition, Ds males were characterized by a reduced early mortality compared to females, which led to the development of sexual dimorphism in the shape of survival curves (which is absent in control Dn flies).
Analysis of survival curves and fecundity dynamics of F1 hybrids obtained by crossing Dn and Ds flies has shown that hybrids demonstrate not the state intermediate in relation to parental lines, but a mosaic of traits of parental lines. In terms of average lifespan, hybrids show no difference from the simultaneously tested Dn flies; increased early fecundity and accelerated reproductive aging bring them closer to Ds flies, whereas sexual dimorphism of survival curves is even more pronounced in hybrids than in Ds flies (hybrid males live longer than females).
Our results are consistent with data on different genetic basis of male and female lifespan, which may lead to the development of sexual dimorphism in lifespan in evolving populations. Furthermore, they are consistent with the idea that the character of an evolutionary response to increased mortality depends on the level of selectivity of this mortality and the presence of inherited variability in the ability to resist factors causing increased mortality in the population. Predictions of Williams’ hypothesis should be fulfilled in case of non-selective mortality, but selective mortality may lead to the opposite results, contributing to the development of resistance and lifespan increase.
Antagonistic pleiotropy of alleles increasing early fecundity at the cost of accelerated reduction of viability and fecundity with age could play an important role in the evolutionary changes in the female life cycle. At the same time, changes registered in males may have been largely due to the accumulation of deleterious recessive mutations with late effect. This allows explanation why lifespan increase in hybrid males compared to Ds males was more pronounced than that of hybrid females compared to Ds females.
The direction of evolutionary changes in Ds line (lifespan reduction, increase in early fecundity, development of sexual dimorphism in lifespan) generally coincides with the direction of modificational changes caused by the transition of naïve Dn flies to depleted starch-based medium. Thus, genetic assimilation of modificational changes took place in Ds line: changes originally caused by adverse environment became hereditary, and they were now reproduced in the adapted Ds line even on favorable N medium. Thus, our results broaden understanding of the mechanisms of genetic assimilation showing that in some cases not only adaptive, but also deleterious modificational changes (such as lifespan reduction caused by adverse environmental conditions) can be subjected to genetic assimilation.
Acknowledgements
This work was financially supported by the program “Scientific Schools” (project NSh-9303.2016.5).
REFERENCES
1.Kawecki, T. J., Lenski, R. E., Ebert, D., Hollis,
B., Olivieri, I., and Whitlock, M. C. (2012) Experimental evolution,
Trends Ecol. Evol., 27, 547-560.
2.Skulachev, V. P. (1999) Phenoptosis: programmed
death of an organism, Biochemistry (Moscow), 64,
1418-1426.
3.Weismann, A. (1889) Essays upon Heredity and
Kindred Biological Problems, Clarendon, Oxford.
4.Longo, V. D., Mitteldorf, J., and Skulachev, V. P.
(2005) Programmed and altruistic ageing, Nat. Rev. Genet.,
11, 866-872.
5.Markov, A. V. (2012) Can kin selection facilitate
the evolution of the genetic program of senescence? Biochemistry
(Moscow), 77, 733-741.
6.Woods, R. J., Barrick, J. E., Cooper, T. F.,
Shrestha, U., Kauth, M. R., and Lenski, R. E. (2011) Second-order
selection for evolvability in a large Escherichia coli
population, Science, 331, 1433-1436.
7.Hamilton, W. D. (1966) The moulding of senescence
by natural selection, J. Theor. Biol., 12, 12-45.
8.Rose, M. (1991) Evolutionary Biology of
Aging, Oxford University Press, Oxford.
9.Williams, G. C. (1957) Pleiotropy, natural
selection, and the evolution of senescence, Evolution,
11, 398-411.
10.Medawar, P. B. (1952) An Unsolved Problem of
Biology, HK Lewis, London.
11.Haldane, J. B. S. (1941) New Paths in
Genetics, Allen and Unwin, London, UK.
12.Wattiaux, J. M. (1968) Cumulative parental age
effects in Drosophila subobscura, Evolution, 22,
406-421.
13.Rose, M. R., and Charlesworth, B. (1981) Genetics
of life history in Drosophila melanogaster. II. Exploratory
selection experiments, Genetics, 97, 187-196.
14.Rose, M. R. (1984) Laboratory evolution of
postponed senescence in Drosophila melanogaster,
Evolution, 38, 1004-1010.
15.Luckinbill, L. S., Arking, R., Clare, M. J.,
Cirocco, W. C., and Buck, S. A. (1984) Selection for delayed senescence
in Drosophila melanogaster, Evolution, 38,
996-1003.
16.Mueller, L. D. (1987) Evolution of accelerated
senescence in laboratory populations of Drosophila, Proc.
Natl. Acad. Sci. USA, 84, 1974-1977.
17.Partridge, L., and Fowler, K. (1992) Direct and
correlated responses to selection on age at reproduction in
Drosophila melanogaster, Evolution, 46, 76-91.
18.Partridge, L., Prowse, N., and Pignatelli, P.
(1999) Another set of responses and correlated responses to selection
on age at reproduction in Drosophila melanogaster, Proc. R
Soc. Lond. B Biol. Sci., 266, 255-261.
19.Travers, L. M., Garcia-Gonzalez, F., and Simmons,
L. W. (2015) Live fast die young life history in females: evolutionary
trade-off between early life mating and lifespan in female
Drosophila melanogaster, Sci. Rep., 5, 15469.
20.Stearns, S. C., Ackermann, M., Doebeli, M., and
Kaiser, M. (2000) Experimental evolution of aging, growth, and
reproduction in fruitflies, Proc. Natl. Acad. Sci. USA,
97, 3309-3313.
21.Kirkwood, T. B. (1977) Evolution of ageing,
Nature, 270, 301-304.
22.Kirkwood, T. B., and Rose, M. R. (1991) Evolution
of senescence: late survival sacrificed for reproduction, Philos.
Trans. R Soc. Lond. Ser. B Biol. Sci., 332, 15-24.
23.Sgro, C. M., and Partridge, L. (1999) A delayed
wave of death from reproduction in Drosophila, Science,
286, 2521-2524.
24.Linnen, C., Tatar, M., and Promislow, D. (2001)
Cultural artifacts: a comparison of senescence in natural,
laboratory-adapted and artificially selected lines of Drosophila
melanogaster, Evol. Ecol. Res., 3, 877-888.
25.Abrams, P. A. (1993) Does increased mortality
favor the evolution of more rapid senescence? Evolution,
47, 877-887.
26.Reznick, D. N., Bryant, M. J., Roff, D.,
Ghalambor, C. K., and Ghalambor, D. E. (2004) Effect of extrinsic
mortality on the evolution of senescence in guppies, Nature,
431, 1095-1099.
27.Williams, P. D., Day, T., Fletcher, Q., and Rowe,
L. (2006) The shaping of senescence in the wild, Trends Ecol.
Evol., 21, 458-463.
28.Chen, H., and Maklakov, A. A. (2012) Longer life
span evolves under high rates of condition-dependent mortality,
Curr. Biol., 22, 2140-2143.
29.Reznick, D. N., Shaw, F. H., Rodd, F. H., and
Shaw, R. G. (1997) Evaluation of the rate of evolution in natural
populations of guppies (Poecilia reticulata), Science,
275, 1934-1937.
30.Williams, P. D., and Day, T. (2003) Antagonistic
pleiotropy, mortality source interactions, and the evolutionary theory
of senescence, Evolution, 57, 1478-1488.
31.Markov, A. V., Ivnitsky, S. B., Kornilova, M. B.,
Naimark, E. B., Shirokova, N. G., and Perfilieva, K. S. (2015) Maternal
effect obscures adaptation to adverse environments and hinders
divergence in Drosophila melanogaster, Zh. Obshch. Biol.,
76, 429-437.
32.Markov, A. V., Naimark, E. B., and Yakovleva, E.
U. (2016) Temporal scaling of age-dependent mortality: dynamics of
aging in Caenorhabditis elegans is easy to speed up or
slow down, but its overall trajectory is stable (on the paper by
Stroustrup et al. entitled “The temporal scaling of
Caenorhabditis elegans ageing” published in
Nature, 530, 103-107 (2016)), Biochemistry
(Moscow), 81, 1145-1152.
33.Mousseau, T. A., Uller, T., Wapstra, E., and
Badyaev, A. V. (2009) Evolution of maternal effects: past and present,
Philos. Trans. R Soc. B, 364, 1035-1038.
34.Francis, A. (2008) Business Mathematics and
Statistics, 6th Edn., Cengage Learning EMEA, UK.
35.Tukey, J. (1949) Comparing individual means in
the analysis of variance, Biometrics, 5, 99-114.
36.R Core Team (2015) R: A Language and
Environment for Statistical Computing, R Foundation for Statistical
Computing, Vienna, Austria.
37.Chen, H., and Maklakov, A. A. (2014) Condition
dependence of male mortality drives the evolution of sex differences in
longevity, Curr. Biol., 24, 2423-2427.
38.Waddington, C. H. (1953) Genetic assimilation of
acquired characters, Evolution, 7, 118-126.
39.Stroustrup, N., Anthony, W. E., Nash, Z. M.,
Gowda, V., Gomez, A., Lopez-Moyado, I. F., Apfeld, J., and Fontana, W.
(2016) The temporal scaling of Caenorhabditis elegans ageing,
Nature, 530, 103-107.
40.Nuzhdin, S. V., Pasyukova, E. G., Dilda, C. L.,
Zeng, Z. B., and Mackay, T. F. (1997) Sex-specific quantitative trait
loci affecting longevity in Drosophila melanogaster, Proc.
Natl. Acad. Sci. USA, 94, 9734-9739.
41.Lehtovaara, A., Schielzeth, H., Flis, I., and
Friberg, U. (2013) Heritability of life span is largely sex limited in
Drosophila, Am. Nat., 182, 653-665.
42.Clarke, J. M., and Smith, M. J. (1955) The
genetics and cytology of Drosophila subobscura, XI. Hybrid vigor
and longevity, J. Genet., 53, 172-180.
43.Rose, M. R., Drapeau, M. D., Yazdi. P. G., Shah,
K. H., Moise, D. B., Thakar, R. R., Rauser, C. L., and Mueller, L. D.
(2002) Evolution of late-life mortality in Drosophila
melanogaster, Evolution, 56, 1982-1991.
44.Gems, D., Sutton, A. J., Sundermeyer, M. L.,
Albert, P. S., King, K. V., Edgley, M. L., Larsen, P. L., and Riddle,
D. L. (1998) Two pleiotropic classes of daf-2 mutation affect larval
arrest, adult behavior, reproduction and longevity in Caenorhabditis
elegans, Genetics, 150, 129-155.
45.Holzenberger, M., Dupont, J., Ducos, B., Leneuve,
P., Geloen, A., Even, P. C., Cervera, P., and Le Bouc, Y. (2003) IGF-1
receptor regulates lifespan and resistance to oxidative stress in mice,
Nature, 421, 182-187.
46.Toivonen, J. M., and Partridge, L. (2009)
Endocrine regulation of ageing and reproduction in Drosophila,
Mol. Cell. Endocrinol., 299, 39-50.
47.Crispo, E. (2007) The Baldwin effect and genetic
assimilation: revisiting two mechanisms of evolutionary change mediated
by phenotypic plasticity, Evolution, 61, 2469-2479.
48.Iordanskij, N. N. (2009) Phenotypic plasticity
and evolution of organisms, Zh. Obshch. Biol., 70,
3-9.
49.Fitzpatrick, B. M. (2012) Underappreciated
consequences of phenotypic plasticity for ecological speciation,
Int. J. Ecol., http://dx.doi.org/10.1155/2012/256017.
50.Ghalambor, C. K., Hoke, K. L., Ruell, E. W.,
Fischer, E. K., Reznick, D. N., and Hughes, K. A. (2015) Non-adaptive
plasticity potentiates rapid adaptive evolution of gene expression in
nature, Nature, 525, 372-375.
51.Grether, G. F. (2005) Environmental change,
phenotypic plasticity, and genetic compensation, Am. Nat.,
166, 115-123.
52.Partridge, L., Alic, N., Bjedov, I., and Piper,
M. D. W. (2011) Ageing in Drosophila: The role of the
insulin/Igf and TOR signaling network, Exp. Gerontol.,
46, 376-381.
53.Pasco, M. Y., and Leopold, P. (2012) High
sugar-induced insulin resistance in Drosophila relies on the
lipocalin Neural Lazarillo, PLoS One, 7, e36583.
54.Rauser, C. L., Tierney, J. J., Gunion, S. M.,
Covarrubias, G. M., Mueller, L. D., and Rose, M. R. (2006) Evolution of
late-life fecundity in Drosophila melanogaster, J. Evol.
Biol., 19, 289-301.