Effect of Alpha-Fetoprotein on Lifespan of Old Mice
V. N. Krut’ko1,2, V. I. Dontsov1*, and A. V. Khalyavkin1
1Institute for Systems Analysis, Federal Research Center “Computer Science and Control”, Russian Academy of Sciences, 117312 Moscow, Russia; E-mail: dontsovvi@mail.ru2Sechenov First Moscow State Medical University, 119991 Moscow, Russia; E-mail: krutkovn@mail.ru
* To whom correspondence should be addressed.
Received May 22, 2016; Revision received July 22, 2016
Alpha-fetoprotein (AFP) is one of the best-known embryo-specific proteins. It is used to diagnose fetal abnormalities and tumors of the gastrointestinal tract and liver. AFP has pronounced immunotropic and detoxifying effect and a direct apoptotic effect on tumor cells. The treatment of mice at the oldest age in our experiments with AFP dramatically increased the survival and markedly increased the relative weight of immunotropic organs, apparently due to the general effect of AFP in improving functions of tissues and detoxifying actions. It also improved appearance and the relative weight of internal organs with a reduced age of autoaggression.
KEY WORDS: age, alpha-fetoprotein, life extensionDOI: 10.1134/S0006297916120087
Alpha-fetoprotein (AFP) was discovered in 1956 as an additional fraction during electrophoresis of albumin in fetal serum. It was identified immunochemically in 1961 as a normal fetal serum antigen. In practice, AFP is primarily known as an oncomarker for tumors of the liver and gastrointestinal tract, but its antitumor effects have also been shown, including stimulation of tumor cell apoptosis [1-3] and pronounced immunotropic effects [4-6]. The second important effect of AFP is the prevention of mother–fetus autoimmune conflict [7, 8], probably by suppressing the expression of antigens of the second class of the main histocompatibility complex, which prevents antigen presentation by macrophages at the earliest stages of the formation of the immune response. In adults, AFP was found in follicular fluid and liver tissue. Liver regeneration is accompanied by temporal and malignant growth and by constant AFP synthesis in liver; p53 protein is involved in this process as a repressor.
Attempts to activate functions of an old organism by introduction of embryonic extracts date back to the beginning of the previous century. They were also used to stimulate various repair processes. Currently, the use of embryonic tissues is a separate rapidly developing scientific and practical area of research.
We studied bioactivating effects of AFP in old animals, keeping in mind the immunoregulatory theory of aging [9] and the presence of geropreventive properties in immunotropic drugs, as well as data on the possible geroprotective properties of AFP [10] and its proven immuno-regulatory activity. It is also known that rat sexual activity increased 30 min after administration of 1 µg/kg AFP. AFP increased cerebral flow and had pronounced anti-hypoxic activity [11].
The purpose of the experiments was to study the general bioactivating effect of AFP and its impact on the lifespan of old mice.
MATERIALS AND METHODS
In the experiment, we used 27 old mice of the BALB/c line, females aged 18 months from the “Stolbovaya” kennel. In the course of the experiments, the mice were kept in vivarium under standard conditions. The drug “Alpha-protein” produced by JSC “Institute of New Medical Technologies” was used as the AFP source. AFP was administered to 12 experimental animals for 2 weeks, in the morning, intraperitoneally, in 0.5 ml saline, 10 µg per kg of body weight. In 15 control animals, the same saline volume was administered without the drug.
In the course of the experiments, we evaluated some parameters of aging as well as survival of animals in the control and experimental groups. We evaluated the appearance of animals based on the state of their hair – its color and gloss, baldness, and the severity of age-hump. The physical state of the animals was evaluated by the time during which the mice could stay on a string; we took into account the weight of the animals and the relative mass of internal organs (mg of organ weight per gram of animal body weight).
After the end of the experiments, the remaining animals were sacrificed and a number of parameters important for assessing the level of aging of the animals were measured. To assess immune status, the following parameters were taken into account: relative weight of the immune organs (spleen and thymus) and degree of age-related autoaggression based on the level of autoimmune complexes after the addition of polyethylene glycol (PEG-6000) to blood serum expressed in units of optical density under spectrophotometric study. The content of intracellular water in tissues was evaluated based on the extent of weight loss (for kidneys) in hypertonic solution (40% sucrose) after 4 h of incubation.
We graphed animal survival during the studied period (percentage of the original number) and evaluated the mean values of the test parameters and standard deviations for control and experimental values; differences were compared by Student’s test, which characterizes the normal distribution of data, and the Mann–Whitney U criterion, which characterizes nonparametric distribution.
RESULTS AND DISCUSSION
In the experiments, we used a group of mice at late stages of aging, which was reflected in the mortality level in the population: half of the mice in the vivarium had died during the previous 2 months.
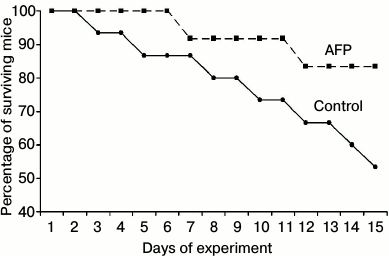
During the experiment, the natural death of animals in the control group was 47%, while AFP administration reduced mortality to 17% in the experimental group (see figure).
Effect of alpha-fetoprotein on survival curves of old mice
We studied the general state of the surviving animals (8 in the control and 10 in the experiment), then the animals were sacrificed, and a number of parameters were examined following the procedure described in the “Materials and Methods” section.
A clear trend towards improvement of the appearance was observed in all the animals treated with AFP: hair loss and bald areas were reduced, hair glistened, and it lost yellowness and patchiness characteristic of old age. Appearance evaluated according to these characteristics was clearly better for all the animals of the experimental group.
Physical activity of animals: the time of retention on a string (measured in seconds) changed from 57.3 ± 54.2 in the control to 77.3 ± 48.9 in the experiment. These differences were significant when processed according to Student’s test (p < 0.05), but not significant for the non-parametric Mann–Whitney parameter. Large variation of the parameter is due to the fact that many animals do not hold on to the string, but immediately jump off the string, and that is why the upper limit and the general distribution of results are more important. In this observation, experimental parameters were higher than control in all cases but one.
Improvement of the physiological state of tissues was also determined based on the increase of intracellular water, which is known to reflect the general condition of cells. Content of intracellular water markedly decreases with age: reduction of organ weight (%) due to the migration of water into hypertonic medium increased from 24.34 ± 1.35 to 25.03 ± 1.44 (p < 0.01).
Statistically significant increase (p < 0.05) was observed for the parameters of relative weight of inner organs (heart) and immunity organs (thymus and spleen). Improvement of immune function was registered also based on the reduction of the number of autoantibodies measured by optical density of the solution in the presence of PEG-6000 – this parameter decreased from 0.163 ± 0.030 to 0.136 ± 0.033 (p < 0.001).
At the same time, relative weight of liver and kidneys were decreased in the experimental group compared to the control (these changes were statistically significant). This can be explained based on the hypothesis on regulating and detoxifying effects of AFP: improvement of the functions of tissues of an aging organism reduces the load of detoxifying animal organs, liver, and kidneys; reduction of age-related autoaggression towards tissues estimated by the number of autoimmune complexes in blood also supports this effect.
Drastic increase in the survival of mice in the experimental group seems to be the most interesting. According to the data presented in the literature, AFP increases survival in various diseases, in tumor processes, chemotherapy, and radiation exposure due to the general stimulating and regulatory effects of AFP as well as favorable immunotropic effects [4-6, 10-12].
In vitro geroprotective effects of AFP are also known: AFP was shown to be an activator of anaerobic catabolism with cytoprotective effect and antioxidant action in biosamples; AFP also reduced cardiac biological age in older patients, beneficially affecting their cardiovascular system [10].
Alpha-fetoprotein affects specific immunity [4-6], suppressing the formation of antibodies and cytotoxic T-lymphocytes to T-dependent antigens, but it has no effect on mature T- and B-lymphocytes, affecting primarily proliferating T-helpers. In mixed lymphocyte culture, AFP can activate T-suppressors inhibiting mitogenesis. AFP suppresses TNF-α and IL-1β synthesis by affecting prostaglandins from group E and reduces the activity of natural killers.
Biological properties of AFP also involve other physiological processes: it regulates homeostasis in normal and pathological states by binding steroids and retinoids, fatty acids, flavonoids, bilirubin, and a number of drugs and toxins. AFP affects the provision of cells with energy and plastic material, enhances the expression of cell receptors, and affects synthesis of simple regulatory molecules – prostaglandins, leukotrienes, and thromboxanes.
The observed general biological and immunotropic effects of AFP may underlie its general bioactivating action and the increase in survival of mice, which is particularly evident for the oldest ages when mortality is high. Detoxifying, general regulatory, and general trophic properties are especially interesting in this regard since they counteract such well-known aging mechanisms as age-related intoxication, dystrophy, and dysregulation. Immunotropic activity of AFP is an important property: it means that AFP can, on one hand, increase immune response and antitumor activity, counteracting age-related immunodeficiency, and, on the other hand, counteract age-related increase in immune complexes (age-related autoaggression). Immunotropic activity of AFP may be of particular interest due to its connection to the special function of immunotropic cells – ability to activate directly regeneration of somatic tissues [13, 14]. Based on this observation, we developed immunoregulatory aging theory: age-related immunodeficiency is directly responsible for the decrease in natural regeneration of renewable somatic tissues and their atrophy with age [9].
Thus, AFP administration drastically increased the survival of the oldest mice and had a pronounced bioactivating effect in our experiments. It increased the relative weight of immunotropic organs, probably due to the general improvement of tissue functions, detoxifying, and direct immunotropic effects. This suggests the necessity for its future more detailed study as a candidate as a geroprotective compound.
REFERENCES
1.Nikogosyan, S. O., Kadagidze, Z. G., Shelepova, V.
M., and Kuznetsov, V. V. (2014) Modern methods of immunodiagnostics of
malignant ovarian tumors, Onkoginekologiya, 3, 49-54.
2.Wong, R. J., Ahmed, A., and Gish, R. G. (2105)
Elevated alpha-fetoprotein: differential diagnosis-hepatocellular
carcinoma and other disorders, Clin. Liver Dis.,
19, 309-323.
3.Zhang, S. Y., Lin, B. D., and Li, B. R. (2015)
Evaluation of the diagnostic value of alpha-l-fucosidase,
alpha-fetoprotein and thymidine kinase 1 with ROC and logistic
regression for hepatocellular carcinoma, FEBS Open Bio,
5, 240-244.
4.Bogdanov, A. Yu., Savvulidi, F. G., Tluelieva, R.
T., and Belyaev, N. N. (2004) Alpha-fetoprotein as inducer of natural
suppressor (NS) bone marrow cells, Biotekhnol. Teor. Prakt.,
3, 83-89.
5.Musatov, O. V., and Kokhanov, A. V. (2009)
Diagnostic abilities of alpha-fetoprotein in assessing reparative and
immunoregulatory processes in liver damages, Int. J.
Immunorehab., 11, 28a.
6.Rybakova, T. M., Nizkorodova, A. S., Zhidaylov, A.
V., Belyaev, N. N., and Bogdanov, A. Yu. (2007) Alpha-fetoprotein
effect on biological activity of hemopoietic stem cells ex vivo.
II. Effect on the expression and production of cuppressor factors,
clonogenic activity, self-renewal and differentiation, Biotekhnol.
Teor. Prakt., 1, 92-114.
7.Alekseeva, M. L., Pustotina, O. A., Fanchenko, N.
D., and Ponkratova, T. S. (2005) AFP as a prognostic indicator of the
state of a newborn, Probl. Reprod., 11, 79-82.
8.Demers, S., Roberge, S., and Bujold, E. (2015)
Low-dose aspirin for the prevention of adverse pregnancy outcomes in
women with elevated alpha-fetoprotein, J. Matern. Fetal Neonatal
Med., 28, 726-726.
9.Dontsov, V. I., Krut’ko, V. N., and
Trukhanov, A. I. (2010) Medicine of Anti-Aging: Fundamentals [in
Russian], URSS, Moscow.
10.Meshchaninov, V. N., Zhiborkin, G. V., Yakovlev,
N. V., and Gavrilov, I. V. (2011) Metabolic Mechanisms of
Heroprophylactic Activity of L-Arginine and alpha-Fetoprotein:
Materials of the I Int. Sci.-Pract. Conf. “Achievements,
Innovative Directions, Perspectives of Development and Problems of
Contemporary Medical Science, Genetics and Biotechnology”
dedicated to the 80th anniversary of the Department of Medical Biology
and Genetics of the Ural State Medical Academy, March 31, 2011, p.
192.
11.Russkikh, A. A., Yushkov, V. V., and Ivashev, M.
N. (2011) On the effect of alpha-fetoprotein on the central nervous
system, Vest. Ural. Med. Akad. Nauk Tem. Vyp. Farmats.,
3/1, 46-48.
12.Zubkova, E. S., Semenkova, L. N., Dudich, E. I.,
Khromykh, L. M., Makarevich, P. I., Parfyonova, Y. V., and Menshikov,
M. Yu. (2012) Recombinant human alpha-fetoprotein as a regulator of
adipose tissue stromal cell activity, Russ. J. Bioorg. Chem.,
38, 459-468.
13.Babaeva, A. G. (1995) The past, present and
future of research on the regulation of nonlymphoid cell proliferation
by lymphoid cells, Bull. Exp. Biol. Med., 120,
869-873.
14.Babaeva, A. G., and Zuev, V. A. (2007) Transfer
of signs of aging to young mice by splenic lymphoid cells from old
syngeneic donors, Bull. Exp. Biol. Med., 144, 89-90.