10-(6′-Plastoquinonyl)decyltriphenylphosphonium (SkQ1) Does Not Increase the Level of Cytochromes P450 in Rat Liver and Human Hepatocyte Cell Culture
K. N. Myasoedova1,2*, D. N. Silachev2,3, and A. D. Petrov4
1Lomonosov Moscow State University, Faculty of Fundamental Medicine, 119991 Moscow, Russia; E-mail: skulach@belozersky.msu.ru2Lomonosov Moscow State University, Institute of Mitoengineering, 119991 Moscow, Russia
3Lomonosov Moscow State University, Belozersky Institute of Physico-Chemical Biology, 119991 Moscow, Russia
4Mitotech S.A., 42 rue de la Vallee, Luxembourg L-2661, Luxembourg; E-mail: antonp@mitotechpharma.com
* To whom correspondence should be addressed.
Received September 16, 2016
Mitochondria-targeted antioxidant SkQ1 did not increase the content of cytochromes P450 in livers of rats that were given SkQ1 in drinking water for 5 days in a dose (2.5 µmol per kg body weight) that exceeded 10 times the SkQ1 therapeutic dose. SkQ1 did not affect the levels of cytochrome P450 forms CYP1A2, CYP2B6, and CYP3A4 in monolayer cultures of freshly isolated human hepatocytes, while specific inducers of these forms (omeprazole, phenobarbital, and rifampicin, respectively) significantly increased expression of the cytochromes P450 under the same conditions. We conclude that therapeutic doses of SkQ1 do not induce cytochromes P450 in liver, and the absence of the inducing effect cannot be explained by poor availability of hepatocytes to SkQ1 in vivo.
KEY WORDS: cytochrome P450, SkQ1, mitochondria-targeted antioxidant, hepatocyte cultureDOI: 10.1134/S0006297916120105
Abbreviations: CYP, cytochrome P450; SkQ1, 10-(6′-plastoquinonyl)decyltriphenylphosphonium.
When xenobiotics (chemical substances foreign to the human organism)
enter a human body as medications or pollutants (narcotics, toxins,
etc.), they undergo oxidation in human liver to facilitate their
excretion from the organism. Biotransformation of xenobiotics is
catalyzed by a group of autooxidizable heme-containing liver proteins
named cytochromes P450 (CYPs) due to the absorption maximum at
450 nm in the spectrum when CYP in its reduced state is complexed
with carbon monoxide. Aside from the liver, CYPs (encoded by over 50
genes) have been found in other human organs and tissues, where they
predominantly oxidize endogenous substrates and are involved in the
biosynthesis and metabolism of a number of physiologically active
compounds [1-6].
Liver CYPs, that are located mostly in the endoplasmic reticulum membranes of hepatocytes, display unusual catalytic properties – a phenomenon that has not yet received a proper explanation. These CYPs exhibit extremely broad substrate specificity and do not follow Michaelis–Menten kinetics. Beside substrate hydroxylation of the monooxygenase type, liver CYPs can catalyze N-, S-, and O-dealkylation, heteroatom oxygenation, cleavage of ester and amide bonds, lipid peroxidation, desaturation, isomerization, etc. Due to these properties, CYP3A4, which is the most studied CYP form, can metabolize up to 50% of drugs that exist on the modern pharmacological market [1, 2, 6]. Xenobiotics are known to increase the levels of cytochromes P450 in liver by stimulating biosynthesis of one or several CYP forms in hepatocytes [5].
For a long time, cytochromes P450 were considered as components of the major detoxifying system in mammals that hydroxylates (hence, makes more hydrophilic) chemical compounds to facilitate their excretion from the organism. In some cases, however, the oxidation products are more toxic that the original compounds. Thus, hydroxylation activates carcinogenic properties of polycyclic aromatic hydrocarbons (for example, benzo(a)pyrene), heterocyclic amines (additives in smoked meat), and aromatic amines (pesticides, tobacco smoke components, many drugs) [1, 2, 6]. Therefore, it is important to study cytochrome P450 induction by newly developed medications before recommending them for treatment of the corresponding pathologies.
Here and in our earlier studies, we investigated induction of cytochromes P450 in liver by the mitochondria-targeted antioxidant SkQ1. SkQ1 was synthesized in our laboratory in 2008 [7]. It is a synthetic derivative of plastoquinone conjugated with the membrane-penetrating cation decyl(triphenyl)phosphonium. SkQ1 had passed preclinical and clinical trials [7-15] and was recommended for the therapy of various pathological conditions; it has been on the market since 2012.
Earlier, we demonstrated that therapeutic doses of SkQ1 did not affect the levels of cytochromes P450 in rat liver. The animals received SkQ1 for 5 days, i.e. for a period commonly used to test cytochrome P450 induction by a studied substance. As a positive control, rats were treated with the classical CYP inducer phenobarbital that considerably increased CYP content in the liver [16]. We also demonstrated that the total amount of cytochromes P450 in liver microsomes isolated from rats that had received therapeutic doses of SkQ1 in drinking water for several (up to 24) months did not differ from the cytochrome P450 content in livers of the control animals [17].
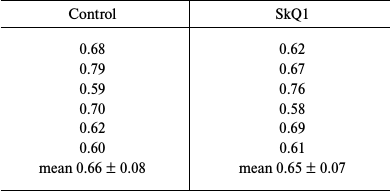
In this work, we repeated our previous experiments described in [16], except that the dose of SkQ1 was increased 10 times. Outbred albino male rats (body weight, 200-220 g) were given SkQ1 in drinking water (2500 nmol per kg body weight) for 5 days. The animals were sacrificed; their livers were perfused with cold physiological solution and then homogenized. Liver microsomes were isolated from the homogenate by differential centrifugation as described in [16, 17]. The total amount of cytochromes P450 in the microsomes was determined by the classical method of Omura and Sato [18] from the absorption of the reduced cytochrome P450 complex with carbon monoxide at 450 nm using the molar extinction coefficient of 91 mM–1·cm–1. Protein was determined by Lowry’s method [19]. Table 1 shows the amounts of cytochromes P450 (nmol/mg protein) in microsomes from the livers of control and experimental rats. The results clearly indicate that SkQ1 did not induce cytochrome P450 biosynthesis even when used is amounts that exceeded many times its therapeutic doses.
Table 1. Cytochrome P450 content
(nmol per mg protein) in liver microsomes from SkQ1-treated and
control rats (daily dose, 2500 nmol per kg body weight; 5
days)
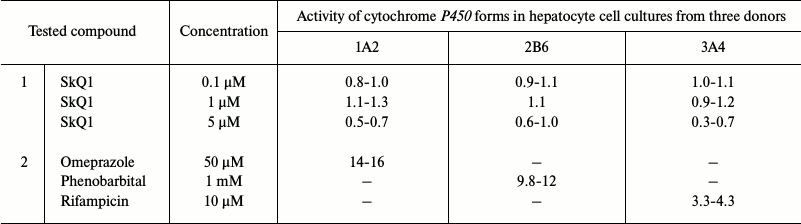
SkQ1 did not induce cytochrome P450 expression when added directly to the monolayer cultures of freshly isolated human hepatocytes [20, 21] from donors. Hepatocytes were incubated with different SkQ1 concentrations for 72 h with daily change of the medium. The SkQ1-containing medium was then removed and replaced with solutions containing standard substrates of the CYP1A2, CYP2B6, and CYP3A4 forms (phenacetin, bupropion, and midazolam, respectively). After 15- or 30-min incubation, the concentrations of the reaction products, i.e. hydroxylated derivatives of the above-mentioned substrates, were measured by the LC-PDA-MS method. The results are presented in Table 2 as the ratios between the amounts of hydroxylated products produced by cytochromes P450 in the treated cells to the amounts of reaction products in the corresponding untreated controls. At all the concentrations tested, SkQ1 did not increase the concentration of hydroxylation products in the hepatocyte cultures from all three donors – the ratios between the amounts of the reaction products in the experimental and control cells were close to 1. Treatment of hepatocytes with CYP inducers considerably increased the ratio between the amounts of the reaction products in the treated and control cells, which proved the ability of studied hepatocyte cultures to respond to CYP inducers with a significant increase in the rates of substrate hydroxylation.
Table 2. Activity of cytochrome P450
forms (CYP1A2, CYP2B6, and CYP3A4) in hepatocyte monolayer cell
cultures (measured using corresponding cytochrome P450 standard
substrates and presented as a ratio between the concentrations of
hydroxylation products in treated and untreated cells): 1) after 72-h
incubation with SKQ1; 2) after incubation with inducers of cytochrome
P450 forms
Based on the results of this work and data from our previous publications, we conclude that the SkQ1 cation does not induce cytochromes P450 in liver when used in therapeutic doses and, therefore, SkQ1 does not exhibit negative (toxic or carcinogenic) side effects. The results obtained in hepatocyte cell culture exclude the possibility that the absence of the SkQ1 effect on liver cytochromes P450 is due to poor delivery of this compound to the cells.
REFERENCES
1.Furge, L. L., and Guengerich, F. P. (2006)
Cytochrome P450 enzymes in drug metabolism and chemical
toxicology: An introduction, Biochem. Mol. Biol. Educ.,
34, 66-74.
2.Coon, M. J. (2002) Enzyme ingenuity in biological
oxidations: A trail leading to cytochrome P450, J. Biol.
Chem., 277, 28351-28363.
3.Ekroos, M., and Sjogren, T. (2006) Structural basis
for ligand promiscuity in cytochrome P450 3A4, Proc. Natl.
Acad. Sci. USA, 103, 13682-13687.
4.Guengerich, F. P. (2006) A malleable catalyst
dominates the metabolism of drugs, Proc. Natl. Acad. Sci. USA,
103, 13565-13566.
5.Lyakhovich, V. V., and Tsyrlov, I. B. (1981)
Induction of Xenobiotics Metabolism Enzymes [in Russian], Nauka,
Novosibirsk.
6.Myasoedova, K. N. (2008) New in studying
cytochromes P450, Biochemistry (Moscow), 73,
1199-1205.
7.Antonenko, Y. N., Avetisyan, A. V., Bakeeva, L. E.,
Chernyak, B. V., Chertkov, V. A., Domnina, L. V., Ivanova, O. Yu.,
Izyumov, D. S., Khailova, L. S., Klishin, S. S., Korshunova, G. A.,
Lyamzaev, K. G., Muntyan, M. S., Nepryakhina, O. K., Pashkovskaya, A.
A., Pletjushkina, O. Yu., Pustovidko, A. V., Roginsky, V. A.,
Rokitskaya, T. I., Ruuge, E. K., Saprunova, V. B., Severina, I. I.,
Simonyan, R. A., Skulachev, I. V., Skulachev, M. V., Sumbatyan, N. V.,
Sviryaeva, I. V., Tashlitsky, V. N., Vassiliev, J. M., Vyssokikh, M.
Yu., Yaguzhinsky, L. S., Zamyatnin, A. A., Jr., and Skulachev, V. P.
(2008) Mitochondria-targeted plastoquinone derivatives as tools to
interrupt execution of the aging program. 1. Cationic plastoquinone
derivatives: synthesis and in vitro studies, Biochemistry
(Moscow), 73, 1273-1287.
8.Skulachev, V. P., Anisimov, V. N., Antonenko, Y.
N., Bakeeva, L. E., Chernyak, B. V., Erichev, V. P., Filenko, O. F.,
Kalinina, N. I., Kapelko, V. I., Kolosova, N. G., Kopnin, B. P.,
Korshunova, G. A., Lichinitser, M. R., Obukhova, L. A., Pasyukova, E.
G., Pisarenko, O. I., Roginsky, V. A., Ruuge, E. K., Senin, I. I.,
Severina, I. I., Skulachev, M. V., Spivak, I. M., Tashlitsky, V. N.,
Tkachuk, V. A., Vyssokikh, M. Y., Yaguzhinsky, L. S., and Zorov, D. B.
(2009) An attempt to prevent senescence: a mitochondrial approach,
Biochim. Biophys. Acta, 1787, 437-461.
9.Severin, F. F., Severina, I. I., Antonenko, Y. N.,
Rokitskaya, T. I., Cherepanov, D. A., Mokhova, E. N., Vyssokikh, M. Y.,
Pustovidko, A. V., Markova, O. V., Yaguzhinsky, L. S., Korshunova, G.
A., Sumbatyan, N. V., Skulachev, M. V., and Skulachev, V. P. (2010)
Penetrating cation/fatty acid anion pair as a mitochondria-targeted
protonophore, Proc. Natl. Acad. Sci. USA, 107,
663-668.
10.Skulachev, M. V., Antonenko, Y. N., Anisimov, V.
N., Chernyak, B. V., Cherepanov, D. A., Chistyakov, V. A., Egorov, M.
V., Kolosova, N. G., Korshunova, G. A., Lyamzaev, K. G., Plotnikov, E.
Y., Roginsky, V. A., Savchenko, A. Y., Severina, I. I., Severin, F. F.,
Shkurat, T. P., Tashlitsky, V. N., Shidlovsky, K. M., Vyssokikh, M. Y.,
Zamyatnin, A. A., Jr., Zorov, D. B., and Skulachev, V. P. (2011)
Mitochondria-targeted plastoquinone derivatives. Effect on senescence
and acute age-related pathologies, Curr. Drug Targ., 12,
800-826.
11.Neroev, V. V., Archipova, M. M., Bakeeva, L. E.,
Fursova, A. Zh., Grigorian, E. N., Grishanova, A. Yu., Iomdina, E. N.,
Ivashchenko, Zh. N., Katargina, L. A., Khoroshilova-Maslova, I. P.,
Kilina, O. V., Kolosova, N. G., Kopenkin, E. P., Korshunov, S. S.,
Kovaleva, N. A., Novikova, Yu. P., Philippov, P. P., Pilipenko, D. I.,
Robustova, O. V., Saprunova, V. B., Senin, I. I., Skulachev, M. V.,
Sotnikova, L. F., Stefanova, N. A., Tikhomirova, N. K., Tsapenko, I.
V., Shchipanova, A. I., Zinovkin, R. A., and Skulachev, V. P. (2008)
Mitochondria-targeted plastoquinone derivatives as tools to interrupt
execution of the aging program. 4. Age-related eye disease. SkQ1
returns vision to blind animals, Biochemistry (Moscow),
73, 1641-1654.
12.Yani, E. V., Katargina, L. A., Chesnokova, N. B.,
Beznos, O. V., Savchenko, A. Yu., Vygodin, E. Yu., Gudkova, E. Yu.,
Zamyatin, A. A., Jr., and Skulachev, M. V. (2012). The first attempt of
Vizomitin application in dry eye syndrome therapy, Prakt. Med.,
1, 134-137.
13.Brzheskiy, V. V., Efimova, E. L., Vorontsova, T.
N., Alekseev, V. N., Gusarevich, O. G., Shaidurova, K. N., Ryabtseva,
A. A., Andryukhina, O. M., Kamenskikh, T. G., Sumarokova, E. S.,
Miljudin, E. S., Egorov, E. A., Lebedev, O. I., Surov, A. V., Korol, A.
R., Nasinnyk, I. O., Bezditko, P. A., Muzhychuk, O. P., Vygodin, V. A.,
Yani, E. V., Savchenko, A. Y., Karger, E. M., Fedorkin, O. N., Mironov,
A. N., Ostapenko, V., Popeko, N. A., Skulachev, V. P., and Skulachev,
M. V. (2015) Results of a multicenter, randomized, double-masked,
placebo-controlled clinical study of the efficacy and safety of
Visomitin eye drops in patients with dry eye syndrome, Adv.
Ther., 32, 1263-1279.
14.Petrov, A., Perekhvatova, N., Skulachev, M.,
Stein, L., and Ousler, G. (2016) SkQ1 ophthalmic solution for dry eye
treatment: results of a phase 2 safety and efficacy clinical study in
the environment and during challenge in the controlled adverse
environment model, Adv. Ther., 33, 96-115.
15.Skulachev, V. P., Bogachev, A. V., and
Kasparinsky, F. O. (2013) Principles of Bioenergetics, Springer,
Berlin, Heidelberg.
16.Myasoedova, K. N., and Silachev, D. N. (2014)
Therapeutic doses of SkQ1 do not induce cytochromes P450 in rat
liver, Biochemistry (Moscow), 79, 1130-1132.
17.Myasoedova, K. N., and Silachev, D. N. (2015)
Effect of long-term treatment with antioxidant SkQ1 added to drinking
water on cytochromes P450 level in rat liver, Biochemistry
(Moscow), 80, 1626-1628.
18.Omura, T., and Sato, R. (1964) Carbon
monoxide-binding pigment of liver microsomes. I. Evidence for its
hemoprotein nature, J. Biol. Chem., 239, 2370-2378.
19.Dawson, R. M. C., Elliott, D. C., Elliott W. H.,
and Jones, K. M. (1986) Data for biochemical research, 3rd Edn., Oxford
University Press, Oxford.
20.Lanford, R. E., Carey, K. D., Estlack, L. E.,
Smith, G. C., and Hay, R. V. (1989) Analysis of plasma protein and
lipoprotein synthesis in long-term primary cultures of baboon
hepatocytes maintained in serum-free medium, In vitro Cell.
Dev. Biol., 2, 174-182.
21.Guguen-Guillouzo, C., and Guillouzo, A. (1986)
Isolated and Cultured Hepatocytes (Guillouzo, A., and Guillouzo,
C., eds.) INSERM, Paris and John Libbey Eurotext, London, pp. 1-12.