REVIEW: Biomedical Applications of Multifunctional Gold-Based Nanocomposites
L. A. Dykman1,2 and N. G. Khlebtsov1,2*
1Institute of Biochemistry and Physiology of Plants and Microorganisms, Russian Academy of Sciences, 410049 Saratov, Russia2Chernyshevsky Saratov State University, 410012 Saratov, Russia; E-mail: khlebtsov@ibppm.ru
* To whom correspondence should be addressed.
Received July 11, 2016; Revision received July 31, 2016
Active application of gold nanoparticles for various diagnostic and therapeutic purposes started in recent decades due to the emergence of new data on their unique optical and physicochemical properties. In addition to colloidal gold conjugates, growth in the number of publications devoted to the synthesis and application of multifunctional nanocomposites has occurred in recent years. This review considers the application in biomedicine of multifunctional nanoparticles that can be produced in three different ways. The first method involves design of composite nanostructures with various components intended for either diagnostic or therapeutic functions. The second approach uses new bioconjugation techniques that allow functionalization of gold nanoparticles with various molecules, thus combining diagnostic and therapeutic functions in one medical procedure. Finally, the third method for production of multifunctional nanoparticles combines the first two approaches, in which a composite nanoparticle is additionally functionalized by molecules having different properties.
KEY WORDS: biomedicine, colloidal gold, nanocomposites, multifunctionalityDOI: 10.1134/S0006297916130125
Abbreviations: BSA, bovine serum albumin; DOX, doxorubicin; GNC, gold nanocages; GNP, gold nanoparticles; GNR, gold nanorods; GNS, gold nanoshells; GNSt, gold nanostars; NC, nanocomposite; PD, photodynamic; PT, photothermal; SERS, surface-enhanced Raman scattering.
Solutions of colloidal gold have been used for medical purposes since
ancient times [1]. However, only in recent decades
have gold nanoparticles (GNP) started to be actively used for various
diagnostic and therapeutic purposes both in experimental biology and
medicine as well as in practice, due to the emergence of new data on
their unique optical and physicochemical properties [2, 3]. Still, although diagnostic
methods using GNP have been applied in medical practice rather often,
only three preparations (Aurasol®, AurImmune™, and
AuraShell®) have passed all phases of clinical trials, and several
preparations are currently undergoing clinical trials. In the last
10-15 years, GNP have been widely used in different biological and
medical applications including chemical and biological sensors,
clinical analytics, genomics and immunology, optical cell, tissue, and
organ bioimaging, photodynamic (PD) and photothermal (PT) therapy of
bacterial infections, cancer cells, and tumors, treatment of various
inflammations, and targeted delivery of drugs, peptides, DNA, antigens,
and other compounds.
Interest in gold and other particles of noble metals is due to their unique optical properties related to excitation of localized plasmon resonances in metal nanoparticles interacting with light [4, 5]. These excitations result in a whole class of plasmon-enhanced linear properties such as resonance absorption, scattering, generation of strong local fields, and surface-enhanced Raman scattering (SERS). In addition, plasmon excitation results in enhancement of different nonlinear effects [6].
Various metal nanoparticles used in nanobiotechnology have different biomacromolecules (such as recognizing molecules – antibodies, aptamers, etc.) attached to their surface via physical adsorption. These nanostructures are termed bioconjugates or simply conjugates [7], and the attachment of biomacromolecules to the surface of nanoparticles is often called “functionalization” [8, 9]. Hence, the probe in the conjugate is used for specific binding to the target, and the metal core – for visualization of this interaction using different types of microscopy [10], as a contrasting agent in optical coherence tomography [11] and photoacoustics [12], for thermal destruction of cancer cells and tumors [13, 14], for targeted delivery of molecules attached to the particle [15], and other purposes.
In addition to GNP conjugates, significant growth in the number of publication devoted to the synthesis and application of the multifunctional composite nanoparticles (often simply called nanocomposites, NC) has been observed in recent years. It is now accepted that multifunctional NC combining analytical, diagnostic, and therapeutic capabilities in one structure comprise a new area in biotechnology that has been termed “theranostics” [16-19]. Theranostics [from Greek thera(peia) – care, attention, healing, and (diag)nostikos – capable of recognizing] is a novel approach in medicine that involves complex solution to therapeutic problems – simultaneous development of therapeutics and means for early diagnostics of disease. Even though the term “theranostics” was introduced relatively recently [20, 21], this area is progressing rapidly as an independent branch of nanoplasmonics and nanomedicine [22].
In our discussion, we focus on applications for theranostics of multifunctional particles that can be produced via three different techniques. The first technique involves the design of composite (or hybrid) nanostructures with different components intended for either diagnostics or therapeutic functions [23]. Most often such hybrid constructs consist of one or multiple plasmon particles (which can be themselves composites, such as SiO2/Au nanoshells or Au-Ag nanocages) embedded into a dielectric shell (biopolymer, mesoporous silica, etc.), which is doped with various reporter molecules and molecules of compounds to be delivered. Another important group comprises composites of gold and magnetic particles, which combine plasmon and magnetic properties supplemented with other modalities using methods for production of hybrids of the first type. Finally, the most promising type of NCs are hybrids of gold and carbon (fullerenes, nanotubes, graphene) nanostructures.
Furthermore, GNPs can be functionalized with various molecules using new conjugation methods that allow combination of both diagnostic and therapeutic functions in one medical treatment [24]. Currently, multifunctionalized nanoparticles find promising applications in theranostics [25], which is why we also discuss recent progress in this area.
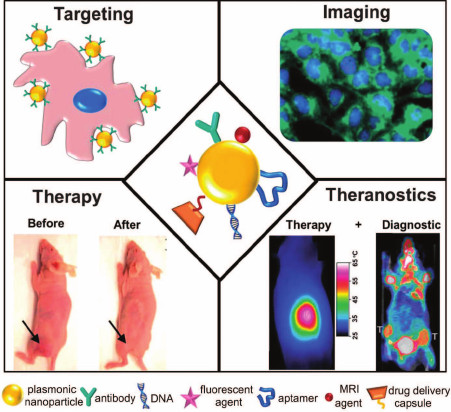
Moreover, a third technique for production of multifunctional nanoparticles involves combination of the first two approaches, in which the hybrid (composite) nanoparticles are additionally functionalized with molecules displaying various properties – multifunctionalized composite nanostructures [26]. A schematic presentation of applications of multifunctional nanostructures in theranostics is shown in Fig. 1.
Fig. 1. Schematic presentation of the use of plasmonic nanostructures conjugated with various therapeutic and diagnostic entities [26] (reproduced with kind permission of the Royal Society of Chemistry).
The number of publications on multifunctional and hybrid nanomaterials is growing rapidly in recent years. That is why, despite the publication of several reviews devoted to certain aspects of application of multifunctional nanomaterials [27-40], there is an urgent need for systematic discussion of continuously emerging new data in this area to help researchers evaluate existing results and planning new studies. In contrast to reviews published earlier, we focus our attention on the discussion of only multifunctional nanocomposites of the three types mentioned above based on data reported mainly during 2010-2016 (173 references of 225 total).
COMPOSITE NANOPARTICLES
Most often the composite GNP used in biomedicine includes polymeric nanoparticles, nanoparticles of other metals, and semiconductors in their composition [41-44]. One of the first reported examples of composite nanoparticles is the composite of GNP with polyaminoamide dendrimer (PAMAM) [45]. These composite GNPs enhance the efficiency of optical imaging and X-ray tomography due to better penetration into cells [46]. Introduction of the 198Au isotopes into composites of PAMAM with GNP increased the efficiency of tumor radiotherapy [47]. Application of polymeric components allows novel analytical and therapeutic functions. For example, GNP composites with polydimethylsiloxane were used by researchers to design new substrates for SERS analysis [48]. Composites of GNP with thermosensitive polymers are commonly used in PT therapy of tumors [49-51]. The conjugate of a GNP+PAMAM composite with the antitumor preparation doxorubicin (DOX) was used for combined PT and chemotherapy [52].
Another example of available NCs are composites of GNPs with nanoparticles of chitosan – cationic biodegradable polyaminosaccharide. High antioxidative potential of such NC exceeding the antioxidative properties of each component was demonstrated [53]. This NC was used [54] for electrochemical investigation of cancer cells, and [55] for electrochemical detection of myoglobin. Gold nanorods (GNR) coated with chitosan doped with antitumor preparation cisplatin enhanced antitumor effect of cisplatin and provided additionally an option for tumor PT therapy [56]. Chitosan-coated GNP with the encapsulated PD dye indocyanine green were used for combined PT and PD therapy [57]. GNP coated with chitosan and polyacrylic acid and doped with cisplatin were used as a tool for efficient delivery of the drug into the cell and cell nucleus, as well as a contrast agent for tumor cell imaging [58].
Other biodegradable polymers have been tested along with chitosan. For example, GNPs encapsulated into chondroitin sulfate with insulin were used for peroral treatment of diabetes in laboratory animals [59]. NC comprising GNP coated with collagen stimulated differentiation of endothelial cells and facilitated regeneration of blood vessels [60]. A plasmonic fluorescent NC consisting of a gold nucleus coated with polyacrylamide with incorporated fluorescein was suggested [61]. The possibility of simultaneous use of its plasmonic and fluorescent properties provides significant advantages.
The “nanobeacon” composite consisting of small (2-3 nm) GNPs embedded into a lipid or polymer matrix was suggested for photoacoustic detection of tumor cells [62]. Interesting results on IR-imaging of tumor cells were obtained using a NC consisting of 5-nm GNPs enclosed into a biodegradable polyethylene glycol (PEG)/polylactone capsule [63]. A similar NC containing GNRs were used for SERS detection and PT therapy of cancer cells [64] or targeted delivery of DOX [65]. NCs were developed for combining ultrasound imaging and PT therapy comprising gold nanoshells (GNS) encapsulated into polylactide [66] or PEG/polycaprolactone [67] capsules. Similar nanostructures containing photosensitizer Ce6 were used for fluorescence detection and combined PT and PD therapy of tumors [68]. Polymeric biocompatible micelles doped with DOX and coated with GNPs were used for combined PT and chemotherapy, as well as an efficient contrast agent for computer and photoacoustic tomography in vivo [69]. With the same objective in mind, it was suggested to encapsulate gold nanocages (GNC) into lipid capsules [70], GNRs into polymerosomes [71], or GNSs into liposomes [72]. In addition, vice versa, liposomes containing medicinal preparation and enclosed into gold shell were used for delivery of drugs without undesirable leakage of the medicinal ingredient outside of biotarget. These structures were termed nanocontainers [73].
Multifunctional biodegradable polylactide-glycolide nanoparticles with encapsulated rhodamine (a drug model) and coated sequentially by magnetic and gold shells [74] allowed PT-controlled drug delivery and enhancing contrast of magnetic resonance. The targeted drug delivery in combination with PT therapy was realized using polylactide-glycolide nanoparticles with encapsulated DOX and coated with gold half-shell [75]. Biocompatible GNPs coated with an albumin (bovine serum albumin, BSA) capsule with antibodies to vascular endothelial growth factor incorporated via the avidin-biotin system were used to enhance efficiency of PT therapy [76]. Three types of GNPs were incorporated in the albumin capsule as reported [77], including the following: nanospheres, nanorods, and nanoshells. These NC exhibited pronounced PT properties. All these examples clearly demonstrate the increase in functionality and, in many cases, synergism of NCs due to the rational selection of polymer components.
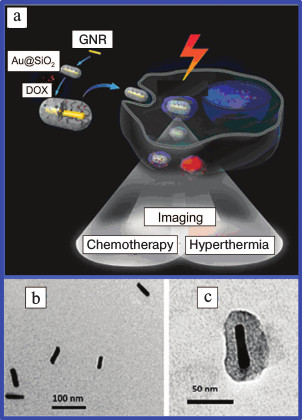
The design of NCs using a plasmonic nucleus coated with mesoporous silica shell comprises a separate direction of research. Interesting data has been published [78]. The authors synthesized a NC consisting of silicon nanowires coated with gold nanoclusters and efficiently used it for photothermolysis of circulating tumor cells. NCs were suggested [79] comprising gold nucleus doped with antitumor preparation camptothecin. This composite was used for combined photo- and chemotherapy of tumors. A similar approach was demonstrated [39, 80, 81], which used GNRs and doxorubicin (Fig. 2).
Fig. 2. a) Schematic presentation of use in theranostics of GNRs coated with a mesoporous silica shell and functionalized with DOX. Electron microscope images of GNRs (b) and GNRs coated with SiO2 (c) [39, 81] (reproduced with permission of the Royal Society of Chemistry and Elsevier Publishers).
Constructs consisting of GNPs coated with a mesoporous silica shell doped with PD dyes can be used for PD therapy, fluorescence microscopy, and SERS [82-86]. For example, GNRs and GNCs coated with mesoporous silica shell doped with PD dye hematoporphyrin were successfully used for combined PD and PT inactivation of antibiotic-resistant Staphylococcus aureus strains [87, 88] and for therapy of large tumors in rats [89, 90]. GNRs embedded into a silica shell doped with folate were used for both computer tomography and photothermolysis in vivo [91].
A new composite was suggested [92] that was called gold nanomatryoshka. It comprised GNPs coated with a silica shell, which, in turn, was coated with a gold shell. Such nanocomposites demonstrated more clearly pronounced photothermal effect in comparison with the common GNSs.
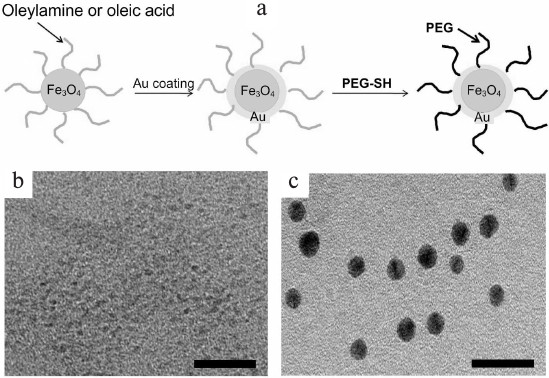
Along with “polymeric” and “silica” NCs, composites consisting of GNPs and magnetic iron oxide nanoparticles received wide recognition [93]. Such NCs combine magnetic properties of a ferromagnetic material and optical properties of plasmon resonant particles, which make their application in various biomedical studies very efficient [94]. GNPs coated with a magnetic shell or nanoparticles with a magnetic core coated with a gold shell have been reported. Hybrid magnetic gold nanoshells were used among the first. The SiO2 nuclei were first coated with magnetite (Fe3O4), and the gold shell was synthesized over them [95]. The produced NCs were successfully used for both magnetic resonance tomography and photothermolysis of tumor cells. With the same objective, spherical magnetic NPs were synthesized from Fe2O3 or Fe3O4 coated with a gold shell [96, 97], or first with a silica shell followed by a gold shell [98]. Similar NCs coated with PEG [99] (Fig. 3) or amphiphilic polymers [100] were used as contrast agents in magnetic resonance and computer tomography, as well as for magneto-acoustic imaging of tumor cells [101]. Gold-magnetic NCs based on GNRs [102] or GNSs [103] were suggested for enhancing the photothermolysis effect.
Fig. 3. a) Scheme for preparation of Fe3O4 nanoparticles coated with gold shell and PEG. b) Electron microscope image of Fe3O4 nanoparticles and (c) prepared NCs. Scale bar, 20 nm [99] (reproduced with permission of the Korean Chemical Society).
A NC consisting of GNRs (nucleus) coated with polypyrrole and a shell from Fe3O4-nanoparticles was developed [104] for simultaneous application in magnetic resonance and computer tomography and photothermolysis of tumor cells. For the same purpose, the authors [105] suggested composite nanostars consisting of a magnetic nucleus placed within a gold shell functionalized with hyaluronic acid, which interacted with CD44 receptors overexpressed on the surface of cancer cells.
GNPs with shells from Fe3O4 were used [106, 107] for detection of point mutations in DNA molecules using a piezoelectric microbalance and for determination of C-reactive protein with a solid-phase immunoassay. GNRs coated with a magnetic shell were applied during the design of an immune biosensor for determination of the level of immunoglobulins in serum [108].
The capabilities of the NC described above can be extended by functionalization with recognizing molecules. For example, GNRs were coated with a SiO2 layer with magnetic nanoparticles and conjugated with folic acid. Folate receptors are known for being overexpressed on the surface of most cancer cells, so the NC described above was used for developing a “magnetic trap” and optoacoustic imaging of circulating tumor cells [109]. Combined effect of chemotherapy and magnetic fields on a tumor was investigated [110, 111] using an Fe3O4/Au nanocomposite coated with DOX.
Even more complex NCs have been developed. In particular, a composite was suggested consisting of a magnetic nucleus coated with a silica shell doped with GNRs [112]. This NC was used for a combination of PT and chemotherapy of tumors coupled with magnetic resonance tomography and IR thermal imaging. Another no less multifaceted composite consisted of a magnetic nucleus (MnFe2O4) coated sequentially with silica and gold shells [113]. The use of this NC was shown be more efficient and provide more information during magnetic resonance testing coupled with therapy.
If a superparamagnetic nucleus is coated successively with silica and gold shells, the produced nanoparticle can be suitable for either PT therapy or magnetic resonance imaging of tumors [114]. A NC consisting of small nanoparticles of iron oxide and lanthanoid nanocrystals coated with a gold shell was found to be effective for fluorescence imaging and magnetically targeted PT of tumors [115].
It is advisable to have magnetic resonance monitoring during all stages of PT treatment of tumor cells. Synthesized NCs consisting of GNPs and magnetite nanoparticles coated with silica shells [116] as well as iron-gold nanoparticles embedded into a polymeric capsule [117, 118] provide examples of this approach. A NC consisting of GNRs and Fe3O4 nanoparticles encapsulated into phospholipid membranes was suggested for the same purposes [119]. Nanomicelles composed of GNSs, Fe3O4 nanoparticles, and DOX were used for combining magnetic resonance imaging, magnetically targeted drug delivery, and PT therapy [120]. The GNPs and superparamagnetic iron nanoparticles coated with polycaprolactone capsule were used for computer and magnetic resonance tomography in combination with in vivo radiotherapy of mice with multiple glioblastoma [121].
Magnetic glyconanoparticles coated with gold were successfully used as a contrast agent simultaneously in the methods of magnetic resonance tomography, X-ray computer tomography, positron-emission tomography, and ultrasound imaging [122].
Interesting data were presented in [123]. The authors designed a NC consisting of a magnetic nucleus and a gold shell coated with the antibiotic vancomycin. This composite was used for magnetic separation of pathogenic bacteria with attached vancomycin followed by PT destruction of the pathogens. A similar NC conjugated with S6 aptamer labeled with Cy3 fluorescent dye was used for targeted diagnostics, isolation, and PT therapy of tumors [124].
Magnetic nanoparticles encapsulated into a silica shell coated with PEGylated GNPs were functionalized by antitumor preparation curcumin and used for either targeted drug delivery or magnetic resonance imaging of tumor cells [125].
A very complex NC preparation was suggested in [126]. It was composed of a polymeric capsule coated with anticancer preparation paclitaxel that also simultaneously contained GNRs, quantum dots, and magnetic nanoparticles. Using this construct, it became possible to conduct combined chemo- and PT therapy under the control of fluorescence microscopy and magnetic resonance tomography.
Other metals, such as palladium, cobalt, gadolinium, manganese, platinum, hafnium, and titanium or their oxides are occasionally used in NCs containing GNPs. Until now, they have been mainly used in various diagnostic methods such as SERS, magnetic resonance tomography, optoacoustic imaging, and positron-emission tomography. It is our opinion that they can find application in theranostics as well. GNPs coated with a palladium shell of different thickness were suggested for developing an efficient SERS platform [127]. Similar particles were used during development of an ethanol-specific amperometric sensor [128]. Ferromagnetic cobalt nanoparticles coated with gold shell were developed to increase sensitivity of magnetic resonance tomography and photoacoustic imaging [129]. GNSs and polymer-coated gold nanospheres doped with gadolinium [130, 131] or manganese oxide [132] were used for the same purpose. Composite gold–platinum nanoparticles were suggested for use in the methods of photoacoustic imaging and positron-emission tomography [133].
NCs with sophisticated composition NaY/GdF4:Yb, Er, Tm@SiO2–Au@PEG5000 were developed for simultaneous use as a contrast agent in magnetic resonance and computer tomography and fluorescence imaging [134]. A rather interesting NC was described in [135]. It comprises PEG coated quantum dots encapsulated together with GNPs and antitumor preparation temozolomide into thermosensitive poly(methyl)methacrylate capsules. This nanocomplex was effectively used both in confocal microscopy of tumor cells and for combined chemo- and PT therapy. Hollow GNPs surrounded by quantum dots and encapsulated into silica shell have been developed [136]. This NC was suggested for use in luminescence detection and PT therapy of tumors.
Moreover, an NC can comprise GNPs of various sizes and shapes that are embedded into polymer films, sponges, or gels [137-139]. Such nanocomposites have been used as supports for SERS, in the composition of analytical sensors [140, 141], and for PT therapy [142, 143].
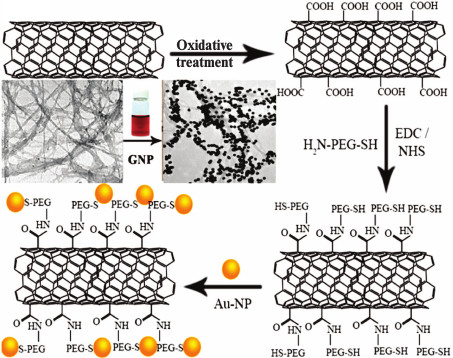
NCs based on gold and carbon nanoparticles (graphene, fullerenes, nanotubes) have been very actively developed in recent years [144-149]. In addition to catalysis and microelectronics, these NCs have been used in biomedicine. For example, it was suggested to use hybrid nanostructures based on carbon nanotubes [150] or graphene [151] and GNPs for efficient delivery of DOX and tumor cell imaging (Fig. 4). A composite consisting of GNPs and carbon nanotubes coated with PEG and folic acid was used for visualization and PT therapy of cancer cells in vitro [152]. More sophisticated NCs consisted of GNPs, magnetic nanoparticles, and graphene and coated with PEG was used [153] for magnetic resonance tomography and PT tumor therapy in vivo. A composite was suggested [154] that consisted of a gold nucleus and grapheme shell coated with PD dye. This composite was used simultaneously for SERS, PT, and PD therapy. A NC based of graphene oxide coated with mesoporous silica shell doped with GNP and folic acid was used for detection and selective killing of tumor cells [155].
Fig. 4. Scheme of preparation of carbon nanotubes coated with GNPs [149] (reproduced with permission of the American Chemical Society).
MULTIFUNCTIONALIZED GNPs
Multifunctionalized GNPs were first used to increase the efficiency of targeted drug delivery to tumor cells and tissues. Double functionalization of nanoparticles with medicinal preparation and recognizing molecules were used most often to design such GNPs. The double conjugation of GNPs with antitumor preparation gemcitabine and antibodies to vascular endothelial growth factor, whose receptors are overexpressed on the surface of cancer cells, provides an example of such multifunctional constructs [156]. The possibility of functionalizing GNPs simultaneously with anticancer drug paclitaxel and tumor necrosis factor was also reported [157]. This double conjugate demonstrated high efficiency both in vitro and in vivo. Good efficiency was also revealed in the case of double conjugation of GNPs with paclitaxel and biotin [158]. Furthermore, a GNP conjugate concurrently with antitumor preparation cetuximab and antibodies to folate receptors of tumor cells [159] and a GNP conjugate with folate and cisplatin [160] were suggested. Double functionalization of GNPs with antitumor and cell penetrating peptides, which facilitate more efficient delivery of the intended compound to the target, also shows considerable promise [161-163].
Considering that the tyrosine kinase receptors are overexpressed on the surface of tumor cells, hollow GNPs conjugated simultaneously with tyrosine kinase and DOX were synthesized [164]. This conjugate exhibited high efficacy during PT and chemotherapy, in part due to the fact that hollow GNPs absorb emission in the near IR range 50-fold more efficiently than solid ones. Conjugates of GNPs with DOX and folate were suggested for tumor treatment under monitoring by multiphoton spectroscopy [165]. Conjugates of DNA-coated [166] and BSA-coated GNPs [167] with DOX were also synthesized, which were used for combined chemo- and PT therapy. To increase the efficiency of intracellular penetration, a triple conjugate of GNPs with DNA, folic acid, and DOX was used [168].
A conjugate of GNPs with aptamers to prostate-specific antigen and DOX was suggested for simultaneous diagnostics with computer tomography and prostate cancer therapy [169]. A GNP conjugate with DOX and cell penetrating peptide A54 was developed for combined chemo- and PT therapy [170]. Conjugates of GNPs with DOX [171] or trastuzumab [172] together with cell-penetrating peptide cRGD were synthesized for use of GNPs simultaneously for therapy and positron-emission tomography. Moreover, the issue of blocking of the NC surface to prevent nonspecific adsorption of serum proteins on the particle (protein corona effect) was considered [172]. Formation of protein corona can affect the functional properties of NCs. It was suggested to protect NCs from nonspecific adsorption using such compounds as dextran, polyoxazoline, polyglycerol, and PEG (most popular). In particular, it was shown using X-ray photoelectron spectroscopy that formation of the protein corona was reduced significantly on coating the surface of nanoparticles with PEG.
A conjugate of GNRs with indocyanine green and antibodies to epidermal growth factor receptor was synthesized for simultaneous application in PT and PD therapy and monitoring of tumor cells with IR imaging [173]. A conjugate of GNPs with tumor-specific antibodies and the PD dye phthalocyanine was described, which was used for effective PD therapy [174].
GNPs coated simultaneously with the cell-penetrating peptide RGD and heparin labeled with fluorochrome efficiently penetrated metastasizing cells allowing their visualization by fluorescence microscopy and caused cell death due to apoptotic effect of heparin [175]. The gold–silver nanorods conjugated simultaneously with rhodamine 6G and a phage hybrid protein specific to colorectal cancer cells were used for fluorescence imaging and PT therapy of tumor cells [176].
A multifunctional conjugate based on GNSt was developed for application in combined PT, PD, and chemotherapy of tumors and IR imaging [177]. The GNSt were functionalized with three ligands: cell penetrating peptide, DOX, and indocyanine green. The efficiency of application of GNRs also labeled with three types of probes including: (i) scFv antibody fragment to epidermal growth factor receptor; (ii) amino-terminal fragment of the peptide recognizing the urokinase plasminogen activator; and (iii) cyclic RGD-peptide recognizing ανβ3 integrin receptor [178] was investigated. One important result of this study was that the total efficiency of particle delivery into cells depended only slightly on the availability of probe molecules, but it affected strongly the distribution of particles in the intercellular space.
A NC consisting of GNPs coated with conjugate of BSA with rifampicin and with cell-penetrating cRGD peptide was synthesized with the objectives to increase efficiency of the PT tumor therapy and to enhance intracellular penetration of the drug [179]. The multifunctionalized GNPs conjugated sequentially with recognizing antibodies and cell-penetrating peptides and coated with PEG demonstrated increased circulation time of the nanoparticles in the bloodstream [180], which resulted in increased accumulation of the composite into cells.
A rather sophisticated multifunctionalized construct was reported [181]. GNPs were sequentially functionalized with oligonucleotides, chelated 64Cu, and fluorophore Cy5. This allowed using the nanoconjugates for positron-emission tomography and fluorescence imaging of tumor cells.
Multifunctionalized GNPs conjugated with fluorescein isothiocyanate, boronophenylalanine, and folic acid were developed for simultaneous application in boron neutron capture therapy and fluorescence tumor biodetection [182]. These multiconjugates demonstrated high therapeutic potential against several tumor cell lines. Conjugates of GNPs with DOX and CpG immunomodulator were synthesized for use in combination immuno-, chemo-, and PT therapy [183]. These conjugates exhibited high efficiency in in vitro experiments (higher degree of inhibition of tumor cell growth in comparison with the use of non-conjugated DOX) and in vivo (triple combination of chemotherapy, thermotherapy, and immunotherapy normalized the volume of the experimental tumor in mice within one day).
In addition to targeted delivery of drugs, multifunctional GNPs are often used for transport of genetic material into the cell nuclei. A double conjugate of GNPs with siRNA and folic acid as a target molecule was used for efficient intracellular delivery of siRNA [184]. Cell-penetrating peptides have also been used for this purpose [185]. To protect siRNA from intracellular endonucleases and to enhance its cell penetration, it was suggested to coat the GNS+siRNA conjugate additionally with TAT lipid [186]. The nanoparticles were conjugated with siRNA labeled with Gd+ to enable monitoring of intracellular and intra-tissue penetration of the GNPs by the magnetic resonance technique [187]. Simultaneous delivery to the tumor of siRNA and DOX conjugated with GNRs coated with folate was suggested [188] for photoacoustic imaging of tumor cells and enhancing efficiency of chemotherapy. In all the above-mentioned examples, the target compounds (drugs, siRNA) served as the main therapeutic agent, the auxiliary compounds (antibodies, aptamers, cell penetrating peptides) served as means for targeted delivery of the NC, while the GNPs themselves and dyes served for diagnostic purposes as well as for PT and PD therapy.
In addition to targeted delivery of drugs and genetic material, multifunctionalized GNPs have been used for developing vaccines. For example, gold glyconanoparticles were synthesized that contained simultaneously two tumor antigens and a T-helper activating peptide in the hydrocarbon shell [189]. This glycoconjugate was suggested for creating an anticancer vaccine. A similar approach was used for producing corpuscular immunogens to other types of tumors [190] and to Streptococcus pneumoniae [191], as well as for producing a prototype drug for treating HIV infection [192]. Furthermore, gold glyconanoparticles intended for various biomedical applications can have drugs, siRNA, fluorophores, and other ligands in their composition [193, 194].
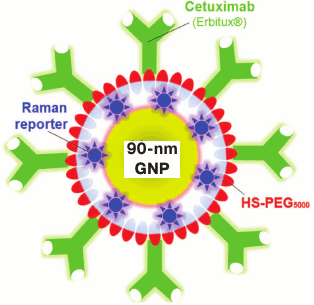
Moreover, multifunctionalized GNPs were developed specifically for application in computer tomography [195], SERS [196, 197] (Fig. 5), photoacoustic imaging [198], and solid-phase immunoassay [199].
Fig. 5. Schematic presentation of SERS-nanoantenna: 90-nm GNP coated sequentially with SERS-reporter, PEG, and cetuximab [197] (reproduced with permission of Elsevier Publisher).
MULTIFUNCTIONALIZED COMPOSITE GNPs
In this section, we will consider the most (in our opinion) interesting and promising nanoconstructs – composite nanoparticles conjugated with several functional probes (multifunctionalized composite GNPs). There are still only few data available on such nanostructures, but the possibilities opened by application of such structures in theranostics are impressive.
One of the first successful applications of multifunctional composite nanoparticles was published [200]. The authors synthesized two-segment gold–nickel nanorods. Plasmid DNA was attached to the nickel segment of the rod, and transferrin labeled with fluorescent dye rhodamine – to the gold segment. This nanocomposite was used for efficient transfection of plasmid DNA into HEK293 cells; furthermore, the transfection process was facilitated by the availability of transferrin, and monitoring of the process could be conducted using confocal microscopy identifying rhodamine penetrated into the cell. Moreover, it was possible to control the process of transfection using magnetic field due to the magnetic properties of the nickel segment. This NC provides significant promise for production of transgenes, gene therapy, and DNA vaccination.
A new multifunctional NC was suggested by the authors [201, 202]. It comprises GNPs stabilized with PAMAM dendrimer, which were preliminarily conjugated with folic acid and fluorescein isothiocyanate. This NC was used for combined detection of tumor cells by flow cytometry, confocal microscopy, inductively coupled plasma mass spectrometry, and computer tomography.
Composite nanomicelles consisting of GNPs coated with amphiphilic block-copolymer conjugated with folic acid and DOX were used for efficient targeted delivery of DOX into tumor cells [203]. GNPs coated with silica shell doped with silver nanoparticles, aptamer, and PD dye were successfully used for targeted delivery of nanoparticles into cancer cells followed by combined PD and PT therapy [204]. NC that incorporated GNPs and iron oxide nanoparticles coated with block copolymer doped with paclitaxel was used for combined chemo- and PT therapy and magnetic resonance imaging of tumor cells [205].
The group of N. Halas at Rice University contributed significantly to the development of multifunctionalized GNPs and their application in theranostics. In 2010, they designed a sophisticated NC consisting of gold shells on silicon dioxide nuclei coated with silica epilayer with incorporated magnetic nanoparticles and conjugated with antitumor antibodies and indocyanine green [206]. Thus, the nanocomposite displayed four modalities: plasmon resonance features of gold, magnetic properties of Fe3O4 nanoparticles, recognizing functions of antibodies, and PD capabilities of the dye. That is why the suggested composite can be used either for diagnostics (fluorescent methods, magnetic resonance tomography) or for tumor therapy (PT and PD). In the same year, a similar NC (with antibodies conjugated via the avidin–biotin system) was tested in vivo in mice with transplanted breast tumor [207]. The efficiency of diagnostics by IR fluorescence and magnetic resonance tomography was demonstrated. Biodistribution of nanoparticles in organs and systems was determined during 72 h after injection, selective accumulation of GNPs in tumor was demonstrated, and effective PT therapy was conducted. In the next study, the efficacy of the conjugate for ovarian cancer was shown [208].
Another multifunctional composite was termed “nanorattle” [209]. It consisted of several silica spheres loaded with the antitumor preparation docetaxel enclosed into one common gold shell coated with PEG. The nanocomplex offered significant promise for combined chemo- and PT therapy and exhibited high biocompatibility. The authors tested the “magic bullet” in vitro and in vivo in a hepatocellular carcinoma model and demonstrated pronounced synergetic effect of the NC.
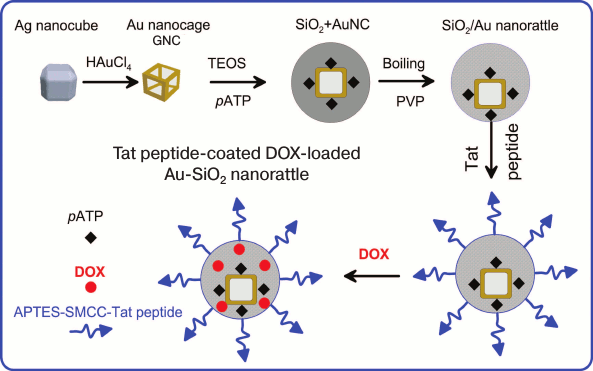
Another “nanorattle” was reported [210]. It comprised GNCs loaded with Raman reporter p-aminothiophenol and coated with hollow silica shell functionalized simultaneously with cell-penetrating peptide and DOX (Fig. 6). The nanocomposite was used for SERS imaging, targeted drug delivery, and PT therapy.
Fig. 6. Scheme of Au-SiO2 nanorattle synthesis that is coated with TAT peptide and doped with DOX. Adapted from [210].
Another version of “nanorattle” has been described [211]. GNPs with diameter of ~7 nm were placed into a hollow silica shell with gold nanoclusters (gold quantum dots) with diameter <2 nm incorporated into it that displayed magnetic and fluorescent properties. The overall size of the “nanorattle” was ~150 nm. The NC was used for delivery of DOX conjugated with the nanocomposite surface into tumor cells, PT therapy, as well as for fluorescence, photoacoustic, and magnetic resonance imaging.
In general, it must be mentioned that along with the GNPs, gold nanoclusters are finding ever-increasing use in theranostics [212, 213]. In particular, they have been used in composition of multifunctional NCs based on polymer micelles conjugated with transferrin, which were developed for targeted delivery of docetaxel into cells and tumor bioimaging [214].
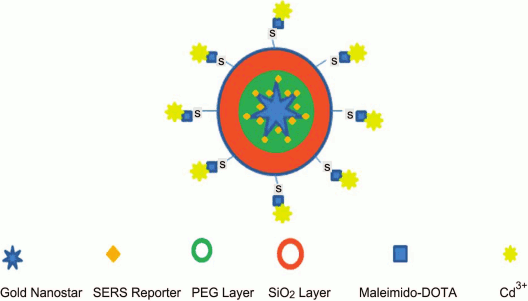
Development of a unique NC with five functional modalities was reported [215]. The NC comprised PEGylated GNSt conjugated with p-mercaptobenzoic acid (SERS reporter) encapsulated into silica shell linked to gadolinium complex. Thus, the nanocomplex could be applied in all methods such as (i) SERS, (ii) magnetic resonance and (iii) computed tomography, (iv) two-photon luminescence, and (v) PT therapy (Fig. 7).
Fig. 7. Schematic presentation of NC with five functional modalities [215] (reproduced with permission of the Royal Society of Chemistry Publisher).
Another interesting example of multifunctionalized composite GNPs – gold nanoprisms enclosed in biodegradable gelatin capsules doped with paclitaxel and folic acid linked to the surface of the capsules [216]. Paclitaxel penetrated effectively into tumor cells, causing their death. A no less interesting NC consisted of biodegradable polylactide-co-glycolide nanoparticles with encapsulated DOX [217]. The surface of the particles was coated with gold shell and functionalized with human serum albumin conjugated with indocyanine green and folic acid. The virus-like nanoplatform allowed conducting fluorescence microscopy of tumor cells and fluorescence imaging in vivo, as well as combined targeted chemo- and PT therapy. The NC demonstrated high synergistic effect in in vivo experiments with mice with transplanted breast carcinoma.
CONCLUSION
Due to development and improvement of technologies for synthesis of GNPs in the last decade, researchers are now provided with a great diversity of available particles with required parameters in size, shape, structure, and optical properties. Moreover, the task was recently set for primary modeling of nanoparticles possessing required properties that would be followed by development of a synthesis technique for the modeled nanostructure. From the point of view of medical applications, the development of efficient technologies for functionalization of GNPs with different classes of molecules is essential as this could ensure stability of nanoparticles under in vivo conditions, homing interaction with biological targets, and, consequently, targeted delivery of medicinal preparations or diagnostics markers.
It is now generally accepted that GNP conjugates are perfect labels for bioimaging by different technologies including optical imaging, photoacoustic imaging, SERS, computer tomography, etc. In addition to published examples of clinical diagnostics of cancer, Alzheimer’s disease, HIV, hepatitis, tuberculosis, diabetes, and others, new diagnostic applications for GNPs should be expected.
The development of SERS platforms using plasmonic nanoparticles and NCs is one of the hot areas in analytics and biomedicine. It is our opinion that platforms based on self-assembly of nanoparticles [218, 219] or chemical synthesis of nano-islands [220] offer the most promise. Such platforms have low cost; demonstrate high signal amplification with high signal reproducibility in different spots of the same platform and in platforms synthesized independently. In most cases, the use of silver or gold/silver nanostructures provides advantage in terms of sensitivity of the analyte determination. The SERS platforms produced by electron beam nanolithography exhibit the highest reproducibility of controlled properties [221]. However, this technology is quite expensive, and signal amplification is often even lower than that observed with self-assembled or island-type structures. A new type of composite particles with Raman molecules inside the plasmonic nanostructure offers promise for diagnostics [222-224]. The main advantage of these particles is high signal amplification (one order of magnitude higher than for molecules on the surface of particles) and independence of stable signal on environmental conditions such as those outside and inside the cell.
Targeted delivery of DNA, antigens, and medicinal preparations using nanoparticles appears as the most promising approach in biomedicine. Functionalization of GNPs with molecular vectors to cancer cell receptors increases significantly the delivery of nanoparticles to cell targets. Respectively, loading of GNPs with anticancer preparation will enhance its targeted delivery. In addition to chemotherapeutic actions, these conjugates can be used for thermal therapy of tumors.
Analysis of literature data shows that the development of a universal support for all types of delivered compounds and biotargets is unlikely. It is more likely and seems more appropriate to design a support that has been optimized for both loading with a certain compound and efficiency of delivery to a certain target. In particular, the stability of the conjugate support in the bloodstream and weak interaction with nontarget and immune cells must be accompanied by effective penetration into the target cells. It is quite possible that these properties can be generated using not statically, but dynamically controlled nanosystems, whose functions can be switched via a particular signal (optical, magnetic, acoustic, etc.). In this connection, high hopes are associated with fast progress in the development of technologies for synthesis of multifunctional NCs combining the controlled physical properties (magnetic, optical, photodynamic, radioactive, etc.) with improved technologies of molecular surface targeting. Precisely these constructs can ensure targeted delivery of nanoparticles for both visualization and targeted therapy. Multifunctional nanoparticles allow simultaneous delivery of several target agents, which results in effective combined therapeutic regimes for cancer treatment.
Plasmonic PT laser cancer therapy with GNPs first reported in 2003 is now undergoing clinical trials. The modern state of laboratory studies and potential for their transfer to clinical practice was reviewed recently [225]. The available experimental data indicate that further progress in the development of nano-oncology should be expected in combination of various technologies including photodynamics, chemotherapy, gene therapy, and other approaches of this type. The delivery of genetic material that can suppress the aggressive growth of cancer cells and their metastasizing in combination with other nanobiotechnologies and surgical approaches could be the most efficient direction. Furthermore, the potential for the use of multifunctional composites as a platform for nanovaccine development is expanding.
In this review, we considered different variants of application of multifunctional GNPs. It is exactly the availability of multiple functions provided via different component composition of nanoparticles (NCs) or via different functional groups on their surface (multifunctionalized GNPs), or via a combination of these properties (multifunctionalized NCs) that explains active application of this type of nanoparticles in theranostics. Multifunctionality ensures both diagnostic and therapeutic (chemical and physical action) possibilities of application of these nanocomplexes. Moreover, very often multifunctionality is manifested through synergetic effect of GNP action either in vitro or in vivo. In our opinion multifunctional GNPs have great therapeutic and diagnostic potential in various medical studies, and, most importantly, in practical personalized medicine.
Progress in genomics and proteomics has resulted in the growth of information related to the molecular biomarkers of different types of cancer. This information will help in development of novel multifunctional GNPs that can identify tumor target cells and exert very precise and specific action on them. Novel, more complex functions of “smart” multimodal GNPs will ensure early diagnostic of diseases and monitoring of treatment of patients in real time.
Acknowledgements
This work was supported by the Russian Science Foundation (projects Nos. 14-13-01167, NGK; and 15-14-00002, LAD).
REFERENCES
1.Dykman, L. A., Bogatyrev, V. A., Shchegolev, S.
Yu., and Khlebtsov, N. G. (2008) Gold Nanoparticles: Synthesis,
Properties, and Biomedical Applications [in Russian], Nauka,
Moscow, p. 319.
2.Dykman, L. A., and Khlebtsov, N. G. (2012) Gold
nanoparticles in biomedical applications: recent advances and
perspectives, Chem. Soc. Rev., 41, 2256-2282.
3.Dreaden, E. C., Alkilany, A. M., Huang, X., Murphy,
C. J., and El-Sayed, M. A. (2012) The golden age: gold nanoparticles
for biomedicine, Chem. Soc. Rev., 41, 2740-2779.
4.Khlebtsov, N. G. (2008) Optics and biophotonics of
nanoparticles with a plasmon resonance, Quant. Electron.,
38, 504-529.
5.Quinten, M. (2011) Optical Properties of
Nanoparticle Systems, Wiley-VCH, Weinheim, p. 502.
6.Stockman, M. I. (2011) Nanoplasmonics: past,
present, and glimpse into future, Opt. Express, 19,
22029-22106.
7.Khlebtsov, N. G., and Dykman, L. A. (2010) Optical
properties and biomedical applications of plasmonic nanoparticles,
J. Quant. Spectr. Radiat. Transfer, 111, 1-35.
8.Glomm, W. R. (2005) Functionalized gold
nanoparticles for application in biotechnology, J. Dispers. Sci.
Technol., 26, 389-414.
9.Sapsford, K. E., Algar, W. R., Berti, L., Gemmill,
K. B., Casey, B. J., Oh, E., Stewart, M. H., and Medintz, I. L. (2013)
Functionalizing nanoparticles with biological molecules: developing
chemistries that facilitate nanotechnology, Chem. Rev.,
113, 1904-2074.
10.Jaque, D., Richard, C., Viana, B., Soga, K., Liu,
X., and Sole, J. G. (2016) Inorganic nanoparticles for optical
bioimaging, Adv. Opt. Photonics, 8, 1-103.
11.Cang, H., Sun, T., Li, Z.-Y., Chen, J. Y., Wiley,
B. J., Xia, Y. N., and Li, X. D. (2005) Gold nanocages as contrast
agents for spectroscopic optical coherence tomography, Opt.
Lett., 30, 3048-3050.
12.Wang, L. V. (2009) Photoacoustic Imaging and
Spectroscopy, CRC Press, Boca Raton, p. 518.
13.Bardhan, R., Lal, S., Joshi, A., and Halas, N. J.
(2011) Theranostic nanoshells: from probe design to imaging and
treatment of cancer, Acc. Chem. Res., 44, 936-946.
14.Kennedy, L. C., Bickford, L. R., Lewinski, N. A.,
Coughlin, A. J., Hu, Y., Day, E. S., West, J. L., and Drezek, R. A.
(2011) A new era for cancer treatment: gold-nanoparticle-mediated
thermal therapies, Small, 7, 169-183.
15.Pissuwan, D., Niidome, T., and Cortie, M. B.
(2011) The forthcoming applications of gold nanoparticles in drug and
gene delivery systems, J. Control. Release, 149,
65-71.
16.Picard, F. J., and Bergeron, M. G. (2002) Rapid
molecular theranostics in infectious diseases, Drug Discov.
Today, 7, 1092-1101.
17.Wagner, D. S., Delk, N. A., Lukianova-Hleb, E.
Y., Hafner, J. H., Farach-Carson, M. C., and Lapotko, D. O. (2010) The
in vivo performance of plasmonic nanobubbles as cell theranostic
agents in zebrafish hosting prostate cancer xenografts,
Biomaterials, 31, 7567-7574.
18.Lukianova-Hleb, E., Oginsky, A. O., Samaniego, A.
P., Shenefelt, D. L., Wagner, D. S., Hafner, J. H., Farach-Carson, C.,
and Lapotko, D. O. (2011) Tunable plasmonic nanoprobes for theranostics
of prostate cancer, Theranostics, 1, 3-17.
19.Lammers, T., Aime, S., Hennink, W. E., Storm, G.,
and Kiessling, F. (2011) Theranostic nanomedicine, Acc. Chem.
Res., 44, 1029-1038.
20.Funkhouser, J. (2002) Reinventing pharma: the
theranostic revolution, Curr. Drug Discov., 2, 17-19.
21.Warner, S. (2004) Diagnostics +
therapy = theranostics, Scientist, 18, 38-39.
22.Kelkar, S. S., and Reineke, T. M. (2011)
Theranostics: combining imaging and therapy, Bioconjug. Chem.,
22, 1879-1903.
23.Motl, N. E., Smith, A. F., DeSantis, C. J., and
Skrabalak, S. E. (2014) Engineering plasmonic metal colloids through
composition and structural design, Chem. Soc. Rev., 43,
3823-3834.
24.Jiao, P. F., Zhou, H. Y., Chen, L. X., and Yan,
B. (2011) Cancer-targeting multifunctionalized gold nanoparticles in
imaging and therapy, Curr. Med. Chem., 18, 2086-2102.
25.Liang, R., Wei, M., Evans, D. G., and Duan, X.
(2014) Inorganic nanomaterials for bioimaging, targeted drug delivery
and therapeutics, Chem. Commun. (Camb.), 50,
14071-14081.
26.Webb, J. A., and Bardhan, R. (2014) Emerging
advances in nanomedicine with engineered gold nanostructures,
Nanoscale, 6, 2502-2530.
27.Pellegrino, T., Kudera, S., Liedl, T., Javier, A.
M., Manna, L., and Parak, W. J. (2005) On the development of colloidal
nanoparticles towards multifunctional structures and their possible use
for biological applications, Small, 1, 48-63.
28.Minelli, C., Lowe, S. B., and Stevens, M. M.
(2010) Engineering nanocomposite materials for cancer therapy,
Small, 6, 2336-2357.
29.Conde, J., Doria, G., and Baptista, P. (2012)
Noble metal nanoparticles applications in cancer, J. Drug
Deliv., 2012, 751075.
30.Kim, D., and Jon, S. (2012) Gold nanoparticles in
image-guided cancer therapy, Inorg. Chim. Acta, 393,
154-164.
31.Sotiriou, G. A. (2013) Biomedical applications of
multifunctional plasmonic nanoparticles, Wiley Interdiscip. Rev.
Nanomed. Nanobiotechnol., 5, 19-30.
32.Ahmad, M. Z., Akhter, S., Rahman, Z., Akhter, S.,
Anwar, M., Mallik, N., and Ahmad, F. J. (2013) Nanometric gold in
cancer nanotechnology: current status and future prospect, J. Pharm.
Pharmacol., 65, 634-651.
33.Le Trequesser, Q., Seznec, H., and Delville,
M.-H. (2013) Functionalized nanomaterials: their use as contrast agents
in bioimaging: mono- and multimodal approaches, Nanotechnol.
Rev., 2, 125-169.
34.Chatterjee, K., Sarkar, S., Jagajjanani Rao, K.,
and Paria, S. (2014) Core/shell nanoparticles in biomedical
applications, Adv. Colloid Interface Sci., 209, 8-39.
35.Saleh, N. B., Afrooz, A. R. M. N., Bisesi, J. H.,
Jr., Aich, N., Plazas-Tuttle, J., and Sabo-Attwood, T. (2014) Emergent
properties and toxicological considerations for nanohybrid materials in
aquatic systems, Nanomaterials, 4, 372-407.
36.Jin, Y. (2014) Multifunctional compact hybrid Au
nanoshells: a new generation of nanoplasmonic probes for biosensing,
imaging, and controlled release, Acc. Chem. Res., 47,
138-148.
37.Srinivasan, M., Rajabi, M., and Mousa, S. A.
(2015) Multifunctional nanomaterials and their applications in drug
delivery and cancer therapy, Nanomaterials, 5,
1690-1703.
38.El-Toni, A. M., Habila, M. A., Labis, J. P.,
AlOthman, Z. A., Alhoshan, M., Elzatahry, A. A., and Zhang, F. (2016)
Design, synthesis and applications of core-shell, hollow core, and
nanorattle multifunctional nanostructures, Nanoscale, 8,
2510-2531.
39.Lim, W. Q., Phua, S. Z. F., Xu, H. V., Sreejith,
S., and Zhao, Y. (2016) Recent advances in multifunctional silica-based
hybrid nanocarriers for bioimaging and cancer therapy,
Nanoscale, 8, 12510-12519.
40.Shahbazi, R., Ozpolat, B., and Ulubayram, K.
(2016) Oligonucleotide-based theranostic nanoparticles in cancer
therapy, Nanomedicine (Lond.), 11, 1287-1308.
41.Sounderya, N., and Zhang, Y. (2008) Use of
core/shell structured nanoparticles for biomedical applications,
Recent Patents Biomed. Eng., 1, 34-42.
42.Cortie, M. B., and McDonagh, A. M. (2011)
Synthesis and optical properties of hybrid and alloy plasmonic
nanoparticles, Chem. Rev., 111, 3713-3735.
43.Pomogailo, A. D., and Kestelman, V. N. (2005)
Metallopolymer Nanocomposites, Springer, Berlin, p.
564.
44.Pereira, S. O., Barros-Timmons, A., and Trindade,
T. (2014) Biofunctionalization of colloidal gold nanoparticles via
polyelectrolytes assemblies, Colloid Polym. Sci., 292,
33-50.
45.Bielinska, A., Eichman, J. D., Lee, I., Baker, J.
R., Jr., and Balogh, L. (2002) Imaging {Au0-PAMAM} gold-dendrimer
nanocomposites in cells, J. Nanopart. Res., 4,
395-403.
46.Kojima, C., Umeda, Y., Ogawa, M., Harada, A.,
Magata, Y., and Kono, K. (2010) X-ray computed tomography contrast
agents prepared by seeded growth of gold nanoparticles in PEGylated
dendrimer, Nanotechnology, 21, 245104.
47.Balogh, L. P., Nigavekar, S. S., Cook, A. C.,
Minc, L., and Khan, M. K. (2003) Development of dendrimer-gold
radioactive nanocomposites to treat cancer microvasculature,
PharmaChem., 2, 94-99.
48.Giesfeldt, K. S., Connatser, R. M., De Jesus, M.
A., Dutta, P., and Sepaniak, M. J. (2005) Gold-polymer nanocomposites:
studies of their optical properties and their potential as SERS
substrates, J. Raman Spectrosc., 36, 1134-1142.
49.Sershen, S. R., Westcott, S. L., Halas, N. J.,
and West, J. L. (2000) Temperature-sensitive polymer-nanoshell
composites for photothermally modulated drug delivery, J. Biomed.
Mater. Res., 51, 293-298.
50.Young, J. K., Figueroa, E. R., and Drezek, R. A.
(2012) Tunable nanostructures as photothermal theranostic agents,
Ann. Biomed. Eng., 40, 438-459.
51.Doane, T. L., and Burda, C. (2012) The unique
role of nanoparticles in nanomedicine: imaging, drug delivery and
therapy, Chem. Soc. Rev., 41, 2885-2911.
52.Li, X., Takashima, M., Yuba, E., Harada, A., and
Kono, K. (2014) PEGylated PAMAM dendrimere doxorubicin
conjugate-hybridized gold nanorod for combined photothermal
chemotherapy, Biomaterials, 35, 6576-6584.
53.Esumi, K., Takei, N., and Yoshimura, T. (2003)
Antioxidant-potentiality of gold–chitosan nanocomposites,
Colloids Surf. B, 32, 117-123.
54.Ding, L., Hao, C., Xue, Y., and Ju, H. (2007) A
bio-inspired support of gold nanoparticles–chitosan
nanocomposites gel for immobilization and electrochemical study of K562
leukemia cells, Biomacromolecules, 8, 1341-1346.
55.Zhao, X. J., Mai, Z. B., Kang, X. H., Dai, Z.,
and Zou, X. Y. (2008) Clay-chitosan-gold nanoparticle nanohybrid:
preparation and application for assembly and direct electrochemistry of
myoglobin, Electrochim. Acta, 53, 4732-4739.
56.Chen, R., Zheng, X., Qian, H., Wang, X., Wang,
J., and Jiang, X. (2013) Combined near-IR photothermal therapy and
chemotherapy using gold-nanorod/chitosan hybrid nanospheres to enhance
the antitumor effect, Biomater. Sci., 3, 285-293.
57.Chen, R., Wang, X., Yao, X., Zheng, X., Wang, J.,
and Jiang, X. (2013) Near-IR-triggered photothermal/photodynamic
dual-modality therapy system via chitosan hybrid nanospheres,
Biomaterials, 34, 8314-8322.
58.Hu, Y., Chen, Q., Ding, Y., Li, R., Jiang, X.,
and Liu, B. (2009) Entering and lighting up nuclei using hollow
chitosan–gold hybrid nanospheres, Adv. Mater., 21,
3639-3643.
59.Cho, H.-J., Oh, J., Choo, M.-K., Ha, J.-I., Park,
Y., and Maeng, H.-J. (2014) Chondroitin sulfate-capped gold
nanoparticles for the oral delivery of insulin, Int. J. Biol.
Macromol., 63, 15-20.
60.Hung, H.-S., Chang, C.-H., Chang, C.-J., Tang,
C.-M., Kao, W.-C., Lin, S.-Z., Hsieh, H.-H., Chu, M.-Y., Sun, W.-S.,
and Hsu, S.-H. (2014) In vitro study of a novel
nanogold–collagen composite to enhance the mesenchymal stem cell
behavior for vascular regeneration, PLoS One, 9,
e104019.
61.Saha, A., Basiruddin, S. K., Sarkar, R., Pradhan,
N., and Jana, N. R. (2009) Functionalized plasmonic-fluorescent
nanoparticles for imaging and detection, J. Phys. Chem. C,
113, 18492-18498.
62.Pan, D. P. J., Pramanik, M., Senpan, A., Ghosh,
S., Wickline, S. A., Wang, L. V., and Lanza, G. M. (2010) Near infrared
photoacoustic detection of sentinel lymph nodes with gold nanobeacons,
Biomaterials, 31, 4088-4093.
63.Tam, J. M., Tam, J. O., Murthy, A., Ingram, D.
R., Ma, L. L., Travis, K., Johnston, K. P., and Sokolov, K. V. (2010)
Controlled assembly of biodegradable plasmonic nanoclusters for
near-infrared imaging and therapeutic applications, ACS Nano,
4, 2178-2184.
64.Song, J., Pu, L., Zhou, J., Duan, B., and Duan,
H. (2013) Biodegradable theranostic plasmonic vesicles of amphiphilic
gold nanorods, ACS Nano, 7, 9947-9960.
65.Song, J., Zhou, J., and Duan, H. (2012)
Self-assembled plasmonic vesicles of SERS-encoded amphiphilic gold
nanoparticles for cancer cell targeting and traceable intracellular
drug delivery, J. Am. Chem. Soc., 134, 13458-13469.
66.Ke, H., Wang, J., Dai, Z., Jin, Y., Qu, E., Xing,
Z., Guo, C., Yue, X., and Liu, J. (2011) Gold-nanoshelled
microcapsules: a theranostic agent for ultrasound contrast imaging and
photothermal therapy, Angew. Chem. Int. Ed., 50,
3017-3021.
67.Huang, P., Lin, J., Li, W., Rong, P., Wang, Z.,
Wang, S., Wang, X., Sun, X., Aronova, M., Niu, G., Leapman, R. D., Nie,
Z., and Chen, X. (2013) Biodegradable gold nanovesicles with an
ultrastrong plasmonic coupling effect for photoacoustic imaging and
photothermal therapy, Angew. Chem. Int. Ed., 52,
13958-13964.
68.Lin, J., Wang, S., Huang, P., Wang, Z., Chen, S.,
Niu, G., Li, W., He, J., Cui, D., Lu, G., Chen, X., and Nie, Z. (2013)
Photosensitizer-loaded gold vesicles with strong plasmonic coupling
effect for imaging-guided photothermal/photodynamic therapy, ACS
Nano, 7, 5320-5329.
69.Deng, H., Dai, F., Ma, G., and Zhang, X. (2015)
Theranostic gold nanomicelles made from biocompatible comb-like
polymers for thermochemotherapy and multifunctional imaging with rapid
clearance, Adv. Mater., 27, 3645-3653.
70.Gao, L., Fei, J., Zhao, J., Li, H., Cui, Y., and
Li, J. (2012) Hypocrellin-loaded gold nanocages with high two-photon
efficiency for photothermal/photodynamic cancer therapy in
vitro, ACS Nano, 6, 8030-8040.
71.Liao, J., Li, W., Peng, J., Yang, Q., Li, H.,
Wei, Y., Zhang, X., and Qian, Z. (2015) Combined cancer
photothermal-chemotherapy based on doxorubicin/gold nanorod-loaded
polymerosomes, Theranostics, 5, 345-356.
72.Wu, G., Mikhailovsky, A., Khant, H. A., and
Zasadzinski, J. A. (2009) Synthesis, characterization, and optical
response of gold nanoshells used to trigger release from liposomes,
Methods Enzymol., 464, 279-307.
73.Jin, Y. D., and Gao, X. H. (2009) Spectrally
tunable leakage-free gold nanocontainers, J. Am. Chem. Soc.,
131, 17774-17776.
74.Park, H., Yang, J., Seo, S., Kim, K., Suh, J.,
Kim, D., Haam, S., and Yoo, K.-H. (2008) Multifunctional nanoparticles
for photothermally controlled drug delivery and magnetic resonance
imaging enhancement, Small, 4, 192-196.
75.Lee, S.-M., Park, H., and Yoo, K.-H. (2010)
Synergistic cancer therapeutic effects of locally delivered drug and
heat using multifunctional nanoparticles, Adv. Mater.,
22, 4049-4053.
76.Wang, Y.-H., Chen, S.-P., Liao, A.-H., Yang,
Y.-C., Lee, C.-R., Wu, C.-H., Wu, P.-C., Liu, T.-M., Wang, C.-R. C.,
and Li, P.-C. (2014) Synergistic delivery of gold nanorods using
multifunctional microbubbles for enhanced plasmonic photothermal
therapy, Sci. Rep., 4, 5685.
77.Peralta, D. V., He, J., Wheeler, D. A., Zhang, J.
Z., and Tarr, M. A. (2014) Encapsulating gold nanomaterials into
size-controlled human serum albumin nanoparticles for cancer therapy
platforms, J. Microencapsul., 31, 824-831.
78.Park, G.-S., Kwon, H., Kwak, D. W., Park, S. Y.,
Kim, M., Lee, J.-H., Han, H., Heo, S., Li, X. S., Lee, J. H., Kim, Y.
H., Lee, J.-G., Yang, W., Cho, H. Y., Kim, S. K., and Kim, K. (2012)
Full surface embedding of gold clusters on silicon nanowires for
efficient capture and photothermal therapy of circulating tumor cells,
Nano Lett., 12, 1638-1642.
79.Botella, P., Ortega, I., Quesada, M., Madrigal,
R. F., Muniesa, C., Fimia, A., Fernandez, E., and Corma, A. (2012)
Multifunctional hybrid materials for combined photo and chemotherapy of
cancer, Dalton Trans., 41, 9286-9296.
80.Zhang, Z., Wang, L., Wang, J., Jiang, X., Li, X.,
Hu, Z., Ji, Y., Wu, X., and Chen, C. (2012) Mesoporous silica-coated
gold nanorods as a light-mediated multifunctional theranostic platform
for cancer treatment, Adv. Mater., 24, 1418-1423.
81.Monem, A. S., Elbialy, N., and Mohamed, N. (2014)
Mesoporous silica coated gold nanorods loaded doxorubicin for combined
chemo-photothermal therapy, Int. J. Pharm., 470, 1-7.
82.Zhang, Y., Qian, J., Wang, D., Wang, Y., and He,
S. (2013) Multifunctional gold nanorods with ultrahigh stability and
tunability for in vivo fluorescence imaging, SERS detection, and
photodynamic therapy, Angew. Chem., Int. Ed., 52,
1148-1151.
83.Fales, A. M., Yuan, H., and Vo-Dinh, T. (2011)
Silica-coated gold nanostars for combined surface-enhanced Raman
scattering (SERS) detection and singlet-oxygen generation: a potential
nanoplatform for theranostics, Langmuir, 27,
12186-12190.
84.Vankayala, R., Lin, C.-C., Kalluru, P., Chiang,
C.-S., and Hwang, K. C. (2014) Gold nanoshells-mediated bimodal
photodynamic and photothermal cancer treatment using ultra-low doses of
near infra-red light, Biomaterials, 35, 5527-5538.
85.Ouhenia-Ouadahi, K., Yasukuni, R., Yu, P.,
Laurent, G., Pavageau, C., Grand, J., Guerin, J., Leaustic, A., Felidj,
N., Aubard, J., Nakatani, K., and Metivier, R. (2014)
Photochromic–fluorescent–plasmonic nanomaterials: towards
integrated three-component photoactive hybrid nanosystems, Chem.
Commun., 50, 7299-7302.
86.Fang, S., Li, C., Lin, J., Zhu, H., Cui, D., Xu,
Y., and Li, Z. (2016) Gold nanorods-based theranostics for simultaneous
fluorescence/two-photon luminescence imaging and synergistic
phototherapies, J. Nanomater., 2016, 1082746.
87.Khlebtsov, B. N., Tuchina, E. S., Khanadeev, V.
A., Panfilova, E. V., Petrov, P. O., Tuchin, V. V., and Khlebtsov, N.
G. (2013) Enhanced photoinactivation of Staphylococcus aureus
with nanocomposites containing plasmonic particles and hematoporphyrin,
J. Biophotonics, 6, 338-351.
88.Khlebtsov, B., Panfilova, E., Khanadeev, V.,
Bibikova, O., Terentyuk, G., Ivanov, A., Rumyantseva, V., Shilov, I.,
Ryabova, A., Loshchenov, V., and Khlebtsov, N. (2011) Nanocomposites
containing silica-coated gold-silver nanocages and
Yb-2,4-dimethoxyhematoporphyrin: multifunctional capability of
IR-luminescence detection, photosensitization, and photothermolysis,
ACS Nano, 5, 7077-7089.
89.Khlebtsov, B. N., Panfilova, E. V., Khanadeev, V.
A., Markin, A. V., Terentyuk, G. S., Rumyantseva, V. D., Ivanov, A. V.,
Shilov, I. P., and Khlebtsov, N. G. (2011) Composite multifunctional
nanoparticles based on silica-coated gold-silver nanocages
functionalized by Yb-hematoporphyrin, Nanotechnol. Russia,
6, 496-503.
90.Terentyuk, G., Panfilova, E., Khanadeev, V.,
Chumakov, D., Genina, E., Bashkatov, A., Tuchin, V., Bucharskaya, A.,
Maslyakova, G., Khlebtsov, N., and Khlebtsov, B. (2014) Gold nanorods
with a hematoporphyrin-loaded silica shell for dual-modality
photodynamic and photothermal treatment of tumors in vivo,
Nano Res., 7, 325-337.
91.Huang, P., Bao, L., Zhang, C., Lin, J., Luo, T.,
Yang, D., He, M., Li, Z., Gao, G., Gao, B., Fu, S., and Cui, D. (2011)
Folic acid-conjugated silica-modified gold nanorods for X-ray/CT
imaging-guided dual-mode radiation and photothermal therapy,
Biomaterials, 32, 9796-9809.
92.Ayala-Orozco, C., Urban, C., Knight, M. W.,
Urban, A. S., Neumann, O., Bishnoi, S. W., Mukherjee, S., Goodman, A.
M., Charron, H., Mitchell, T., Shea, M., Roy, R., Nanda, S., Schiff,
R., Halas, N. J., and Joshi, A. (2014) Au nanomatryoshkas as efficient
near-infrared photothermal transducers for cancer treatment:
benchmarking against nanoshells, ACS Nano, 8,
6372-6381.
93.Leung, K. C., and Xuan, S. (2016) Noble
metal-iron oxide hybrid nanomaterials: emerging applications, Chem.
Rec., 16, 458-472.
94.Wu, C.-H., Cook, J., Emelianov, S., and Sokolov,
K. (2014) Multimodal magneto-plasmonic nanoclusters for biomedical
applications, Adv. Funct. Mater., 24, 6862-6871.
95.Kim, J., Park, S., Lee, J. E., Jin, S. M., Lee,
J. H., Lee, I. S., Yang, I., Kim, J. S., Kim, S. K., Cho, M. H., and
Hyeon, T. (2006) Designed fabrication of multifunctional magnetic gold
nanoshells and their application to magnetic resonance imaging and
photothermal therapy, Angew. Chem. Int. Ed., 45,
7754-7758.
96.Larson, T. A., Bankson, J., Aaron, J., and
Sokolov, K. (2007) Hybrid plasmonic magnetic nanoparticles as molecular
specific agents for MRI/optical imaging and photothermal therapy
of cancer cells, Nanotechnology, 18, 325101.
97.Zhou, T., Wu, B., and Xing, D. (2012)
Bio-modified Fe3O4 core/Au shell nanoparticles
for targeting and multimodal imaging of cancer cells, J. Mater.
Chem., 22, 470-477.
98.Ji, X., Shao, R., Elliott, A. M., Stafford, R.
J., Esparza-Coss, E., Bankson, J. A., Liang, G., Luo, Z.-P., Park, K.,
Markert, J. T., and Li, C. (2007) Bifunctional gold nanoshells with a
superparamagnetic iron oxide–silica core suitable for both MR
imaging and photothermal therapy, J. Phys. Chem. C, 111,
6245-6251.
99.Kim, D., Kim, J. W., Jeong, Y. Y., and Jon, S.
(2009) Antibiofouling polymer coated gold-iron oxide nanoparticle
(GION) as a dual contrast agent for CT and MRI, Bull. Korean Chem.
Soc., 30, 1855-1857.
100.Kim, D., Yu, M. K., Lee, T. S., Park, J. J.,
Jeong, Y. Y., and Jon, S. (2011) Amphiphilic polymer-coated hybrid
nanoparticles as CT/MRI dual contrast agents, Nanotechnology,
22, 155101.
101.Jin, Y. D., Jia, C. X., Huang, S.-W.,
O’Donnell, M., and Gao, X. H. (2010) Multifunctional
nanoparticles as coupled contrast agents, Nat. Commun.,
1, 41.
102.Wang, C., Chen, J., Talavage, T., and
Irudayaraj, J. (2009) Gold nanorod/Fe3O4
nanoparticle “nano-pearl-necklace” for simultaneous
targeting, dual-mode imaging and photothermal ablation of cancer cells,
Angew. Chem. Int. Ed., 48, 2759-2763.
103.Mohammad, F., Balaji, G., Weber, A., Uppu, R.
M., and Kumar, C. S. S. R. (2010) Influence of gold nanoshell on
hyperthermia of superparamagnetic iron oxide nanoparticles, J. Phys.
Chem. C, 114, 19194-19201.
104.Feng, W., Zhou, X., Nie, W., Chen, L., Qiu, K.,
Zhang, Y., and He, C. (2015) Au/polypyrrole@Fe3O4
nanocomposites for MR/CT dual-modal imaging guided-photothermal
therapy: an in vitro study, ACS Appl. Mater. Interfaces,
7, 4354-4367.
105.Li, J., Hu, Y., Yang, J., Wei, P., Sun, W.,
Shen, M., Zhang, G., and Shi, X. (2015) Hyaluronic acid-modified
Fe3O4@Au core/shell nanostars for multimodal
imaging and photothermal therapy of tumors, Biomaterials,
38, 10-21.
106.Pang, L.-L., Li, J.-S., Jiang, J.-H., Le, Y.,
Shen, G. L., and Yu, R.-Q. (2007) A novel detection method for DNA
point mutation using QCM based on Fe3O4/Au
core/shell nanoparticle and DNA ligase reaction, Sens. Actuators
B, 127, 311-316.
107.Zhang, H., and Meyerhoff, M. E. (2006)
Gold-coated magnetic particles for solid-phase immunoassays: enhancing
immobilized antibody binding efficiency and analytical performance,
Anal. Chem., 78, 609-616.
108.Zhang, H., Sun, Y., Gao, S., Zhang, H., Zhang,
J., Bai, Y., and Song, D. (2014) Studies of gold nanorod-iron oxide
nanohybrids for immunoassay based on SPR biosensor, Talanta,
125, 29-35.
109.Hu, X. G., Wei, C.-W., Xia, J. J., Pelivanov,
I., O’Donnell, M., and Gao, X. H. (2013) Trapping and
photoacoustic detection of CTCs at the single cell per milliliter level
with magneto-optical coupled nanoparticles, Small, 9,
2046-2052.
110.Chao, X., Shi, F., Zhao, Y. Y., Li, K., Peng,
M. L., Chen, C., and Cui, Y. L. (2010) Cytotoxicity of
Fe3O4/Au composite nanoparticles loaded with
doxorubicin combined with magnetic field, Pharmazie, 65,
500-504.
111.Kayal, S., and Ramanujan, R. V. (2010)
Anti-cancer drug loaded iron-gold core-shell nanoparticles (Fe@Au) for
magnetic drug targeting, J. Nanosci. Nanotechnol., 10,
5527-5539.
112.Ma, M., Chen, H., Chen, Y., Wang, X., Chen, F.,
Cui, X., and Shi, J. (2012) Au capped magnetic core/mesoporous silica
shell nanoparticles for combined photothermo-/chemo-therapy and
multimodal imaging, Biomaterials, 33, 989-998.
113.Lee, J., Yang, J., Ko, H., Oh, S. J., Kang, J.,
Son, J.-H., Lee, K., Lee, S.-W., Yoon, H.-G., Suh, J.-S., Huh, Y.-M.,
and Haam, S. (2008) Multifunctional magnetic gold nanocomposites: human
epithelial cancer detection via magnetic resonance imaging and
localized synchronous therapy, Adv. Funct. Mater., 18,
258-264.
114.Melancon, M. P., Elliott, A., Ji, X., Shetty,
A., Yang, Z., Tian, M., Taylor, B., Stafford, R. J., and Li, C. (2011)
Theranostics with multifunctional magnetic gold nanoshells:
photothermal therapy and t2* magnetic resonance imaging, Invest.
Radiol., 46, 132-140.
115.Cheng, L., Yang, K., Li, Y., Zeng, X., Shao,
M., Lee, S.-T., and Liu, Z. (2012) Multifunctional nanoparticles for
upconversion luminescence/MR multimodal imaging and magnetically
targeted photothermal therapy, Biomaterials, 33,
2215-2222.
116.Sotiriou, G. A., Starsich, F., Dasargyri, A.,
Wurnig, M. C., Krumeich, F., Boss, A., Leroux, J.-C., and Pratsinis, S.
E. (2014) Photothermal killing of cancer cells by the controlled
plasmonic coupling of silica-coated Au/Fe2O3
nanoaggregates, Adv. Funct. Mater., 24, 2818-2827.
117.Kim, D. H., Rozhkova, E. A., Rajh, T., Bader,
S. D., and Novosad, V. (2009) Synthesis of hybrid gold/iron oxide
nanoparticles in block copolymer micelles for imaging, drug delivery,
and magnetic hyperthermia, IEEE Trans. Magn., 45,
4821-4824.
118.Yang, H. W., Liu, H. L., Li, M. L., His, I. W.,
Fan, C. T., Huang, C. Y., Lu, Y. J., Hua, M. Y., Chou, H. Y., Liaw, J.
W., Ma, C. C., and Wei, K. C. (2013) Magnetic gold-nanorod/PNIPAAmMA
nanoparticles for dual magnetic resonance and photoacoustic imaging and
targeted photothermal therapy, Biomaterials, 34,
5651-5660.
119.Ohulchanskyy, T. Y., Kopwitthaya, A., Jeon, M.,
Guo, M., Law, W. C., Furlani, E. P., Kim, C., and Prasad, P. N. (2013)
Phospholipid micelle-based magneto-plasmonic nanoformulation for
magnetic field-directed, imaging-guided photo-induced cancer therapy,
Nanomedicine, 9, 1192-1202.
120.Ma, Y., Liang, X., Tong, S., Bao, G., Ren, Q.,
and Dai, Z. (2013) Gold nanoshell nanomicelles for potential magnetic
resonance imaging, light-triggered drug release, and photothermal
therapy, Adv. Funct. Mater., 23, 815-822.
121.Sun, L., Joh, D., Al-Zaki, A., Stangl, M.,
Murty, S., Davis, J. J., Baumann, B. C., Alonso-Basanta, M., Kao, G.
D., Tsourkas, A., and Dorsey, J. F. (2016) Theranostic application of
mixed gold and superparamagnetic iron oxide nanoparticle micelles in
glioblastoma multiforme, J. Biomed. Nanotechnol., 12,
347-356.
122.Carril, M., Fernandez, I., Rodriguez, J.,
Garcia, I., and Penades, S. (2014) Gold-coated iron oxide
glyconanoparticles for MRI, CT, and US multimodal imaging, Part.
Part. Syst. Charact., 31, 81-87.
123.Huang, W. C., Tsai, P.-J., and Chen, Y.-C.
(2009) Multifunctional Fe3O4@Au nanoeggs as
photothermal agents for selective killing of nosocomial and
antibiotic-resistant bacteria, Small, 4, 51-56.
124.Fan, Z., Shelton, M., Singh, A. K., Senapati,
D., Khan, S. A., and Ray, P. C. (2012) Multifunctional plasmonic
shell-magnetic core nanoparticles for targeted diagnostics, isolation,
and photothermal destruction of tumor cells, ACS Nano, 6,
1065-1073.
125.Chen, W., Xu, N. F., Xu, L. G., Wang, L. B.,
Li, Z. K., Ma, W., Zhu, Y. Y., Xu, C. L., and Kotov, N. A. (2010)
Multifunctional magnetoplasmonic nanoparticle assemblies for cancer
therapy and diagnostics (theranostics), Macromol. Rapid Commun.,
31, 228-236.
126.Cheng, F.-Y., Su, C.-H., Wu, P.-C., and Yeh,
C.-S. (2010) Multifunctional polymeric nanoparticles for combined
chemotherapeutic and near-infrared photothermal cancer therapy in
vitro and in vivo, Chem. Commun., 46,
3167-3169.
127.Hu, J. W., Li, J. F., Ren, B., Wu, D. Y., Sun,
S. G., and Tian, Z. Q. (2007) Palladium-coated gold nanoparticles with
a controlled shell thickness used as surface-enhanced Raman scattering
substrate, J. Phys. Chem. C, 111, 1105-1112.
128.Li, C., Su, Y., Lv, X., Zuo, Y., Yang, X., and
Wang, Y. (2012) Au@Pd core-shell nanoparticles: a highly active
electrocatalyst for amperometric gaseous ethanol sensors, Sens.
Actuators B, 171-172, 1192-1198.
129.Bouchard, L.-S., Anwar, M. S., Liu, G. L.,
Hann, B., Xie, Z. H., Gray, J. W., Wang, X., Pines, A., and Chen, F. F.
(2009) Picomolar sensitivity MRI and photoacoustic imaging of cobalt
nanoparticles, Proc. Natl. Acad. Sci. USA, 106,
4085-4089.
130.Sharma, P., Brown, S. C., Bengtsson, N., Zhang,
Q., Walter, G. A., Grobmyer, S. R., Santra, S., Jiang, H., Scott, E.
W., and Moudgil, B. M. (2008) Gold-speckled multimodal nanoparticles
for noninvasive bioimaging, Chem. Mater., 20,
6087-6094.
131.Beija, M., Li, Y., Duong, H. T., Laurent, S.,
Vander, E. L., Muller, R. N., Lowe, A. B., Davis, T. P., and Boyer, C.
(2012) Polymer-gold nanohybrids with potential use in bimodal MRI/CT:
enhancing the relaxometric properties of Gd(III) complexes, J.
Mater. Chem., 22, 21382-21386.
132.Schladt, T. D., Shukoor, M. I., Schneider, K.,
Tahir, M. N., Natalio, F., Ament, I., Becker, J., Jochum, F. D., Weber,
S., Kohler, O., Theato, P., Schreiber, L. M., Sonnichsen, C., Schroder,
H. C., Muller, W. E. G., and Tremel, W. (2010) Au@MnO nanoflowers:
hybrid nanocomposites for selective dual functionalization and imaging,
Angew. Chem. Int. Ed., 49, 3976-3980.
133.Cheng, K., Kothapalli, S.-R., Liu, H., Koh, A.
L., Jokerst, J. V., Jiang, H., Yang, M., Li, J., Levi, J., Wu, J. C.,
Gambhir, S. S., and Cheng, Z. (2014) Construction and validation of
nano gold tripods for molecular imaging of living subjects, J. Am.
Chem. Soc., 136, 3560-3571.
134.Xing, H., Bu, W., Zhang, S., Zheng, X., Li, M.,
Chen, F., He, Q., Zhou, L., Peng, W., Hua, Y., and Shi, J. (2012)
Multifunctional nanoprobes for upconversion fluorescence, MR and CT
trimodal imaging, Biomaterials, 33, 1079-1089.
135.Wu, W., Shen, J., Banerjee, P., and Zhou, S.
(2011) A multifunctional nanoplatform based on responsive fluorescent
plasmonic ZnO-Au@PEG hybrid nanogels, Adv. Funct. Mater.,
21, 2830-2839.
136.Lin, A. Y., Young, J. K., Nixon, A. V., and
Drezek, R. A. (2014) Synthesis of a quantum nanocrystal-gold nanoshell
complex for near-infrared generated fluorescence and photothermal decay
of luminescence, Nanoscale, 6, 10701-10709.
137.Matteini, P., Ratto, F., Rossi, F., Centi, S.,
Dei, L., and Pini, R. (2010) Chitosan films doped with gold nanorods as
laser-activatable hybrid bioadhesives, Adv. Mater., 22,
4313-4316.
138.Perez-Juste, J., Rodriguez-Gonzalez, B.,
Mulvaney, P., and Liz-Marzán, L. M. (2005) Optical control and
patterning of gold-nanorod-poly(vinyl alcohol) nanocomposite films,
Adv. Funct. Mater., 15, 1065-1071.
139.Matteini, P., Martina, M. R., Giambastiani, G.,
Tatini, F., Cascella, R., Ratto, F., Cecchi, C., Caminati, G., Dei, L.,
and Pini, R. (2013) Light-responsive nanocomposite sponges for on
demand chemical release with high spatial and dosage control, J.
Mater. Chem. B, 1, 1096-1100.
140.Oishi, M., Tamura, A., Nakamura, T., and
Nagasaki, Y. (2009) A smart nanoprobe based on fluorescence-quenching
PEGylated nanogels containing gold nanoparticles for monitoring the
response to cancer therapy, Adv. Funct. Mater., 19,
827-834.
141.Zhang, H., and Hu, N. (2007) Assembly of
myoglobin layer-by-layer films with poly(propyleneimine)
dendrimer-stabilized gold nanoparticles and its application in
electrochemical biosensing, Biosens. Bioelectron., 23,
393-399.
142.Nakamura, T., Tamura, A., Murotani, H., Oishi,
M., Jinji, Y., Matsuishi, K., and Nagasaki, Y. (2010) Large payloads of
gold nanoparticles into the polyamine network core of
stimuli-responsive PEGylated nanogels for selective and noninvasive
cancer photothermal therapy, Nanoscale, 2, 739-746.
143.Kim, J.-Y., Choi, W. I., Kim, M., and Tae, G.
(2013) Tumor-targeting nanogel that can function independently for both
photodynamic and photothermal therapy and its synergy from the
procedure of PDT followed by PTT, J. Control. Rel., 171,
113-121.
144.Li, Y., Fan, X., Qi, J., Ji, J., Wang, S.,
Zhang, G., and Zhang, F. (2010) Gold nanoparticles-graphene hybrids as
active catalysts for Suzuki reaction, Mater. Res. Bull.,
45, 1413-1418.
145.Kong, B. S., Jung, D. H., Oh, S. K., Han, C.
S., and Jung, H. T. (2007) Single-walled carbon nanotube gold
nanohybrids: application in highly effective transparent and conductive
films, J. Phys. Chem. C, 111, 8377-8382.
146.Kim, J.-W., Galanzha, E. I., Shashkov, E. V.,
Moon, H.-M., and Zharov, V. P. (2009) Golden carbon nanotubes as
multimodal photoacoustic and photothermal high-contrast molecular
agents, Nat. Nanotechnol., 4, 688-694.
147.Modugno, G., Menard-Moyon, C., Prato, M., and
Bianco, A. (2015) Carbon nanomaterials combined with metal
nanoparticles for theranostic applications, Br. J. Pharm.,
172, 975-991.
148.Lu, Q., Hu, H., Wu, Y., Chen, S., Yuan, D., and
Yuan, R. (2014) An electrogenerated chemiluminescence sensor based on
gold nanoparticles@C60 hybrid for the determination of phenolic
compounds, Biosens. Bioelectron., 60, 325-331.
149.Tchounwou, C., Sinha, S. S., Viraka Nellore, B.
P., Pramanik, A., Kanchanapally, R., Jones, S., Chavva, S. R., and Ray,
P. C. (2015) Hybrid theranostic platform for second near-IR window
light triggered selective two-photon imaging and photothermal killing
of targeted melanoma cells, ACS Appl. Mater. Interfaces,
7, 20649-20656.
150.Minati, L., Antonini, V., Dalla Serra, M., and
Speranza, G. (2012) Multifunctional branched gold-carbon nanotube
hybrid for cell imaging and drug delivery, Langmuir, 28,
15900-15906.
151.Ma, X., Qu, Q., Zhao, Y., Luo, Z., Zhao, Y.,
Ng, K. W., and Zhao, Y. (2013) Graphene oxide wrapped gold
nanoparticles for intracellular Raman imaging and drug delivery, J.
Mater. Chem. B, 1, 6495-6500.
152.Wang, X., Wang, C., Cheng, L., Lee, S. T., and
Liu, Z. (2012) Noble metal coated single-walled carbon nanotubes for
applications in surface enhanced Raman scattering imaging and
photothermal therapy, J. Am. Chem. Soc., 134,
7414-7422.
153.Shi, X., Gong, H., Li, Y., Wang, C., Cheng, L.,
and Liu, Z. (2013) Graphene-based magnetic plasmonic nanocomposite for
dual bioimaging and photothermal therapy, Biomaterials,
34, 4786-4793.
154.Kim, Y.-K., Na, H.-K., Kim, S., Jang, H.,
Chang, S.-J., and Min, D.-H. (2015) One-pot synthesis of
multifunctional Au@graphene oxide nanocolloid core@shell nanoparticles
for Raman bioimaging, photothermal, and photodynamic therapy,
Small, 11, 2527-2535.
155.Maji, S. K., Mandal, A. K., Nguyen, K. T.,
Borah, P., and Zhao, Y. (2015) Cancer cell detection and therapeutics
using peroxidase-active nanohybrid of gold nanoparticle-loaded
mesoporous silica-coated graphene, ACS Appl. Mater. Interfaces,
7, 9807-9816.
156.Mukherjee, P., Bhattacharya, R., and
Mukhopadhyay, D. (2005) Gold nanoparticles bearing functional
anti-cancer drug and anti-angiogenic agent: a “2 in 1”
system with potential application in cancer therapeutics, J. Biomed.
Nanotech., 1, 224-228.
157.Paciotti, G. F., Kingston, D. G. I., and
Tamarkin, L. (2006) Colloidal gold nanoparticles: a novel nanoparticle
platform for developing multifunctional tumor-targeted drug delivery
vectors, Drug Dev. Res., 67, 47-54.
158.Heo, D. N., Yang, D. H., Moon, H.-J., Lee, J.
B., Bae, M. S., Lee, S. C., Lee, W. J., Sun, I.-C., and Kwon, I. K.
(2012) Gold nanoparticles surface-functionalized with paclitaxel drug
and biotin receptor as theranostic agents for cancer therapy,
Biomaterials, 33, 856-866.
159.Bhattacharyya, S., Khan, J. A., Curran, G. L.,
Robertson, J. D., Bhattacharya, R., and Mukherjee, P. (2011) Efficient
delivery of gold nanoparticles by dual receptor targeting, Adv.
Mater., 23, 5034-5038.
160.Patra, C. R., Bhattacharya, R., and Mukherjee,
P. (2010) Fabrication and functional characterization of gold
nanoconjugates for potential application in ovarian cancer, J.
Mater. Chem., 20, 547-554.
161.Hosta-Rigau, L., Olmedo, I., Arbiol, J., Cruz,
L. J., Kogan, M. J., and Albericio, F. (2010) Multifunctionalized gold
nanoparticles with peptides targeted to gastrin-releasing peptide
receptor of a tumor cell line, Bioconjug. Chem., 21,
1070-1078.
162.Kumar, A., Ma, H., Zhang, X., Huang, K., Jin,
S., Liu, J., Wei, T., Cao, W., Zou, G., and Liang, X.-J. (2012) Gold
nanoparticles functionalized with therapeutic and targeted peptides for
cancer treatment, Biomaterials, 33, 1180-1189.
163.Kang, B., Mackey, M. A., and El-Sayed, M. A.
(2010) Nuclear targeting of gold nanoparticles in cancer cells induces
DNA damage, causing cytokinesis arrest and apoptosis, J. Am. Chem.
Soc., 132, 1517-1519.
164.You, J., Zhang, R., Xiong, C., Zhong, M.,
Melancon, M., Gupta, S., Nick, A. M., Sood, A. K., and Li, C. (2012)
Effective photothermal chemotherapy using doxorubicin-loaded gold
nanospheres that target EphB4 receptors in tumors, Cancer Res.,
72, 4777-4786.
165.Book Newell, B., Wang, Y., and Irudayaraj, J.
(2012) Multifunctional gold nanorod theranostics probed by multi-photon
imaging, Eur. J. Med. Chem., 48, 330-337.
166.Wang, D., Xu, Z., Yu, H., Chen, X., Feng, B.,
Cui, Z., Lin, B., Yin, Q., Zhang, Z., Chen, C., Wang, J., Zhang, W.,
and Li, Y. (2014) Treatment of metastatic breast cancer by combination
of chemotherapy and photothermal ablation using doxorubicin-loaded DNA
wrapped gold nanorods, Biomaterials, 35, 8374-8384.
167.Chen, H., Chi, X., Li, B., Zhang, M., Ma, Y.,
Achilefud, S., and Gu, Y. (2014) Drug loaded multilayered gold nanorods
for combined photothermal and chemotherapy, Biomater. Sci.,
2, 996-1006.
168.Alexander, C. M., Hamner, K. L., Maye, M. M.,
and Dabrowiak, J. C. (2014) Multifunctional DNA-gold nanoparticles for
targeted doxorubicin delivery, Bioconjug. Chem., 25,
1261-1271.
169.Kim, D., Jeong, Y. Y., and Jon, S. (2010) A
drug-loaded aptamer-gold nanoparticle bioconjugate for combined CT
imaging and therapy of prostate cancer, ACS Nano, 4,
3689-3696.
170.Liang, Z., Li, X., Xie, Y., and Liu, S. (2014)
“Smart” gold nanoshells for combined cancer chemotherapy
and hyperthermia, Biomed. Mater., 9, 025012.
171.Xiao, Y., Hong, H., Matson, V. Z., Javadi, A.,
Xu, W., Yang, Y., Zhang, Y., Engle, J. W., Nickles, R. J., Cai, W.,
Steeber, D. A., and Gong, S. (2012) Gold nanorods conjugated with
doxorubicin and cRGD for combined anti-cancer drug delivery and PET
imaging, Theranostics, 2, 757-768.
172.Avvakumova, S., Colombo, M., Tortora, P., and
Prosperi, D. (2014) Biotechnological approaches toward nanoparticle
biofunctionalization, Trends Biotechnol., 32, 11-20.
173.Kuo, W. S., Chang, C. N., Chang, Y. T., Yang,
M. H., Chien, Y. H., Chen, S. J., and Yeh, C. S. (2010) Gold nanorods
in photodynamic therapy, as hyperthermia agents, and in near-infrared
optical imaging, Angew. Chem. Int. Ed., 49,
2711-2715.
174.Stuchinskaya, T., Moreno, M., Cook, M. J.,
Edwards, D. R., and Russell, D. A. (2011) Targeted photodynamic therapy
of breast cancer cells using antibody–phthalocyanine–gold
nanoparticle conjugates, Photochem. Photobiol. Sci., 10,
822-831.
175.Lee, K., Lee, H., Bae, K. H., and Park, T. G.
(2010) Heparin immobilized gold nanoparticles for targeted detection
and apoptotic death of metastatic cancer cells, Biomaterials,
31, 6530-6536.
176.Wang, F., Liu, P., Sun, L., Li, C., Petrenko,
V. A., and Liu, A. (2014) Biomimetic nanostructure self-assembled from
Au@Ag heterogeneous nanorods and phage fusion proteins for targeted
tumor optical detection and photothermal therapy, Sci. Rep.,
4, 6808.
177.Chen, H., Zhang, X., Dai, S., Ma, Y., Cui, S.,
Achilefu, S., and Gu, Y. (2013) Multifunctional gold nanostar
conjugates for tumor imaging and combined photothermal and
chemo-therapy, Theranostics, 3, 633-649.
178.Huang, X., Peng, X., Wang, Y., Wang, Y., Shin,
D. M., El-Sayed, M. A., and Nie, S. (2010) A reexamination of active
and passive tumor targeting by using rod-shaped gold nanocrystals and
covalently conjugated peptide ligands, ACS Nano, 4,
5887-5896.
179.Ali, M. R. K., Panikkanvalappil, S. R., and
El-Sayed, M. A. (2014) Enhancing the efficiency of gold nanoparticles
treatment of cancer by increasing their rate of endocytosis and cell
accumulation using rifampicin, J. Am. Chem. Soc., 136,
4464-4467.
180.Kumar, S., Harrison, N., Richards-Kortum, R.,
and Sokolov, K. (2007) Plasmonic nanosensors for imaging intracellular
biomarkers in live cells, Nano Lett., 7, 1338-1343.
181.Zhang, Z., Liu, Y., Jarreau, C., Welch, M. J.,
and Taylor, J.-S. A. (2013) Nucleic acid-directed self-assembly of
multifunctional gold nanoparticle imaging agents, Biomater.
Sci., 1, 1055-1064.
182.Mandal, S., Bakeine, G. J., Krol, S., Ferrari,
C., Clerici, A. M., Zonta, C., Cansolino, L., Ballarini, F.,
Bortolussi, S., Stella, S., Protti, N., Bruschi, P., and Altieri, S.
(2011) Design, development and characterization of multifunctionalized
gold nanoparticles for biodetection and targeted boron delivery in BNCT
applications, Appl. Radiat. Isot., 69, 1692-1697.
183.Tao, Y., Ju, E., Liu, Z., Dong, K., Ren, J.,
and Qu, X. (2014) Engineered, self-assembled near-infrared photothermal
agents for combined tumor immunotherapy and chemo-photothermal therapy,
Biomaterials, 35, 6646-6656.
184.Lu, W., Zhang, G., Zhang, R., Flores, L. G.,
Huang, Q., Gelovani, J. G., and Li, C. (2010) Tumor site-specific
silencing of NF-kappaB p65 by targeted hollow gold nanosphere-mediated
photothermal transfection, Cancer Res., 70,
3177-3188.
185.Conde, J., Ambrosone, A., Sanz, V., Hernandez,
Y., Marchesano, V., Tian, F., Child, H., Berry, C. C., Ibarra, M. R.,
Baptista, P. V., Tortiglione, C., and De la Fuente, J. M. (2012) Design
of multifunctional gold nanoparticles for in vitro and in
vivo gene silencing, ACS Nano, 6, 8316-8324.
186.Braun, G. B., Pallaoro, A., Wu, G., Missirlis,
D., Zasadzinski, J. A., Tirrell, M., and Reich, N. O. (2009)
Laser-activated gene silencing via gold nanoshell–siRNA
conjugates, ACS Nano, 3, 2007-2015.
187.Jensen, S. A., Day, E. S., Ko, C. H., Hurley,
L. A., Luciano, J. P., Kouri, F. M., Merkel, T. J., Luthi, A. J.,
Patel, P. C., Cutler, J. I., Daniel, W. L., Scott, A. W., Rotz, M. W.,
Meade, T. J., Giljohann, D. A., Mirkin, C. A., and Stegh, A. H. (2013)
Spherical nucleic acid nanoparticle conjugates as an RNAi-based therapy
for glioblastoma, Sci. Transl. Med., 5, 209ra152.
188.Zang, Y., Wei, Y., Shi, Y., Chen, Q., and Xing,
D. (2016) Chemo/photoacoustic dual therapy with mRNA-triggered DOX
release and photoinduced shockwave based on a DNA–gold
nanoplatform, Small, 12, 756-769.
189.Ojeda, R., de Paz, J. L., Barrientos, A. G.,
Martin-Lomas, M., and Penades, S. (2007) Preparation of multifunctional
glyconanoparticles as a platform for potential carbohydrate-based
anticancer vaccines, Carbohydr. Res., 342, 448-459.
190.Brinãs, R. P., Sundgren, A., Sahoo, P.,
Morey, S., Rittenhouse-Olson, K., Wilding, G. E., Deng, W., and Barchi,
J. J., Jr. (2012) Design and synthesis of multifunctional gold
nanoparticles bearing tumor-associated glycopeptide antigens as
potential cancer vaccines, Bioconjug. Chem., 23,
1513-1523.
191.Safari, D., Marradi, M., Chiodo, F., Dekker, H.
A. T., Shan, Y., Adamo, R., Oscarson, S., Rijkers, G. T., Lahmann, M.,
Kamerling, J. P., Penades, S., and Snippe, H. (2012) Gold nanoparticles
as carriers for a synthetic Streptococcus pneumoniae type 14
conjugate vaccine, Nanomedicine (Lond.), 7, 651-662.
192.Chiodo, F., Marradi, M., Calvo, J., Yuste, E.,
and Penades, S. (2014) Glycosystems in nanotechnology: gold
glyconanoparticles as carrier for anti-HIV prodrugs, Beilstein J.
Org. Chem., 10, 1339-1346.
193.Reichardt, N. C., Martin-Lomas, M., and
Penades, S. (2013) Glyconanotechnology, Chem. Soc. Rev.,
42, 4358-4376.
194.Li, X., and Chen, G. (2015) Glycopolymer-based
nanoparticles: synthesis and application, Polym. Chem.,
6, 1417-1430.
195.Sun, I., Eun, D., Na, J., Lee, S., Kim, I.,
Youn, I., Ko, C., Kim, H., Lim, D., Choi, K., Messersmith, P., Park,
T., Kim, S., Kwon, I., Kim, K., and Ahn, C. (2009) Heparin-coated gold
nanoparticles for liver-specific CT imaging, Chem. Eur. J.,
15, 13341-13347.
196.Qian, X., Peng, X.-H., Ansari, D. O., Yin-Goen,
Q., Chen, G. Z., Shin, D. M., Yang, L., Young, A. N., Wang, M. D., and
Nie, S. (2008) In vivo tumor targeting and spectroscopic
detection with surface-enhanced Raman nanoparticle tags, Nat.
Biotech., 26, 83-90.
197.Conde, J., Bao, C., Cui, D., Baptista, P. V.,
and Tian, F. (2014) Antibody-drug gold nanoantennas with Raman
spectroscopic fingerprints for in vivo tumor theranostics, J.
Control. Rel., 183, 87-93.
198.Kim, C., Cho, E. C., Chen, J., Song, K. H., Au,
L., Favazza, C., Zhang, Q., Cobley, C. M., Gao, F., Xia, Y., and Wang,
L. V. (2010) In vivo molecular photoacoustic tomography of
melanomas targeted by bioconjugated gold nanocages, ACS Nano,
4, 4559-4564.
199.Li, Y. S., Zhou, Y., Meng, X. Y., Zhang, Y. Y.,
Liu, J. Q., Zhang, Y., Wang, N. N., Hu, P., Lu, S. Y., Ren, H. L., and
Liu, Z. S. (2014) Enzyme–antibody dual labeled gold nanoparticles
probe for ultrasensitive detection of κ-casein in bovine milk
samples, Biosens. Bioelectron., 61, 241-244.
200.Salem, A. K., Searson, P. C., and Leong, K. W.
(2003) Multifunctional nanorods for gene delivery, Nat. Mater.,
2, 668-671.
201.Shi, X., Wang, S. H., Van Antwerp, M. E., Chen,
X., and Baker, J. R., Jr. (2009) Targeting and detecting cancer cells
using spontaneously formed multifunctional dendrimer-stabilized gold
nanoparticles, Analyst, 134, 1373-1379.
202.Zhu, J., Zheng, L., Wen, S., Tang, Y., Shen,
M., Zhang, G., and Shi, X. (2014) Targeted cancer theranostics using
alpha-tocopheryl succinate-conjugated multifunctional
dendrimer-entrapped gold nanoparticles, Biomaterials, 35,
7635-7646.
203.Prabaharan, M., Grailer, J. J., Pilla, S.,
Steeber, D. A., and Gong, S. (2009) Gold nanoparticles with a monolayer
of doxorubicin-conjugated amphiphilic block copolymer for
tumor-targeted drug delivery, Biomaterials, 30,
6065-6075.
204.Zhang, Z., Liu, C., Bai, J., Wu, C., Xiao, Y.,
Li, Y., Zheng, J., Yang, R., and Tan, W. (2015) Silver nanoparticle
gated, mesoporous silica coated gold nanorods (AuNR@MS@AgNPs): low
premature release and multifunctional cancer theranostic platform,
ACS Appl. Mater. Interfaces, 7, 6211-6219.
205.Zhang, M., Yilmaz, T., Boztas, A. O., Karakuzu,
O., Bang, W. Y., Yegin, Y., Luo, Z., Lenox, M., Cisneros-Zevallos, L.,
and Akbulut, M. (2016) A multifunctional nanoparticulate theranostic
system with simultaneous chemotherapeutic, photothermal therapeutic,
and MRI contrast capabilities, RSC Adv., 6,
27798-27806.
206.Bardhan, R., Chen, W., Perez-Torres, C.,
Bartels, M., Huschka, R. M., Zhao, L. L., Morosan, E., Pautler, R. G.,
Joshi, A., and Halas, N. J. (2009) Nanoshells with targeted
simultaneous enhancement of magnetic and optical imaging and
photothermal therapeutic response, Adv. Funct. Mater.,
19, 3901-3909.
207.Bardhan, R., Chen, W., Bartels, M.,
Perez-Torres, C., Botero, M. F., McAninch, R. W., Contreras, A.,
Schiff, R., Pautler, R. G., Halas, N. J., and Joshi, A. (2010) Tracking
of multimodal therapeutic nanocomplexes targeting breast cancer in
vivo, Nano Lett., 10, 4920-4928.
208.Chen, W., Bardhan, R., Bartels, M.,
Perez-Torres, C., Pautler, R. G., Halas, N. J., and Joshi, A. (2010) A
molecularly targeted theranostic probe for ovarian cancer, Mol.
Cancer Ther., 9, 1028-1038.
209.Liu, H., Chen, D., Li, L., Liu, T., Tan, L.,
Wu, X., and Tang, F. (2011) Multifunctional gold nanoshells on silica
nanorattles: a platform for the combination of photothermal therapy and
chemotherapy with low systemic toxicity, Angew. Chem. Int. Ed.,
50, 891-895.
210.Hu, F., Zhang, Y., Chen, G., Li, C., and Wang,
Q. (2015) Double-walled Au nanocage/SiO2 nanorattles:
integrating SERS imaging, drug delivery and photothermal therapy,
Small, 11, 985-993.
211.Hembury, M., Chiappini, C., Bertazzo, S.,
Kalber, T. L., Drisko, G. L., Ogunlade, O., Walker-Samuel, S., Krishna,
K. S., Jumeaux, C., Beard, P., Kumar, C. S., Porter, A. E., Lythgoe, M.
F., Boissière, C., Sanchez, C., and Stevens, M. M. (2015)
Gold-silica quantum rattles for multimodal imaging and therapy,
Proc. Natl. Acad. Sci. USA, 112, 1959-1964.
212.Chen, L. Y., Wang, C. W., Yuan, Z., and Chang,
H. T. (2015) Fluorescent gold nanoclusters: recent advances in sensing
and imaging, Anal. Chem., 87, 216-229.
213.Li, N., Li, T., Liu, Chen., Ye, S., Liang, J.,
and Han, H. (2016) Folic acid-targeted and cell penetrating
peptide-mediated theranostic nanoplatform for high-efficiency tri-modal
imaging-guided synergistic anticancer phototherapy, J. Biomed.
Nanotechnol., 12, 878-893.
214.Muthu, M. S., Kutty, R. V., Luo, Z., Xie, J.,
and Feng, S. S. (2015) Theranostic vitamin E TPGS micelles of
transferrin conjugation for targeted co-delivery of docetaxel and
ultra-bright gold nanoclusters, Biomaterials, 39,
234-248.
215.Liu, Y., Chang, Z., Yuan, H., Fales, A. M., and
Vo-Dinh, T. (2013) Quintuple-modality (SERS-MRI-CT-TPL-PTT) plasmonic
nanoprobe for theranostics, Nanoscale, 5,
12126-12131.
216.Movia, D., Gerard, V., Maguire, C. M., Jain,
N., Bell, A. P., Nicolosi, V., O’Neill, T., Scholz, D.,
Gun’ko, Y., Volkov, Y., and Prina-Mello, A. (2014) A
safe-by-design approach to the development of gold nanoboxes as
carriers for internalization into cancer cells, Biomaterials,
35, 2543-2557.
217.Topete, A., Alatorre-Meda, M., Iglesias, P.,
Villar-Alvarez, E. M., Barbosa, S., Costoya, J. A., Taboada, P., and
Mosquera, V. (2014) Fluorescent drug-loaded, polymeric-based, branched
gold nanoshells for localized multimodal therapy and imaging of tumoral
cells, ACS Nano, 8, 2725-2738.
218.Khlebtsov, B. N., Khanadeev, V. A., Tsvetkov,
M. Y., Bagratashvili, V. N., and Khlebtsov, N. G. (2013)
Surface-enhanced Raman scattering substrates based on self-assembled
PEGylated gold and gold–silver core-shell nanorods, J. Phys.
Chem. C, 117, 23162-23171.
219.Hamon, C., Novikov, S., Scarabelli, L.,
Basabe-Desmonts, L., and Liz-Marzán, L. M. (2014) Hierarchical
self-assembly of gold nanoparticles into patterned plasmonic
nanostructures, ACS Nano, 8, 10694-10700.
220.Khlebtsov, B. N., Khanadeev, V. A., Panfilova,
E. V., Bratashov, D. N., and Khlebtsov, N. G. (2015) Gold nano-island
films as reproducible SERS substrates for highly sensitive detection of
fungicides, ACS Appl. Mater. Interfaces, 7,
6518-6529.
221.Fan, M. K., Andrade, G. F. S., and Brolo, A. G.
(2011) A review on the fabrication of substrates for surface enhanced
Raman spectroscopy and their applications in analytical chemistry,
Anal. Chim. Acta, 693, 7-25.
222.Lin, L., Gu, H., and Ye, J. (2015) Plasmonic
multi-shell nanomatryoshka particles as highly tunable SERS tags with
built-in reporters, Chem. Commun., 51, 17740-17743.
223.Khlebtsov, B., Khanadeev, V., and Khlebtsov, N.
(2016) Surface-enhanced Raman scattering inside Au@Ag core/shell
nanorods, Nano Res., 9, 2303-2318.
224.Khlebtsov, B. N., and Khlebtsov, N. G. (2016)
Surface morphology of a gold core controls the formation of hollow or
bridged nanogaps in plasmonic nanomatryoshkas and their SERS responses,
J. Phys. Chem. C, 120, 15385-15394.
225.Abadeer, N. S., and Murphy, C. J. (2016) Recent
progress in cancer thermal therapy using gold nanoparticles, J.
Phys. Chem. C, 120, 4691-4716.