Effects of Seeding on Lysozyme Amyloid Fibrillation in the Presence of Epigallocatechin and Polyethylene Glycol
Li-Xiu Kong and Cheng-Ming Zeng*
Shaanxi Normal University, School of Chemistry and Chemical Engineering, Key Laboratory of Analytical Chemistry for Life Science of Shaanxi Province, 710119 Xi’an, China; E-mail: chengmingzeng@snnu.edu.cn* To whom correspondence should be addressed.
Received August 7, 2016; Revision received October 5, 2016
Preformed amyloid fibrils can act as seeds for accelerating protein fibrillation. In the present study, we examined the effects of preformed seeds on lysozyme amyloid fibrillation in the presence of two distinct inhibitors – epigallocatechin (EGC) and polyethylene glycol 2000 (PEG). The results demonstrated that the effects of fibrillar seeds on the acceleration of lysozyme fibrillation depended on the aggregation pathway directed by an inhibitor. EGC inhibited lysozyme fibrillation and modified the peptide chains with quinone moieties in a concentration-dependent manner. The resulting aggregates showed amorphous off-pathway morphology. Preformed fibril seeds did not promote lysozyme fibrillation in the presence of EGC. PEG also inhibited lysozyme fibrillation, and the resulting aggregates showed on-pathway protofibrillar morphology. In contrast, the addition of fibril seeds into the mixture of lysozyme and PEG significantly stimulated fibril growth. Assays of cell viability showed that both EGC and PEG inhibited the formation of cytotoxic species. In accordance with thioflavine T data, the seeds failed to alter the cell-damaging potency of the EGC-directed off-pathway aggregates, but increased the cytotoxicity of the PEG-directed on-pathway fibrils. We suggest that the pattern of interaction between lysozyme and an inhibitor determines the pathway of aggregation and therefore the effects of seeding on amyloid formation. EGC covalently modified lysozyme chains with quinones, directing the aggregation to proceed through an off-pathway, whereas PEG affected the protein in a noncovalent manner, and fibril growth could be stimulated under seeding through an on-pathway.
KEY WORDS: lysozyme, amyloid fibrillation, seeding, epigallocatechin, polyethylene glycol, cytotoxicityDOI: 10.1134/S0006297917020079
Abbreviations: EGC, epigallocatechin; EGCG, epigallocatechin-3-gallate; MTT, thiazolyl blue tetrazolium bromide; NBT, nitroblue tetrazolium; PEG, polyethylene glycol; TEM, transmission electron microscopy; ThT, thioflavine T.
Amyloid fibrillation of protein molecules is associated with a variety
of amyloid-related diseases, including Alzheimer’s disease, type
2 diabetes, and several systemic amyloidoses [1-3]. These proteins, despite their unrelated amino acid
sequences and tertiary structures, can unfold and assemble into fibrils
with similar morphologies and β-sheet enriched structures.
Accumulating evidence has strongly suggested that amyloid fibrils are
cytotoxic due to their abilities to disrupt cellular membranes, induce
oxidative stress, and trigger a cascade of cellular events leading to
apoptosis [4, 5].
Amyloid fibrillation of a protein generally consists of several phases including association/dissociation of protein monomers, nucleation, oligomer formation, elongation, and maturation of the fibrils. Of these, nucleation is the rate-limiting step in amyloid formation. After nucleation is completed, the aggregation process then proceeds to fibril elongation, wherein the fibril grows via a self-replicating mechanism by utilizing the formed amyloid structure as a template. Coincubation of a protein with its preformed fibril seeds significantly accelerates fibril growth by skipping over the nucleation phase. Recent reports have demonstrated that amyloid seeds form in cells after the cells take up an amyloidogenic peptide [6], and that preformed superoxide dismutase fibrils function as seeds to trigger amyloid formation of the protein in cells [7].
The process of amyloid formation may, however, comprise parallel steps that are competing with the fibril growth [8, 9]. These furcating aggregation pathways may result in the formation of off-pathway aggregates that are less cytotoxic than amyloid species. Besides, numerous investigations have demonstrated that small molecules can be used to manipulate the aggregation pathway of a protein [10-13]. Exploration of the role of preformed amyloid seeds on protein aggregation through different pathways is of importance to shed light on the molecular mechanism of protein fibrillation under different conditions.
Mutated human lysozyme has been reported to be associated with non-neuropathic systemic amyloidosis [14]. The amyloid fibrils formed by wild-type lysozyme in vitro have ultrastructures and biochemical properties similar to those of lysozyme extracted from pathological deposits in tissues [15]. Under in vitro conditions of low pH and elevated temperature, lysozyme transforms into amyloid fibrils with typical amyloid structure and biochemical properties. Recent studies have shown that the lysozyme fibrils prepared in vitro exhibit nonenzymatic cytotoxicity, including induction of hemolysis in human erythrocytes, triggering cross-linking of cytoskeletal proteins, and reducing the viability of neuroblastoma cells through apoptotic and necrotic pathways [5, 16, 17].
A common property of the interior of cells is dense crowding with a high concentration of macromolecules. A number of hydrophilic polymers, including polyethylene glycol (PEG), polysaccharides, and albumin, have been utilized to simulate the internal environment of living cells for exploring the molecular mechanism of protein fibrillation under crowded conditions. A crowding reagent can exert two opposing effects on protein fibrillation. Several reports have demonstrated that protein fibrillation is enhanced under crowded conditions [18-20], but crowding reagents have also been found to inhibit protein amyloid fibrillation [21-23].
Polyphenols have been reported to have inhibitory effects on protein fibrillation [24-26]. Epigallocatechin (EGC), belonging to the flavonoid family, is abundant in green tea and is a potent antioxidant that has been widely investigated. Its gallate derivative, epigallocatechin-3-gallate (EGCG), has been reported to inhibit amyloid formation in various proteins and peptides [11, 12]. The anti-amyloidogenic role of EGCG involves covalent modification of peptide chains and remodeling of fibrillar species into off-pathway aggregates. EGC may also function through pathways similar to those of EGCG in the inhibition of amyloid formation and destabilization of existing amyloid fibrils.
In the present study, by utilizing lysozyme as an in vitro model, we investigated the role of seeding on amyloid fibril growth in the presence of two distinct inhibitors – EGC and PEG 2000. The results demonstrated that EGC and PEG directed lysozyme aggregation into different pathways. The seeding effects of preformed fibrils on the acceleration of lysozyme fibrillation depended on the inhibitor-determined aggregate pathway. The seeds attenuated the inhibitory role of PEG on lysozyme fibrillation proceeding through an on-pathway. In contrast, the seeds exerted little effect on fibril growth proceeding through an off-pathway induced by EGC.
MATERIALS AND METHODS
Chemicals. Hen egg-white lysozyme (14.3 kDa), EGC (306 Da), PEG 2000, nitroblue tetrazolium (NBT), thiazolyl blue tetrazolium bromide (MTT), thioflavine T (ThT), and dimethylsulfoxide (DMSO) were purchased from Sigma Aldrich (USA). Electrophoresis-related reagents were from Bio-Rad (USA). Rat pheochromocytoma PC12 cells were obtained from ATCC (USA). Cell culture reagents were purchased from HyClone (USA). Other reagents were of analytical grade.
Preparation and characterization of lysozyme fibrils. Lysozyme fibrils were prepared according to previous reports [5, 25] with minor modifications. Briefly, hen egg-white lysozyme was dissolved in 10 mM HCl solution (pH 2.0) with or without an inhibitor to a final concentration of 10 mg/ml (0.7 mM). The samples were incubated at 65°C in a water-bath without agitation. ThT fluorescence was measured in a mixture of 33 µg/ml lysozyme and 10 µM ThT, with excitation at 440 nm and emission at 484 nm in a Perkin Elmer LS55 spectrofluorimeter (Waltham, USA). For analysis by transmission electron microscopy (TEM), an aliquot of lysozyme solution was diluted 20-fold with water and dropped onto copper-mesh grids. Samples were negatively stained with 2% (w/v) uranyl acetate and air-dried at room temperature. Observations were carried out using a JEM-1400 (Japan) or a Hitachi H-600 (Japan) electron microscope with accelerating voltage of 80 kV.
Seeding assay. For seeding experiments, mature lysozyme fibrils aged 12 days were sonicated (20 kHz) 20 times for 5 s with a time interval of 5 s in a water bath at 37°C. The resulting fibrils seeds were added to lysozyme solutions at ratio of 2% (seeds/lysozyme; w/w).
Gel electrophoresis and NBT staining assay. Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) was performed in Tricine buffer (pH 8.2) using a 5% stacking gel and a 15% separating gel. Twenty micrograms of protein was applied to each lane, and bands were visualized by Coomassie brilliant blue R-250 staining. For western blotting, the gel bands were transferred onto polyvinylidene fluoride membranes (0.45 µm; Millipore, USA) with a mini transfer cell (GE Healthcare, USA). Quinoproteins were detected by staining the membranes with NBT (0.24 mM in 2 M potassium glycinate, pH 10). The membranes were then immersed in the glycinate/NBT solution for 45 min in the dark, resulting in a blue-purple stain of quinoprotein bands and no staining of other peptides.
Cytotoxicity assay. Cell viability was assessed as the inhibition of the ability of cells to reduce the metabolic dye MTT to a blue formazan product. PC12 cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum, penicillin (100 U/ml), and streptomycin (100 µg/ml) and incubated at 37°C in a 5% CO2 in a humidified atmosphere. Cells were harvested and plated in 96-well plates with approximately 10,000 cells per well. The plates were incubated at 37°C for 24 h to allow cells to attach. For the MTT reduction assays, stock sample solutions were diluted with RPMI 1640 medium. The diluted samples were added to the wells and incubated for 24 h. To the cell samples, MTT (5 mg/ml in PBS) was added and incubated for 4 h. Then, the supernatants were discarded, and 150 µl of DMSO was added to each plate to dissolve formazan crystals. The absorbance of each sample was recorded at 570 nm with a Model 680 Microplate Reader (Bio-Rad). Averages from six replicate wells were used for each sample and control. Percentage viability of cells in comparison to untreated control cells could then be calculated. All data are presented as means ± standard deviations. Student’s t-test was used for comparisons between two samples.
RESULTS AND DISCUSSION
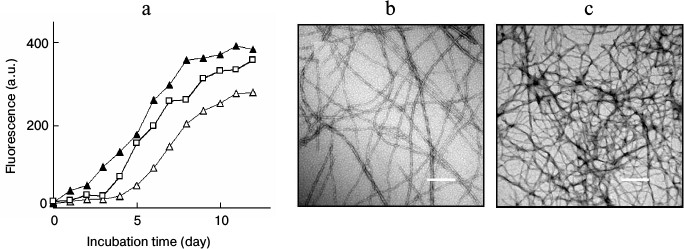
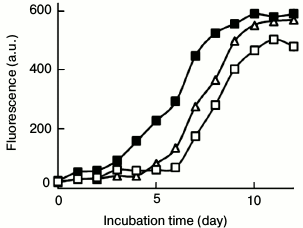
Lysozyme amyloid fibrillation in the presence of fibrillar seeds. Incubation of lysozyme under conditions of low pH and elevated temperature resulted in the formation of amyloid fibrils. The growth of amyloid fibrils was monitored and characterized by ThT fluorescence and TEM. A typical fluorescent profile of ThT bound to lysozyme fibrillar species exhibited a lag phase followed by a sigmoid-like elongation phase and a saturation phase, corresponding to the processes of nucleation, fibril elongation, and maturation, respectively, as shown in Fig. 1a. Mature fibrils harvested after 12 days of incubation showed a typical amyloid morphology characterized as long, straight, dense fibrils with diameters of 8-35 nm (Fig. 1b). Amyloid seeds were prepared by sonicating the mature fibrils. When fresh lysozyme was incubated with fibril seeds, the ThT fluorescence increased without a lag phase and reached a plateau after approximately 8 days of incubation, as shown in Fig. 1a. To determine the effects of seeding on different stages of fibril growth, the seeds were introduced and mixed with lysozyme after incubation was initiated. Fibril growth was accelerated by introduction of the seeds after 3 days of incubation. Once the fibril growth started (after 5 days of incubation), the addition of fibril seeds did not affect lysozyme fibrillation (data not shown), suggesting that the seeds played a role in fibril acceleration only in the early stages of amyloid formation. Figure 1c shows a TEM image of mature fibrils prepared in the presence of fibril seeds. The coincubation of preformed seeds with lysozyme resulted in a relatively dense fibrillar structure in comparison with that of the non-seeded sample.
Fig. 1. a) ThT fluorescence of lysozyme fibril growth in the absence (open triangle) or presence of fibril seeds. The seeds were introduced into the samples at day 0 (closed triangle) or day 3 (open square) after incubation was initiated. TEM images of mature fibrils aged 12 days prepared in the absence (b) or presence (c) of seeds. Scale bars represent 200 nm.
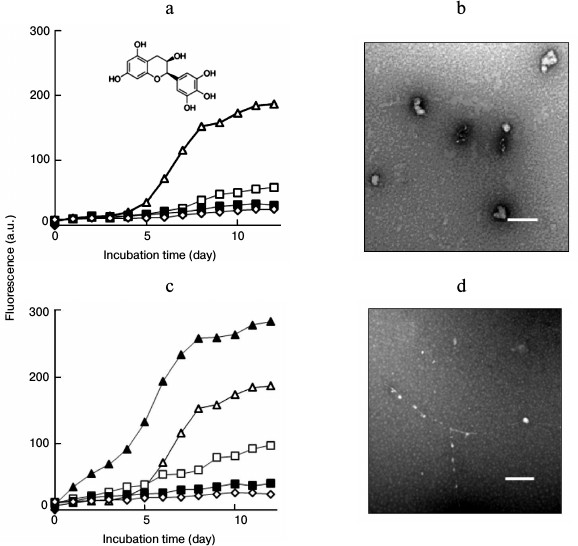
Effects of seeding on lysozyme fibrillation in the presence of EGC. EGC exhibited an inhibitory role on lysozyme fibrillation. As shown in Fig. 2a, EGC inhibited the growth of lysozyme fibrils in a dose-dependent manner, resulting in a significant decrease in the final intensity of ThT fluorescence. No sigmoidal curve was observed when lysozyme was incubated with 70 or 100 µg/ml EGC, indicating that fibril formation was inhibited completely. Changes in the kinetics of aggregation usually lead to alterations in the morphology of resulting aggregates. A TEM image demonstrated that incubation of fresh lysozyme with EGC resulted in the formation of amorphous aggregates. As shown in Fig. 2b, amorphous aggregates were predominant in the day-12 sample containing 100 µg/ml EGC. We categorized these amorphous assemblies as “off-pathway” aggregates, as described in previous reports [11, 12].
Fig. 2. a) Concentration-dependent effects of EGC on amyloid inhibition. ThT curves of lysozyme fibril growth in the absence (open triangle) or presence of 30 (open square), 70 (closed square), and 100 µg/ml EGC (open rhombus), respectively. The inset shows the molecular structure of EGC. b) TEM image of lysozyme fibrils aged 12 days prepared in the presence of 100 µg/ml EGC. c) Amyloid inhibition by EGC under seeding. The seeds were introduced before incubation into the samples containing 0 (closed triangle), 30 (open square), 70 (closed square), or 100 µg/ml EGC (open rhombus), respectively; open triangle designates the control sample without seeds and EGC. d) TEM image of lysozyme fibrils aged 12 days prepared in the presence of 100 µg/ml EGC + seeds. Scale bars represent 200 nm.
An acceleration of fibril growth was observed when the seeds were introduced into the mixture of fresh lysozyme and 30 µg/ml EGC, as depicted in Fig. 2c. However, there was almost no increase in ThT fluorescence when lysozyme was incubated with the seeds in the presence of 70 or 100 µg/ml EGC, indicating that the seeds were not able to accelerate fibril growth in the presence of high doses of EGC. Consistent with ThT data, the TEM image demonstrated that addition of fibril seeds did not promote much fibril formation, although a few “beads-on-string” aggregates were observed (Fig. 2d), suggesting that the seeding reaction was unable to compete with the “off-pathway” aggregation of lysozyme induced by 100 µg/ml EGC.
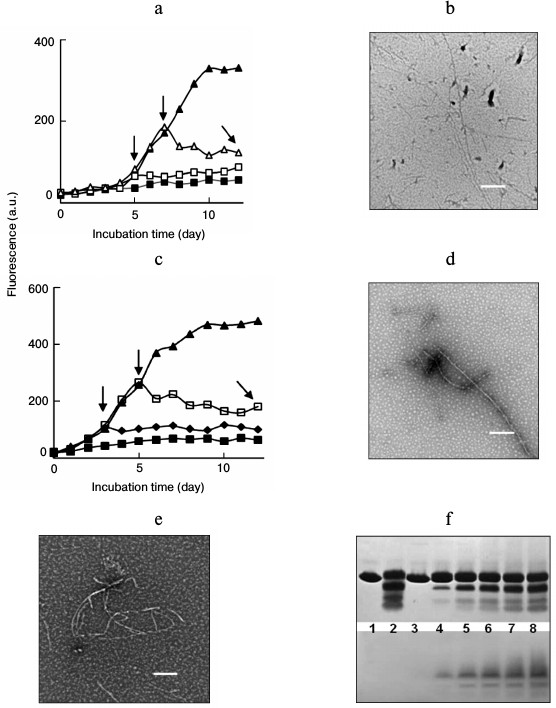
To explore the role of EGC on amyloid formation after fibril assembly has commenced, EGC was introduced into the samples at later time points of incubation. After fibril growth was triggered, the addition of EGC caused decreases in ThT fluorescence and changes in fibrillar morphology, indicating that fibril growth was interrupted by EGC under both non-seeding (Fig. 3, a and b) and seeding (Fig. 3, c and d) conditions. This suggested that EGC inhibited lysozyme fibrillation not only through suppressing the formation of amyloid species, but also through interrupting the assembly of amyloid species on the existing fibrillar templates and destabilizing the formed amyloid structures. To explore these possibilities, the destabilizing role of EGC on the fibrils was examined. TEM data showed that treatment of preformed lysozyme fibrils (12 days) with EGC resulted in the transformation of the amyloid fibrils to short filaments and amorphous aggregates (Fig. 3e). This fact confirms that EGC has disruptive activity on amyloid fibrils.
Fig. 3. Inhibition of lysozyme fibrillation in the absence or presence of amyloid seeds by introduction of EGC at different time points of incubation. EGC (100 µg/ml) was introduced into the samples at 0 (closed square), 3 (closed rhombus), 5 (open square), and 7 days (open triangle) after incubation was initiated, as indicated by the vertical arrows. For curve (closed triangle), no EGC was added. a) Incubation under non-seeding. b) TEM image of the sample indicated by a tilted arrow in panel (a). c) Incubation under seeding. d) TEM image of the sample indicated by a tilted arrow in panel (c). e) Short filaments and amorphous aggregates induced by treating preformed lysozyme fibrils (5 mg/ml) with EGC (50 µg/ml) at 65°C for 48 h. Scale bars in (b), (d), and (e) represent 200 nm. f) SDS-PAGE (upper panel) and NBT staining assay (lower panel) of lysozyme assemblies prepared in the presence of EGC (100 µg/ml). Lanes: 1) native lysozyme (14.3 kDa) without incubation; 2) lysozyme incubated without EGC for 12 days; 3-8) lysozyme incubated with EGC for 1, 3, 5, 7, 9, and 12 days, respectively.
Both noncovalent binding and covalent modifications are thought to be involved in mediating the inhibitory effects of polyphenols on protein fibrillation [25, 27, 28]. Moreover, considerable evidence has shown that polyphenols can be transformed into reactive quinonoid species that covalently bind with peptide chains to form quinoproteins [25, 29]. To examine whether EGC was transformed into quinone intermediates during the incubation, NBT staining assays were performed to detect quinone-modified peptides. At an alkaline pH, quinones and related quinonoid substances can catalyze a redox cycling reaction during which NBT is reduced to blue-purple insoluble formazan, allowing the detection of quinoproteins on a blotting membrane [30]. Figure 3f shows the SDS-PAGE and NBT staining patterns of lysozyme incubated for 0-12 days in the absence or presence of EGC. In the presence of SDS, the fibrillar species could be disaggregated and separated into lysozyme monomers and small-sized peptide fragments originating from acidic hydrolysis of the protein. In the presence of 100 µg/ml EGC, the SDS-PAGE patterns were similar to those of lysozyme fibrils aged 12 days.
A parallel SDS-PAGE experiment was performed, and the gel bands were electrically transferred onto a polyvinylidene fluoride membrane prior to detection of quinoproteins by NBT staining. As shown in the lower panel, quinone-modified peptides were observed in lanes 4-8, indicating that incubation of EGC with lysozyme resulted in the formation of reactive quinones that bound to the peptide chains. The quinopeptides formed by EGC and lysozyme were stable under electrophoresis, electric blotting, and dialysis, suggesting that the peptides were modified covalently by EGC. The formation of reactive quinone is thought to be a prerequisite for inhibition of amyloid fibrillation of lysozyme by EGCG [29] and other polyphenolic compounds [25]. Thus, modification of peptides altered the interacting forces within intra- and interpeptide chains, causing amyloid fibrillation to proceed through an off-pathway.
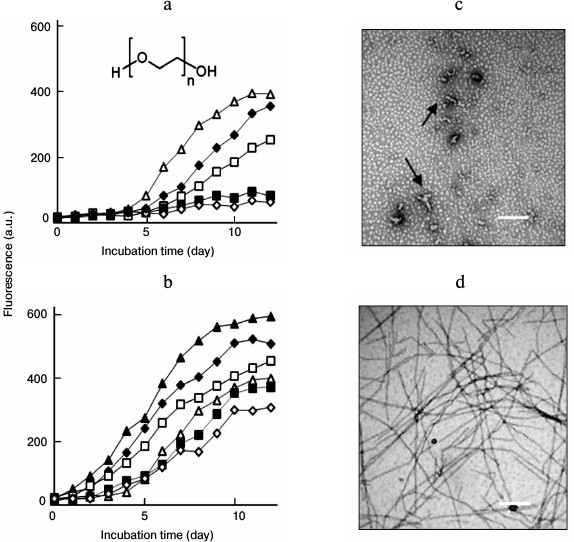
Effects of seeding on lysozyme fibrillation in the presence of PEG. PEG inhibited lysozyme fibrillation in a concentration-dependent manner, as shown in Fig. 4a. When PEG was applied at high concentrations (50 or 100 mg/ml), no increase in the final intensity of ThT fluorescence was observed, indicating that fibril formation was inhibited completely. To explore the effects of seeding on lysozyme aggregation in the presence of PEG, fibril seeds were introduced into the mixture of fresh lysozyme and PEG before the onset of incubation. As depicted in Fig. 4b, the intensity of ThT fluorescence increased with the incubation time, regardless of whether samples contained PEG. This suggested that fibril seeds could stimulate lysozyme fibrillation under a crowded environment.
Fig. 4. Effects of seeding on lysozyme fibrillation in the presence of PEG. a) Inhibitory effects of PEG on lysozyme amyloid fibrillation. Concentrations of PEG were 0 (open triangle), 10 (closed rhombus), 20 (open square), 50 (closed square), and 100 mg/ml (open rhombus), respectively. The inset shows the molecular structure of PEG. b) Amyloid inhibition by PEG under seeded conditions. The seeds were introduced before incubation into the samples containing PEG at 0 (closed triangle), 10 (closed rhombus), 20 (open square), 50 (closed square), and 100 mg/ml (open rhombus), respectively. The control sample without seeds and PEG (open triangle). TEM images of the lysozyme assemblies aged 12 days prepared in the presence of 100 mg/ml PEG (c) or 100 mg/ml PEG + seeds (d). Protofibrils are indicated by arrows in (c). Scale bars represent 200 nm.
We next examined the morphologies of lysozyme assemblies obtained in the presence of PEG and fibrillar seeds by TEM. Consistent with the ThT data, fibril formation was inhibited by PEG. In contrast to the effects of EGC, addition of fibril seeds to a mixture of fresh lysozyme and PEG significantly increased the density and diameters of the fibrils, including the samples containing high doses of PEG. Under crowded conditions (100 mg/ml PEG), a few vermicular aggregates, similar to so-called protofibrils [12, 13, 31], were observed on day 12 of incubation (Fig. 4c). The addition of fibril seeds to the PEG-crowded sample obviously promoted fibril growth, and the resulting fibrils showed typical fibrillar morphology (Fig. 4d). We categorize the protofibrils as “on-pathway aggregates” because these entities could assemble into mature fibrils following seeding conditions and promote amyloid formation of fresh lysozyme (Fig. 5).
Fig. 5. Effects of PEG- and EGC-directed aggregates on lysozyme amyloid growth. Lysozyme aggregates prepared in the presence of 100 mg/ml PEG (closed square) or 100 µg/ml EGC (open square) were sonicated and added as seeds (2%) into a solution of fresh lysozyme (10 mg/ml) prior to incubation at 65°C. The PEG-directed on-pathway species significantly promoted the growth of lysozyme fibrils. Curve (open triangle) is the control sample of lysozyme without seeds.
Thus, two distinct seeding roles were observed during lysozyme fibrillation. When EGC was applied at a high concentration (70 or 100 µg/ml), lysozyme fibrillation was inhibited completely, and only amorphous aggregates were formed. The addition of preformed fibril seeds failed to promote fibril growth. When PEG was applied at high concentrations (50 or 100 mg/ml), lysozyme fibrillation was also inhibited completely. In contrast, the addition of preformed fibril seeds significantly promoted fibril growth. The protofibrils shown in Fig. 4c suggested that PEG inhibited the assembly of lysozyme into large fibrillar species, and aggregation was restrained in the early stages of amyloid fibrillation. In other words, PEG inhibited lysozyme fibrillation by interrupting assembly of the on-pathway fibrillar species. Because the seeds can provide templates for fibril assembly, lysozyme fibrillation through an on-pathway can be accelerated by seeding.
The PEG-directed on-pathway aggregates possess seeding capacity accelerating lysozyme fibrillation. As shown in Fig. 4a, 100 mg/ml PEG inhibited completely the assembly of on-pathway fibrillar species into mature fibrils. To further prove the amyloid property of the PEG-directed species, the protofibrils harvested on day 12 were sonicated and added as seeds (2%) into solution of fresh lysozyme prior to incubation at 65°C. The ThT profile demonstrated that the PEG-directed on-pathway species significantly promoted fibril growth (Fig. 5). In contrast, the EGC-directed off-pathway aggregates (2%) showed a weak inhibitory role on lysozyme fibrillation. These samples obtained on day 12 showed a typical amyloid morphology under TEM (data not shown). These results confirm that the PEG-directed on-pathway species, but not the EGC-directed off-pathway aggregates, possess seeding capacity in accelerating lysozyme amyloid fibrillation.
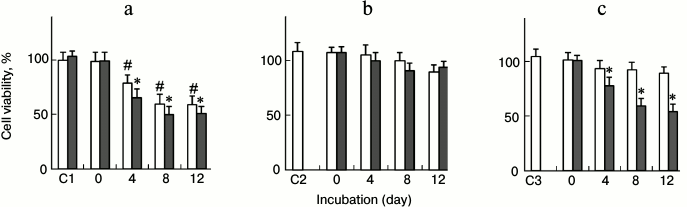
Cytotoxicity induced by lysozyme assemblies prepared in the presence of inhibitors and seeds. Lysozyme amyloid oligomers and fibrils have been reported to induce cell damage through both apoptotic and necrotic pathways [16]. Previous investigations [5, 26] demonstrated that lysozyme fibrils cause hemolysis and aggregation of human erythrocytes in a dose- and age-dependent manner. In this study, MTT assays were performed to evaluate the cytotoxicity of lysozyme assemblies prepared in the absence or presence of an inhibitor and fibrillar seeds. As shown in Fig. 6a, fibrillar species (0.5 mg/ml) exerted a prominent cytotoxicity on PC12 cells compared with the untreated control cells (p < 0.01). Upon treating by the fibrillar species aging 4, 8, and 12 days, the percentages of cell viability were 78.7, 60.0, and 59.3%, respectively. Coincubation of fresh lysozyme with seeds significantly increased the fibrillar cytotoxicity on PC12 cells. The seeded-fibrillar species on days 4, 8, and 12 decreased the percentage of cell viability to 65.1, 51.6, and 51.9%, respectively.
Fig. 6. Evaluation of cytotoxicity of lysozyme assemblies by the MTT reduction assay. Lysozyme (10 mg/ml) was incubated with or without an inhibitor (100 µg/ml EGC or 100 mg/ml PEG) under non-seeded (open columns) or seeded (filled columns) conditions. The cells were treated with lysozyme assemblies (0.5 mg/ml) harvested after different incubation times before MTT assays. a) Lysozyme only; b) lysozyme + EGC; c) lysozyme + PEG. C1 values are the control cell viability of culture medium with (filled column) or without (open column) seeds. C2 and C3 are the cell viability of culture medium with 5 µg/ml EGC or 5 mg/ml PEG, respectively. * p < 0.05 versus the value of corresponding non-seeded samples; # p < 0.01 versus the control cell viability of culture medium.
In the presence of 100 µg/ml EGC, the resulting lysozyme assemblies on days 4, 8, and 12 did not show significant cytotoxicity on PC12 cells (p > 0.05), the resultant percentages of cell viability being 95.8, 92.6, and 89.3%, respectively (Fig. 6b). The addition of fibrillar seeds into the mixture of fresh lysozyme and EGC did not change the cell-damaging capacity of lysozyme assemblies. As shown in Fig. 6b, there was no significant difference between the cell viability of the seeded (filled columns) and non-seeded samples (open columns), suggesting that the seeds were not able to stimulate the growth of cytotoxic species, in accordance with the ThT data. This observation indicated that the EGC-directed off-pathway aggregates lacked the capacity to develop into effective cell-damaging species under seeding.
PEG also exhibited a strong inhibitory role on the formation of cytotoxic assemblies. In the presence of 100 mg/ml PEG, the resulting aggregates on days 4, 8, and 12 did not show significant cytotoxic role on PC12 cells (p > 0.05). The rates of cell viability were 93.4, 92.6, and 89.1%, respectively (Fig. 6c). In contrast to the case of EGC, the addition of fibrillar seeds into a mixture of fresh lysozyme and PEG significantly increased the cell-damaging capacity of the lysozyme assemblies. The resulting percentages of cell viability were 78.0, 59.5, and 54.1% (Fig. 6c) for the seeded samples incubated for 4, 8, and 12 days, respectively.
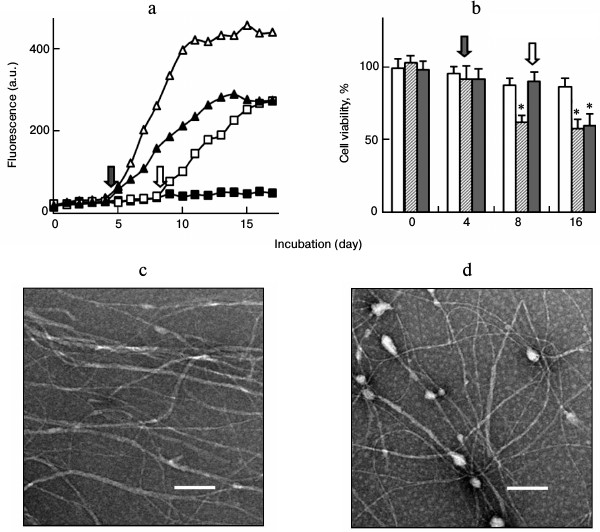
To further explore the effects of seeding on fibril growth, the seeds were introduced into the mixture of lysozyme and PEG at later time points after the onset of incubation. As shown in Fig. 7a, the intensity of ThT fluorescence increased after the seeds were introduced, suggesting that amyloid formation was stimulated in the aging lysozyme solution. MTT assays showed that, in the absence of seeds, PEG inhibited the formation of cell-damaging species, resulting in similar cell viability for the day-8 and day-16 samples (Fig. 7b, open columns). Addition of seeds into the sample at day 4 significantly increased the growth of cell-damaging species (Fig. 7b, hatched columns). The percentages of cell viability of the samples harvested on days 8 and 16 were 61.8 and 57.6%, respectively. Addition of seeds into the sample on day 8 also significantly promoted fibril growth and increased cell-damaging capacity of the assemblies (Fig. 7b, closed columns). The resulting sample on day 16 decreased the cell viability to 56.8%. Both of the two later-seeded samples obtained on day 16 showed a typical amyloid morphology under TEM (Fig. 7, c and d).
We also introduced the seeds into the mixture of lysozyme and EGC on days 4 and 8 of incubation. As expected, no changes were observed in ThT fluorescence and cytotoxicity of the samples after the seeds were introduced (data not shown).
Fig. 7. Fibril seeds stimulate the on-pathway fibrillation of lysozyme (10 mg/ml) in the presence of PEG (100 mg/ml). Seeds were introduced into the samples on days 4 and 8 of incubation, as indicated by the arrows. a) ThT fluorescence of fibril growth: (open triangle) lysozyme only; (closed square) lysozyme + PEG; (closed triangle) lysozyme + PEG + seeds (day 4); (open square) lysozyme + PEG + seeds (day 8). b) MTT assay of the cells after exposure to the lysozyme fibrils (0.5 mg/ml) prepared in (a): open columns, lysozyme + PEG; hatched columns, lysozyme + PEG + seeds (day 4); closed columns, lysozyme + PEG + seeds (day 8); * p < 0.05 versus the value of corresponding non-seeded sample. c, d) TEM images of lysozyme fibrils at day 16: c) lysozyme + PEG + seeds (day 4); d) lysozyme + PEG + seeds (day 8). Scale bars represent 200 nm.
These cytotoxic data correlated well with the number of accumulated fibrils as detected by the corresponding ThT and TEM measurements. EGC directed the aggregation of lysozyme through an off-pathway, resulting in the formation of assemblies with low cytotoxicity under both seeded and non-seeded conditions. In contrast, PEG did not alter the on-pathway pattern of lysozyme assembly; therefore, the formation of cytotoxic fibrils could be accelerated under seeding. We conclude that the effects of seeding on lysozyme amyloid fibrillation depend on the pathway of aggregate formation.
It is worth noting that PEG [32] and EGC [33] have been reported to inhibit lysozyme fibrillation in an alkali-salt medium (pH 12.75) instead of the acidic medium (pH 2.0) used in the present work. Typically, lysozyme fibrils are prepared at pH 2.0 to study fibrillar properties and cytotoxicity [5, 16, 31, 34-37]. Fibrils prepared in an acidic medium can be disassembled by SDS into lysozyme monomers and hydrolysis-originated fragments [25, 29, 31]. In contrast, at a high pH, lysozyme transforms into aggregates by intermolecular disulfide cross-linking [38, 39]. Although the molecular mechanisms of lysozyme fibrillation were different under acidic and alkaline conditions, PEG and EGC exhibited an inhibitory effect on amyloid formation under both conditions.
Several mechanisms have been used to elucidate fibril growth, including nucleated conformational transition [40] and template-based assembly [41, 42]. In template-dependent assembly, the association of monomers and oligomers with the fibrillar template follows a so-called dock-lock mechanism. Monomers or oligomers can be added on the existed template reversibly in the dock phase. In contrast, in the lock phase, the added monomers or oligomers associate irreversibly to the template, accompanied by the conformational transition into a β-sheet enriched structure and subsequent fibril elongation. In addition, recent reports demonstrate that ring-like oligomers are the basic building units of amyloid fibrils [43, 44]. During an early stage of fibril formation, such oligomers associate with each other by a ring-to-ring or ring-on-ring pattern [44].
The distinct roles of seeding established in this study may be attributed to different patterns of interactions between the inhibitors and lysozyme. Upon incubation with lysozyme, ECG transforms to active quinone intermediates and covalently binds to the peptide chains. The modification of peptide chains alters the interacting forces of lysozyme monomers and/or oligomers, leading to interruption of on-pathway fibrillation. Alternatively, the formation of amorphous aggregates through the off-pathway becomes predominant. In the presence of fibril seeds and EGC, lysozyme fibrillation through the on-pathway assembly proceeds competitively with off-pathway aggregation. When EGC is applied at a high concentration, off-pathway aggregation becomes predominant, while on-pathway fibrillation is inhibited.
Polymers with high hydrophilicity, such as PEG, Ficoll, dextran, and other polysaccharides, have been used to create crowded conditions for mimicking the intracellular environment. These polymers are highly soluble in water and form network-like structures with high viscosity at high concentrations [45, 46]. Several properties, such as nonspecific interactions, excluded volume, and increased viscosity are thought to drive the crowding effects of these polymers on protein fibrillation [47-49]. In general, the effects of excluded volume are to accelerate the rate of amyloid formation of peptides, while increased viscosity plays an opposite role on fibril growth due to a reduction in the diffusional mobility of aggregation-prone intermediates.
In this study, we showed that PEG inhibited lysozyme fibrillation in a concentration-dependent manner. The inhibition of amyloid formation by a low concentration of PEG (10 or 20 mg/ml) indicates that direct nonspecific interactions between lysozyme and PEG may play a more important role than the effects of excluded volume and viscosity. Oligomers and protofibrils are generally considered short-lived intermediates of the assembly process but can be stabilized under some environments [50-52]. The peptide–PEG interaction stabilizes the aggregation-prone intermediates and decreases the rate of fibril assembly, thereby resulting in the observed inhibition of lysozyme fibrillation. Because PEG is chemically inert, the fibrillar species of lysozyme are confined in their on-pathway forms in crowded environments. These species may be kept in the “dock” phase where the monomers/oligomers associate with large species reversibly. When preformed seeds are introduced into the crowding system, the seeds provide many templates, triggering the assembly of on-pathway species into amyloid fibrils and leading to acceleration of fibril growth. Moreover, the on-pathway species can also act as seeds to accelerate fibril growth of fresh lysozyme monomers under a non-crowded condition. This information may be important for exploring the role of seeding in amyloidogenesis in intracellular medium.
In conclusion, we investigated the effects of seeding on lysozyme fibrillation in the presence of two distinct inhibitors. The results suggest that the effects of seeding of preformed fibrils on acceleration of lysozyme fibrillation depends on the type and concentration of inhibitor. EGC inhibited lysozyme fibrillation and covalently modified the peptide chains with quinone moieties in a concentration-dependent manner. The addition of preformed fibril seeds did not stimulate fibril growth because lysozyme aggregation in the presence of EGC proceeded through an off-pathway. PEG also inhibited lysozyme fibrillation. The addition of preformed fibril seeds significantly accelerated fibril growth because lysozyme fibrillation within the PEG-crowded system proceeded through an on-pathway assembly. In accordance with the ThT and TEM data, cytotoxic assays showed that fibrillar seeds failed to alter the cell-damaging effects of the EGC-directed off-pathway aggregates, but did increase the cytotoxicity induced by the PEG-directed on-pathway fibrils.
REFERENCES
1.Stefani, M. (2004) Protein misfolding and
aggregation: new examples in medicine and biology of the dark side of
the protein world, Biochim. Biophys. Acta, 1739,
5-25.
2.Dobson, C. M. (2003) Protein folding and
misfolding, Nature, 426, 884-890.
3.Nizhnikov, A. A., Antonets, K. S., and
Inge-Vechtomov, S. G. (2015) Amyloids: from pathogenesis to function,
Biochemistry (Moscow), 80, 1127-1144.
4.Bucciantini, M., Giannoni, E., Chiti, F., Baroni,
F., Formigli, L., Zurdo, J., Taddei, N., Ramponi, G., Dobson, C. M.,
and Stefani, M. (2002) Inherent toxicity of aggregates implies a common
mechanism for protein misfolding diseases, Nature, 416,
507-511.
5.Huang, B., He, J., Ren, J., Yan, X. Y., and Zeng,
C. M. (2009) Cellular membrane disruption by amyloid fibrils involved
intermolecular disulfide cross-linking, Biochemistry, 48,
5794-5800.
6.Hu, X., Crick, S. L., Bu, G., Frieden, C., Pappu,
R. V., and Lee, J. M. (2009) Amyloid seeds formed by cellular uptake,
concentration, and aggregation of the amyloid-beta peptide, Proc.
Natl. Acad. Sci. USA, 106, 20324-20329.
7.Furukawa, Y., Kaneko, K., Watanabe, S., Yamanaka,
K., and Nukina, N. (2013) Intracellular seeded aggregation of mutant
Cu,Zn-superoxide dismutase associated with amyotrophic lateral
sclerosis, FEBS Lett., 587, 2500-2505.
8.Hall, D., Kardos, J., Edskes, H., Carver, J. A.,
and Goto, Y. (2015) A multi-pathway perspective on protein aggregation:
implications for control of the rate and extent of amyloid formation,
FEBS Lett., 589, 672-679.
9.Crespo, R., Villar-Alvarez, E., Taboada, P., Rocha,
F. A., Damas, A. M., and Martins, P. M. (2016) What can the kinetics of
amyloid fibril formation tell about off-pathway aggregation? J.
Biol. Chem., 291, 2018-2032.
10.Ehrnhoefer, D. E., Bieschke, J., Boeddrich, A.,
Herbst, M., Masino, L., Lurz, R., Engemann, S., Pastore, A., and
Wanker, E. E. (2008) EGCG redirects amyloidogenic polypeptides into
unstructured, off-pathway oligomers, Nature Struct. Mol. Biol.,
15, 558-566.
11.Bieschke, J., Russ, J., Friedrich, R. P.,
Ehrnhoefer, D. E., Wobst, H., Neugebauer, K., and Wanker, E. E. (2010)
EGCG remodels mature alpha-synuclein and amyloid-beta fibrils and
reduces cellular toxicity, Proc. Natl. Acad. Sci. USA,
107, 7710-7715.
12.Williams, A. D., Sega, M., Chen, M., Kheterpal,
I., Geva, M., Berthelier, V., Kaleta, D. T., Cook, K. D., and Wetzel,
R. (2005) Structural properties of Aβ protofibrils stabilized by a
small molecule, Proc. Natl. Acad. Sci. USA, 102,
7115-7120.
13.Necula, M., Breydo, L., Milton, S., Kayed, R.,
Van der Veer, W. E., Tone, P., and Glabe, C. G. (2007) Methylene blue
inhibits amyloid Abeta oligomerization by promoting fibrillization,
Biochemistry, 46, 8850-8860.
14.Pepys, M. B., Hawkins, P. N., Booth, D. R.,
Vigushin, D. M., Tennent, G. A., Soutar, A. K., Totty, N., Nguyen, O.,
Blake, C. F., Terry, C. J., Feest, T. G., Zalin, A. M., and Hsuan, J.
J. (1993) Human lysozyme gene mutations cause hereditary systemic
amyloidosis, Nature, 362, 553-557.
15.Swaminathan, R., Ravi, V. K., Kumar, S., Kumar,
M. V. S., and Chandra, N. (2011) Lysozyme: a model protein for amyloid
research, Adv. Protein Chem. Struct. Biol., 84,
63-111.
16.Gharibyan, A. L., Zamotin, V., Yanamandra, K.,
Moskaleva, O. S., Margulis, B. A., Kostanyan, I. A., and
Morozova-Roche, L. A. (2007) Lysozyme amyloid oligomers and fibrils
induce cellular death via different apoptotic/necrotic pathways, J.
Mol. Biol., 365, 1337-1349.
17.Frare, E., De Laureto, P. P., Zurdo, J., Dobson,
C. M., and Fontana, A. (2004) A highly amyloidogenic region of hen
lysozyme, J. Mol. Biol., 340, 1153-1165.
18.Munishkina, L. A., Cooper, E. M., Uversky, V. N.,
and Fink, A. L. (2004) The effect of macromolecular crowding on protein
aggregation and amyloid fibril formation, J. Mol.
Recognit., 17, 456-464.
19.Ghahghaei, A., Divsalar, A., and Faridi, N.
(2010) The effects of molecular crowding on the amyloid fibril
formation of α-lactalbumin and the chaperone action of
α-casein, Protein J., 29, 257-264.
20.Hatters, D. M., Minton, A. P., and Howlett, G. J.
(2002) Macromolecular crowding accelerates amyloid formation by human
apolipoprotein C-II, J. Biol. Chem., 277, 7824-7830.
21.Seeliger, J., Werkmuller, A., and Winter, R.
(2013) Macromolecular crowding as a suppressor of human IAPP fibril
formation and cytotoxicity, PLoS One, 8, e69652.
22.Sukenik, S., Politi, R., Ziserman, L., Danino,
D., Friedler, A., and Harries, D. (2011) Crowding alone cannot account
for cosolute effect on amyloid aggregation, PLoS One, 6,
e15608.
23.Mittal, S., and Singh, L. R. (2014)
Macromolecular crowding decelerates aggregation of a β-rich
protein, bovine carbonic anhydrase: a case study, J. Biochem.,
156, 273-282.
24.Porat, Y., Abramowitz, A., and Gazit, E. (2006)
Inhibition of amyloid fibril formation by polyphenols: structural
similarity and aromatic interactions as a common inhibition mechanism,
Chem. Biol. Drug Des., 67, 27-37.
25.Feng, S., Song, X. H., and Zeng, C. M. (2012)
Inhibition of amyloid fibrillation of lysozyme by phenolic compounds
involves quinoprotein formation, FEBS Lett., 586,
3951-3955.
26.He, J., Xing, Y. F., Huang, B., Zhang, Y. Z., and
Zeng, C. M. (2009) Tea catechins induced the conversion of preformed
lysozyme amyloid fibrils to amorphous aggregates, J. Agr. Food
Chem., 57, 11391-11396.
27.Kim, J., Lee, H. J., and Lee, K. W. (2010)
Naturally occurring phytochemicals for the prevention of
Alzheimer’s disease, J. Neurochem., 112,
1415-1430.
28.Shoval, H., Lichtenberg, D., and Gazit, E. (2007)
The molecular mechanisms of the anti-amyloid effects of phenols,
Amyloid, 14, 73-87.
29.Cao, N., Zhang, Y. J., Feng, S., and Zeng, C. M.
(2015) Quinopeptide formation associated with the disruptive effect of
epigallocatechin gallate on lysozyme fibrils, Int. J. Biol.
Macromol., 78, 389-395.
30.Paz, M. A., Fluckiger, R., Boak, A., Kagan, H.
M., and Gallop, P. M. (1991) Specific detection of quinoproteins by
redox-cycling staining, J. Biol. Chem., 266, 689-692.
31.Mishra, R., Sorgjerd, K., Nystrom, S.,
Nordigarden, A., Yu, Y. C., and Hammarstrom, P. (2007) Lysozyme
amyloidogenesis is accelerated by specific nicking and fragmentation
but decelerated by intact protein binding and conversion, J. Mol.
Biol., 366, 1029-1044.
32.Ghosh, S., Pandey, N. K., and Dasgupta, S. (2014)
Crowded milieu prevents fibrillation of hen egg white lysozyme with
retention of enzymatic activity, J. Photochem. Photobiol. B,
138, 8-16.
33.Ghosh, S., Pandey, N. K., and Dasgupta, S. (2013)
Epicatechin gallate prevents alkali-salt mediated fibrillogenesis of
hen egg white lysozyme, Int. J. Biol. Macromol., 54,
90-98.
34.Mossuto, M. F., Bolognesi, B., Guixer, B.,
Dhulesia, A., Agostini, F., Kumita, J. R., Tartaglia, G. G., Dumoulin,
M., Dobson, C. M., and Salvatella, X. (2011) Disulfide bonds reduce the
toxicity of the amyloid fibrils formed by an extracellular protein,
Angew. Chem. Int. Ed., 50, 7048-7051.
35.Hill, S. E., Miti, T., Richmond, T., and Muschol,
M. (2011) Spatial extent of charge repulsion regulates assembly
pathways for lysozyme amyloid fibrils, PLoS One, 6,
e18171.
36.Wawer, J., Krakowiak, J., Szocinski, M., Lustig,
Z., Olszewski, M., and Szostak, K. (2014) Inhibition of amyloid fibril
formation of hen egg white lysozyme by trimethylamine N-oxide at low
pH, Int. J. Biol. Macromol., 70, 214-221.
37.Harada, A., Azakami, H., and Kato, A. (2008)
Amyloid fibril formation of hen lysozyme depends on the instability of
the C-helix (88-99), Biosci. Biotechnol. Biochem., 72,
1523-1530.
38.Kumar, S. V., Ravi, K., and Swaminathan, R.
(2008) How do surfactants and DTT affect the size, dynamics, activity
and growth of soluble lysozyme aggregates? Biochem. J.,
415, 275-288.
39.Ravi, V. K., Swain, T., Chandra, N., and
Swaminathan, R. (2014) On the characterization of intermediates in the
isodesmic aggregation pathway of hen lysozyme at alkaline pH, PLoS
One, 9, e87256.
40.Serio, T. R., Cashikar, A. G., Kowal, A. S.,
Sawicki, G. J., Moslehi, J. J., Serpell, L., Arnsdorf, M. F., and
Lindquist, S. L. (2000) Nucleated conformational conversion and the
replication of conformational information by a prion determinant,
Science, 289, 1317-1321.
41.Esler, W. P., Stimson, E. R., Jennings, J. M.,
Vinters, H. V., Ghilardi, J. R., Lee, J. P., Mantyh, P. W., and Maggio,
J. E. (2000) Alzheimer’s disease amyloid propagation by a
template-dependent dock-lock mechanism, Biochemistry, 39,
6288-6295.
42.Nguyen, P. H., Li, M. S., Stock, G., Straub, J.
E., and Thirumalai, D. (2007) Monomer adds to preformed structured
oligomers of Aβ-peptides by a two-stage dock-lock mechanism,
Proc. Natl. Acad. Sci. USA, 104, 111-116.
43.Grigorashvili, E. I., Selivanova, O. M.,
Dovidchenko, N. V., Dzhus, U. F., Mikhailina, A. O., Suvorina, M. Y.,
Marchenkov, V. V., Surin, A. K., and Galzitskaya, O. V. (2016)
Determination of size of folding nuclei of fibrils formed from
recombinant Aβ(1-40) peptide, Biochemistry (Moscow),
81, 538-547.
44.Selivanova, O. M., Glyakina, A. V., Gorbunova, E.
Y., Mustaeva, L. G., Suvorina, M. Y., Grigorashvili, E. I., Nikulin, A.
D., Dovidchenko, N. V., Rekstina, V. V., Kalebina, T. S., Surin, A. K.,
and Galzitskaya, O. V. (2016) Structural model of amyloid fibrils for
amyloidogenic peptide from Bgl2p-glucantransferase of S.
cerevisiae cell wall and its modifying analog. New morphology of
amyloid fibrils, Biochim. Biophys. Acta, 1864,
1489-1499.
45.Wenner, J. R., and Bloomfield, V. A. (1999)
Crowding effects on EcoRV kinetics and binding, Biophys. J.,
77, 3234-3241.
46.Hirata, Y., Sano, Y., Aoki, M., Shohji, H.,
Katoh, S., Abe, J., Hitsukuri, S., and Yamamoto, H. (2003) Small-angle
X-ray scattering studies of moderately concentrated dextran solution,
Carbohydr. Polym., 53, 331-335.
47.Breydo, L., Reddy, K. D., Piai, A., Felli, I. C.,
Pierattelli, R., and Uversky, V. N. (2014) The crowd you’re in
with: effects of different types of crowding agents on protein
aggregation, Biochim. Biophys. Acta, 1844, 346-357.
48.Phillip, Y., and Schreiber, G. (2013) Formation
of protein complexes in crowded environments – from in
vitro to in vivo, FEBS Lett., 587,
1046-1052.
49.Gaharwar, B., Gour, S., Kaushik, V., Gupta, N.,
Kumar, V., Hause, G., and Yadav, J. K. (2015) Assessment of the effect
of macromolecular crowding on aggregation behavior of a model
amyloidogenic peptide, Protein Pept. Lett., 22,
87-93.
50.Necula, M., Kayed, R., Milton, S., and Glabe, C.
(2007) Small molecule inhibitors of aggregation indicate that
amyloid-β oligomerization and fibrillization pathways are
independent and distinct, J. Biol. Chem.,
282, 10311-10324.
51.Moores, B., Drolle, E., Attwood, S. J., Simons,
J., and Leonenko, Z. (2011) Effect of surfaces on amyloid fibril
formation, PLoS One, 6, e25954.
52.Pellarin, R., Schuetz, P., Guarnera, E., and
Caflisch, A. (2010) Amyloid fibril polymorphism is under kinetic
control, J. Am. Chem. Soc., 132, 14960-14970.