Chronic Alcohol Intoxication Is Not Accompanied by an Increase in Calpain Proteolytic Activity in Cardiac Muscle of Rats
Yu. V. Gritsyna1, N. N. Salmov1, A. G. Bobylev1, I. S. Fadeeva1, N. I. Fesenko1, D. G. Sadikova2, N. I. Kukushkin2, Z. A. Podlubnaya1,3, and I. M. Vikhlyantsev1,3*
1Institute of Theoretical and Experimental Biophysics, Russian Academy of Sciences, 142290 Pushchino, Moscow Region, Russia; E-mail: vikhlyantsev@iteb.ru, ivanvikhlyantsev@gmail.com2Institute of Cell Biophysics, 142290 Pushchino, Moscow Region, Russia
3Pushchino State Institute of Natural Sciences, 142290 Pushchino, Moscow Region, Russia
* To whom correspondence should be addressed.
Received August 9, 2016; Revision received October 25, 2016
Enzymatic activity of Ca2+-dependent calpain proteases as well as the content and gene expression of μ-calpain (activated by micromolar calcium ion concentrations), calpastatin (inhibitor of calpains), and titin (substrate for calpains) were investigated in cardiac muscles of rats subjected to chronic alcoholization for 3 and 6 months. There was no increase in the “heart weight/body weight” parameter indicating development of heart hypertrophy in the alcoholized rats, while a decreasing trend was observed for this parameter in the rats after 6-month modeling of alcoholic cardiomyopathy, which indicated development of atrophic changes in the myocardium. Fluorometric measurements conducted using the Calpain Activity Assay Kit did not reveal any changes in total calpain activity in protein extracts of cardiac muscles of the rats alcoholized for 3 and 6 months. Western blot analysis did not show reliable changes in the contents of μ-calpain and calpastatin, and SDS-PAGE did not reveal any decrease in the titin content in the myocardium of rats after the chronic alcohol intoxication. Autolysis of μ-calpain was also not verified, which could indicate that proteolytic activity of this enzyme in myocardium of chronically alcoholized rats is not enhanced. Using Pro-Q Diamond staining, changes in phosphorylation level of titin were not detected in cardiac muscle of rats after chronic alcoholization during three and six months. A decrease in µ-calpain and calpastatin mRNA content (~1.3-fold, p ≤ 0.01 and ~1.9-fold, p ≤ 0.01, respectively) in the myocardium of rats alcoholized for 3 months and decrease in calpastatin mRNA (~1.4-fold, p ≤ 0.01) in animals alcoholized for 6 months was demonstrated using real-time PCR. These results indicate negative effect of chronic alcohol intoxication on expression of the abovementioned genes.
KEY WORDS: cardiac muscle, μ-calpain, calpastatin, titin, chronically alcoholized ratsDOI: 10.1134/S0006297917020080
Abbreviations: MHC, myosin heavy chains; T1, intact titin molecule located between the M-band and Z-disk of a sarcomere; T2, proteolytic fragment of the intact T1 linked to myosin filaments in A-disk of a sarcomere.
Calpains belong to the family of cytosolic Ca2+-dependent
cysteine proteases [1]. Sixteen components of the
mammalian calpain system are now known: 15 calpain proteases and one
inhibitor – calpastatin [2, 3]. In the animal’s striated
muscles, μ-calpain (activated by micromolar concentrations of
calcium ions – 2-200 μM) and m-calpain (activated by
submillimolar concentrations of calcium ions –
400-800 μM) have the highest content and are ubiquitous [2, 4]. Other calpains are also
expressed in mammalian muscles, such as calpain 3 in skeletal muscles
[5] and calpains 9 and 10 in cardiac muscle [6]. It is known that several myofibrillar proteins of
thick and thin filaments in muscle sarcomeres, including titin and
nebulin, are subjected to proteolysis by calpains such
as μ-calpain in vitro [2, 7]. It has been suggested that protein turnover in
muscle cells is initiated by calpain-mediated proteolysis of titin,
nebulin, and other sarcomeric proteins followed by degradation of their
fragments to amino acid residues via the ubiquitin–proteasome
pathway [3]. It was shown that an increase in
activity of calpains such as μ-calpain in mammalian skeletal
muscles under conditions of gravitational unloading [8] contributed to development of atrophy, which was
accompanied by enhanced proteolysis of giant proteins of the sarcomere
cytoskeleton titin and nebulin [9-13], as well as by an increased level of titin
phosphorylation [13]. In previously conducted
studies, we showed that alcohol-induced atrophy of rat m. soleus
was also accompanied by increase in μ-calpain activity,
decrease in titin content, and by increase in the level of
phosphorylation of this protein [14]. On the
contrary, the development of alcoholic cardiomyopathy is manifested by
the development of cardiac hypertrophy. It has been shown that the
development of cardiac muscle hypertrophy in spontaneously hypertensive
rats (SHR) is accompanied by a decrease in the content of N2BA- and
N2B-isoforms of titin [15, 16]. It cannot be ruled out that similar changes
would occur in rat cardiac muscle after chronic alcohol intoxication,
which in turn could be caused by the increased activity of calpains.
In this work, we studied whether changes in proteolytic activity of calpains occurred in rat cardiac muscle after 3- and 6-month alcoholic myopathy modeling as well as if there were any changes in the content and gene expression of μ-calpain, calpastatin, and titin during this process.
MATERIALS AND METHODS
Chronic alcoholization of rats. Male rats of the Wistar line were used in the study. The body weight of each animal at the beginning of experiment was 150 ± 5 g. The animals were contained in separate cages in the vivarium of the Institute of Theoretical and Experimental Biophysics of the Russian Academy of Sciences (Pushchino). The protocol for use of the animals was approved by the Biomedical Ethics Committee of the Institute of Theoretical and Experimental Biophysics of the Russian Academy of Sciences. Chronic alcoholization of animals was performed using two procedures: (i) according to method [17] using 10% ethanol solution in water, as well as agar blocks containing 30% ethanol, for 3 and 6 months (five animals in each control and alcohol-treated group, average daily consumption of ethanol was 26.6 ± 3.0 and 23.4 ± 2.0 g/kg per day, respectively); (ii) according to method [18] using nutritionally balanced liquid food containing 5% ethanol for 3 months (four rats in each control and alcohol-treated groups, average daily ethanol consumption was 19.1 ± 2.0 g/kg per day). When cardiomyopathy was modeled according to [17], adaptation of the animals to alcohol was developed by gradually increasing ethanol content in drinking water and agar. The ethanol content in water was 5% during the first 3 days, and after that it was 10% until the completion of the experiment. The ethanol content in agar was increased by 5% each 3 days (to 30% final alcohol concentration). After completion of the period of chronic alcoholization, the animals were weighed and euthanized using 25 mg of Zoletil preparation (Virbac Sante Animale, France) per kg body weight, which was 3-fold higher than the therapeutic dose (7-8 mg/kg). The heart was removed and weighed, and then left ventricle was isolated, frozen, and stored at –75°C.
Calpain activities in protein extracts of cardiac muscle were determined using fluorescence spectrophotometry with Calpain Activity Assay Kit (Abcam, USA) according to the manufacturer’s protocol. Measurements were conducted on Infinite F200 multifunctional plate reader (Tecan, Austria).
Analysis of calpain and calpastatin content using SDS gel electrophoresis and Western blot. The μ-calpain from cardiac muscle was extracted as reported [19]. Extraction of calpastatin and GAPDH (reference protein) was conducted in lysing buffer containing 12 mM Tris-HCl, 1.2% SDS, 5 mM EGTA, 10% glycerol, 2% β-mercaptoethanol, and 5 µg/ml of leupeptin or E64, pH 6.8-7.0. Total protein concentration was determined spectrophotometrically using a NanoDrop ND-1000 device (NanoDrop Technologies, USA). Protein samples were heated at 95°C for 4 min. Equal volumes of protein samples with equal total protein concentrations were loaded onto gel lanes. SDS-PAGE was conducted according to [20] in 6.5% polyacrylamide gel for μ-calpain and in 9.5% polyacrylamide gel for calpastatin and GAPDH. Proteins were transferred from the gel to PVDF membrane. Primary antibodies to: μ-calpain (Abcam; ab28258, 1 : 4000), calpastatin (Abcam; ab28252, 1 : 3000), and GAPDH (Abcam; ab37168, 1 : 2000) were used. Antibodies conjugated with alkaline phosphatase (Abcam; ab6722, 1 : 3000) were used as the secondary antibodies. NBT/BCIP solution (Roche, Germany) was used for visualization of the protein bands on the PVDF membranes.
SDS-PAGE of titin. Polyacrylamide (2%) slab gels strengthened with agarose were prepared according to the Tatsumi–Hattori method [21] with modifications [11] and used for separation of high molecular weight (3000-3400 kDa) T1 isoforms (N2B, N2BA, NT) from the rat cardiac muscle. Samples were prepared for electrophoresis using a method excluding heating above 40°C [11] to prevent titin destruction at high temperatures.
Determination of titin phosphorylation level. Titin phosphorylation level was estimated using the fluorescent phosphoprotein stain Pro-Q Diamond (Invitrogen, USA) according to [22] with a slight modification described below. The gel was fixed for 12-18 h in solution containing 50% ethanol and 10% acetic acid after completion of electrophoresis. Next, the gel was washed for 30 min in distilled water and stained for 1.5 h with Pro-Q Diamond stain. The stained gel was washed in Pro-Q Diamond phosphoprotein gel destaining solution (Invitrogen) for 1.5 h. Protein bands containing phosphate were viewed using Pharos FX Plus Molecular Image (Bio-Rad, USA). Then gels were stained with Coomassie Brilliant Blue G-250 and R-250 mixed in ratio 1 : 1, to control protein content.
RNA was isolated from muscle tissue using Aurum™ Total RNA Fatty and Fibrous Tissue Kit (Bio-Rad) according to the manufacturer’s protocol. RNA quality was evaluated from preservation of the 18S and 28S rRNA by electrophoresis in 1% agarose gel. RNA concentration was determined with a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, USA). The RNA solution was stored at −75°C.
Reverse transcription and real-time PCR. The reverse transcription reaction was conducted with the total RNA preparation (concentration 0.2 μg/μl) using M-MLV reverse transcriptase (Evrogen, Russia) and a standard oligo-dT12 primer. The resulting cDNA was used in PCR with primers specific to the genes of the investigated proteins. Nucleotide sequences of the primers specific to the genes of μ-calpain (CAPN1), calpastatin (CAST), GAPDH (reference gene) (Table 1), as well as of titin (TTN, for the sum of N2BA and N2B-isoforms) were synthesized by Evrogen. Gene primers were designed using the Vector NTI Advance 11 software. Titin specific primers described in [23] were used. Real-time PCR was carried out in a DT-322 amplifier (DNK Tekhnologiya, Russia) using Taq-polymerase (Evrogen) and SYBR Green I (Invitrogen) as a fluorescent dye. The following PCR cycling parameters were used: (1) hot start – 95°C, 5 min; (2) denaturation – 95°C, 15 s; (3) primer annealing – 64°C, 20 s; (4) elongation – 72°C, 20 s. Steps 2-4 were repeated 35 times. The gene of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a housekeeping gene. Change in gene expression was calculated using the 2–ΔΔCt method [24]. The ΔΔCt values were calculated using the equation ΔΔCt = ΔCt (control) – ΔCt (test), and each ΔCt value was calculated using the equation ΔCt = Ct (muscle protein gene) – Ct (housekeeping gene). Products of amplification were separated in 2% agarose gel. Amplicons were isolated from the gel according to the Cleanup Standard protocol (Evrogen). DNA fragments were sequenced by Evrogen.
Table 1. Primers used for real-time PCR
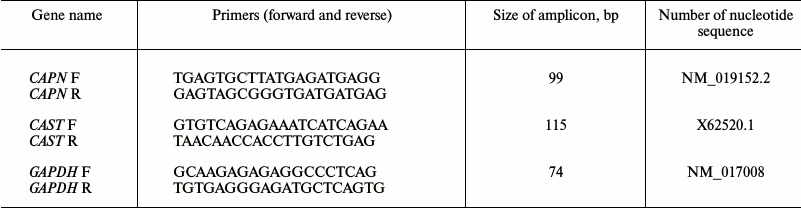
Note: F, forward primer; R, reverse primer. Titin specific primers
described in [23] were used for PCR.
Densitometry and statistical analysis of data. Washed gels and blot membranes were scanned and digitized, and the data were processed with Total Lab v1.11 software. The titin content was evaluated relative to the content of myosin heavy chains. It is known that there is certain ratio of these proteins in A-disk of the sarcomere (six titin molecules per each half of a myosin filament in a sarcomere), hence this method for estimation of the titin content is widely used and is more accurate than the estimation of titin content based on the total protein content in the sample. Statistical processing was conducted using the non-parametric Mann–Whitney U-criterion. In the tables depicting changes in the content and level of phosphorylation of T1 and T2, control values were taken as 100%. For each individual experiment, the average value of at least three control samples present in the gel were taken into consideration. Standard deviations were no more than 7% for the values related to the T1 and T2 contents, and no more than 10% for the values related to the level of phosphorylation of T1 and T2. The control values of expression of genes investigated in this study and of calpain activities were taken as 100% on the graphs.
RESULTS AND DISCUSSION
Data on the change in body weight and cardiac muscle weight of rats alcoholized for 3 and 6 months are presented in Table 2. No reliable changes of the cardiac muscle weight and total body weight of rats was observed after 3 months of chronic alcoholization according to [18] using liquid food (Table 2). No changes were observed in the “heart weight/body weight” parameter that indicated on developing of hypertrophy (in the case of ratio increase) or atrophy (in the case of ratio decrease) of the myocardium. A reliable decrease in the body weight of the animals after 3 and 6 months chronic alcoholization conducted according to [17] (by ~10%, p ≤ 0.05, and ~20%, p ≤ 0.01, respectively) was observed, as well as a decrease in the cardiac muscle weight (by ~15%, p ≤ 0.05, and ~22.5%, p ≤ 0.01, respectively) (Table 2), which was in agreement with the data in [17] where similar changes of the abovementioned parameters in rats were found after 14 weeks of chronic alcoholization. At the same time, we did not observe any reliable change in the “heart weight/body weight” ratio (Table 2), which also did not contradict data from [17]. However, a decreasing trend for the heart muscle weight relative to the whole body weight of rats alcoholized for 3 and 6 months according to [17] was observed in our experiments (Table 2). Hence, our results indicate on tendency for the development of atrophic rather than hypertrophic changes in the myocardium of chronically alcoholized rats.
Table 2. Parameters of cardiac muscle weight
and body weight of control and chronically alcoholized rats
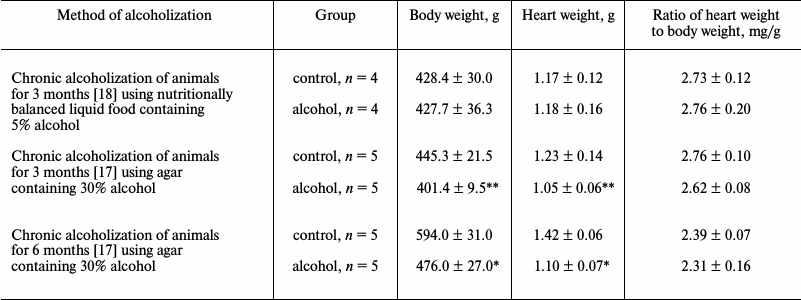
* p ≤ 0.01.
** p ≤ 0.05.
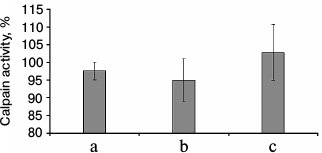
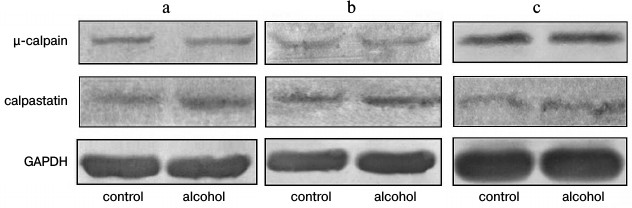
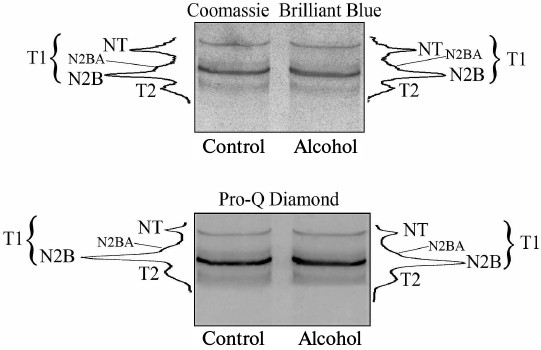
The results of fluorometric measurements carried out using Calpain Activity Assay Kit (Abcam) are presented in Fig. 1. No changes in the total calpain activity in the protein extracts of the cardiac muscle in all groups of the alcoholized rats were revealed (Fig. 1). These results explain why reliable atrophic changes in the myocardium of these animals were not observed (Table 2). However, considering the data on the tendency of development of alcohol-induced cardiac muscle atrophy in rats, the probability of changes in the proteolytic activity of individual forms of calpain such as μ-calpain must not be ruled out. To test this hypothesis, we conducted Western-blot analysis of content of μ-calpain and its fragments formed by protein processing (autolysis). It is known that μ-calpain is subjected to autolysis in the presence of Ca2+ [4, 19, 25]. Data of in vitro studies showed an increase of proteolytic activity of µ-calpain observed during this process [4]. It was established using Western-blot analysis that the autolysis of μ-calpain (MW 80 kDa) in rat and human skeletal muscles resulted in formation of two fragments with MW 78 and 76 kDa [19, 25]. In our earlier studies, we showed the availability of one fragment of μ-calpain (with MW 78 kDa) in skeletal muscles (m. soleus, m. gastrocnemius, m. longissimus dorsi) of rats [14]. Moreover, the alcohol-induced atrophic changes in m. soleus of these animals was accompanied by enhanced autolysis of μ-calpain, decrease in titin content, and increase in the level of phosphorylation of this protein [14]. Taking into consideration the trend for the development of atrophic changes in the cardiac muscle of chronically alcoholized rats (Table 2), it was expected to find enhanced autolysis of μ-calpain. However, according to the data of Western blot analysis, the presence of autolyzed fragments of μ-calpain was not observed in the cardiac muscle of both control and chronically alcoholized rats (Fig. 2). These results agree with the data of Western blot analysis published by other authors, which also found no autolyzed fragments of μ-calpain in rat myocardium [26]. In our study, we also observed no change in the content of μ-calpain in the rat myocardium (Fig. 2) despite a reliable decrease (by 23.4%, p ≤ 0.01) of μ-calpain mRNA in the cardiac muscle of these animals after 3-month alcoholization according to [17] (Fig. 3b). There was no significant change observed for the content of calpastatin – an inhibitor of calpains (Fig. 2), despite the decrease in mRNA of this protein by 47% (p ≤ 0.01) and by 31.3% (p ≤ 0.01) in the cardiac muscle of rats after 3 and 6 months of alcoholization according to [17] (Fig. 3, b and c). Hence, the data of Western blot analysis showed the absence of changes in the content of μ-calpain and calpastatin in cardiac muscle of rats after a prolonged period of alcoholic intoxication. The results also suggest the absence of changes in proteolytic activity of μ-calpain in the cardiac muscle of chronically alcoholized rats. This suggestion agrees with data on the content of titin (μ-calpain substrate) in the rat myocardium. No decrease in T1 content as well as increase in content of proteolytic T2 fragments in the myocardium of all investigated groups of chronically alcoholized rats was observed (Table 3 and Fig. 4). Also, no reliable change in the level of phosphorylation of T1 and T2 in the cardiac muscle of chronically alcoholized animals was detected (Table 4 and Fig. 4). The following must be mentioned during discussion of these results. The work we conducted previously showed that atrophic changes in skeletal muscles of animals were accompanied not only by decrease in T1 content, but also by increase in its level of phosphorylation [13, 14, 27]. The results of these studies suggested that the hyperphosphorylation of titin, especially of the T2 part of its molecules, increased its susceptibility to calpain-mediated proteolysis [27]. The data produced in the present study indicating on both the absence of titin content decrease and of increase in its level of phosphorylation in the myocardium of alcoholized rats (Tables 3 and 4 and Fig. 4) could be considered as corroboration of the abovementioned suggestion.
Fig. 1. Total calpain activity in protein extracts of cardiac muscle of chronically alcoholized rats. a) The animals were chronically alcoholized for 3 months according to [18] using nutritionally balanced liquid food containing 5% alcohol. b, c) The animals were chronically alcoholized for 3 and 6 months, respectively, according to [17] using agar containing 30% alcohol. Data are presented in percent relative to the control values taken as 100%.
Fig. 2. Western blot analysis of μ-calpain and calpastatin in myocardium of control and chronically alcoholized rats. a) The animals were chronically alcoholized for 3 months according to [18] using nutritionally balanced liquid food containing 5% alcohol. b, c) The animals were chronically alcoholized for 3 and 6 months, respectively, according to [17] using agar containing 30% alcohol. Content of μ-calpain and calpastatin in myocardium of the three groups of alcoholized rats relative to the content of these proteins in the myocardium of control rats was: 97.1 ± 7.2, 103.2 ± 5.0, 99.2 ± 9.5, and 111.6 ± 14.5, 100.9 ± 3.8, 110.7 ± 16.7, respectively.
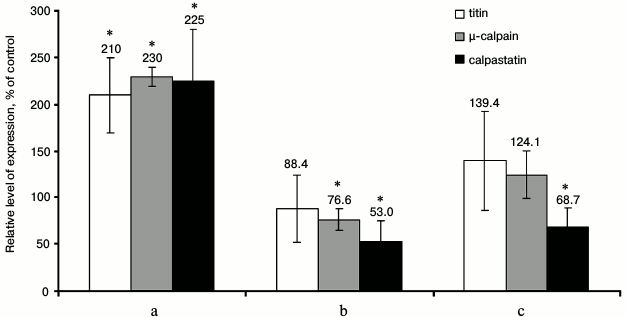
Fig. 3. Changes in gene expression of titin, μ-calpain, and calpastatin in the cardiac muscle of chronically alcoholized rats. a) The animals were chronically alcoholized for 3 months according to [18] using nutritionally balanced liquid food containing 5% alcohol. b, c) The animals were chronically alcoholized for 3 and 6 months, respectively, according to [17] using agar containing 30% alcohol. * p ≤ 0.01.
Fig. 4. Determination of content (Coomassie Brilliant Blue) and level of phosphorylation (Pro-Q Diamond) of titin in myocardium of control and 6-month chronically alcoholized rats. Bands of T1 isoforms (NT, N2BA, N2B) and of T2-fragment of titin are indicated.
Table 3. Titin content in myocardium of
chronically alcoholized rats (percentage relative to control)
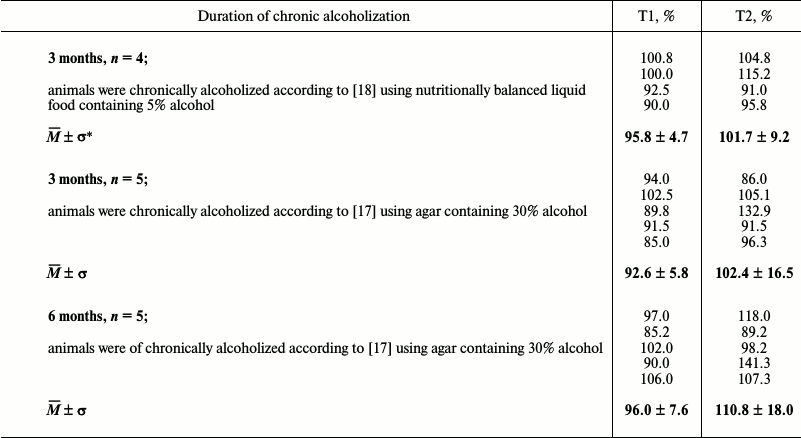
* Mean ± standard deviation.
Table 4. Changes in level of titin
phosphorylation in myocardium of chronically alcoholized rats
(percentage relative to control)
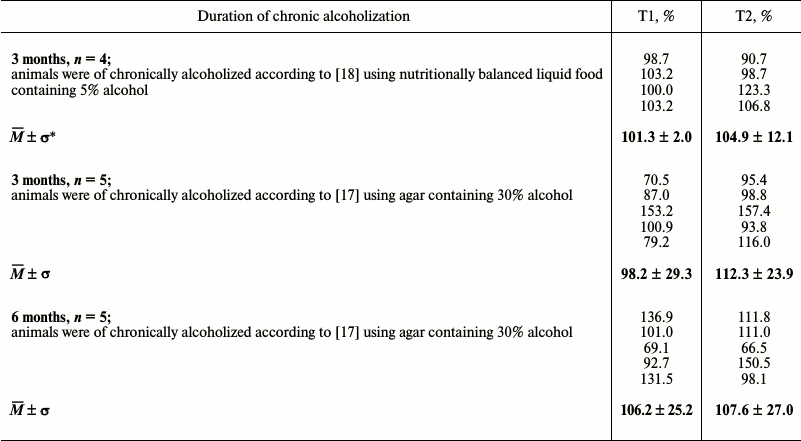
* Mean ± standard deviation.
Based on results about changes in expression of genes of the investigated proteins in cardiac muscle of chronically alcoholized rats, it followed that on the background of insignificant increase in titin gene expression (Fig. 3c), a reliable decrease in μ-calpain and calpastatin mRNA after 3-month chronic alcoholization (Fig. 3b), as well as significant decrease in calpastatin mRNA after 6-month chronic alcoholization of rats (Fig. 3c) were observed [17]. The results agree with data on the decrease in MHC mRNA in the skeletal muscles of chronically alcoholized rats [28], while they conflict with data on increase in MHC β-isoform mRNA in rat myocardium after 16-week alcoholization of rats carried out with agar blocks containing alcohol [29]. Hence, our data indicate on negative effect of chronic alcoholic intoxication on the expression of the μ-calpain and calpastatin genes in the rat cardiac muscle. No decrease in the content of μ-calpain and calpastatin in the myocardium of rats in the “alcohol” group (Fig. 2) could be related most likely to decreased proteolysis of these proteins. Further studies are required to elucidate the cause of this phenomenon.
When alcoholic myopathy was modeled for 3 months according to [18] using liquid food, increase (2.1-2.3-fold, p ≤ 0.01) in expression of μ-calpain, calpastatin, and titin genes was observed (Fig. 3a). On one hand, it is possible that not so “harsh” conditions of chronic alcoholization are created by this method of alcoholic myopathy modeling, so as not to cause decrease in gene expression of the investigated proteins. On the other hand, the revealed changes could be aimed at an increase in protein synthesis, which is inhibited in muscles due to the alcohol-induced disruptions of translation initiation [17] and elongation [29]. Other causes of the revealed changes are also possible, and further studies are needed to determine them.
The following conclusions can be made from this work. No hypertrophic or pronounced atrophic changes were detected in the cardiac muscle of rats alcoholized for 3 and 6 months. Changes in the total activity of calpains and in the content of titin, μ-calpain, and calpastatin were not observed in the myocardium of chronically alcoholized rats in all three investigated groups, and the levels of titin phosphorylation were not changed. However, the alcohol-induced decrease in μ-calpain and calpastatin mRNA in the myocardium of rats alcoholized according to the method described by Lang and coauthors [17] was detected. These results indicate on negative effect of chronic alcohol intoxication on the expression of the abovementioned genes. The reasons for enhanced expression of titin, μ-calpain, and calpastatin genes in the myocardium of rats alcoholized according to the method described by Lieber and DeCarli [18] are not clear. Further studies are needed for their elucidation.
Acknowledgements
This work was financially supported by the Russian Foundation for Basic Research (projects Nos. 14-04-00112 and 14-04-00283).
Equipment for collective use of the Institute of Theoretical and Experimental Biophysics and of the Institute of Cell Biology (Russian Academy of Sciences) was used in this work.
REFERENCES
1.Suzuki, K. (1990) The structure of the calpains and
the calpain gene, in Intracellular Calcium-Dependent Proteolysis
(Mellgren, R. L., and Murachi, T., eds.) Boca Raton, CRC Press, FL, pp.
25-35.
2.Goll, D. E., Thompson, V. F., Li, H., Wei, W., and
Cong, J. (2003) The calpain system, Physiol. Rev., 83,
731-801.
3.Goll, D. E., Neti, G., Mares, S. W., and Thompson,
V. F. (2008) Myofibrillar protein turnover: the proteasome and the
calpains, J. Anim. Sci., 86, E19-35.
4.Baki, A., Tompa, P., Alexa, A., Molnar, O., and
Friedrich, P. (1996) Autolysis parallels activation of mu-calpain,
Biochem. J., 318, 897-901.
5.Grishina, D. A., Suponeva, N. A., Shvedkov, V. V.,
and Belopasova, A. V. (2015) Inherited progressive limb-girdle muscular
dystrophy type 2A (calpainopathy): a review of literature, Nerv.
Mysh. Bol., 1, 25-36.
6.Campbell, R. L., and Davies, P. L. (2012)
Structure-function relationships in calpains, Biochem. J.,
447, 335-351.
7.Mohrhauser, D. A., Underwood, K. R., and Weaver, A.
D. (2011) In vitro degradation of bovine myofibrils is caused
by μ-calpain, not caspase-3, J. Anim. Sci., 89,
798-808.
8.Enns, D. L., Raastad, T., Ugelstad, I., and
Belcastro, A. N. (2007) Calpain/calpastatin activities and substrate
depletion patterns during hindlimb unweighting and reweighting in
skeletal muscle, Eur. J. Appl. Physiol., 100,
445-455.
9.Shenkman, B. S., Podlubnaya, Z. A., Vikhlyantsev,
I. M., Litvinova, K. S., Udal’tsov, S. N., Nemirovskaya, T. L.,
Lemesheva, Yu. S., Mukhina, A. M., and Kozlovskaya, I. B. (2004)
Contractile characteristics and proteins of sarcomeric cytoskeleton of
human m. soleus fibers under conditions of gravitational
unloading. Role of support stimulus, Biofizika, 49,
881-890.
10.Udaka, J., Ohmori, S., Terui, T., Ohtsuki, I.,
Ishiwata, S., Kurihara, S., and Fukuda, N. (2008) Disuse-induced
preferential loss of the giant protein titin depresses muscle
performance via abnormal sarcomeric organization, J. Gen.
Physiol., 131, 33-41.
11.Vikhlyantsev, I. M., and Podlubnaya, Z. A. (2012)
New titin (connectin) isoforms and their functional role in striated
muscles of mammals: facts and suppositions, Biochemistry
(Moscow), 77, 1515-1535.
12.Okuneva, A. D., Vikhlyantsev, I. M., Shpagina, M.
D., Rogachevskii, V. V., Khutzyan, S. S., Podlubnaya, Z. A., and
Grigoriev, A. I. (2012) Changes in titin and myosin heavy chain isoform
composition in skeletal muscles of Mongolian gerbil (Meriones
unguiculatus) after 12-day spaceflight, Biophysics,
57, 581-586.
13.Ulanova, A., Gritsyna, Y., Vikhlyantsev, I.,
Salmov, N., Bobylev, A., Abdusalamova, Z., Rogachevsky, V., Shenkman,
B., and Podlubnaya, Z. (2015) Isoform composition and gene expression
of thick and thin filament proteins in striated muscles of mice after
30-day space flight, Biomed Res. Int., doi:
10.1155/2015/104735.
14.Gritsyna, Y. V., Vikhlyantsev, I. M., Salmov, N.
N., Bobylev, A. G., Kukushkin, N. I., and Podlubnaya, Z. A. (2016) The
role of calpain system in atrophy of skeletal muscles of alcohol-fed
rats, in Biological Motility, pp. 79-85.
15.Warren, C. M., Jordan, M. C., Roos, K. P.,
Krzesinski, P. R., and Greaser, M. L. (2003) Titin isoform expression
in normal and hypertensive myocardium, Cardiovasc. Res.,
59, 86-94.
16.Karaduleva, E. V., Vikhlyantsev, I. M., and
Podlubnaya, Z. A. (2010) Expression of titin in the myocardium of
spontaneously hypertensive rats during development of hypertrophy,
Biophysics, 55, 550-554.
17.Lang, C. N., Wu, D., Frost, R. A., Jefferson, L.
S., Kimball, S. R., and Vary, T. C. (1999) Inhibition of muscle protein
synthesis by alcohol is associated with modulation of eIF2B and eIF4E,
Am. J. Physiol. Endocrinol. Metab., 277, E268-E276.
18.Lieber, C. S., and DeCarli, L. M. (1989) Liquid
diet technique of ethanol administration: 1989 update, Alcohol
Alcohol., 24, 197-211.
19.Murphy, R. M., Snow, R. J., and Lamb, G. D.
(2006) mu-Calpain and calpain-3 are not autolyzed with exhaustive
exercise in humans, Am. J. Physiol. Cell. Physiol., 290,
C116-C122.
20.Laemmli, U. K. (1970) Cleavage of structural
proteins during the assembly of the head of bacteriophage T4,
Nature, 227, 680-685.
21.Tatsumi, R., and Hattori, A. (1995) Detection of
giant myofibrillar proteins connectin and nebulin by electrophoresis in
2% polyacrylamide slab gels strengthened with agarose, Anal.
Biochem., 224, 28-31.
22.Borbely, A., Falcao-Pires, I., van Heerebeek, L.,
Hamdani, N., Edes, I., Gavina, C., Leite-Moreira, A. F., Bronzwaer, J.
G., Papp, Z., van der Velden, J., Stienen, G. J., and Paulus, W. J.
(2009) Hypophosphorylation of the Stiff N2B-titin isoform raises
cardiomyocyte resting tension in failing human myocardium, Circ.
Res., 104, 780-786.
23.Opitz, C. A., Leake, M. C., Makarenko, I., Benes,
V., and Linke, W. A. (2004) Developmentally regulated switching of
titin size alters myofibrillar stiffness in the perinatal heart,
Circ. Res., 94, 967-975.
24.Livak, K. J., and Schmittgen, T. D. (2001)
Analysis of relative gene expression data using real-time quantitative
PCR and the 2(–Delta Delta C(T)) method, Methods,
25, 402-408.
25.Murphy, R. M., Verburg, E., and Lamb, G. D.
(2006) Ca2+ activation of diffusible and bound pools of
mu-calpain in rat skeletal muscle, J. Physiol., 576,
595-612.
26.Sandmann, S., Yu, M., and Unger, T. (2001)
Transcriptional and translational regulation of calpain in the rat
heart after myocardial infarction-effects of AT(1) and AT(2) receptor
antagonists and ACE inhibitor, Br. J. Pharmacol., 132,
767-777.
27.Salmov, N. N., Gritsyna, Yu. V., Ulanova, A. D.,
Vikhlyantsev, I. M., and Podlubnaya, Z. A. (2015) On the role of titin
phosphorylation in the development of muscular atrophy,
Biophysics, 60, 684-686.
28.Reilly, M. E., McKoy, G., Mantle, D., Peters, T.
J., Goldspink, G., and Preedy, V. R. (2000) Protein and mRNA levels of
the myosin heavy chain isoforms Ibeta, IIa, IIx and IIb in type I and
type II fibre-predominant rat skeletal muscles in response to chronic
alcohol feeding, J. Muscle Res. Cell Motil., 21,
763-773.
29.Vary, T. C., and Deiter, G. (2005) Long-term
alcohol administration inhibits synthesis of both myofibrillar and
sarcoplasmic proteins in heart, Metabolism, 54,
212-219.