REVIEW: Mechanisms of Long-Term Plasticity of Hippocampal GABAergic Synapses
A. V. Rozov1,2, F. F. Valiullina1, and A. P. Bolshakov3,4*
1Kazan Federal University, OpenLab of Neurobiology, 420008 Kazan, Russia2Division of Neuro- and Sensory Physiology, Institute of Physiology and Pathophysiology, Heidelberg University, 69120 Heidelberg, Germany
3Institute of Higher Nervous Activity and Neurophysiology, Russian Academy of Sciences, 117485 Moscow, Russia; E-mail: al230679@yandex.ru, ocrachek@yahoo.com
4Russian National Research Medical University, 117997 Moscow, Russia
* To whom correspondence should be addressed.
Received October 19, 2016; Revision received December 5, 2016
Long-term potentiation and depression of synaptic transmission have been considered as cellular mechanisms of memory in studies conducted in recent decades. These studies were predominantly focused on mechanisms underlying plasticity at excitatory synapses. Nevertheless, normal central nervous system functioning requires maintenance of a balance between inhibition and excitation, suggesting existence of similar modulation of glutamatergic and GABAergic synapses. Here we review the involvement of G-protein-coupled receptors in the generation of long-term changes in synaptic transmission of inhibitory synapses. We considered the role of endocannabinoid and glutamate systems, GABAB and opioid receptors in the induction of long-term potentiation and long-term depression in inhibitory synapses. The pre- and postsynaptic effects of activation of these receptors are also discussed.
KEY WORDS: long-term synaptic plasticity, GABAergic synapses, endocannabinoids, GABAB receptor, G-protein-coupled receptors, hippocampus, opioid receptorsDOI: 10.1134/S0006297917030038
Abbreviations: AMPA receptors/channels, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid-sensitive glutamate receptors/channels; CaMKII, calmodulin-dependent protein kinase II; CB1 and CB2, cannabinoid receptors of types 1 and 2, respectively; CNS, central nervous system; DSI, depolarization-induced suppression of inhibition; GABA, gamma-aminobutyric acid; LTD, long-term depression; LTP, long-term potentiation; NMDA receptors/channels, glutamate N-methyl-D-aspartate receptors/channels; PKA, cAMP-dependent protein kinase A.
The ability of synapses to change the efficiency of synaptic
transmission in response to either frequency stimulation or under the
action of metabolically active agents is recognized as synaptic
plasticity. The first experimental indications for the possibility of
changing the efficiency of synaptic transmission were demonstrated in
the rabbit hippocampus, where high-frequency activation of excitatory
synapses resulted in an increase in postsynaptic responses that could
last for several hours or even days [1, 2]. This phenomenon was termed long-term potentiation
(LTP). For half a century, LTP and its opposing process, long-term
depression (LTD), has attracted the attention of numerous scientific
groups, because both these phenomena could represent cellular
mechanisms of memory. It has been suggested that LTD plays the central
role in memory consolidation – the ability of the brain to
convert a short-term memory into a long-term memory [3, 4].
At the present time, LTP and LTD are intensely studied at hippocampal excitatory synapses. At the synapses formed by Schaffer collaterals on the spines of pyramidal neurons in the CA1 area of the hippocampus, both processes involve synaptic glutamate N-methyl-D-aspartate (NMDA) receptors [5].
It has been firmly established that the induction of LTP in the CA1 area of the hippocampus requires not only activation of NMDA receptors, but also significant depolarization of postsynaptic cells, which results in a decrease in voltage-dependent magnesium block of these channels and, respectively, an increase in the Ca2+ influx through NMDA channels. The significant NMDA receptor-mediated increase in calcium concentration in dendritic spines results, in turn, in activation of an intracellular signaling cascade that includes: (i) binding of free Ca2+ with calmodulin; (ii) binding of Ca2+-calmodulin complex to calmodulin-dependent protein kinase II (CaMKII); (iii) autophosphorylation of CaMKII causing a multifold increase of its kinase activity; (iv) phosphorylation of a subtype of glutamate receptors (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-sensitive); (v) increase in the number of AMPA channels in postsynaptic density due to mobilization of phosphorylated AMPA receptors, which, in turn, increases amplitude of the postsynaptic response. LTD emerges in response to low-frequency stimulation, which causes significantly lower depolarization of postsynaptic spines and, respectively, results in lesser weakening of the magnesium block of NMDA channels. Hence, the Ca2+ influx through NMDA channels in this case is significantly lower than during high-frequency stimulation and is likely insufficient for CaMKII activation. Nevertheless, it is recognized that Ca2+ binds to calcineurin and activates Ca2+-sensitive phosphatase I. Activation of the phosphatase causes dephosphorylation of the synaptic AMPA receptors and decrease in their density on the postsynaptic membrane [5].
Even though the clear majority of studies on long-term plasticity in recent decades were devoted to the excitatory synapses, an important role is played by similar changes in the efficiency of transmission in GABAergic inhibitory synapses, since a fine balance between excitation and inhibition is required for normal brain functioning. However, unlike the excitatory synapses formed by Schaffer collaterals on the pyramidal cells in the CA1 area of the hippocampus, the GABAergic synapses exhibit a wide diversity determined by the type of presynaptic GABAergic interneurons. For example, three types of excitatory cells have been identified in the hippocampus (granule cells of fascia dentata, pyramidal neurons of the CA3-CA4 area, and pyramidal neurons of the CA1-CA2 area), while there are more than 20 types of GABAergic interneurons [6]. Moreover, all these interneurons differ from each other not only by morphological features, but also by the type of inhibition (dendritic, perisomatic, etc.), expression of specific peptide or protein markers (such as Ca2+-binding proteins) and metabotropic receptors capable of initiation of various intercellular cascades. It is most likely that the polymorphism of interneurons defines impressive variability of mechanisms underlying synaptic plasticity of GABAergic synapses [7-11]. Here, we review the participation and role of G-protein-coupled receptors in the emergence of long-term changes in the efficiency of synaptic transmission at GABAergic synapses formed by different types of hippocampal interneurons.
MECHANISMS OF LONG-TERM SYNAPTIC PLASTICITY AT GABAergic SYNAPSES
IN THE HIPPOCAMPUS
Unlike excitatory synapses, long-term changes in synaptic transmission in GABAergic synapses involve G-protein-coupled signaling systems; moreover, participation of these systems is essential for the development of LTP and LTD. Nevertheless, the mechanism underlying long-term synaptic changes in various inhibitory synapses is similar to the one described for the excitatory connections. For example, high-frequency stimulation caused the NMDA-dependent activation of the calcineurin phosphatase in the synapses formed by interneurons localized in stratum radiatum onto the CA1 pyramidal neurons. Activation of this cascade resulted in long-term depression of postsynaptic inhibitory responses at these synapses [11]; furthermore, it was shown later that the LTD emerged not due to change in the protein phosphorylation, but rather due to the direct interaction of calcineurin with the γ2-subunit of the GABAA channel [12]. It is likely that the release of glutamate required for activation of NMDA channels occurred from the neighboring excitatory synapses similarly to a process described in [13], but the experimental protocol did not allow either rejection or confirmation of this hypothesis. Meanwhile, the mechanisms of long-term synaptic changes induction in most GABAergic synapses involve intracellular cascades different from the ones participating in excitatory synapses.
In this review, we will discuss the involvement of the following G-protein-coupled cascades in the generation of long-term plasticity of GABAergic transmission: (i) endocannabinoid; (ii) glutamate; (iii) GABAB; and (iv) opioid. In many cases, the synergetic interaction of these systems is required for the induction of long-term synaptic changes; moreover, this interaction occurs not only inside one cell, but also involves spatially segregated signaling systems in pre- and postsynaptic neurons.
ENDOCANNABINOID AND GLUTAMATE SIGNALING SYSTEMS
The endocannabinoid system plays an important role in the central nervous system (CNS) functioning. Several endocannabinoids were identified and the effects of anandamide and 2-arachidonoylglycerol have been well studied. All known endocannabinoids are synthesized from cell lipids. The anandamide and 2-arachidonoylglycerol are synthesized via several cascades that often are functioning simultaneously in the cell and activated in neurons by an increase in intracellular Ca2+ level. It was shown that cannabinoids activate cannabinoid receptors of types 1 and 2 (CB1 and CB2).
The effects of endocannabinoids (2-arachidonoylglycerol, anandamide, N-arachidonoyl dopamine) in the CNS are generally mediated by the type 1 cannabinoid receptors (CB1). These receptors are expressed mainly in interneurons [14], and moreover, 95% of hippocampal interneurons expressing CB1 receptors also synthesize cholecystokinin. These receptors are coupled with Gi proteins, whose activation inhibits adenylate cyclase activity and decreases the cyclic adenosine monophosphate (cAMP) concentration. In addition, activation of CB1 receptors results in dissociation of the Gβγ-subunit of the receptor, which can block voltage-gated Ca2+ channels via direct interaction [15]. Unlike many other receptors, these metabotropic receptors are located exclusively on presynaptic neurons [16].
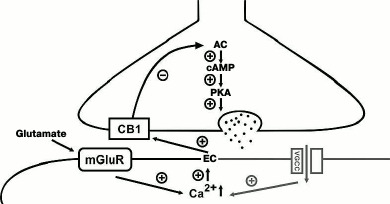
Endocannabinoids significantly modulate the release of mediator from perisomatic synapses formed by CB1-expressing interneurons in all areas of the hippocampus. One form of the endocannabinoid-dependent short-term plasticity is depolarization-induced suppression of inhibition (DSI). DSI was first demonstrated in pyramidal neurons of the CA1 area of the hippocampus [17, 18]. However, the endocannabinoid system participates not only in short-term plasticity, but it is also involved in the formation of various forms of long-term synaptic changes at inhibitory synapses in many regions of the CNS [19-22]. Unlike neuromediators, which are released from presynaptic terminals, endocannabinoids are synthesized in postsynaptic neurons in response to an increase in intracellular Ca2+ concentration and play a role of retrograde messenger activating presynaptic CB1 receptors, which results in the suppression of transmitter release [23]. The endocannabinoid-mediated LTD in many brain areas also requires an increase in intracellular Ca2+ concentration and in addition to that activation of the postsynaptic metabotropic glutamate receptors (Fig. 1). Chevaleyre and Castillo demonstrated that high-frequency stimulation in the stratum radiatum in CA1 region activated postsynaptic metabotropic type 1 glutamate receptors and Ca2+-dependent synthesis and release of endocannabinoids from postsynaptic neurons, which, in turn, caused long-term reduction of the GABA release via activation of presynaptic CB1 receptors [24]. Activation of CB1 receptors in this case is required for induction, but not for maintenance of LTD, which indicates involvement of CB1-mediated inhibition of adenylate cyclase activity in the presynaptic neuron. The authors made this conclusion because the blockade of CB1 receptors prior to the high-frequency stimulation blocked generation of LTD, while application of the CB1 receptor antagonist 20 min after the onset of LTD did not affect the response amplitude. It must be noted that these experiments were conducted using extracellular stimulation, which resulted in activation of both inhibitory and excitatory synapses. Activation of the latter ensured background level of glutamate needed for activation of metabotropic receptors; hence, in this case LTD induction was heterosynaptic.
The CB1 receptors are expressed at the presynaptic terminals of two subgroups of GABAergic interneurons in hippocampus: 1) cells innervating apical dendrites of pyramidal cells; 2) interneurons providing perisomatic inhibition of hippocampal excitatory neurons.
Fig. 1. Endocannabinoid-dependent mechanisms of LTD induction at GABAergic synapses. The postsynaptic cascade (indicated with gray color) is activated during depolarization of the postsynaptic neuron and activation of voltage-gated Ca2+ channels (VGCC), which triggers synthesis of endocannabinoids (ECs). ECs diffuse through the synaptic cleft and activate presynaptic CB1 receptors on the terminals of the GABAergic interneuron. This sequentially inhibits adenylate cyclase (AC) activity and decreases the level of cAMP leading to suppression of protein kinase A (PKA) activity. A similar reaction cascade is induced during activation of type I metabotropic glutamate receptors (mGluR) in the postsynaptic neuron (shown on the left). “+”, activating effect; “–”, inhibiting effect.
The phenomenon described above involved interneurons contacting dendrites of excitatory neurons, which was confirmed by the low basal level of DSI at CB1-positive synapses demonstrating endocannabinoid-dependent LTD [25, 26]. The mechanism of endocannabinoid-dependent LTD was further investigated in a study of Heifets and coauthors, where it was shown that the endocannabinoid-dependent inhibition of adenylate cyclase also resulted in the suppression of the function of PKA shifting the balance between phosphorylation and dephosphorylation towards dephosphorylation of PKA presynaptic targets. The latter notion was corroborated by experiments showing that blockade of the calcineurin phosphatase activity abolished the LTD induction [27]. The endocannabinoid-dependent LTD at the striatal GABAergic synapses does not require activation of metabotropic glutamate receptors, and the Ca2+ influx is provided via postsynaptic voltage-dependent L-type channels [28]. It is worth mentioning that the involvement of the endocannabinoid system in the induction of LTP at GABAergic synapses has not yet been demonstrated.
ROLE OF GABAB RECEPTORS IN LONG-TERM PLASTICITY AT
GABAergic SYNAPSES
The release of GABA at CNS synapses activates not only ionotropic GABAA receptors, but also metabotropic GABAB receptors. The GABAB receptor is a heterodimer consisting of GABAB1- and GABAB2-subunits and is coupled with Gi/o-proteins, whose activation decreases adenylate cyclase activity. Like the activation of CB1 receptors, activation of GABAB receptors might result in the blocking of presynaptic voltage-gated Ca2+ channels and opening of the postsynaptic K+ channels due to the direct interaction of the Gβγ-subunit with these channels, which dissociates upon receptor activation. However, in contrast to endocannabinoid receptors, GABAB receptors can be expressed both pre- and/or postsynaptically [29].
High-frequency (100 Hz) extracellular stimulation in stratum radiatum induces only short-term depression of GABAA- and GABAB-receptor-mediated responses recorded from the CA1 pyramidal neurons. However, theta frequency stimulation induces selective long-term potentiation of the GABAA-mediated responses in monosynaptic connections between interneurons located in the lacunosum-moleculare layer and in pyramidal neurons in the CA1 area [30]. Combined activation of postsynaptic GABAB and glutamate type I/II metabotropic receptors is required for generation of LTP. It is likely that the activation of glutamate metabotropic receptors results in an increase in intracellular Ca2+ level, which has been shown to be essential for LTP. The role of GABAB receptors in this case remains unclear. Similar data were obtained for the inhibitory synapses in the hippocampus formed on GABAergic interneurons located in the stratum radiatum [31]. The presynaptic GABAB receptors can also be involved in the induction of LTP in response to high-frequency stimulation (100 Hz, 2 s) [32]. Surprisingly, the necessity of activation of the cAMP-dependent protein kinase A is postulated by the authors of this study, which contradicts the GABAB-dependent decrease in the cAMP level and indicates the possible participation of additional cascades in the LTP induction at these synapses.
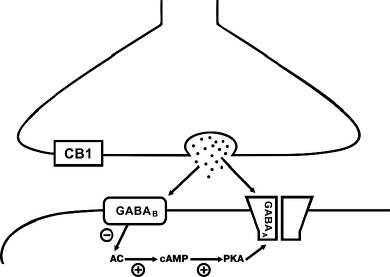
Unlike the endocannabinoid system, GABAB receptors can be involved in both LTP and LTD generation, depending on the type of inhibitory synapse. For instance, combined stimulation at theta-gamma frequencies of the perisomatic synapses formed by cholecystokinin-positive basket cells onto the CA1 pyramidal cells results in a long-term decrease in the amplitude of postsynaptic responses [33]. LTD at these synapses has postsynaptic nature, and activation of GABAB receptors is required exclusively in the stage of induction of synaptic changes (Fig. 2). Even though these synapses are characterized by high expression of CB1 receptors, involvement of the endocannabinoid signaling system is not required for LTD induction, in contrast to CB1-positive interneurons innervating dendrites. Interestingly, combined theta-gamma stimulation causes LTP at the CB1-negative GABAergic synapses [33]. The two types of perisomatic synapses differ in the subunit composition of GABAA receptors, which might be a basis for the different response to the same stimulation protocol. The synaptic transmission at synapses formed by CB1-positive interneurons is mediated by channels containing α2- and β3-subunits, while the currents in the CB1-negative contacts are conducted via channels containing α1- and β2(1)-subunits [34]. As discussed above, activation of metabotropic Gi-coupled receptors decreased the adenylate cyclase activity and, consequently, suppressed the function of PKA. The latter shifts the balance of phosphorylation and dephosphorylation of intercellular protein targets towards the dephosphorylated state. This may explain the differential effect of activation of GABAB receptors at two different types of synapses. Dephosphorylation of the β1-containing receptors results in a significant increase in single channel conductance and, respectively, to the enhancement of the postsynaptic responses amplitudes. On the contrary, the β3-containing channels have higher conductance in the phosphorylated state [35, 36]. Hence, the type of plasticity at GABAergic synapses is determined not only by the involved signaling cascades, but also by the subunit composition of GABAA receptors mediating the synaptic transmission. In particular, some data indicate that the LTP caused by high-frequency stimulation of GABAergic synapses in stratum radiatum results in structural modulation of postsynaptic receptors [37].
Fig. 2. Postsynaptic GABAB-dependent mechanism of LTD induction at GABAergic synapses in the CA1 area of hippocampus. Release of GABA from presynaptic terminal activates GABAA and GABAB receptors. Activation of GABAB receptors suppresses adenylate cyclase (AC) activity and, as a result, decreases cAMP level and PKA activity controlling the level of phosphorylation of GABAA channels. The consequences of the PKA activity decrease depend on the subunit composition of GABAA channels (see text). At synapses formed by CB1-expressing interneurons, the decrease in phosphorylation of GABAA channels leads to reduction of the single channel conductance. “+”, activating effect; “–”, inhibiting effect.
ROLE OF OPIOID RECEPTORS IN LONG-TERM PLASTICITY AT GABAergic
SYNAPSES
The opioid signaling system involves a set of opioid peptides and different types of their receptors. Three genes are known that encode precursors of opioid peptides: preproopiomelanocortin, preproenkephalin, and preprodynorphin. The peptides encoded by these genes are subjected to complex posttranslational modifications that result in formation of various active peptides. These compounds share the same sequence Tyr-Gly-Gly-Phe-(Met or Leu) termed the “opioid motif”. The main opioid peptide encoded by the pre-proopiomelanocortin gene is β-endorphin. The preproenkephalin gene encodes met-enkephalin and leu-enkephalin. The preprodynorphin gene encodes three opioid peptides of different lengths: dynorphin A, dynorphin B, and neoendorphin, and all of those include the leu-enkephalin sequence [38]. All the above-mentioned peptides can activate all types of opioid receptors; however, their affinity varies significantly depending on the receptor type [38].
Three major types of opioid receptors are known: µ-opioid, δ-opioid, and κ-opioid. All these receptors can be localized either pre- or postsynaptically and are coupled with Gi/o-protein. Binding of agonists to these receptors suppresses adenylate cyclase activity and decreases cAMP concentration [38, 39]. It was shown that the opioid signaling system participates in plasticity processes in CNS and can modulate either excitatory or inhibitory neurotransmission [40].
Even though activation of CB1, GABAB, and opioid receptors induces similar intracellular signaling cascades such as inhibition of presynaptic voltage-gated calcium channels, activation of postsynaptic potassium transport, and suppression of adenylate cyclase activity both in pre- and postsynaptic cells, activation of these metabotropic receptors have multidirectional impact on induction of long-term synaptic changes. As established above, activation of presynaptic GABAB receptors causes LTP, while involvement of the presynaptic endocannabinoid cascade is required for induction of LTD. The available data indicate that the blocking of δ-opioid receptors facilitates induction of LTP in response to high-frequency stimulation of GABAergic inputs to granular cells in dentate gyrus that are localized in the outer molecular layer [41]. It was found that the induction of LTP in this case was most likely heterosynaptic; hence, it requires the increase in intracellular Ca2+ via activation of glutamate NMDA channels. The authors did not clarify the source of glutamate, but it was reasonable to suggest that it was released during simultaneous stimulation of glutamatergic fibers located in the outer molecular layer of the dentate gyrus. It must be noted that the described work was devoted not to the process of activation of opioid receptors leading to the development of long-term changes in the inhibitory synaptic transmission, but rather to the fact that the opioid system controlled the probability of development of such changes. Direct observation of opioid-dependent long-term depression was described for the CA2 area of the hippocampus [42]. The authors showed that high-frequency stimulation at GABAergic synapses formed by parvalbumin-expressing interneurons resulted in the development of LTD. It was found that the LTD at these synapses developed as a result of activation of δ-, but not µ-receptors at presynaptic terminals. Activation of the δ-receptors was required only in the stage of LTD induction because their blocking 10 min after the induction of plastic changes did not affect the amplitude of inhibitory responses. It is important to note that this type of synaptic plasticity was specific to GABAergic synapses formed by parvalbumin-positive interneurons in the CA2 area. High-frequency stimulation of synapses formed by interneurons of the same type in the CA1 area or application of δ-receptor agonists did not result in the development of long-term synaptic changes. It is not clear what the differences between the synapses formed by interneurons of this type are. It is also not known what intracellular mechanisms are activated during high-frequency stimulation that lead to development of δ-receptors-dependent LTD in the CA2 area.
The main difference in the induction mechanisms of LTP and LTD at GABAergic synapses from excitatory synapses is the differential involvement of multiple G-protein-coupled signaling systems. Furthermore, while at most excitatory synapses the LTP and LTD have a postsynaptic nature, and metabotropic receptors exert only modulating action, at GABAergic synapses the initiation locus for the long-term changes can be either pre- or postsynaptic, the direction of changes depends on the involvement of the particular signaling system, and, in a number of cases, on the subunit composition of GABAA channels. It must be noted that despite the obvious importance of modulation of the inhibitory transmission for normal network functioning, the mechanisms of LTP and LTD at GABAergic synapses remain poorly understood, which most likely is due to the uniqueness of each type of synapses determined primarily by the nature of the presynaptic neuron.
Acknowledgements
A. P. Bolshakov was financially supported by the Russian Foundation for Basic Research (project No. 15-04-06115). A. V. Rozov was financially supported by the program for Enhancing Competitiveness of the Kazan Federal University.
REFERENCES
1.Bliss, T. V. P., and Lomo, T. (1973) Long-lasting
potentiation of synaptic transmission in the dentate area of the
anaesthetized rabbit following stimulation of the perforant path, J.
Physiol., 232, 331-356.
2.Vinogradova, O. S. (2001) Hippocampus as
comparator: role of the two input and two output systems of the
hippocampus in selection and registration of information,
Hippocampus, 11, 578-598.
3.Pastalkova, E., Serrano, P., Pinkhasova, D.,
Wallace, E., Fenton, A. A., and Sacktor, T. C. (2006) Storage of
spatial information by the maintenance mechanism of LTP,
Science, 313, 1141-1144.
4.Whitlock, J. R., Heynen, A. J., Shuler, M. G., and
Bear, M. F. (2006) Learning induces long-term potentiation in the
hippocampus, Science, 313, 1093-1097.
5.Citri, A., and Malenka, R. C. (2007) Synaptic
plasticity: multiple forms, functions, and mechanisms,
Neuropsychopharmacology, 33, 18-41.
6Somogyi, P., and Klausberger, T. (2005) Defined types of cortical
interneurone structure space and spike timing in the hippocampus, J.
Physiol., 562, 9-26.
7.McLean, H. A., Caillard, O., Ben-Ari, Y., and
Gaiarsa, J. L. (1996) Bidirectional plasticity expressed by GABAergic
synapses in the neonatal rat hippocampus, J. Physiol.,
496, 471-477.
8.Caillard, O., Ben-Ari, Y., and Gaiarsa, J. L.
(1999) Long-term potentiation of GABAergic synaptic transmission in
neonatal rat hippocampus, J. Physiol., 518, 109-119.
9.Caillard, O., Ben-Ari, Y., and Gaiarsa, J. L.
(2000) Activation of presynaptic and postsynaptic ryanodine-sensitive
calcium stores is required for the induction of long-term depression at
GABAergic synapses in the neonatal rat hippocampus amphetamine, J.
Neurosci., 20, RC94.
10.Ouardouz, M., and Sastry, B. R. (2000) Mechanisms
underlying LTP of inhibitory synaptic transmission in the deep
cerebellar nuclei, J. Neurophysiol., 84, 1414-1421.
11.Lu, Y. M., Mansuy, I. M., Kandel, E. R., and
Roder, J. (2000) Calcineurin-mediated LTD of GABAergic inhibition
underlies the increased excitability of CA1 neurons associated with
LTP, Neuron, 26, 197-205.
12.Wang, J., Liu, S., Haditsch, U., Tu, W.,
Cochrane, K., Ahmadian, G., Tran, L., Paw, J., Wang, Y., Mansuy, I.,
Salter, M. M., and Lu, Y. M. (2003) Interaction of calcineurin and
type-A GABA receptor gamma 2 subunits produces long-term depression at
CA1 inhibitory synapses, J. Neurosci., 23, 826-836.
13.Semyanov, A., and Kullmann, D. M. (2000)
Modulation of GABAergic signaling among interneurons by metabotropic
glutamate receptors, Neuron, 25, 663-672.
14.Steindel, F., Lerner, R., Haring, M., Ruehle, S.,
Marsicano, G., Lutz, B., and Monory, K. (2013) Neuron-type specific
cannabinoid-mediated G protein signalling in mouse hippocampus, J.
Neurochem., 124, 795-807.
15.Wilson, R. I., Kunos, G., and Nicoll, R. A.
(2001) Presynaptic specificity of endocannabinoid signaling in the
hippocampus, Neuron, 31, 453-462.
16.Freund, T. F., and Katona, I. (2007) Perisomatic
inhibition, Neuron, 56, 33-42.
17.Pitler, T. A., and Alger, B. E. (1992)
Postsynaptic spike firing reduces synaptic GABA A responses in
hippocampal pyramidal cells, J. Neurosci., 12,
4122-4132.
18.Pitler, T. A., and Alger, B. E. (1994)
Depolarization-induced suppression of GABAergic inhibition in rat
hippocampal pyramidal cells: G protein involvement in a presynaptic
mechanism, Neuron, 13, 1447-1455.
19.Alger, B. E. (2002) Retrograde signaling in the
regulation of synaptic transmission: focus on endocannabinoids,
Prog. Neurobiol., 68, 247-286.
20.Wilson, R. I., and Nicoll, R. A. (2002)
Endocannabinoid signaling in the brain, Science, 296,
678-682.
21.Chevaleyre, V., Takahashi, K. A., and Castillo,
P. E. (2006) Endocannabinoid-mediated synaptic plasticity in the CNS,
Annu. Rev. Neurosci., 29, 37-76.
22.Kano, M., Ohno-Shosaku, T., Hashimotodani, Y.,
Uchigashima, M., and Watanabe, M. (2009) Endocannabinoid-mediated
control of synaptic transmission, Physiol. Rev., 89,
309-380.
23.Freund, T. F., Katona, I., and Piomelli, D.
(2003) Role of endogenous cannabinoids in synaptic signaling,
Physiol. Rev., 83, 1017-1066.
24.Chevaleyre, V., and Castillo, P. E. (2003)
Heterosynaptic LTD of hippocampal GABAergic synapses, Neuron,
38, 461-472.
25.Ali, A. B., and Todorova, M. (2010) Asynchronous
release of GABA via tonic cannabinoid receptor activation at identified
interneuron synapses in rat CA1, Eur. J. Neurosci., 31,
1196-1207.
26.Zhu, P. J., and Lovinger, D. M. (2007) Persistent
synaptic activity produces long-lasting enhancement of endocannabinoid
modulation and alters long-term synaptic plasticity, J.
Neurophysiol., 97, 4386-4389.
27.Heifets, B. D., Chevaleyre, V., and Castillo, P.
E. (2008) Interneuron activity controls endocannabinoid-mediated
presynaptic plasticity through calcineurin, Proc. Natl. Acad. Sci.
USA, 105, 10250-10255.
28.Adermark, L., Talani, G., and Lovinger, D. M.
(2009) Endocannabinoid-dependent plasticity at GABAergic and
glutamatergic synapses in the striatum is regulated by synaptic
activity, Eur. J. Neurosci., 29, 32-41.
29.Bettler, B., Kaupmann, K., Mosbacher, J., and
Gassmann, M. (2004) Molecular structure and physiological functions of
GABA B receptors, Physiol. Rev., 84, 835-867.
30.Patenaude, C., Chapman, C. A., Bertrand, S.,
Congar, P., and Lacaille, J.-C. (2003) GABA B receptor- and
metabotropic glutamate receptor-dependent cooperative long-term
potentiation of rat hippocampal GABA A synaptic transmission, J.
Physiol., 553, 155-167.
31.Evstratova, A., Chamberland, S., and Topolnik, L.
(2011) Cell-type-specific and activity-dependent dynamics of
action-potential-evoked Ca2+ signals in dendrites of
hippocampal inhibitory interneurons, J. Physiol., 8,
1957-1977.
32.Shew, T., Yip, S., and Sastry, B. R. (2000)
Mechanisms involved in tetanus-induced potentiation of fast IPSCs in
rat hippocampal CA1 neurons, J. Neurophysiol., 83,
3388-3401.
33.Jappy, D., Valiullina, F., Draguhn, A., and
Rozov, A. (2016) GABA(B)R-dependent long-term depression at hippocampal
synapses between CB1-positive interneurons and CA1 pyramidal cells,
Front. Cell. Neurosci., 10, 4.
34.Nyíri, G., Freund, T. F., and Somogyi, P.
(2001) Input-dependent synaptic targeting of
α2-subunit-containing GABAA receptors in synapses of hippocampal
pyramidal cells of the rat, Eur. J. Neurosci., 13,
428-442.
35.McDonald, B. J., Amato, A., Connolly, C. N.,
Benke, D., Moss, S. J., and Smart, T. G. (1998) Adjacent
phosphorylation sites on GABAA receptor beta-subunits determine
regulation by cAMP-dependent protein kinase, Nat. Neurosci.,
1, 23-28.
36.Vithlani, M., Terunuma, M., and Moss, S. J.
(2011) Receptor trafficking and its role in regulating the plasticity
of inhibitory synapses, Physiol. Rev., 91, 1009-1022.
37.Xu, J.-Y., and Sastry, B. R. (2005)
Benzodiazepine involvement in LTP of the GABA-ergic IPSC in rat
hippocampal CA1 neurons, Brain Res., 1062, 134-143.
38.McNally, G., and Akil, H. (2002) Opioid peptides
and their receptors: overview and function in pain modulation, in
Neuropsychopharmacology: The Fifth Generation of Progress
(Davis, K. L., Charney, D., Coyle, J. T., and Nemeroff, C., eds.)
Lippincott, Williams & Wilkins, Philadelphia, pp. 35-46.
39.Al-Hasani, R., and Bruchas, M. R. (2011)
Molecular mechanisms of opioid receptor-dependent signaling and
behavior, Anesthesiology, 115, 1363-1381.
40.Dacher, M., and Nugent, F. S. (2011) Opiates and
plasticity, Neuropharmacology, 61, 1088-1096.
41.Xie, C. W., and Lewis, D. V. (1995) Endogenous
opioids regulate long-term potentiation of synaptic inhibition in the
dentate gyrus of rat hippocampus, J. Neurosci.,
15, 3788-3795.
42.Piskorowski, R. A., and Chevaleyre, V. (2013)
Delta-opioid receptors mediate unique plasticity onto
parvalbumin-expressing interneurons in area CA2 of the hippocampus,
J. Neurosci., 33, 14567-14578.