Brain Mitochondrial Subproteome of Rpn10-Binding Proteins and Its Changes Induced by the Neurotoxin MPTP and the Neuroprotector Isatin
A. E. Medvedev1*, O. A. Buneeva1, A. T. Kopylov1, O. V. Tikhonova1, M. V. Medvedeva2, L. N. Nerobkova3, I. G. Kapitsa3, and V. G. Zgoda1
1Institute of Biomedical Chemistry, 119121 Moscow, Russia; E-mail: professor57@yandex.ru2Lomonosov Moscow State University, Biological Faculty, 119991 Moscow, Russia
3Zakusov Institute of Pharmacology, 125315 Moscow, Russia
* To whom correspondence should be addressed.
Received September 27, 2016; Revision received October 21, 2016
Mitochondria play an important role in molecular mechanisms of neuroplasticity, adaptive changes of the brain that occur in the structure and function of its cells in response to altered physiological conditions or development of pathological disorders. Mitochondria are a crucial target for actions of neurotoxins, causing symptoms of Parkinson’s disease in various experimental animal models, and also neuroprotectors. Good evidence exists in the literature that mitochondrial dysfunction induced by the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) influences functioning of the ubiquitin-proteasomal system (UPS) responsible for selective proteolytic degradation of proteins from various intracellular compartments (including mitochondria), and neuroprotective effects of certain antiparkinsonian agents (monoamine oxidase inhibitors) may be associated with their effects on UPS. The 19S proteasomal Rpn10 subunit is considered as a ubiquitin receptor responsible for delivery of ubiquitinated proteins to the proteasome proteolytic machinery. In this study, we investigated proteomic profiles of mouse brain mitochondrial Rpn10-binding proteins, brain monoamine oxidase B (MAO B) activity, and their changes induced by a single-dose administration of the neurotoxin MPTP and the neuroprotector isatin. Administration of isatin to mice prevented MPTP-induced inactivation of MAO B and influenced the profile of brain mitochondrial Rpn10-binding proteins, in which two pools of proteins were clearly recognized. The constitutive pool was insensitive to neurotoxic/neuroprotective treatments, while the variable pool was specifically influenced by MPTP and the neuroprotector isatin. Taking into consideration that the neuroprotective dose of isatin used in this study can result in brain isatin concentrations that are proapoptotic for cells in vitro, the altered repertoire of mitochondrial Rpn10-binding proteins may thus represent a part of a switch mechanism from targeted elimination of individual (damaged) proteins to more efficient (“global”) elimination of damaged organelles and whole damaged cells.
KEY WORDS: neuroplasticity, MPTP-induced parkinsonism, neuroprotection, isatin, Rpn10-binding proteins, brain mitochondrial fraction, subproteomeDOI: 10.1134/S0006297917030117
Abbreviations: a.u., arbitrary units; GO, Gene Ontology; i.p., intraperitoneally; MAO B, monoamine oxidase B; MAPK, mitogen activated protein kinase; MPP+, 1-methyl-4-phenylpyridinium ion; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; UPS, ubiquitin-proteasomal system.
Mitochondria play an important role in molecular mechanisms of
neuroplasticity, adaptive changes of the brain that occur in the
structure and function of its cells in response to altered
physiological conditions or development of pathological disorders [1]. These intracellular organelles are a crucial
target for actions of neurotoxins, causing symptoms of
Parkinson’s disease [1-3]. In the context of parkinsonism induced by
administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP),
the protoxin undergoes catalytic conversion by monoamine oxidase B (MAO
B), which is self-inactivated during this process. The resultant
neurotoxin MPP+ (1-methyl-4-phenylpyridinium) inhibits
complex I of the respiratory chain and causes development of symptoms
typical for Parkinson’s disease [3, 4]. Administration of MAO B inhibitors (e.g. deprenyl
or isatin [5, 6]) or substrates
competing for the active site of this enzyme (e.g. phenylethylamine)
[7] prevented not only metabolic activation of
MPTP, but also deficiency of the neurotransmitter dopamine and
locomotor impairments typical for this disease.
Thus, brain mitochondria obviously represent not only the principle subcellular organelles where “the first act of the scenario” of MPTP-induced Parkinsonism is realized, but also crucial targets for endogenous and exogenous neuroprotectors. Good evidence exists in the literature that neuroprotector mechanisms of certain antiparkinsonian agents, monoamine oxidase inhibitors, may be associated with their effects on the ubiquitin-proteasomal system (UPS) [8] responsible for selective proteolytic degradation of proteins from various intracellular compartments including mitochondria. Mitochondrial dysfunction induced by MPTP has a significant impact on functioning of the UPS [9, 10].
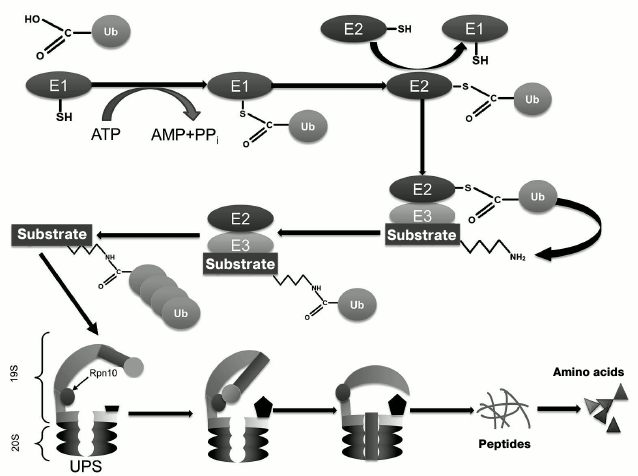
Ubiquitin, a 76-residue protein, is widely distributed in all eukaryotic cells, where it targets proteins for subsequent degradation [11-14]. The ubiquitination process includes several sequential stages that involve several enzymes (Fig. 1): ubiquitin activating enzyme (E1), ubiquitin-conjugating enzyme (E2), and ubiquitin ligase (E3) [11-14]. The major function of ubiquitin is (poly)ubiquitination of proteins for their subsequent proteasomal degradation. In the context of UPS functioning, the 19S proteasome Rpn10 subunit (as well as extraproteasomal Rpn10) plays a role as the ubiquitin receptor [15] responsible for delivery of client proteins to the 20S proteolytically active proteasome, where their subsequent proteolytic degradation occurs (Fig. 1) [16].
Fig. 1. Scheme of functioning of the ubiquitin-proteasomal system (UPS).
Isatin (indoledione-2,3) is an endogenous neuroprotector found in mammalian brain, peripheral tissues, and body fluids [17-19]. Besides reversible inhibition of MAO B and receptor guanylate cyclases [17-19], isatin interacts with numerous isatin-binding proteins located in various subcellular organelles including mitochondria [20, 21]. Proteomic profiling of brain isatin-binding proteins revealed several enzymes directly involved in UPS functioning [22]. Taking into consideration that some isatin derivatives act as proteasome inhibitors [23, 24], in this study we investigated the effect of a single-dose administration of MPTP and isatin to mice on MAO B activity and the subproteome of brain mitochondrial Rpn10-binding proteins.
MATERIALS AND METHODS
Male C57BL/6 mice (20-25 g) obtained from the Stolbovaya nursery (Moscow Region) were used in experiments, which were performed at least one week after their arrival from the nursery. The animals were maintained under natural illumination and received standard laboratory chow (full ration extruded briquette feed, GOST 50258-92) and water ad libitum. MPTP was injected intraperitoneally (i.p., 30 mg/kg). Isatin (100 mg/kg, i.p.) was injected 30 min before MPTP. Control mice were treated with intraperitoneal injection of saline (0.1 ml/10 g of weight). Each group contained 6-8 mice. Behavioral changes induced by MPTP or isatin were analyzed 90 min after the last administration by means of the open field test [25] and the rotarod and static rod tests [26]. The exploratory reaction of mice in the open field test was defined as the sum of horizontal activity (units) and vertical activity (units). All procedures were approved by local authorities for animal research.
Isolation of the brain mitochondrial fraction and determination of MAO B activity were carried out as described previously [27, 28].
All procedures related to isolation of mouse brain Rpn-binding proteins, sample preparation for mass spectrometric analysis including liquid chromatography, high resolution mass spectrometry, and data analysis are given in the Supplementary materials (see Supplement to this report on the site of the journal http://protein.bio.msu.ru/biokhimiya and Springer site Link.springer.com).
Generation and prediction of an interactome/protein interaction network. Protein annotation in terms of gene ontology (GO) using categories “Biological process” and “Molecular function” was performed using UniProt/SwissProt IDs [29] (release 2016_08 updated on 7.09.2016) as input data and applied for interactive network processing. Interactions of proteins were predicted using the open access STRING version 10.0 software platform [30]. A list of positive identifications obtained during proteomic profiling was used for generation and prediction of the protein interaction network. The network of physically and functionally interacting proteins was visualized using a high confidence level (C ≥ 0.7). The visualization was performed using information about proteins from three sources: experimental data, databases, and analysis of available texts (e.g. joint references in annotations to PubMed publications).
RESULTS
Effect of MPTP and isatin on behavioral activity of mice and on brain MAO B activity. In accordance with previous studies [3, 31, 32], a single-dose administration of MPTP caused appearance of movement disorders typical for animal models of Parkinson’s disease (Table 1). Pretreatment of mice with isatin before MPTP attenuated the locomotor impairments induced by the neurotoxin. In the control mice, isatin administration also decreased locomotor activity, which may be attributed to its sedative effect known from the literature [17, 18]. Under these conditions, MAO B activity assayed in the brain mitochondrial fraction of MPTP-treated mice demonstrated a statistically significant decrease compared with the control group (18.0 ± 2.6% versus control; p < 0.01; n = 6 in each group). After sequential treatment of mice with isatin and MPTP, brain mitochondrial MAO B activity was basically the same as in the control group. This suggests that the reversible MAO B inhibitor isatin effectively prevents interaction of this enzyme with MPTP and is then effectively washed out of brain mitochondria during their isolation.
Table 1. Effect of MPTP and isatin on
behavioral activity of C57BL/6 mice
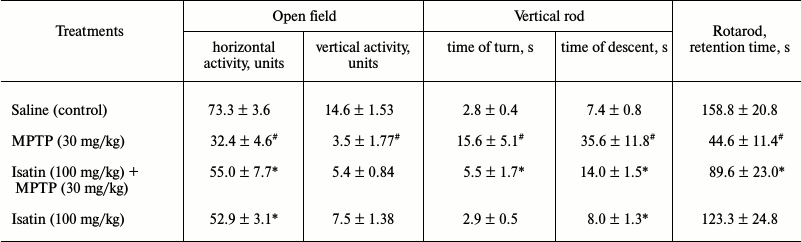
Note: Behavioral activity of mice was evaluated 90 min after MPTP
administration. Isatin was injected 30 min before MPTP
administration. Data represent mean ± SEM. Each group contained
6-8 animals. Statistical significance of differences: #
p < 0.05 versus control; * p < 0.05 versus MPTP.
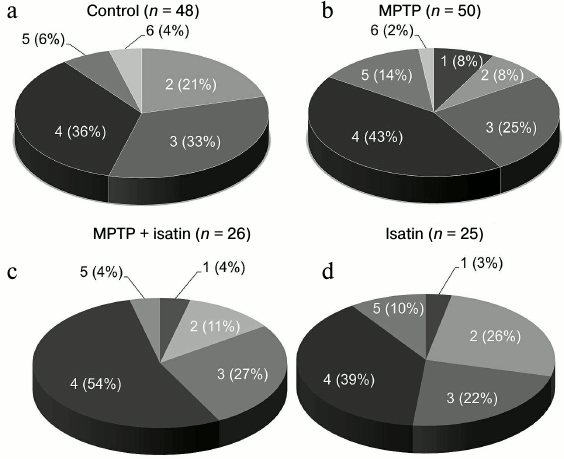
Proteomic profiling of brain mitochondrial Rpn10-binding proteins. Affinity-based profiling of Rpn10-binding proteins of control brain mitochondrial samples resulted in confident identification of 48 individual proteins (Fig. 2a). Functionally, they can be subdivided into the following groups: (i) proteins involved in energy generation and carbohydrate metabolism; (ii) proteins involved in cytoskeleton formation and exocytosis/trafficking; (iii) proteins involved in regulation of gene expression, cell division, and differentiation; (iv) proteins involved in signal transduction and regulation of enzyme activity; (v) antioxidant and protection proteins/enzymes; (vi) enzymes involved in lipid metabolism. Most of these proteins (including those traditionally identified in cytosol or nucleus) are associated with internal or external mitochondrial compartments, and functional links have been already established for several groups of the identified proteins (Table S1 in Supplement). For example, histones identified in this study (Table S1 and Fig. S1 in Supplement) have been previously found in different mitochondrial sub-compartments [33]. In the internal mitochondrial compartment, histones H1, H2A, H2B, H3, and H4 were identified among so-called D-loop DNA-binding proteins [33]. In the external mitochondrial compartment, they were identified in the outer mitochondrial membrane; it is even suggested that “H2A and H2B must be integral outer membrane proteins protruding towards the cytoplasm” [33]. In vitro, histones H2A, H2B, H3, and H4 bind to isolated mitochondria and increase outer membrane permeabilization followed by release of proapoptotic intermembrane space proteins [34].
Fig. 2. Distribution of Rpn10-binding proteins of the brain mitochondrial fraction into functional groups: 1) proteins/enzymes involved in energy generation and carbohydrate metabolism; 2) proteins involved in cytoskeleton formation and exocytosis; 3) protein regulators of gene expression, cell division, and differentiation; 4) proteins involved in signal transduction and regulation of enzyme activity; 5) antioxidant and protective proteins/enzymes; 6) enzymes of lipid metabolism.
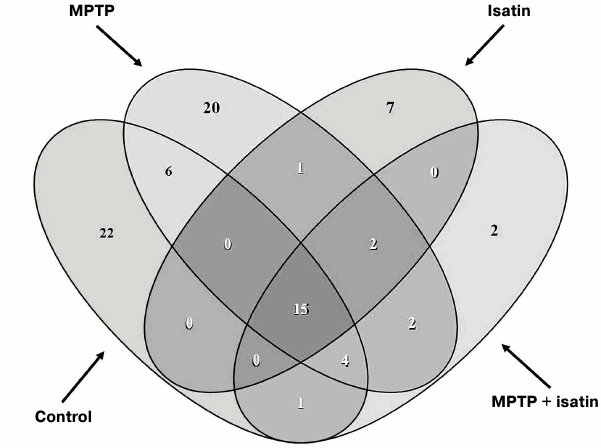
Effect of neurotoxin MPTP and neuroprotector isatin on the subproteome of brain mitochondrial Rpn10-binding proteins. Development of the MPTP-induced parkinsonian disorder insignificantly influenced the total number of identified Rpn10-binding proteins (50 versus 48 control proteins), but it dramatically changed the profile of Rpn10-binding proteins (Figs. 2b and 3). In contrast to the control, the Rpn10-binding proteins of the MPTP-treated animals were characterized by almost complete disappearance of cytoskeletal proteins and a reciprocal increase in proteins referred to the antioxidation/protection group (Fig. 2). It is especially interesting that Rpn10-binding proteins specific for MPTP-treated animals include Cul5-RING ubiquitin ligase complex (G3X914) directly involved in ubiquitin-dependent catabolic processes and cerebral cortex cell functioning [35], protein disulfide isomerase, mitochondrial heat shock proteins, and caspase 3, which is crucial for the development of MPTP-induced Parkinson’s disease in mice [36, 37] (Table S2 in Supplement). Interestingly, heat shock proteins and caspase 3 are in the cluster of interacting proteins (Fig. S2 in Supplement).
Fig. 3. Venn diagram showing the number of common Rpn10-binding proteins in brain mitochondria of investigated groups of mice.
Administration of isatin to intact mice significantly reduced the number of Rpn10-binding proteins to 25 and also altered the profile of Rpn10-binding proteins qualitatively (Figs. 2c and 3). Some of the Rpn10-binding proteins specific for isatin-treated mice (cytochrome P450 1A2, cytochrome P450 2D26) (Table S3 in Supplement) and destined to proteasomal degradation may be involved in metabolism of isatin itself [17, 18].
Pretreatment of mice with isatin before administration of MPTP also reduced the number of Rpn10-binding proteins to 26 (Fig. 2d; Table S4 in Supplement), i.e. to the level observed after a single administration of isatin, however, their profiles demonstrated clear differences (Figs. 2 and 3). It is especially important that the sedative decrease in behavioral activity of intact mice treated with isatin and oligokinesia of MPTP-treated mice are associated with different proteomic profiles and therefore are realized by different mechanisms.
Thus, it appears that the repertoire of Rpn10-binding proteins is influenced not only by MPTP, but also by isatin, which significantly reduces the number of Rpn10-binding proteins and influences the proteomic profile of brain mitochondrial proteins interacting with the proteasomal Rpn10 subunit.
DISCUSSION
Previously it was found that isatin administration to rats at a dose 50-100 mg/kg resulted in a level of brain isatin of 9 µg/g [38]. Taking into consideration brain water content of 0.78 µl/g [39], this corresponds to 77 µM (provided the administered isatin uniformly distributed in the brain). Such concentration effectively protected MAO B against irreversible inhibition by specific mechanism-based inhibitors [40]. Earlier, it was also demonstrated that administration of a MAO B substrate prevented MPTP-induced toxicity by competitively inhibiting MPTP conversion into MPP+ [41]. A smaller (but statistically significant) decrease in brain mitochondrial MAO B activity compared with values reported in the literature [41] may be attributed to the use of different brain preparations for assay of MAO B activity. We determined MAO B activity in the total fraction of brain mitochondria, while others assayed MAO B activity in homogenates of brain regions representing the main MPTP targets (striatum) [41]. Nevertheless, taken together all these results indicate that pretreatment of mice with a large dose of isatin (100 mg/kg) is sufficient for inhibition of MPTP biotransformation into MPP+. In this context, it should be noted that isatin concentrations 50-100 µM induce apoptosis in various cell cultures [42-45]. Moreover, for some cell lines it was demonstrated that apoptosis induced by isatin involved the mitochondrial pathway [45].
Besides MAO B, isatin can possibly interact with numerous isatin-binding proteins [21, 22]. Recent proteomic profiling of brain isatin-binding proteins revealed several UPS-related enzymes [22]. These included probable E3 ubiquitin protein ligase MYCBP2 (Q9TPH6), ubiquitin carboxyl terminal hydrolase 24 (B1AY13), E3 ubiquitin protein ligase MIB2 (Q8R516), E3 ubiquitin protein ligase HUWE1 (Q7TMY8), ubiquitin conjugating enzyme E2 variant 1 (Q9CZY3), and polyubiquitin B (P0CG49). Mapping of identified isatin-binding proteins to known pathways suggests that some of them participate in the parkin (E3 ubiquitin-protein ligase)-associated pathway (Gene ontology reference CH000000947) [22]. Since the probability that hits in this pathway represent accidental events is as low as 2.5·10–5 [22], it appears that isatin may interfere with various components constituting the UPS.
The 19S proteasome Rpn10 subunit plays an important role in recognition of substrates destined to proteasomal degradation [15, 16]. Results of the present study indicate that the Rpn10 subunit can bind certain mitochondrial proteins and thus participate in their delivery to targeted proteasomal degradation.
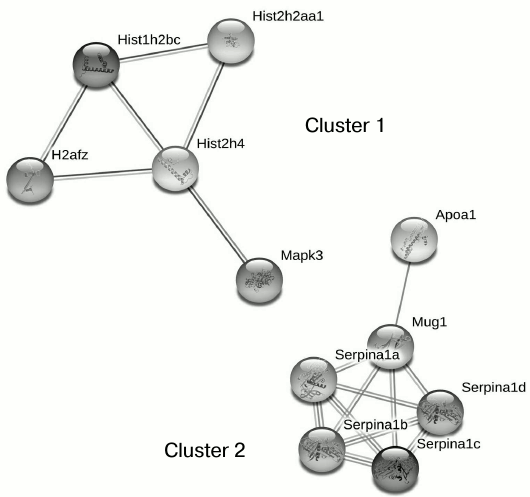
Proteomic profiling of mitochondrial fractions from animals of all groups (control, MPTP-treated mice, isatin-treated mice, and mice treated with isatin and MPTP) revealed existence of a constitutive pool of mitochondrial Rpn10-binding proteins. This pool insensitive to either the neurotoxin MPTP or the neuroprotector isatin (Table 2 and Fig. 4) includes serpins/protease inhibitors, histones, MAPK (mitogen-activated protein kinase), and apolipoprotein A-I. There is increasing evidence that serpins (serine protease inhibitors) not only control activity of certain proteases, but also regulate cell proliferation and apoptosis [46]. Mutant serpins are degraded by UPS enzymes [47]; a conjugate of serpin 2a with a ubiquitin homolog was found in activated macrophages [48]. Recently, it has been demonstrated that apolipoprotein A-I, the principal HDL (high-density lipoproteins) protein, is associated with mitochondria [49], and glycated HDL induced apoptosis of cells via mitochondrial dysfunction [50]. Taking into consideration MAPK association with mitochondria [51], submitochondrial localization of histones [34, 35], and an important role of MAPK-dependent phosphorylation of histones for development of apoptosis [52], functional links between proteins identified in this study (Fig. 4) suggest that the constitutive pool of mitochondrial Rpn10-binding proteins play a role in regulation of apoptosis.
Fig. 4. Clusters of interactions of brain mitochondrial Rpn10-binding proteins (the constitutive pool). Clusters of physical and functional interactions of proteins were generated by results of identification obtained during proteomic profiling. Names of genes coding the protein products correspond to those listed Table 2.
Table 2. Brain mitochondrial Rpn10-binding
common for all groups of proteins
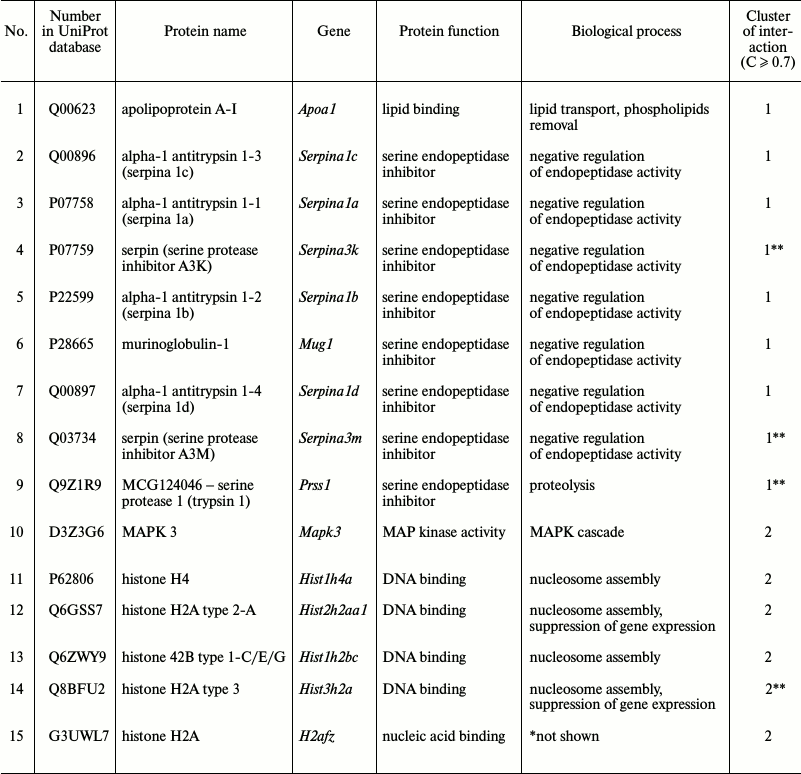
* Biological process not shown in available databases.
** Referred to this cluster at moderate confidence level (C ≥
0.4).
The repertoire of the variable pool of brain mitochondrial Rpn10-binding proteins is sensitive to effects of both neurotoxin MPTP and neuroprotector isatin (Figs. 2 and 3; Figs. S3 and S4 in Supplement). Considering that isatin stimulates apoptosis in various cells [42-45] and its analogs act as apoptosis-inducing inhibitors of UPS [23, 24], there are reasons to believe that the altered repertoire of mitochondrial Rpn10-binding proteins induced by isatin may represent a part of a switch mechanism from targeted elimination of individual (damaged) proteins to more efficient (“global”) elimination of damaged organelles and whole damaged cells.
Thus, in the context of molecular mechanisms of neuroplasticity that are associated with regulatory effects of isatin on brain mitochondria under conditions of MPTP-induced experimental parkinsonism, the neuroprotector effect of isatin may be associated with several interrelated actions:
– inhibition of MAO (B)-dependent conversion of MPTP to MPP+;
– interaction with isatin-binding proteins and modulation of their functions;
– altered repertoire of mitochondrial proteins interacting with the receptor, Rpn10, responsible for proteasomal delivery of (damaged) proteins.
Acknowledgements
This work was supported by the Russian Foundation for Basic Research (project No. 15-04-01545a; experimental modeling of MPTP-induced parkinsonism and behavioral analysis; project No. 16-04-00173a; isolation of mitochondrial Rpn10-binding proteins of the brain of mice), and the Russian Science Foundation (project No. 14-25-00,132; mass spectrometric analysis of Rpn10-binding proteins and their identification).
REFERENCES
1.Cheng, A., Hou, Y., and Mattson, M. P. (2010)
Mitochondria and neuroplasticity, ASN Neuro, 2,
e00045.
2.Cookson, M. R. (2005) The biochemistry of
Parkinson’s disease, Ann. Rev. Biochem., 74,
9-52.
3.Buneeva, O. A., and Medvedev, A. E. (2011)
Mitochondrial dysfunction in Parkinson’s disease, Biomed.
Khim., 57, 246-281.
4.Maret, G., Testa, B., Jenner, P., El Tayar, N., and
Carrupt, P. A. (1990) The MPTP story: MAO activates tetrahydropyridine
derivatives to toxins causing parkinsonism, Drug Metab. Rev.,
22, 291-332.
5.Zhou, Y., Zhao, Z. Q., and Xie, J. X. (2001) Effect
of isatin on rotational behavior and DA levels in caudate putamen in
Parkinsonian rats, Brain Res., 917, 127-132.
6.Hamaue, N., Minami, M., Terado, M., Hirafuji, M.,
Endo, T., Machida, M., Hiroshige, T., Ogata, A., Tashiro, K., Saito,
H., and Parvez, S. H. (2004) Comparative study of the effects of
isatin, an endogenous MAO-inhibitor, and selegiline on bradykinesia and
dopamine levels in a rat model of Parkinson’s disease induced by
the Japanese Encephalitis virus, Neurotoxicology, 25,
205-213.
7.Melamed, E., and Youdim, M. B. H. (1985) Prevention
of dopaminergic toxicity of MPTP in mice by phenylethylamine, a
specific substrate of type B monoamine oxidase, Br. J.
Pharmacol., 86, 529-531.
8.Youdim, M., Edmondson, D., and Tipton, K. (2006)
The therapeutic potential of monoamine oxidase inhibitors, Nat. Rev.
Neurosci., 7, 295-308.
9.Hoglinger, G. U., Carrard, G., Michel, P. P.,
Medja, F., Lombes, A., Ruberg, M., Friguet, B., and Hirsch, E. C.
(2003) Dysfunction of mitochondrial complex I and the proteasome:
interactions between two biochemical deficits in a cellular model of
Parkinson’s disease, J. Neurochem., 86,
1297-1307.
10.Fornai, F., Schluter, O. M., Lenzi, P., Gesi, M.,
Ruffoli, R., Ferrucci, M., Lazzeri, G., Busceti, C. L., Pontarelli, F.,
Battaglia, G., Pellegrini, A., Nicoletti, F., Ruggieri, S., Paparelli,
A., and Sudhof, T. C. (2006) Parkinson-like syndrome induced by
continuous MPTP infusion: convergent roles of the ubiquitin proteasome
system and α-synuclein, Proc. Natl. Acad. Sci. USA,
102, 3413-3418.
11.Hershko, A., and Ciechanover, A. (1998) The
ubiquitin system, Annu. Rev. Biochem., 67, 425-479.
12.Hershko, A., Ciechanover, A., and Varshavsky, A.
(2000) The ubiquitin system, Nat. Med., 6, 1073-1081.
13.Schwartz, A. L., and Ciechanover, A. (2009)
Targeting proteins for destruction by the ubiquitin system:
implications for human pathobiology, Annu. Rev. Pharmacol.
Toxicol., 49, 73-96.
14.Kravtsova-Ivantsiv, Y., and Ciechanover, A.
(2012) Non-canonical ubiquitin-based signals for proteasomal
degradation, J. Cell Sci., 125, 539-548.
15.Deveraux, Q., Ustrell, V., Pickart, C., and
Rechsteiner, M. (1994) A 26S protease subunit that binds ubiquitin
conjugates, J. Biol. Chem., 269, 7059-7061.
16.Hamazaki, J., Sasaki, K., Kawahara, H., Hisanaga,
S., Tanaka, K., and Murata, S. (2007) Rpn10-mediated degradation of
ubiquitinated proteins is essential for mouse development, Mol.
Cell. Biol., 19, 6629-6638.
17.Medvedev, A. E., Clow, A., Sandler, M., and
Glover, V. (1996) Isatin – a link between natriuretic peptides
and monoamines? Biochem. Pharmacol., 52, 385-391.
18.Medvedev, A., Igosheva, N., Crumeyrolle-Arias,
M., and Glover, V. (2005) Isatin: role in stress and anxiety,
Stress, 8, 175-183.
19.Medvedev, A., Buneeva, O., and Glover, V. (2007)
Biological targets for isatin and its analogues: implications for
therapy, Biologics, 1, 151-162.
20.Crumeyrolle-Arias, M., Buneeva, O., Zgoda, V.,
Kopylov, A., Cardona, A., Tournaire, M. C., Pozdnev, V., Glover, V.,
and Medvedev, A. (2009) Isatin binding proteins in rat brain: in
situ imaging, quantitative characterization of specific
[3H]isatin binding, and proteomic profiling, J. Neurosci.
Res., 87, 2763-2772.
21.Buneeva, O., Gnedenko, O., Zgoda, V., Kopylov,
A., Glover, V., Ivanov, A., Medvedev, A., and Archakov, A. (2010)
Isatin binding proteins of rat and mouse brain: proteomic
identification and optical biosensor validation, Proteomics,
10, 23-37.
22.Buneeva, O. A., Kopylov, A. T., Tikhonova, O. V.,
Zgoda, V. G., Medvedev, A. E., and Archakov, A. I. (2012) Effect of
affinity sorbent on proteomic profiling of isatin-binding proteins of
mouse brain, Biochemistry (Moscow), 77, 1326-1338.
23.Hirayama, K., Aoki, S., Nishikawa, K., Matsumoto,
T., and Wada, K. (2007) Identification of novel chemical inhibitors for
ubiquitin C-terminal hydrolase-L3 by virtual screening, Bioorg. Med.
Chem., 15, 6810-6818.
24.Zhang, P., Bi, C., Schmitt, S. M., Li, X., Fan,
Y., Zhang, N., and Dou, Q. P. (2014) Metal-based 2,3-indolinedione
derivatives as proteasome inhibitors and inducers of apoptosis in human
cancer cells, Int. J. Mol. Med., 34, 870-879.
25.Prut, L., and Belzung, C. (2003) The open field
as a paradigm to measure the effects of drugs on anxiety-like
behaviors: a review, Eur. J. Pharmacol., 463, 3-33.
26.Deacon, R. M. (2013) Measuring motor coordination
in mice, J. Vis. Exp., 75, e2609.
27.Medvedev, A. E., Rajgorodskaya, D. I., Gorkin, V.
Z., Fedotova, I. B., and Semiokhina, A. F. (1992) The role of lipid
peroxidation in the possible involvement of membrane-bound monoamine
oxidases in gamma-aminobutyric acid and glucosamine deamination in rat
brain: focus on chemical pathogenesis of experimental audiogenic
seizures, Mol. Chem. Neuropathol., 16, 187-201.
28.Medvedev, A. E., Kirkel, A. Z., Kamyshanskaya, N.
S., Axenova, L. N., Moskvitina, T. A., Gorkin, V. Z., Andreeva, N. I.,
Golovina, S. M., and Mashkovsky, M. D. (1994) Inhibition of monoamine
oxidase by novel antidepressant tetrindole, Biochem. Pharmacol.,
47, 303-308.
29.The UniProt Consortium (2015) UniProt: a hub for
protein information, Nucleic Acids Res., 43,
D204-D212.
30.Jensen, J. L., Kuhn, M., Stark, M., Chaffron, S.,
Creevey, C., Muller, J., Doerks, T., Julien, P., Roth, A., Simonovic,
M., Bork, P., and Von Mering, C. (2009) STRING 8 – a global view
on proteins and their functional interactions in 630 organisms,
Nucleic Acids Res., 37, D412-416.
31.Ayala, A., Venero, J. L., Cano, J., and Machado,
A. (2007) Mitochondrial toxins and neurodegenerative diseases,
Front. Biosci., 12, 986-1007.
32.Cannon, J. R., and Greenamyre, J. T. (2010)
Neurotoxic in vivo models of Parkinson’s disease recent
advances, Prog. Brain Res., 184, 17-33.
33.Choi, Y. S., Hoon Jeong, J., Min, H. K., Jung, H.
J., Hwang, D., Lee, S. W., and Pak, Y. K. (2011) Shot-gun proteomic
analysis of mitochondrial D-loop DNA binding proteins: identification
of mitochondrial histones, Mol. BioSystems, 7,
1523-1536.
34.Cascone, A., Bruelle, C., Lindholm, D., Bernardi,
P., and Eriksson, O. (2012) Destabilization of the outer and inner
mitochondrial membranes by core and linker histones, PLoS One,
7, e35357.
35.Simo, S., and Cooper, J. A. (2013) Rbx2 regulates
neuronal migration through different Cullin 5-RING ligase adaptors,
Dev. Cell, 27, 399-411.
36.Yamada, M., Kida, K., Amutuhaire, W., Ichinose,
F., and Kaneki, M. (2010) Gene disruption of caspace-3 prevents
MPTP-induced Parkinson’s disease in mice, Biochem. Biophys.
Res. Commun., 402, 312-318.
37.Conn, K. J., Gao, W., McKee, A., Lane, M. S.,
Ullmana, M. D., Eisenhauera, P. B., Finea, R. E., and Wellsa, J. M.
(2004) Identification of the protein disulfide isomerase family member
PDIp in experimental Parkinson’s disease and Lewy body pathology,
Brain Res., 1022, 164-172.
38.Bhattacharya, S. K., Clow, A., Przyborowska, A.,
Halket, J., Glover, V., and Sandler, M. (1991) Effect of aromatic
acids, pentylene tetrazole and yohimbine on isatin and tribulin
activity in rat brain, Neurosci. Lett., 132, 44-46.
39.Reinoso, R. F., Telfer, B. A., and Rowland, M.
(1997) Tissue water content in rats measured by desiccation, J.
Pharmacol. Toxicol. Methods, 38, 87-92.
40.Panova, N. G., Zemskova, M. A., Axenova, L. N.,
and Medvedev, A. E. (1997) Does isatin interact with rat brain
monoamine oxidases in vivo? Neurosci. Lett., 233,
58-60.
41.Melamed, E., Youdim, M. B. H., Rosenthal, J.,
Spanier, I., Uzzan, A., and Globus, M. (1985) In vivo effect of
MPTP on monoamine oxidase activity in mouse striatum, Brain
Res., 359, 360-363.
42.Cane, A., Tournaire, M. C., Barritault, D., and
Crumeyrolle-Arias, M. (2000) The endogenous oxindoles 5-hydroxyoxindole
and isatin are antiproliferative and proapoptotic, Biochem. Biophys.
Res. Commun., 276, 379-384.
43.Igosheva, N., Lorz, C., O’Conner, E.,
Glover, V., and Mehmet, H. (2005) Isatin, an endogenous monoamine
oxidase inhibitor, triggers a dose- and time-dependent switch from
apoptosis to necrosis in human neuroblastoma cells, Neurochem.
Int., 47, 216-224.
44.Song, J., Hou, L., Ju, C., Zhang, J., Ge, Y., and
Yue, W. (2013) Isatin inhibits proliferation and induces apoptosis of
SH-SY5Y neuroblastoma cells in vitro and in vivo, Eur.
J. Pharmacol., 702, 235-241.
45.Ma, Z., Hou, L., Jiang, Y., Chen, Y., and Song,
J. (2014) The endogenous oxindole isatin induces apoptosis of MCF-7
breast cancer cells through a mitochondrial pathway, Oncol.
Rep., 32, 2111-2117.
46.Gatto, M., Iaccarino, L., Ghirardello, A., Bassi,
N., Pontisso, P., Punzi, L., Shoenfeld, Y., and Doria, A. (2013)
Serpins, immunity and autoimmunity: old molecules, new functions,
Clin. Rev. Allergy Immunol., 45, 267-280.
47.Kroeger, H., Miranda, E., MacLeod, I., Perez, J.,
Crowther, D. C., Marciniak, S. J., and Lomas, D. A. (2009) Endoplasmic
reticulum-associated degradation (ERAD) and autophagy cooperate to
degrade polymerogenic mutant serpins, J. Biol. Chem.,
284, 22793-22802.
48.Hamerman, J. A., Hayashi, F., Schroeder, L. A.,
Gygi, S. P., Haas, A. L., Hampson, L., Coughlin, P., Aebersold, R., and
Aderem, A. (2002) Serpin 2a is induced in activated macrophages and
conjugates to a ubiquitin homolog, J. Immunol., 168,
2415-2422.
49.Zhang, H., Wang, Y., Li, J., Yu, J., Pu, J., Li,
L., Zhang, S., Peng, G., Yang, F., and Liu, P. (2011) Proteome of
skeletal muscle lipid droplet reveals association with mitochondria and
apolipoprotein A-I, J. Proteome Res., 10, 4757-4768.
50.Matsunaga, T., Iguchi, K., Nakajima, T., Koyama,
I., Miyazaki, T., Inoue, I., Kawai, S., Katayama, S., Hirano, K.,
Hokari, S., and Komoda, T. (2001) Glycated high-density lipoprotein
induces apoptosis of endothelial cells via a mitochondrial dysfunction,
Biochem. Biophys. Res. Commun., 287, 714-720.
51.Baines, C. P., Zhang, J., Wang, G., Zheng, Y.,
Xiu, J. X., Cardwell, E. M., Bolli, R., and Ping, P. (2002)
Mitochondrial PKCε and MAPK form signaling modules in the
murine heart, Circ. Res., 90, 390-397.
52.Kikuchi, H., Yuan, B., Yuhara, E., Takagi, N.,
and Toyoda, H. (2013) Involvement of histone H3 phosphorylation through
p38 MAPK pathway activation in casticin-induced cytocidal effects
against the human promyelocytic cell line HL-60, Int. J. Oncol.,
43, 2046-2056.
Supplementary Materials (PDF)